Abstract
Purpose
Surgical repair of varicocele has long been a procedure to correct spermatogenesis. However, the outcome has been reported to be inadequate. We combined varicocelectomy with supplement therapy to evaluate the concurrent effect of these procedures.
Methods
A prospective randomized controlled study was undertaken to investigate the effects of zinc sulfate, folic acid and zinc sulfate/folic acid on sperm quality, protamine content and acrosomal integrity following surgical repair of varicocele. Male subjects with palpable varicocele were included in the study and randomized into four groups. Subjects received Zinc sulfate, Follic acid, Zinc sulfate/Follic acid or placebo for 6 months. A semen sample was obtained before surgery and 3 and 6 months after surgical repair. Semen samples were evaluated for sperm parameters as well as chromatin content and acrosomal integrity.
Results
Most of the evaluated parameters showed a mild improvement after varicocelectomy in the placebo group. Interestingly, co-administration of Zinc sulfate and folic acid improved most factors significantly. Folic acid administration but not zinc sulfate could increase sperm number. Hence, Zinc sulfate was better than folic acid when change in morphology was assessed, and none of them was significantly effective in sperm motility. In Zinc sulfate and Follic acid groups, protamine content and halo formation rate significantly improved.
Conclusions
We may conclude that co-administration of zinc and folic acid significantly improved sperm parameters and increased varicocelectomy outcomes. So, medical treatment with compatible drugs after surgery might be advantageous for obtaining acceptable results.
Keywords: Zinc sulfate, Folic acid, Sperm parameters, Protamine, Acrosomal integrity, Varicocelectomy
Introduction
Varicocele, a vascular lesion of the pampiniform plexus in the spermatic cord caused by melancholic blood was first described by the French surgeon Ambroise Pare in the 16th century. Later on a British surgeon, Barfield proposed a relationship between infertility and varicocele [1], and there is currently much debate about pathophysiology of deleterious effects of varicocele on testes and epididymis [2]. However several possible mechanisms have been proposed, such as failure of testicular growth and development of testes, impairment of spermatogenesis due to increase in intrascrotal temperature, oxidative stress, and leydig cell dysfunction [3].
Varicocele can be treated by surgical intervention (varicocelectomy) which is still the most popular treatment [4]. Varicocelectomy can restore testicular volumes and semen parameters [5]. However, there is no strong evidence whether the treatment of varicocele with surgery in subfertile males could improve the spontaneous pregnancy rates. Molecular and genetic studies have provided further insights into the pathogenesis of infertility in patients with varicocele [6]. Recent molecular studies have put great emphasis on the role of oxidative stress on the pathogenesis of infertility in patients with varicocele [7]. Alterations in the testicular microenvironment [8] and hemodynamic can increase production of reactive oxygen species (ROS) and decrease the local antioxidant capacity, resulting in oxidative stress [7]. Infertile men with varicocele were found to have higher levels of ROS than healthy fertile individuals [9]. Human spermatozoa are particularly vulnerable to ROS injury owing to the excess polyunsaturated fatty acids in these cells [10]. Furthermore, lipid peroxidation of the plasma membrane affects the fluidity of the sperm plasma membrane and causes functional defects in sperm-oocyte fusion. Also, 4-hydroxy-2-nonenal, an aldehyde end product of lipid peroxidation, is an alkylating agent that damages DNA and forms adducts with proteins, playing a role in inducing apoptosis [11]. Additionally, a significant increase in abnormal sperm chromatin condensation was observed in infertile men with varicocele which could occur as a consequence of protamine deficiency [12]. Some studies have reported improved semen parameters [12, 13] and density [14] after varicocelectomy. However, the actual effect of adult varicocelectomy on male fertility still remains controversial [15, 16].
