Abstract
Purpose
To compare the expression profiles of Tektin 2 and CatSper 2 motility proteins in the spermatozoa of normozoospermic and oligoasthenozoospermic men and determine its correlation with sperm motility, fertilization rate, embryo quality and pregnancy rate.
Methods
Tektin 2 and CatSper 2 protein expression was studied using Western Blotting and immunofluorescence. Tektin 2 and CatSper 2 protein levels were quantified by ELISA.
Results
Oligoasthenozoospermic men were found to have lower fertilization rates, poor embryo quality and lower pregnancy rates as compared to normozoospermic men. The levels of Tektin 2 and CatSper 2 are significantly lower in spermatozoa of oligoasthenozoospermic men as compared to normozoospermic controls; the levels were also lower in immotile fraction as compared to motile fraction of spermatozoa obtained from normozoospermic individuals. The levels of Tektin 2 and CatSper 2 were higher in individuals demonstrating sperm motility >60 % as compared to sperm motility <30 %. Tektin 2 but not CatSper 2 levels were positively associated with fertilization rate, embryo quality and pregnancy rate.
Conclusion
Levels of Tektin 2 and CatSper 2 proteins are positively associated with sperm motility parameters. Measurements of Tektin 2 levels can be correlated with the clinical outcome of ICSI.
Keywords: Tektin, CatSper, Embryo quality, Fertilization, Pregnancy
Introduction
Infertility is observed in 15 % of all couples in reproductive age with the male partner being affected in approximately 50 % cases [47]. Assisted Reproductive Technologies (ART) like In vitro fertilization (IVF) and Intra cytoplasmic sperm injection (ICSI) has revolutionized our approach to male infertility and now most infertile men can be helped to have biological parenthood [12].
It is generally accepted that good sperm motility is a central component for normal male fertility [43]. Evidence suggests that during IVF, the fertilization rate, number of embryos transferred and pregnancy rate increases directly with the total number of motile sperm in a given sample [10, 27, 42, 45, 46]. Cleavage rates of embryo are high when motile spermatozoa are used for ICSI rather than an immotile spermatozoon [14]. ART outcomes have demonstrated that the fertilization rates, embryo qualities and pregnancy rates are poor when spermatozoa with low motility have been used for fertilization as compared to those with normal motility [14, 17, 28]. A retrospective analysis of the data from our clinic in the last 5 years have reconfirmed the notion that the ART outcome is generally poor in couples with oligoasthenozoospermia despite the use of ICSI (Table 2). These observations suggest that there must exist some components in the spermatozoa which when lost or altered would lead not only to asthenozoospermia but is also responsible for poor ART clinical outcome. Indeed proteomic comparisons of sperm from normozoospermic and asthenozoospermic men have identified a plethora of candidate molecules that are differentially expressed, however the identity of the factors in association to clinical outcome remains elusive [6, 8, 11, 38, 49]. A recent study has revealed that despite ICSI, pregnancy rates are low in oligoasthenozoospermic men, because of low centrin (centrosomal protein) levels [13]. Since centrin is associated with sperm motility, we hypothesize that there may be other motility proteins that may be altered and affect the success rate of ART.
Table 2.
