Abstract
Purpose
There has been an increasing interest in the evaluation of metal ion concentration, present in different body fluids. It is known that metal ions, especially zinc play vital role in the fertility of human semen.
Objective
The main objective of the study is to evaluate the Zn concentration in Normospermia samples by Atomic absorption spectroscopy (AAS) and to predict the same by artificial neural network (ANN).
Materials and methods
Normospermia semen samples were collected from the patients who came to attend semen analysis at Bangalore assisted conception centre, Bangalore, India. Semen analysis was done according to World Health Organization (WHO) guidance. Atomic absorption spectroscopy was used to estimate the total Zn in these samples, while the Back propagation neural network algorithm (BPNN) was used to predict the Zn levels in these samples.
Results
Zinc concentration obtained by AAS and BPNN indicated that there was a good correlation between the estimated and predicted values and was also found to be statistically significant.
Conclusion
The BPNN algorithm developed in this study could be used for the prediction of Zn concentration in human Normospermia samples.
Future perspective
The algorithm could be further developed to predict the concentration of all the trace elements present in human seminal plasma of different infertile categories.
Keywords: Spermatozoa, Seminal plasma, Zinc, Artificial neural network, Atomic absorption spectroscopy, Back propagation neural network
Introduction
Analysis of trace elements, especially zinc (Zn), in different body fluids has been one of the areas of focus because of their correlation to the human health. It is known that certain elements play foremost role in a variety of biochemical process that determine the welfare of living organisms. Depending on the concentration of these trace metal ions, they could be either beneficial or injurious [24]. Seminal plasma is the secretion by the sexual accessory glands at the time of ejaculation to support spermatozoa. It contain proteins, including enzymes like acid phosphatase, alanine transaminase, alkaline phosphatase, aspartate transaminase in addition to lipids, macroelements like sodium, potassium, calcium, magnesium, phosphate, chloride, and microelements like copper, iron and zinc [14]. Zn has been shown to be obligatory to maintain the structure and function of a large number of macromolecules and more than 300 enzymes [25]. This divalent metal ion has both catalytic and structural role in enzymes [23]. Zn is present at high concentrations in human seminal plasma at a mean concentration of 2 mM which is 100 times higher than the concentration in serum [7]. The concentration of Zn in human seminal plasma is higher than in any other tissues [22]. Evaluation of Zn concentration in human seminal plasma was found to be one of the diagnostic measures for human male infertility. Zinc has an imperative responsibility in testis development, sperm as well as semen physiologic functions. Decrease in the level of Zn concentration causes hypogonadism, inadequate development of secondary sexual characteristics, and atrophy of semniferous tubules that could possibly lead to failure in spermatogenesis [10,13].
Total content of Zn in mammalian semen is elevated and has been found to be decisive to Spermatogenesis, but there have been contradictory reports on the effect of seminal Zn on sperm quality. Some studies indicated that there is no significant difference between Zn content in fertile and infertile men [3,5,11,27]. Zn in seminal plasma was proposed to stabilize the cell membrane and nuclear chromatin of sperm [6]. There is extensive evidence that human seminal Zn has an important role in the physiologic functions of sperm and that reduced levels result in low quality of sperm and reduced chances of fertilization [19].
The objective of the current study is to develop a method to predict the concentration of Zn in human seminal plasma. An Artificial Neural Network (ANN) consists of networks of interrelated neural computing rudiments that symbolize the manoeuvre of the human central nervous system. ANNs have found all-embracing use as highly flexible modeling tools due to their malleability and non-linear universal mapping approximations. ANNs find use in the study of an ample range of struggle that includes the modelling, inference, forecast, prediction, optimization, diagnosis, and adaptive control of complex non-linear system. Back propagation Neural networks (BPNN), are particularly useful for application to problems that are amenable to biological samples. An MLP (multilayer perceptron) consists of multiple layers of neurons; generally three with an input layer that receives external inputs, one hidden layer, and an output layer which generates the classification results [4,20]. In this article we describe the construction of an artificial neural network (ANN), specifically back propagation neural network (BPNN), which could be used for predicting the Zn concentration in human seminal plasma with special reference to Normospermia samples.