Another approach against varicocelectomy could be drug-base treatments [17] but according to our knowledge combine treatment of drug and varicocelectomy have not yet been investigated in subfertile men. Zinc is a micronutrient that serves as a cofactor for more than 80 metalloenzymes involved in DNA transcription and protein synthesis [18]. Moreover, zinc finger proteins are implicated in the genetic expression of steroid hormone receptors [19], and they also have antioxidant [20] and antiapoptotic properties [21]. Zinc concentrations are very high in the male genital organs particularly in the prostate which is largely responsible for the high zinc content of seminal plasma [18]. The impaired sex gland secretions in varicocele could influence seminal plasma zinc levels and the motility of ejaculated spermatozoa [22]. The micronutrient folate is also important for the synthesis of DNA and transfer of RNA. It has been reported that folic acid, the synthetic form of folate, effectively scavenges oxidizing free radicals and inhibits lipid peroxidation [23].
We co-administered zinc and folic acid following varicocelectomy in a double blind placebo controlled clinical trial study to investigate whether concurrent treatment with these drugs may affect sperm quality and improves the outcome of varicocelectomy considering sperm function.
Materials and methods
Materials
All the chemicals were purchased from Sigma-Aldrich Company (St. Louis, MO, USA) except those otherwise indicated.
Participants
This prospective randomized study was carried out at a university based laboratory and a private clinic, between May 2008 and November 2010. Approval for this investigation was obtained from the ethics committee for research involving human subjects at Kerman University of Medical Sciences, Kerman, Iran and a written informed consent was fulfilled and signed by each patient before participating in the study. The study was also registered at Iranian Registry of Clinical Trials (Irct registration number: IRCT138802261910N1 ). One hundred and sixty infertile subjects were enrolled in the present study who had already consulted with an urologist for varicocelectomy. The presence of a grade III varicocele, on I-III scale, was the criteria to enter the study. It was assessed by clinical parameters and was confirmed by Doppler ultrasound scanning. Patients with the evidence of leukocytospermia, low testicular volume <15 mL, congenital urogenital abnormalities and urogenital infections were excluded from the study.
Study design
Every participant was randomly allocated into one of the four experimental groups. Our randomization schedule was Zinc sulfate (ZS), Folic acid (FA), Zinc sulfate/Folic acid (ZF), and placebo (PL). The patients underwent surgical repair of varicocele, before which at least a semen sample was obtained for laboratory assessments. Patients in each group took orally one capsule per day after dinner following varicocelectomy for 6 month. The dosage of the zinc sulfate (Alhavi Pharmaceutical Co, Tehran, Iran) and folic acid (Iran Daru, Tehran, Iran) was 66 and 5 mg per capsule, respectively as suggested by Wong et al [24]. Patients in placebo group received the same capsules without the effective drug.
Semen collection and evaluation
Preoperatively, one sample was obtained from each participant for standard semen analysis according to WHO guidelines [25] and also 3 and 6 months postoperatively the other two samples were collected. The semen samples were provided by masturbation after an abstinence period of 3–5 days and delivered to the laboratory within 30 min after production. After liquefaction, the semen parameters including sperm number, sperm morphology, sperm motility and progressive sperm motility were assessed. An improved Neubauer haemacytometer was used for sperm count and was expressed as 106/mL of semen. Motility was evaluated by direct microscopic examination (magnification = ×400) and expressed as the percentage of motile sperm. Papanicolaou staining technique was used for evaluating sperm morphology according to WHO criteria. All sperm analyses were carried out by the same technician. Interobserver variation was evaluated several times during and before the study. Coefficient of variation was within the acceptable range of 0.5–10 %.