Five years (2006–2011) clinical characteristics of normozoospermic and oligoasthenozoospermic men attending the infertility clinic (INKUS IVF Centre, Mumbai)
| Normozoospermic men | Oligoasthenozoospermic men | |
|---|---|---|
| Total number of patients (n) | 337 | 330 |
| Maternal age | 30.2 ± 0.8 | 33.4 ± 02 |
| Paternal age | 31.5 ± 0.4 | 34.1 ± 0.3 |
| Sperm count | 39.5 ± 0.8 | 10.3 ± 0.4a |
| Sperm motility | 36.7 ± 0.7 | 7.5 ± .0.4a |
| Total number of cycles | 337 | 330 |
| Number of oocytes retrieved | 4478 ± 0.4 | 4443 ± 0.7 |
| Oocytes retrieved/cycle | 11.23 ± 5.72 | 12.23 ± 5.81 |
| Number of oocytes injected | 4400 ± 0.4 | 4378 ± 0.5 |
| % of oocytes injected | 98.25 | 98.53 |
| Fertilization rate (%) | 75.71 % | 39.2 %a |
| Number of embryos transferred | 1011 ± 0.6 | 990 ± 0.8 |
| Grade I embryos (%) | 86 % | 42 %a |
| Grade II embryos (%) | 12.4 % | 55.9 %a |
| Pregnancy rate | 43.02 % (337/145) | 12.12 %(330/40)a |
| Number of abortions | – | 5 |
| Number of live births | 145 | 35 |
p value < 0.001 is considered significant
Values are expressed as Mean ± SE
aSignificant difference as compared to normozoospermic men
Sperm motility proteins belong to diverse classes including ion channels, cytoskeletal proteins, cell signaling proteins and glycolytic enzymes [43]. While a formal proof for the motility related functions of these proteins in humans is required, two proteins that have been experimentally proven to be indispensable for spermatozoa motility are Tektin and CatSper. Tektins (Tektin 1–4) are the constitutive proteins of microtubules in cilia, flagella, basal bodies and centrioles and contribute to the stability and structural complexity of the axonemal microtubules [9, 15, 32, 40]. CatSper channels (CatSper 1–4) are sperm specific six-transmembrane voltage gated Ca2+ permeant channels exclusively expressed in the testis. Ca2+ entry through the CatSper channels has been implicated in the activation of hyperactivated motility required for fertility [7, 33, 35]. CatSper 1 and 2 are required for hyperactivated motility whereas CatSper 3 and 4 are needed for acrosome reaction [16]. Gene knockout for Tektin and CatSper in mouse models have shown normal epididymal sperm counts with reduced or almost absent sperm motility and hyperactivated motility [7, 33–36, 41]. Furthermore, genetic screening of asthenozoospermic men has revealed mutations in Tektin 2 gene and screening of asthenoteratozoospermic men has revealed mutations in CatSper 1 and 2 genes in a subset of individuals [2, 3, 50]. These observations suggest that Tektin and CatSper are associated with sperm motility. It is possible that beyond genetic mutations, men with low sperm motility may inherently have reduced expression of Tektin and CatSper in their spermatozoa leading to low motility. Indeed atleast in case of CatSper, the expression is reduced in testicular biopsies of men with reduced sperm motility as compared to controls [29]. These observations tempt us to hypothesize that the spermatozoa with reduced motility may inherently have low expression of these proteins. To the best of our knowledge levels of Tektin 2 and CatSper 2 in the spermatozoa of oligoasthenozoospermic men has not been reported and its relationship to ART outcome has not been investigated.
In the present study we aimed to investigate the expression profiles of Tektin 2 and CatSper 2 in motile and immotile spermatozoa of normozoospermic men; in the spermatozoa of normozoospermic and oligoasthenozoospermic men and determine its correlation with motility, fertilization rate, embryo quality and pregnancy rate. A more complete understanding of the molecular processes that go into the creation of a motile sperm will enable us to address the issue of reduced motility and associated subfertility more effectively.
Materials and methods
The study was approved by the institutional review board and informed consent was obtained from all patients.
Study design: cross-sectional
Patients
The control group (Group I) included normozoospermic (NZ) (≥50 % grade a+b sperm motility, sperm count ≥20 million/ml and >15 % normal forms); the study group (Group II) included male partners of infertile couples with oligoasthenozoospermia (OA) (≤50 % grade c+d sperm motility, sperm count ≤5 million/ml and >15 % normal forms) [47]. There was no evidence of smoking, alcoholism, cryptorchidism, childhood disease, radiation exposure, prescribed drug usage or presence of varicocele. All the female partners in both the groups were normal with no endocrine or other abnormalities associated with infertility, except in group I where they had tubal factor infertility. Couples where more than 20 % of oocytes obtained after pickup were of poor quality were excluded from the study.
In all a total of 52 controls and an equivalent number of study subjects were included in the study. The age of the patients ranged from 28 to 45 years. Mean ages were 33.6 years in Group I and 34.5 years in Group II.
Semen preparation
Semen samples from normozoospermic and oligoasthenozoospermic men were collected by masturbation after 3 days of sexual abstinence. Post liquefaction, sperm count and motility were assessed according to WHO recommendation [47]. Sperm morphology was evaluated using strict Tygerberg criteria [19].
For microscopic examinations, 50 μl sperm fraction was stained for 30 min with an equal volume of 0.4 % eosin solution (Sigma) and was observed under a light microscope to confirm no remaining round cells and cytoplasmic droplets [21]. Samples with >5 × 106 round cells/ml were excluded because round cells of non spermatogenic origin contribute to the loss of motility and hence give a false indication of asthenozoopsermia [18].