Materials and methods
Semen collection
Semen samples were collected from the 41 Normospermia patients, who attended the semen analysis at Bangalore Assisted Conception Centre Private Limited, Bangalore, Karnataka, India. Samples were collected through masturbation in a clean, sterile and wide-mounted container made up of plastic which is confirmed as non-toxic for spermatozoa. The sample container was kept at 37 ºC. After collection, the specimen was labelled with name of the donor, identification number, date and time of collection. The semen container was placed in the incubator at 37 ºC while the semen liquefies.
Normospermia
When the quality of the semen is normal as per WHO guideline it is said to be Normospermia [28]. The criteria to identify the Normospermia condition were as follows: The sample should liquefy within 20–60 min, and the pH should be between 7.2 and 8.0. Volume of the sample was not considered as a factor. Sample with more than 20 mil/ml of sperm concentration with 50 % total motility is considered as Normospermia.
Research ethics
Ethical approval and clearance to work on human semen samples was obtained from VIT Human Ethical Committee (Ref. No. VIT/UHEC-3/NO.11).
Initial macroscopic analysis
Liquefied semen samples were used to measure the semen volume and pH. Volume of the semen sample was calculated from the weight of the sample. The density of the semen was taken as 1 g/ml (WHO). Semen pH was determined by using pH paper strips.
Initial microscopic analysis
Sperm count was done with the help of phase-contrast microscope by placing a drop of semen sample on haemocytometer and the preparation was done under a total magnification of 100. Sperm motility within semen was assessed immediately after liquefaction of the sample by using Computer-Aided Sperm Analysis (CASA). The semen samples were mixed well and allowing no time for spermatozoa to settle out of suspension. Remix the semen sample before removing a replicate aliquot. Wait for the sample to stop drifting. Examine the slide with phase-contrast optics at ×400 magnifications. Approximately 200 spermatozoa were assessed per replicate for determining the percentage of different motile categories. Spermatozoa moving actively, either linearly or in a large circle, regardless of speed were counted as progressive motile sperm. All other patterns of motility with an absence of progression, which swims in small circles, were counted as non-progressive motile sperm. The sperm without any movement were counted as immotile.
To examine the morphology of sperm present in semen sample, feathering technique was employed as per WHO protocol. The glass slides were cleaned with 70 % ethanol and dried. About 5–10 μl of semen sample was placed at one end of a slide whereby the edge of second slide was used to drag semen along the surface of first slide. The slides were allowed to dry in air for 3 min and then fixed with Diff Quick fixative. The stained slide was examined under microscope with 100X magnification by oil immersion.
Hypo-osmotic swelling test (HOS) was used to access the vitality. Swelling solution was prepared by dissolving 0.735 g of sodium citrate dehydrate and 1.351 g of D-fructose in 100 ml of purified, sterile water. One ml of swelling solution was incubated in a closed micro centrifuge tube at 37 ° C for 5 min. An aliquot (10 μl) was transferred to a clean slide and examined using a phase- contrast microscope (200× magnification). The number of unswollen and swollen cells was counted with the help of a laboratory counter.
Evaluation of Zn concentration in seminal plasma
Initially semen samples were centrifuged at 800 g for 10 min at 4 º C. The supernatant was transferred to the other Eppendrorf tube and centrifuged at 800 g for 10 min to completely remove any debris or spermatozoa. The samples were then taken for the estimation of Zn concentration by using Atomic Absorption Spectroscopy (AAS). ZnCl2 (Hi-Media) was used to plot the standard curve. Dilution of the test solutions, if needed, were done using Millipore distilled water and then used for evaluation of its Zn concentration. All graphs and statistical analysis were done using Graph pad Prism version 5.0.
Artificial neural network
Artificial Neural Network (ANN) is an advanced computing tool that processes information using neurocomputing technique. This is different from conventional computation. ANN has been shown to be highly flexible modelling tool with capability of learning the mathematical mapping between input and output. ANN is composed of layers of neurons. The input layer of neurons is connected to the output layers of neurons through one or more hidden layers of neurons. Initially, ANN was trained and tested with experimental data (obtained from the results of atomic absorption spectroscopy) to reach at an optimum topology and weights. A multilayer perceptron (MLP) is feed forward neural network with one or more hidden layers. During the training process ANN adjusts its weights to minimize the errors between the predicted result and actual output by using back-propagation algorithm.