Chromomycin A3 (CMA3) staining
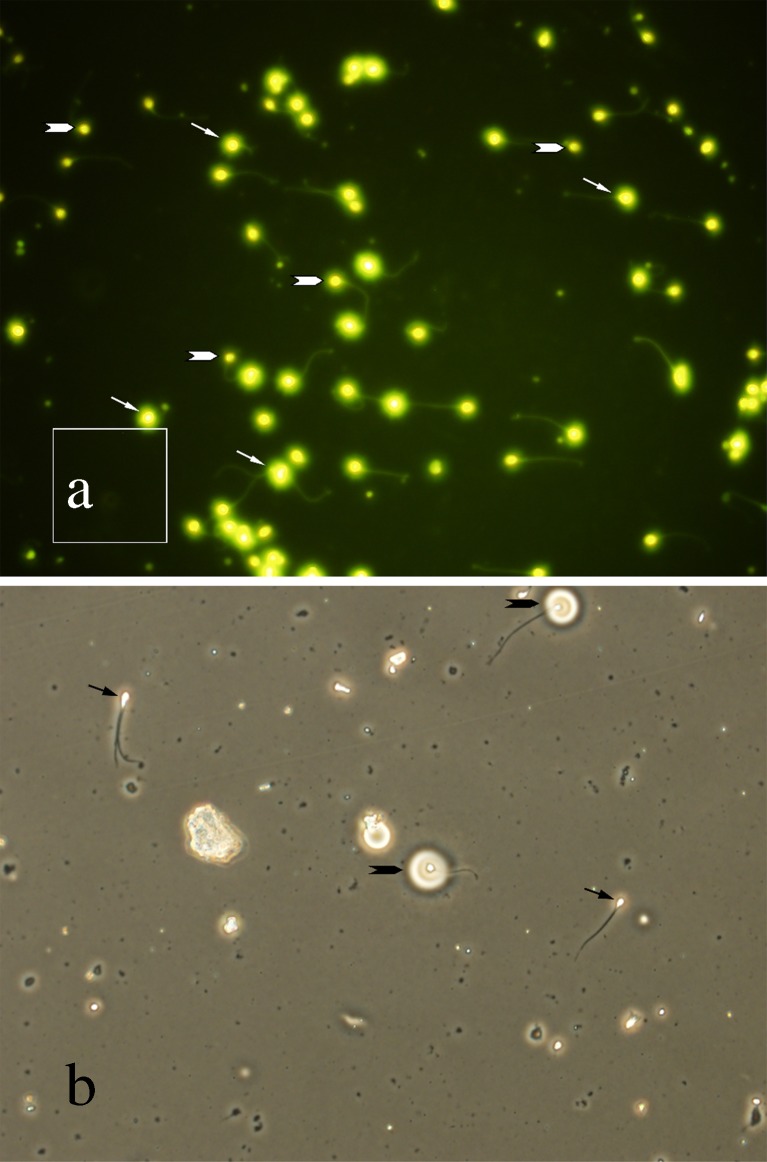
Semen smears were prepared from each samples and fixed in Carnoy’s solution [methanol: glacial acetic acid 3:1 (Merck, Germany)] at 4 °C for 5 min. Each slide was treated for 20 min with 100 μL of CMA3 solution (0.25 mg/mL in McIlvaine buffer, PH 7.0, containing 10 mM MgCl2). The slides were then rinsed in buffer and mounted with buffered glycerol (1:1). Microscopic analysis of the slides was performed using an Olympus inverted fluorescent microscope (IX71, Tokyo, Japan) with green filter. On each slide, 100 sperm cells were evaluated and those with bright fluorescence (Fig. 1a) were considered as CMA3 positive or with protamine deficiency [26].
Fig. 1.
a Sperm cells were stained by chromomycin A3 to evaluate protamine content of sperms. Arrows show CMA3 positive sperms and arrow heads show CMA3 negative sperms. b Sperms were layered on a thin film of gelatin to evaluate acrosomal integrity. Arrows show sperms with abnormal acrosomal activity and arrow heads show sperm with normal acrosomal activity resulting in a clear halo around sperm head
Gelatinolysis test
This test was carried out according to the method of Henkel et al., (1995). The test is based on the ability of acrosomal enzymes to hydrolyze a high molecular weight gelatin. Briefly, 20 μL of semen samples were diluted 1:10 in phosphate buffered saline (PBS; 34.22 mM NaCl, 20.8 mM Na2HPO4·2H2O, 1.42mM KH2PO4) containing 15.72 mM α-D-glucose (anhydrous; 280 mosmol/kg). Semen samples were smeared on pre-coated gelatin slides and incubated in a moist chamber at 37 °C for 2 h. The halo diameter (Fig. 1b) around any ten spermatozoa was measured under a phase contrast microscope (Olympus IX71) equipped with a digital camera and analysis software (Olympus Corporation, Tokyo, Japan). The halo formation rate was calculated per slide as the percentage of spermatozoa showing a halo. For this purpose 100 spermatozoa were evaluated in each slide.