For protein expression studies (by Western Blot or ELISA) the spermatozoa were washed twice with phosphate buffered saline (PBS). Prior to use the sperm concentration was adjusted to 1 × 106 cells/ml and incubated with protein lysis buffer (2 % SDS containing mixture of protease inhibitors) at 4 °C, overnight, sonicated and centrifuged at 4000 g for 30 min at 4 °C. The protein concentration in the supernatant was estimated by Bradford Protein Assay [5].
Preparation of motile and immotile sperm
Semen samples from twenty normozoospermic men were liquefied and processed for swim up using standard protocols [30]. Briefly, 1 ml of semen sample was added to 1 ml of Earls Balanced Salt Solution (EBSS, Sigma, USA) supplemented with pyruvate and antibiotics (Sigma, USA), mixed and centrifuged at 200 g for 10 min. Supernatant was discarded and 1 ml EBSS added to the pellet, mixed well and centrifuged. Lastly the supernatant was discarded and the pellet overlaid with 0.5 ml of EBSS and kept in incubator (5 % CO2 in air) at 37 °C for 1 h. After incubation the supernatant was carefully collected, centrifuged and the pellet was frozen at −80 °C, until use. This fraction of highly motile spermatozoa was considered as “motile fraction” and defined as group M. The pellet leftover after swim up was washed and considered as “immotile fraction” and defined as group IM. For microscopic examinations, 50 μl sperm fraction was stained for 30 min with an equal volume of 0.4 % eosin solution (Sigma) and was observed under a light microscope to confirm no remaining round cells and cytoplasmic droplets [21]. Prior to use the spermatozoa concentration in both the fractions was adjusted to 1 × 106 cells/ml and protein was extracted and estimated as above.
Immunofluorescence
Immunofluorescence was performed as detailed previously [37]. Briefly, spermatozoa collected from patients by swim up technique were smeared on clean grease free, poly lysine (Sigma, USA) coated slides and fixed with chilled acetone for 20 min at −20 °C. The smears were washed twice with PBS. This is followed by blocking with 1 % BSA (Bovine serum albumin, HiMedia, Mumbai, India) in PBS at room temperature, for 1 h. Thereafter slides were incubated with mouse monoclonal Tektin 2 antibody (Abcam, USA) diluted 1:50 (optimized dilution) and rabbit polyclonal CatSper 2 anibody (Abcam, USA) diluted 1:20 (optimized dilution), in PBS at 4 °C, overnight. Slides were then washed twice with PBS and incubated with fluorescence isothicyanate (FITC) labeled antimouse and antirabbit secondary antibodies (Abcam, USA) respectively diluted 1:100 (optimized dilution) in PBS, for 1 h in dark at room temperature. The slides were washed thrice with PBS, followed by counterstaining with propidium iodide (Sigma, USA) and mounting in Vectashield (Vector laboratories, Burlingame, UK). Specificity of the staining was evaluated by running a negative control (antibody replaced with PBS) in each experiment. The slides were examined using fluorescence microscope (Olympus).
Western blotting
Western blotting was performed as described previously [1]. Briefly, 50 μg/lane protein were separated by 12 % Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) under reducing conditions [20]. The separated proteins were transferred onto a nitrocellulose membrane (GE Healthcare, USA) and blocked with 5 % non fat dried milk (NFDM, Amul, India) in Tris buffered saline (TBS). The blots were incubated overnight at 4 °C with mouse monoclonal Tektin 2 antibody diluted 1:1000 (optimized dilution) in TBS and rabbit polyclonal CatSper 2 anibody diluted 1:20 (optimized dilution) in TBS. The blots were washed with TBS containing 0.1 % Tween 20 (TBS-T) and then incubated with horseradish peroxidase (HRP) conjugated antimouse and antirabbit secondary antibodies respectively (Bangalore Genei, India and Dako, Denmark respectively) diluted 1:5000 (optimized dilution) in TBS for 1 h. The blots were washed with TBS-T and detection was carried out using the chemiluminescence detection system (GE Healthcare, USA). For negative controls, parallel blots were incubated with TBS in place of the monoclonal and polyclonal antibodies.
For normalization, the same blot was stripped and reprobed with mouse monoclonal β Actin antibody (Sigma, USA). For stripping, the membrane was covered with stripping solution (0.19 M glycine, 0.003 M SDS, 10 ml Tween 20, pH 2.2), incubated at room temperature for I hour. This was followed by three washes with PBS and one wash with PBST. Thereafter the blots were blocked with NFDM and probed with β Actin antibody, diluted 1:100 (optimized dilution) (Sigma, USA).