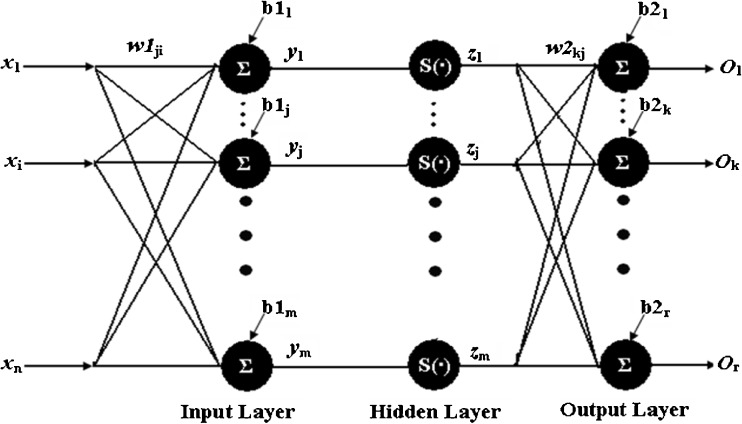
A schematic representation of the Back-Propagation Neural Network (BPNN) with n input nodes, r output nodes and a single hidden layer of m nodes are shown in Fig. 1. Each interconnection between the nodes has a weight associated with it. The input nodes have a transfer function of unity and the activation function of the hidden and output nodes are sigmoidal S(•) and linear, respectively.
Fig. 1.
Schematic Diagram of BPNN. A schematic diagram of a Back-propagation Neural Network (BPNN) with n inputs nodes, r outputs nodes and a single hidden layer of m nodes are shown in Fig. 1. Each interconnection between the nodes has a weight associate with it. The input nodes have a transfer function of unity and the activation function of the hidden and output nodes are sigmoid S(•) and linear, respectively
According to Fig. 1 the net input to the jth hidden neuron is given by
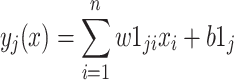 |
1 |
where wlji is the weight between the ith node of input layer and jth node of hidden layer and blj is the bias at jth node of hidden layer. The output of the jth hidden node is defined by
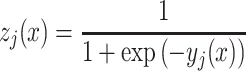 |
2 |
Given an input vector x, the output, value ok(x) of the kth node of output layer is equal to the sum of the weighted outputs of the hidden nodes and the bias of the kth node output layer, and is given by
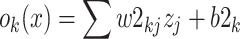 |
3 |
where w2kj is the weight between the jth node of hidden layer and kth node of output layer, b2k is biasing term at the kth output node, b2k is the biasing term at the kth node of output layer.
The output of ANN was determined by giving the inputs and computing the output from various nodes activation and interconnection weights. The output was compared to the experimental output and Mean Squared Error was calculated. The error value was then propagated backwards through the network and changes were made to the weights at each node in each layer. The whole process was repeated, in a iterative fashion, until the overall error value drops below a predetermined threshold. At this point, it is said that the ANN has learnt the system ‘good enough’—the network will never exactly learn the ideal function, but rather it will asymptotically approach the ideal function.
Results and discussion
Preliminary analysis of the semen samples
Semen parameters used for semen analysis were done as per the WHO guidelines [28] and is given in Table 1. The volume of Normospermia samples were ranging from 1.3 ml to 7.7 ml. The pH was found to be slightly alkaline but it was never acidic. Motility on an average was found to be 68 %, out of which about 26 % were found to be progressively moving. As more than 50 % of the samples indicate high motility the samples chosen are definite fertile and are therefore belong to the class of Normospermia. The results of this study correlated with that of Adamopoulos and Deliyiannis [1] with a P-value less than 0.0001, which indicates high significance of the data.
Table 1.