Statistical analysis
Mixed model analysis was applied to investigate difference between treatment groups (between factor variable) and between time points (within factor variable) simultaneously. P-value <0.05 was considered as significance level. All analysis were done using SPSS software version 15 for Windows.
Results
The present investigation is a prospective clinical trial in which semen samples were obtained from 160 candidates being randomly allocated to four trial groups. Seventy percent of the 160 (112) individuals continued with the study and provided the semen and blood samples for assessment up to 6 months after surgery Age ranges and duration of infertility of male partners were from 20 to 43 (mean ± SD: 29.07 ± 6.8) and 1 to 10 years (mean ± SD: 3.32 ± 2.4) respectively. The mean volume of semen was 4 ± 1.9 ml. Other sperm parameters are represented in Tables 1, 2, and 3. The participants who received placebo improved in sperm parameters but the rate of sperm improvement did not reach the level of significance within 3 and 6 months after varicocelectomy compared with values obtained prior to the intervention. Most of the sperm parameters had nonsignificantly improved after 6 months. Folic acid administration significantly increased the sperm number 3 months after varicocelectomy compared to the values obtained before varicocelectomy (46.8 ± 8.3 vs. 27.1 ± 5.3, respectively) but zinc sulfate administration had improved sperm morphology 3 months post varicocelectomy compared with the morphology prior to the intervention (48.0 ± 2.3 vs. 40.0 ± 3.2, respectively). Interestingly, co-administration of zinc and folic acid for 3 months, caused a moderate improvement in sperm number, motility and sperm progression, and after 6 months the values for these parameters were significantly higher than the onset of experiments. This finding is in agreement with the study of Al Bakri et al., 2012 who showed that 6 months is an ideal time for sperm improvement following surgical repair of a varicocele [27].
Table 1.
The mean ± SEM of sperm number before varicocelectomy versus 3 and 6 month after varicocelectomy treatment following supplement therapy in the different randomized trial groups
| Groups | n | Sperm number (106/mL) | ||
|---|---|---|---|---|
| Before | 3 month | 6 month | ||
| ZF | 29 | 30.1 ± 5.5a | 42.6 ± 7.4ab | 47.6 ± 7.5b |
| FA | 26 | 27.1 ± 5.3a | 46.8 ± 8.3b | 49.1 ± 3.3b |
| ZS | 32 | 35.1 ± 6.7a | 41.5 ± 7.1a | 39.6 ± 5.4a |
| PL | 25 | 21.8 ± 3.5a | 24.6 ± 4.4a | 29.9 ± 6.6a |
In each row the values with different superscripts are significantly different (P < 0.05). ZF zinc sulfate and folic acid; FA folic acid; ZS zinc sulfate; PL placebo
Table 2.
The mean ± SEM of normal sperm morphology before varicocelectomy versus 3 and 6 month after varicocelectomy treatment following supplement therapy in the different randomized trial groups
| Groups | n | Normal sperm morphology | ||
|---|---|---|---|---|
| Before | 3 month | 6 month | ||
| ZF | 29 | 46.7 ± 2.7a | 49.9 ± 2.2ab | 56.6 ± 2.2b |
| FA | 26 | 52.1 ± 2.8a | 53.0 ± 2.8a | 53.7 ± 1.7a |
| ZS | 32 | 40.0 ± 3.2a | 48.0 ± 2.3b | 53.2 ± 2.7b |
| PL | 25 | 42.1 ± 4.8a | 38.1 ± 2.5a | 48.4 ± 9.9a |
In each row values with different superscripts are significantly different (P < 0.05). ZF zinc sulfate and folic acid; FA folic acid; ZS zinc sulfate; PL placebo
Table 3.