ELISA
Sperm lysates containing 10 μg of protein were diluted in coating buffer (0.01 M sodium carbonate, 0.03 M sodium bicarbonate, pH-9.2) to a final volume of 100 μl and coated onto maxisorp wells (Nunc, USA) and incubated at 4 °C overnight. Next day the wells were washed with PBS and blocked with 5 % BSA, at 37 °C for 1 h. The wells were incubated with optimized dilution of Tektin 2 antibody (1:2000) and CatSper 2 antibody (1:50), overnight at 4 °C. The wells were washed thrice with PBS containing 0.1 % Tween 20 (PBS–T) and incubated with HRP labeled secondary antibodies, for 1 h in dark at room temperature. The bound peroxidase was then visualized using tetramethylbenzidine (TMB, Bangalore Genei, India) and the reaction was terminated with 4 N H2SO4. Colorimetric readings were taken at 490 nm in a titertek multiscan reader (Titertek, USA). All the measurements were done in triplets and mean of the OD’s was considered as final value. For negative control, primary antibody was replaced with PBS. For normalization, the levels of β Actin (1:500, optimized dilution) were estimated in the same sperm protein lysate.
Assessment of fertilization and embryo quality
All the study subjects (controls and patients) underwent ICSI. Ovarian stimulation and ICSI procedures was performed by skilled and experienced person and as per the standard protocol performed at INKUS IVF Centre (Mumbai, India) which has been detailed elsewhere [26]
A total of 104 cycles were evaluated for fertilization rate, embryo quality and pregnancy rate. Only good quality oocytes, as per published standards (Metaphase II and had the first polar body extruded) were considered for microinjection [48]. The data compiled herein is derived from 1009 oocytes retrieved from 104 cycles. Fertilization was assessed 16–18 h post ICSI. Embryos with two polar bodies and two distinct and opposing pronuclei (PN) with evenly distributed nucleoli were considered as fertilized. In cases where the PN had fused at the time of assessment, the embryos were considered as fertilized if two polar bodies and normal cleavage was observed. Fertilization rate was calculated as the number of oocytes fertilized and undergone first cleavage with respect to the number of oocytes microinjected.
Embryo cleavage and quality was evaluated 40–44 h after ICSI. Embryo quality was defined as Grade 1, Grade 2, Grade 3, Grade 4 and Grade 5 according to the published standards [44]. Briefly Grade 1 embryos were defined as blastomeres of equal size without cytoplasmic fragments. Grade 2 embryos were defined as blastomeres of equal size with minor cytoplasmic fragments or blebs. Grade 3 embryos were defined as blastomeres of distinctly unequal size with few or none cytoplasmic fragments. Grade 4 embryos were defined as blastomeres of equal or unequal size with significant cytoplasmic fragmentation. Grade 5 embryos were defined as few blastomeres of any size with severe or complete fragmentation
A maximum of three embryos were transferred per cycle. Pregnancy was confirmed by measuring serum β hCG concentration 2 weeks after embryo transfer and ultra sonography was done 2 weeks after measuring β hCG levels.
Data analysis
The relationship between Tektin 2 and CatSper 2 levels with spermatozoa motility was analyzed in three defined groups. In this study for each subject mean spermatozoa motility is computed by counting the number of motile and immotile spermatozoa. Group M1 included men with 0 % to ≤30 % motile sperm in their semen sample, group M2 included men with >30 % to ≤60 % motile sperm in their semen sample, group M3 included men with >60 % motile sperm in their semen sample.
The relationship between Tektin 2 and CatSper 2 levels with spermatozoa count was analyzed in two defined groups. Group C1 included men with 2–≤20 million/ml sperm in their semen sample, group C2 included men with >20 million/ml sperm in their semen sample.
The relationship between Tektin 2 and CatSper 2 levels with fertilization rate was analyzed in another three defined groups, in group F1 fertilization rate was 0 % to ≤30 %, in group F2 fertilization rate was >30 % to ≤60 %, in group F3 fertilization rate was >60 % to 100 %.
The relationship between Tektin 2 and CatSper 2 levels with embryo quality was analyzed in four defined groups, group Q1 contained >50 % Grade 1 embryos, group Q2 contained <50 % Grade 1 embryos, group Q3 contained >50 % Grade 2 embryos and group Q4 contained <50 % Grade 2 embryos
The relationship between Tektin 2 and CatSper 2 levels with pregnancy rate was analyzed in two defined groups, group P included male partners of couples conceived after ICSI, group NP included male partners of couples not conceived after ICSI.