Semen parameter analysis for Normospermia Samples (N = 41). The minimial criteria to be satisfied by the semen parameters as given by the WHO guidelines is presented for reference
| Parameter of experiment | Minimal criteria for Normospermia (WHO) | Mean ± Standard error of mean (Present study) |
|---|---|---|
| Volume in ml | 2 | 2.620 ± 0.192 |
| pH | 7.2 | 7.624 ± 0.018 |
| Sperm concentration (mil/ml) | 20 | 79.49 ± 6.311 |
| Total motility (%) | 50 | 69.83 ± 1.620 |
| Progressive motile (%) | 15 | 14.86 ± 1.133 |
| Normal morphology (%) | 15 | 22.71 ± 0.525 |
| Hypo-osmotic swelling (%) | 60 | 75.00 ± 1.523 |
The semen parameters include volume, pH, sperm concentration, total motility, progressive motile, normal morphology, HOS were taken as input parameters and their values were tabulated. The mean with standard error of mean were calculated and tabulated for the 41 population
Estimation of Zn concentration by AAS
The concentration of Zn in the 41 selected Normospermia samples were determined using Atomic absorption spectroscopy. The concentration of Zn was found to lie in the range of 0.23–2.30 mg/ml with a mean value of 0.849 mg/ml (Fig. 2). A small standard deviation associated with the concentration of the Zn present in the Normospermia samples indicates the vital role played by this trace element for the normal function of the human semen. Similar concentrations (0.28–2.5 mg/ml) of Zn have been reported by [7].
Fig. 2.
Comparison of Zn concentration between experimental and ANN (Prediction method). The concentration of Zn was evaluated by Atomic absorption spectroscopy and Artificial Neural Network. The concentration was compared between the groups and shows no significance difference between them. The mean concentration and standard error of mean for experimental was found to be 0.849 ± 0.072 mg/ml and for prediction method it was found to be 0.831 ± 0.077 mg/ml
Back propagation neural network
ANN has been used in the field of reproductive biology, to predict the results of Intrauterine insemination (IUI), Intracytoplasmic sperm injection (ICSI), and In vitro fertilisation (IVF) [15,16,26], and some researchers used to predict and access sperm morphology [17] and to predict the presence of sperm in non-obstructive azoospermia [18]. In the present study, the experimental values obtained for all the parameters were used to train the ANN.
The BPNN consists of three phases—namely the training, validation and test phases. The seven semen physical parameters (volume, pH, sperm concentration, Total motility, Rapid progressive, Normal Morphology, Hypo-osmotic swelling) determined for the samples used in the study were used as the input nodes and concentration of Zn in these samples was used as the output parameter. As there exists no proper rule for setting the exact number of neurons in the hidden layer to avoid over fitting or under fitting of the input parameters and to make the learning phase convergent, number of nodes in the hidden layer was selected through a trial and error method based on the number of epochs needed to train the network. After such iterative procedures it was found that the convergence between the experimental values and predicted values of Zn concentration was achieved with the inclusion of two hidden layers with seven and eight neurons. It could be observed from Fig. 3 that the mean square error values of the training data set could be reduced by the increase in the number of epochs used. However, it was found that a minimum value of 1,000 epochs was sufficient to get a reliable prediction. Such a convergence also indicates the learning behaviour of the proposed ANN model.
Fig. 3.
Learning behavior of BPNN. ANN performed well with 7 and 8 neurons in two hidden layers respectively at epoch 1,000. The figure represents how the developed BPNN was learned well and well in each step like training part, validating part and as well as test part
After the convergence of the experimental and predicted values, the number of hidden layers and epochs were fixed so as to validate the authenticity of the model. It could be seen from Fig. 4 that the percentile error associated with the predicted values in comparison with the experimental values are in the range of – 0.2–0.3, indicating the validity of the proposed model.
Fig. 4.

Error of Predicted Data (N = 41). The error of all predicted data were clearly shown in the figure. Negative error is slightly lower than positive error. The percentile errors for all the data were found to be acceptable
Table 2 lists the randomly chosen test data set (out of the 41 samples). Of the seven data sets, five data sets exhibited less than 10 % error between the predicted and experimental value. Furthermore, the average Zn concentration for the entire 41 test samples used in the study was predicted to be 0.831 with an error value of 0.077, indicating the accuracy of prediction (Fig. 2). The concurrence of the experimental and the predicted values of the Zn concentration were statistically significant with a P-value of less than 0.0001.