The mean ± SEM percentage of sperm motility and forward progressive motility before varicocelectomy versus 3 and 6 month after varicocelectomy treatment following supplement therapy in the different randomized trial groups
| Groups | n | Sperm motility (%) | Forward progressive motility (%) | ||||
|---|---|---|---|---|---|---|---|
| Before | 3 month | 6 month | Before | 3 month | 6 month | ||
| ZF | 29 | 42.6 ± 5.1a | 51.7 ± 3.2a | 52.4 ± 3.3a | 28.7 ± 4.3a | 37.9 ± 5.1b | 43.0 ± 5.6b |
| FA | 26 | 48.8 ± 4.0a | 53.3 ± 3.0a | 51.5 ± 2a | 40.1 ± 6.5a | 48.6 ± 6.4a | 40.0 ± 4.9a |
| ZS | 32 | 55.0 ± 4.3a | 48.9 ± 4.9a | 49.8 ± 2a | 35.2 ± 6.5a | 40.8 ± 6.3a | 42.3 ± 4.1a |
| PL | 25 | 37.6 ± 7.4a | 44.9 ± 6.6a | 49.8 ± 4.8a | 34.0 ± 9.7a | 34.1 ± 7.3a | 40.3 ± 6.8a |
In each row the values with different superscripts are significantly different (P < 0.05). ZF zinc sulfate and folic acid; FA folic acid; ZS zinc sulfate; PL placebo
Effects of zinc and folic acid versus placebo administration on protamine content of spermatozoa are presented in Table 4. Treatment of varicocelectomized subjects with zinc, folic acid and co-administration of zinc and folic acid significantly improved the rate of CMA3 positive sperms after 3 months but no further improvement was achieved after 6 months (ZF: 36.9 ± 2.2vs. 46.9 ± 3.1; FA:36.6 ± 2.6vs. 54.6 ± 4.0; ZS: 43.9 ± 3.0 vs 57.3 ± 3.1; P < 0.05, respectively)
Table 4.
The mean ± SEM percentage of chromomycin A3 positive spermatozoa before varicocelectomy versus 3 and 6 month after varicocelectomy followed by the supplement therapy in the different randomized trial groups
| Groups | n | Chromomycin A3 | ||
|---|---|---|---|---|
| Before | 3 month | 6 month | ||
| ZF | 29 | 46.9 ± 3.1a | 36.9 ± 2.2b | 33.0 ± 2.1b |
| FA | 26 | 54.6 ± 4.0a | 36.6 ± 2.6b | 38.2 ± 2.9b |
| ZS | 32 | 57.3 ± 3.1a | 43.9 ± 3.0b | 37.5 ± 2.0b |
| PL | 25 | 54.3 ± 2.8a | 45.6 ± 4.1a | 41.4 ± 3.3a |
In each row the values with different superscripts are significantly different (P < 0.05). ZF zinc sulfate and folic acid; FA folic acid; ZS zinc sulfate; PL placebo
Table 5 shows the halo formation rate and the mean halo diameters following treatments by zinc, folic acid or zinc/folic acid up to 6 month after varicocelectomy. No significant differences were observed before varicocelectomy compared to after varicocelectomy in any of the treatment groups and time points. However, the halo formation rate significantly increased after 3 month in all treatment groups when compared with placebo group (ZF: 49.2 ± 3.2 vs. 29.7 ± 3.2; FP: 50.8 ± 4.4vs. 31.0 ± 3.5; ZP: 43.3 ± 3.5vs. 23.1 ± 3.5; P < 0.05, respectively). No further improvement was detected after 6 months.
Table 5.