Statistical analysis
Statistical analysis was done (using GraphPad Software, Prism 5.0, GraphPad Software, SanDiego, CA), on raw data by a student t-test and/or one way analysis of variance followed by posthoc analysis using Dunn’s test for all pair wise comparison, as appropriate. p-value < 0.001 was considered statistically significant.
Statistical power calculations were performed using software from DSS research (http://www.dssresearch.com/toolkit/default.asp). It was found to be 100 %, which indicates that the sample size employed has produced clinically significant results.
Results
Expression of CatSper 2 and Tektin 2 protein in spermatozoa
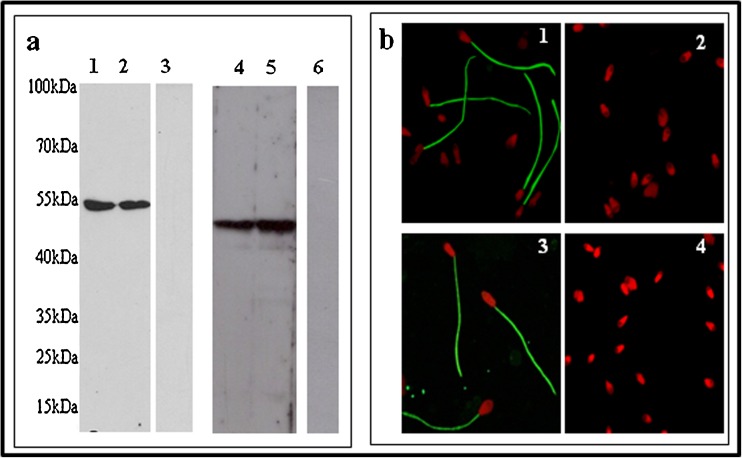
The presence of CatSper 2 and Tektin 2 in the spermatozoa and the specificity of the antibodies used were verified by Western Blotting. As evident from Fig. 1a, a single band of 54 kDa (of the expected size) was observed when Tektin 2 antibody was used to probe sperm protein lysates from normozoospermic men. In case of CatSper 2, as expected a single band of 48 kDa, was observed. No bands were observed in the negative control, demonstrating the specificity of the bands.
Fig. 1.
Expression of Tektin 2 and CatSper 2 in spermatozoa Expression of Tektin 2 and CatSper 2 in spermatozoa by Western blot (a) Lane 1 and 2 - sperm lysates probed with Tektin 2 antibody. Lane 4 and 5 - sperm lysates probed with CatSper 2 antibody. Lane 3 and 6 - negative control without primary antibodies for Tektin 2 and CatSper 2 respectively. Localization of Tektin 2 and CatSper 2 in spermatozoa by immunofluorescence (b) 1 and 3 - cellular localization of Tektin 2 and CatSper 2 respectively to the spermatozoa principle piece. 2 and 4 - Negative control for Tektin 2 and CatSper 2 respectively
The cellular localization of CatSper 2 and Tektin 2 in normozoospermic human spermatozoa was studied by immunofluorescence microscopy. As evident in Fig. 1b, green fluorescence was detected on the principal piece of spermatozoa when the cells were probed with either CatSper 2 or Tektin 2 antibody. No staining was evident in the negative control which shows the antibody specificity.
Tektin 2 and CatSper 2 levels in normozoospermic and oligoasthenozoospermic individuals
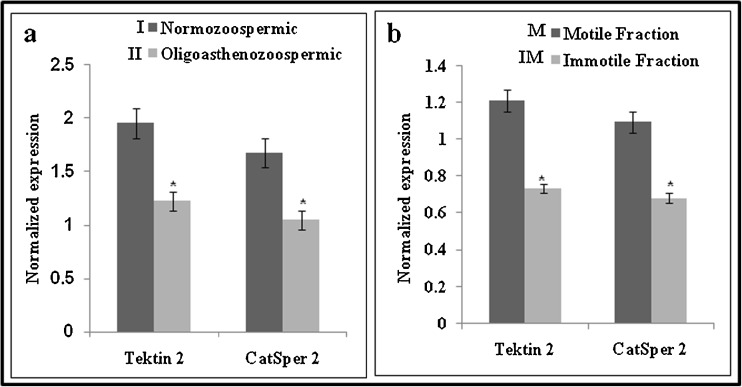
The levels of Tektin 2 and CatSper 2 were estimated by ELISA in the sperm lysate obtained from normozoospermic and oligoasthenozoospermic individuals. Figure 2a, gives the ratio of Tektin 2 by β Actin and CatSper 2 by β Actin in normozoospermic and oligoasthenozoospermic individuals. Both Tektin 2 and CatSper 2 levels were significantly lower in the oligoasthenozoospermic samples as compared to controls. Levels of Tektin 2 were approximately 37 % and CatSper 2 were 20 % lower in oligoasthenozoospermic men as compared to normozoospermic men.