Table 2.
Input, output parameters and their error for testing data
| Input semen parameters | Output parameter (Zn concentration mg/ml) | Error of output | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Volume in ml | pH | Sperm concentration (mil/ml) | Total motility (%) | Rapid progressive (%) | Normal morphology (%) | HOS (%) | Actual value | Predicted value | Absolute error | Error in % |
| 1 | 3 | 7.8 | 35 | 58.9 | 21.6 | 22 | 63 | 0.523 | 0.563 | 0.040 | 7.74 |
| 2 | 2 | 7.5 | 76 | 53.1 | 4.4 | 20 | 58 | 0.891 | 0.948 | 0.057 | 6.46 |
| 3 | 3.4 | 7.6 | 117 | 82.9 | 22.5 | 23 | 87 | 0.861 | 0.861 | 0.0008 | 0.09 |
| 4 | 2 | 7.6 | 47 | 62.2 | 9.2 | 22 | 67 | 0.623 | 0.703 | 0.080 | 12.9 |
| 5 | 2.6 | 7.7 | 70 | 50.4 | 4.6 | 20 | 56 | 0.782 | 0.806 | 0.024 | 3.06 |
| 6 | 1.5 | 7.6 | 45 | 72.5 | 4.9 | 21 | 74 | 0.824 | 0.848 | 0.023 | 2.89 |
| 7 | 2.3 | 7.6 | 35 | 70.1 | 17.9 | 22 | 72 | 0.754 | 0.554 | 0.2 | 26.51 |
Apart from training data, testing data were used to test the neural network, how well it is working. The input parameters, the output parameter with actual and predicted data were tabulated. The error in percentile was calculated between the experimental and predicted method, and the error was found to be acceptable
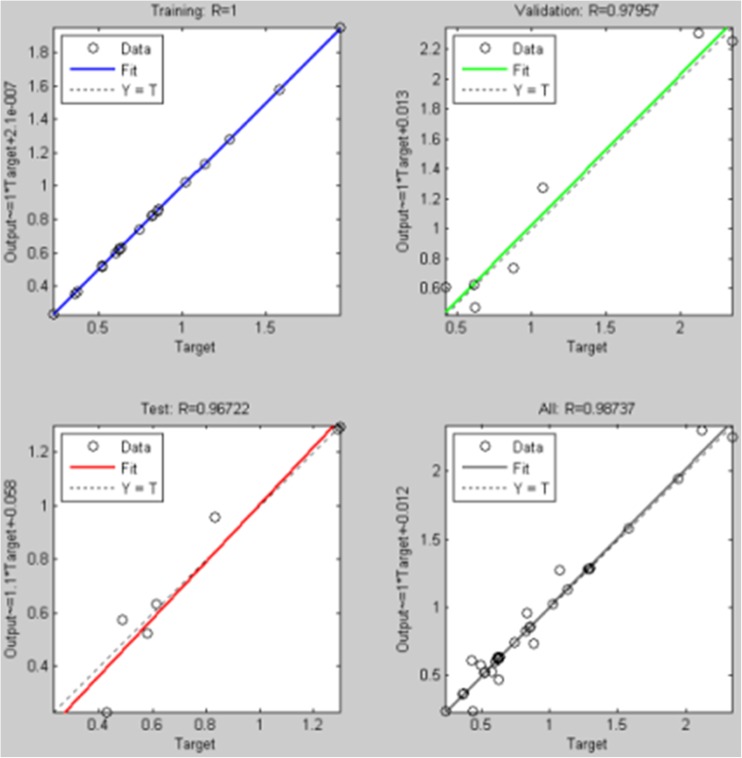
The performance of a trained network can be observed to some extent by the errors on the training, validation and test data sets, but it is often useful to investigate the network response in more detail. We have performed a regression analysis between the network response and the corresponding targets. The entire data set (training, validation and test) is put through the ANN and a linear regression between the network outputs and the corresponding targets was performed. As per Fig. 5, R-value was 1, 0.97957, 0.96722 and 0.98737 for training data set, validation data set, testing data set and entire data set respectively. The outputs seem to track the targets reasonably well and R-value are almost 0.9. Therefore, there is perfect correlation between targets and outputs.