The mean ± SEM percentage of halo formation rate and the mean halo diameter (μm) before varicocelectomy versus 3 and 6 month after varicocelectomy following supplement therapy in the different randomized trial groups
| Groups | n | Halo formation rate (%) | Halo diameter (μm) | ||||
|---|---|---|---|---|---|---|---|
| Before | 3 month | 6 month | Before | 3 month | 6 month | ||
| ZF | 29 | 29.7 ± 3.2a | 49.2 ± 3.2b | 57.3 ± 3.2b | 5.9 ± 1.7a | 4.7 ± 0.2a | 5.3 ± 0.3a |
| FA | 26 | 31.0 ± 3.5a | 50.8 ± 4.4b | 54.1 ± 3.5b | 4.0 ± 0.2a | 5.0 ± 0.3a | 4.7 ± 0.1a |
| ZS | 32 | 23.1 ± 3.5a | 43.3 ± 3.5b | 42.5 ± 3.5b | 4.1 ± 0.3a | 4.3 ± 0.2a | 4.6 ± 0.2a |
| PL | 25 | 29.3 ± 4.0a | 32.1 ± 4.0a | 39.6 ± 4.0a | 3.4 ± 0.2a | 3.5 ± 0.3a | 4.2 ± 0.2a |
In each row the values with different superscripts are significantly different (P < 0.05). ZF zinc sulfate and folic acid; FA folic acid; ZS zinc sulfate; PL placebo
Discussion
Few studies have investigated the outcomes of varicocelectomy based on the clinical trials. In our randomized clinical trial, we found some noticeable impact on sperm parameters following varicocelectomy but it did not reach the level of significance. In contrast, administration of zinc, folic acid and especially zinc/folic acid significantly improved sperm parameters following 3 and 6 months of follow up. Reports on the rate of improvement in sperm parameters following varicocelectomy are somehow controversial. Although some studies have reported improvement in sperm characteristics, DNA fragmentation and pregnancy rate after varicocele repair [12, 13, 28] but some clinical investigations have claimed that varicocelectomy has yielded unsatisfactory results in infertile patients [15]. The challenge on the benefits of surgical repair of varicocele is partly related to the study design because most of these studies are case-control studies rather than clinical randomized ones. However a clinical investigation has reported that the pregnancy rate in a group of subclinical varicocelectomized subjects was not higher than that in an untreated group, although it partially improved seminal parameters [29]. We found a significant improvement following supplement therapy in varicocelectomized patients’ sperm parameters but change in the spontaneous pregnancy rate requires further researches. Disregarding the different believes about varicocelectomy and spermatogenesis, many reproductive endocrinologists offer IVF and ICSI as a clinical alternative treatment [14, 30]. Assisted reproductive technology has undoubtedly improved the rate of child birth in infertile and subfertile couples via IVF and ICSI but the quality of the sperm as the most important parameter in the success of IVF/ICSI remains to be improved [31].
Varicocele-associated infertility can be related to many factors that one of them could be increase in the degree of DNA fragmentation and chromatin damage [4, 16]. The main cause for DNA fragmentation is oxidative stress. Excess production of reactive oxygen species (ROS) or decreased antioxidant defenses in the seminal plasma induces oxidative stress which damages spermatozoa. Sperm plasma membranes are susceptible to oxidative stress owing to their high levels of polyunsaturated fatty acids that readily undergo lipid peroxidation. Moreover, an aldehyde end product of lipid peroxidation, 4-hydroxy-2-nonenal, is an alkylating agent that damages DNA and induces apoptosis [11]. Many of the previous studies had claimed that varicocelectomy improves semen quality not pregnancy rate [15, 29]. Although we found a nonsignificant improvement in protamine content of placebo group, we did not detect any benefits from varicocelectomy. We hypothesis that varicocelectomy is probably capable of controlling ROS formation but prevention from further production of ROS or repair of damaged sperms, specially at the level of DNA and chromatin needs medical treatment. Change in the total antioxidant capacity and SOD has recently been reported in varicocelectomized patients that have received zinc sulfate and folic acid for 6 months [32]. Because DNA fragmentation inhibits normal activation of paternal gene expression at the 4-cell stage of developing embryo [10], correction of DNA fragmentation by supplement therapy may increase pregnancy rate in these patients.
In agreement with previous studies, our results revealed that administration of zinc and folic acid positively affect sperm quality and improve the outcome of varicocelectomy [18]. Deficient folic acid concentration cause elevated homocysteine and impair the remethylation cycle. This metabolism is involved in the methylation of phospholipids, proteins, DNA, and RNA and in the synthesis and repair of DNA. These processes are essential in spermatogenesis [33]. Recently some researchers have shown that a low folate concentration in seminal plasma is associated with more sperm DNA damage in infertile men [34]. During normal repair process, when the removal of the misincorporated uracil fails, double-strand breaks resulting in chromosome instability may occur. Folate shortage also decreases the supply of methyl groups, which are important substances for the protection of DNA against harmful exposures [35]. On the other hand, zinc affects DNA transcription and protein synthesis because serves as a cofactor for more than 80 mehalloenzymes [19], and zinc also has anti-apoptic [21] and antioxidant properties [20]. These above mentioned factors could change sperm quality and improve varicocelectomy outcome as was indicated in our study.
In conclusion, according to our study, varicocelectomy improved sperm parameters to some extent, but varicocelectomy followed by co-administration of zinc and folate significantly improve semen parameters, and also increase protamine content and acrosomal integrity. We believe in spite of beneficial effect of folic acid on semen parameters, the side effects and safety of long term intervention of this drug on male reproductive system need more investigations. Our interpretation of the mechanism of these drugs and their impacts on DNA repair which consequently may influence the pregnancy rate are also of concern.
Acknowledgements
Kerman medical University infertility center members are acknowledged for their warm cooperation in this study
Declaration of interest
The authors have no conflict of interest to declare.
Footnotes
Capsule In a prospective randomized trial, 6 months supplement therapy in varicocelectomized patients improved sperm parameters, acrosomal integrity and chromatin content.
References
- 1.Mohammed A, Chinegwundoh F. Testicular varicocele: an overview. Urol Int. 2009;82(4):373–379. doi: 10.1159/000218523. [DOI] [PubMed] [Google Scholar]
- 2.Ozturk U, et al. The effects of experimental left varicocele on the epididymis. Syst Biol Reprod Med. 2008;54(4–5):177–184. doi: 10.1080/19396360802415752. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, et al. Role of oxidative stress in pathogenesis of varicocele and infertility. Urology. 2009;73(3):461–469. doi: 10.1016/j.urology.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 4.Li F, et al. Significant improvement of sperm DNA quality after microsurgical repair of varicocele. Syst Biol Reprod Med. 2012;58(5):274–277. doi: 10.3109/19396368.2012.692431. [DOI] [PubMed] [Google Scholar]
- 5.Wu AK, et al. Bilateral but not unilateral testicular hypotrophy predicts for severe impairment of semen quality in men with varicocele undergoing infertility evaluation. Urology. 2008;71(6):1114–1118. doi: 10.1016/j.urology.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 6.Marmar JL. The pathophysiology of varicoceles in the light of current molecular and genetic information. Hum Reprod Update. 2001;7(5):461–472. doi: 10.1093/humupd/7.5.461. [DOI] [PubMed] [Google Scholar]
- 7.French DB, Desai NR, Agarwal A. Varicocele repair: does it still have a role in infertility treatment? Curr Opin Obstet Gynecol. 2008;20(3):269–274. doi: 10.1097/GCO.0b013e3282fcc00c. [DOI] [PubMed] [Google Scholar]
- 8.Erkan E, et al. Expression of NOS isoforms in internal spermatic veins of infertile men with varicocele. Syst Biol Reprod Med. 2012;58(5):268–273. doi: 10.3109/19396368.2012.678032. [DOI] [PubMed] [Google Scholar]
- 9.Pasqualotto FF, et al. Semen quality and oxidative stress scores in fertile and infertile patients with varicocele. Fertil Steril. 2008;89(3):602–607. doi: 10.1016/j.fertnstert.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9(4):331–345. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 11.Shiraishi K, Naito K. Effects of 4-hydroxy-2-nonenal, a marker of oxidative stress, on spermatogenesis and expression of p53 protein in male infertility. J Urol. 2007;178(3 Pt 1):1012–1017. doi: 10.1016/j.juro.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Nasr-Esfahani MH, et al. Varicocelectomy: semen parameters and protamine deficiency. Int J Androl. 2009;32(2):115–122. doi: 10.1111/j.1365-2605.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 13.Smit M, et al. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2010;183(1):270–274. doi: 10.1016/j.juro.2009.08.161. [DOI] [PubMed] [Google Scholar]
- 14.Fisher L, Sandlow J. The role of varicocele treatment in the era of assisted reproductive technology. Clin Urol. 2001;27:19–25. [Google Scholar]
- 15.Evers JL, Collins JA. Assessment of efficacy of varicocele repair for male subfertility: a systematic review. Lancet. 2003;361(9372):1849–1852. doi: 10.1016/S0140-6736(03)13503-9. [DOI] [PubMed] [Google Scholar]