Fig. 5.
The performance of individual data sets: The performance of a trained network can be observed to some extent by the errors on the training, validation and test data sets, but it is often useful to investigate the network response in more detail. We have performed a regression analysis for Zn concentration between the ANN response and AAS
Artificial neural networks, with their pattern recognition and modelling capabilities have been successfully used in the field of biomedicine to help in the diagnostic of hepatobilary disorders [12], coronary disorders [2], estimation of lead concentration in grasses [9], prediction of membrane fouling during nanofiltration of ground and surface water [21]. Recently, Gil et al. [8] have evaluated the performance of various artificial networks and its application in the prediction of male fertility potential, aiming at evaluating the semen quality and also used non linear statistical techniques that may allow a better approach to address the complexity of the problem. However, to our knowledge, this is first report on the use of ANN for the accurate prediction of Zn concentration in fertile human semen samples.
Conclusion
The concentration of Zn in human seminal plasma plays an ample role in reproductive biology. The deficiency in Zn concentration leads to lot of complications on physiology of human reproduction. The developed BPNN indicates great potential in predicting the concentration of Zn in human semen for Normospermia samples in terms of sensitivity, precision and speed. The method could be extended for predicting the Zn concentration in infertile samples and could be as will be trained for the prediction of any other parameter in the human semen.
Acknowledgments
The authors wish to acknowledge the management of both the institutes, VIT University and BACC, for support towards the execution of this study.
Footnotes
Capsule
Back propagation neural network can be used for predicting the concentration of Zn in normospermia human semen samples.
References
- 1.Adamopoulos DA, Deliyiannis V. Seminal plasma magnesium, calcium and inorganic phosphate concentration in normozoospermic and subfertile men. Andrologia. 1983;15:648–654. doi: 10.1111/j.1439-0272.1983.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 2.Azuaje F, Dubitzky W, Lopes P, Black N, Adamson K, Wu X, White JA. Artif Intell Med. 1999;15:275. doi: 10.1016/S0933-3657(98)00058-X. [DOI] [PubMed] [Google Scholar]
- 3.Bakalczuk S, Robak-Cholubek D, Jakiel G, Krasucki W. Level of zinc and magnesium in semen taken from male partners of married infertile couples. Ginekol Pol. 1994;65:67–70. [PubMed] [Google Scholar]
- 4.Bishop CM. Neural networks for pattern recognition. Oxford Univ Pr; 2005.
- 5.Carpino A, Siciliano L, Petroni MF, De Stefano C, Aquila S, Ando S, et al. Low seminal zinc bound to high molecular weight proteins in asthenozoospermic patients: evidence of increased sperm zinc content in oligoasthenozoospermic patients. Hum Reprod. 1998;13:111–114. doi: 10.1093/humrep/13.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Caldamone AA, Freytag MK, Cockett AT. Seminal zinc and male infertility. Urology. 1979;13:280–281. doi: 10.1016/0090-4295(79)90421-7. [DOI] [PubMed] [Google Scholar]
- 7.Colagar AH, Marzony ET, Chaichi MJ. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res. 2009;29:82–88. doi: 10.1016/j.nutres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Gil D, Girela JL, De Juan J, Jose Gomez-Torres M, Johnsson M. Predicting seminal quality with artificial intelligence methods. Expert Syst Appl. 2012;39:12564–12573. doi: 10.1016/j.eswa.2012.05.028. [DOI] [Google Scholar]
- 9.Dimopoulos I, Chronopoulos-Serelia JA, Lek S. Neural network models to study relationships between lead concentration in grasses and permanent urban descriptors in Athens city (Greece) Ecol Model. 1999;120:157–165. doi: 10.1016/S0304-3800(99)00099-X. [DOI] [Google Scholar]
- 10.Ebisch IMW, Thomas CMG, Peters WHM, Braat DD, Steegers-Theunissen RPM. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2007;13:163–174. doi: 10.1093/humupd/dml054. [DOI] [PubMed] [Google Scholar]
- 11.Fuse H, Kazama T, Ohta S, Fujiuchi Y. Relationship between zinc concentrations in seminal plasma and various sperm parameters. Int Urol Nephrol. 1999;31:401–408. doi: 10.1023/A:1007190506587. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi Y, Setiono R, Yoshida K. Artif Intell Med. 2000;20:205. doi: 10.1016/S0933-3657(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 13.Ho E, Ames BN. Low intracellular Zinc induces oxidative DNA damage, disrupts P53, NFκB, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Cell Biol. 2002;99:16770–16775. doi: 10.1073/pnas.222679399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston SD, Osborne CA, Lipowitz AJ. Characterization of seminal plasma, prostatic fluid, and bulbourethral gland secretions in the domestic cat. In: Proceedings of the 11th International Congress on Animal Reproduction and Artificial Insemination, Dublin, 1988;4:560.