- 16.Zini A, et al. Beneficial effect of microsurgical varicocelectomy on human sperm DNA integrity. Hum Reprod. 2005;20(4):1018–1021. doi: 10.1093/humrep/deh701. [DOI] [PubMed] [Google Scholar]
- 17.Moskovtsev SI, et al. Cause-specific treatment in patients with high sperm DNA damage resulted in significant DNA improvement. Syst Biol Reprod Med. 2009;55(2):109–115. doi: 10.1080/19396360902787944. [DOI] [PubMed] [Google Scholar]
- 18.Ebisch IMW et al. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod. 2006;p. 163–174. [DOI] [PubMed]
- 19.Favier AE. The role of zinc in reproduction. Hormonal mechanisems. Biol Trace Elem Res. 1992;32:363–382. doi: 10.1007/BF02784623. [DOI] [PubMed] [Google Scholar]
- 20.Zago M, Oteiza PI. The antioxidant properties of zinc: interactions with iron and antioxidants. Free Radic Biol and Med. 2001;31:266–274. doi: 10.1016/S0891-5849(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 21.Chimienti F, et al. Zinc homeostasis regulating proteins: new drug tergets for triggering cell fate. Curr Drug Targets. 2003;4:323–338. doi: 10.2174/1389450033491082. [DOI] [PubMed] [Google Scholar]
- 22.Ando S. et al. Phosphatase and zinc levels in the seminal plasma of varicocele. Int J Fertil. 1990;p. 249–252. [PubMed]
- 23.Joshi R, et al. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radic Biol and Med. 2001;30:1390–1399. doi: 10.1016/S0891-5849(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 24.Wong W, et al. Effect of folic acid and zinc sulphate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Fertil Steril. 2002;77:491–498. doi: 10.1016/S0015-0282(01)03229-0. [DOI] [PubMed] [Google Scholar]
- 25.Laboratory manual for the examination of human semen and semen-cervical mucus interaction. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 26.Nasr-Esfahani MH, Razavi S, Mardani M. Relation between different human sperm nuclear maturity tests and in vitro fertilization. J Assist Reprod Genet. 2001;18:219–225. doi: 10.1023/A:1009412130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al Bakri A. et al. Time for improvement in semen parameters after varicocelectomy. J Urol. 2011.
- 28.Madgar I, et al. Controlled trial of high spermatic vein ligation for varicocele in infertile men. Fertil Steril. 1995;63(1):120–124. doi: 10.1016/s0015-0282(16)57306-3. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, et al. Effect of varicocelectomy on sperm parameters and pregnancy rate in patients with subclinical varicocele: a randomized prospective controlled study. J Urol. 1996;155(5):1636–1638. doi: 10.1016/S0022-5347(01)66149-4. [DOI] [PubMed] [Google Scholar]
- 30.Girardi SK, Goldstein M. Varicocele. Curr Ther Endocrinol Metab. 1997;6:355–358. [PubMed] [Google Scholar]
- 31.Chemes HE, Rawe VY. Sperm pathology: a step beyond descriptive morphology. Origin, characerization and fertility potential of abnormal sperm phenotypes in infertile men. Hum Reprod Update. 2003;9:405–428. doi: 10.1093/humupd/dmg034. [DOI] [PubMed] [Google Scholar]
- 32.Nematollahi-Mahani SN et al. Effect of folic acid and zinc sulphate on endocrine parameters and seminal antioxidant level after varicocelectomy. Andrologia. 2013. doi:10.1111/and.12067. [DOI] [PubMed]
- 33.Ebisch IM, et al. Homocysteine, glutathione and related thiols affect fertility parameters in the (sub)fertile couple. Hum Reprod. 2006;21(7):1725–1733. doi: 10.1093/humrep/del081. [DOI] [PubMed] [Google Scholar]
- 34.Boxmeer JC, et al. Low folate in seminal plasma is associated with increased sperm DNA damage. Fertil Steril. 2009;92(2):548–556. doi: 10.1016/j.fertnstert.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Blount BC, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94(7):3290–3295. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]