- 15.Kaufmann SJ, Eastaugh JL, Snowden S, Smye SW, Sharma V. The application of neural networks in predicting the outcome of in-vitro fertilization. Hum Reprod. 1997;12(7):1454. doi: 10.1093/humrep/12.7.1454. [DOI] [PubMed] [Google Scholar]
- 16.Kshirsagar AV, Murthy L, Chelu L, Lamb D, Ross L, Niederberger C. Predicting outcomes for intracytoplasmic sperm injection. Fertil Steril. 2005;84:274. doi: 10.1016/j.fertnstert.2005.07.712. [DOI] [Google Scholar]
- 17.Linneberg C, Salamon P, Svarer C, Hansen LK, Meyrowitsch J. Towards semen quality assessment using neural networks. In Neural networks for signal processing [1994] IV. Proceedings of the 1994 IEEE workshop (pp. 509–517). IEEE; 1994.
- 18.Ma Y, Chen B, Wang HX, Hu K, Huang YR. Prediction of sperm Retrieval in men with non-obstructive azoospermia using artificial neural networks: leptin is a good assistant diagnostic marker. Hum Reprod. 2011;26(2):294. doi: 10.1093/humrep/deq337. [DOI] [PubMed] [Google Scholar]
- 19.Marmar JL, Katz S, Praiss DE, De Benedictis TJ. Semen zinc levels in infertile and post vasectomy patients and patients with prostatitis. Fertil Steril. 1975;26:1057–1063. doi: 10.1016/s0015-0282(16)41470-6. [DOI] [PubMed] [Google Scholar]
- 20.Ripley BD. Pattern recognition and neural networks. Cambridge University Press; 1996.
- 21.Shetty GR, Chellam S. Predicting membrane fouling during municipal drinking water nanofiltration using artificial neural Networks. J Membrane Sci. 2003;217:69–86. doi: 10.1016/S0376-7388(03)00075-9. [DOI] [Google Scholar]
- 22.Sorensen MB, Stoltenberg M, Danscher G, Ernst E. Chelating of intracellular zinc ions affects human sperm cell motility. Mol Hum Reprod. 1999;5:338–341. doi: 10.1093/molehr/5.4.338. [DOI] [PubMed] [Google Scholar]
- 23.Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003;57:399–411. doi: 10.1016/S0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 24.Tsalev DL, Zaprianov ZK. Atomic absorption spectrometry in occupational and environmental health practice, Analytical Aspects and Health Significance, vol. I. USA: CRC Press Inc; 1983. [Google Scholar]
- 25.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.2466/pr0.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 26.Wald M, Sparks AET, Sandlow J, Van-Voorhis B, Syrop CH, Niederberger CS. Computational models for prediction of ivf/icsi outcomes with surgically retrieved spermatozoa. Reprod BioMed Online. 2005;11(3):325–331. doi: 10.1016/S1472-6483(10)60840-1. [DOI] [PubMed] [Google Scholar]
- 27.Wong WY, Flik G, Groenen PM, Swinkels DW, Thomas CM, Copius-Peereboom JH, et al. The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod Toxicol. 2001;15:131–136. doi: 10.1016/S0890-6238(01)00113-7. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 2010; 5th eds. pp 7–36, 56–102.