Fig. 2.
Association of Tektin 2 and CatSper 2 levels in spermatozoa of normozoospermic (n = 52) and oligoasthenozoospermic (n = 52) men (a) and Association of Tektin 2 and CatSper 2 levels in motile (n = 20) and immotile (n = 20) spermatozoa from normozoospermic men (b). The values on the Y axis are Mean ± SE of the Tektin 2 and CatSper 2 values normalized to β actin. * indicates values significantly different (p < 0.001)
Tektin 2 and CatSper 2 levels in motile and immotile fraction of spermatozoa from normozoospermic individuals
Semen samples from twenty normozoospermic men was processed by swim up technique, wherein, the spermatozoa in the swim up preparation were designated as motile fraction and the pellet was considered as immotile fraction. Figure 2b, gives the ratio of Tektin 2 by β Actin and CatSper 2 by β Actin in the motile and immotile fractions. As evident, the levels of Tektin 2 and CatSper 2 were significantly lower in the immotile fraction as compared to motile fraction. The levels of Tektin 2 were approximately 35 % and CatSper 2 were 38 %, lower in the immotile fraction as compared to the motile fraction of spermatozoa from normozoospermic men.
Correlation of Tektin 2 and CatSper 2 levels with sperm total motility
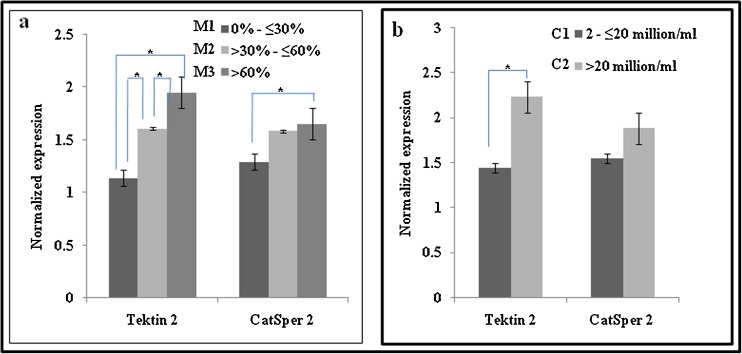
As evident from Fig. 3a, lowest Tektin 2 levels were observed in group of men having 0 % to ≤30 % motile spermatozoa in their semen sample, and the levels were higher in the groups where the number of motile spermatozoa were >30 % and above. Statistically, the level of Tektin 2 were significantly lower in group that had 0 % to ≤30 % motile spermatozoa as compared to group having >30 % to ≤60 % and >60 % motile spermatozoa. Tektin 2 levels were also significantly lower in group having >30 % to ≤60 % motile spermatozoa as compared to group having >60 % motile spermatozoa.
Fig. 3.
Association of Tektin 2 and CatSper 2 levels with sperm motility (a) and sperm count (b). The values on the Y axis are Mean ± SE of the Tektin 2 and CatSper 2 values normalized to β actin. * significant difference between the groups (p < 0.001)
In contrast to Tektin 2, CatSper 2 levels did not show such significant variations in association to total motility. CatSper 2 levels were significantly lower in group having 0 % to ≤30 % motile spermatozoa as compared to group having >60 % motile spermatozoa.
Association of Tektin 2 and CatSper 2 levels with sperm count
As evident from Fig. 3b, the levels of Tektin 2 were significantly lower in group that had 2 to ≤20 million/ml spermatozoa as compared to group having >20 million/ml spermatozoa. CatSper 2 levels did not show statistically significant values with respect to sperm count.
Correlation of Tektin 2 and CatSper 2 levels with fertilization rate
To investigate the association of Tektin 2 and CatSper 2 with fertilization rate, the individuals selected for study were segregated into three defined groups based on their fertilization rate indices.
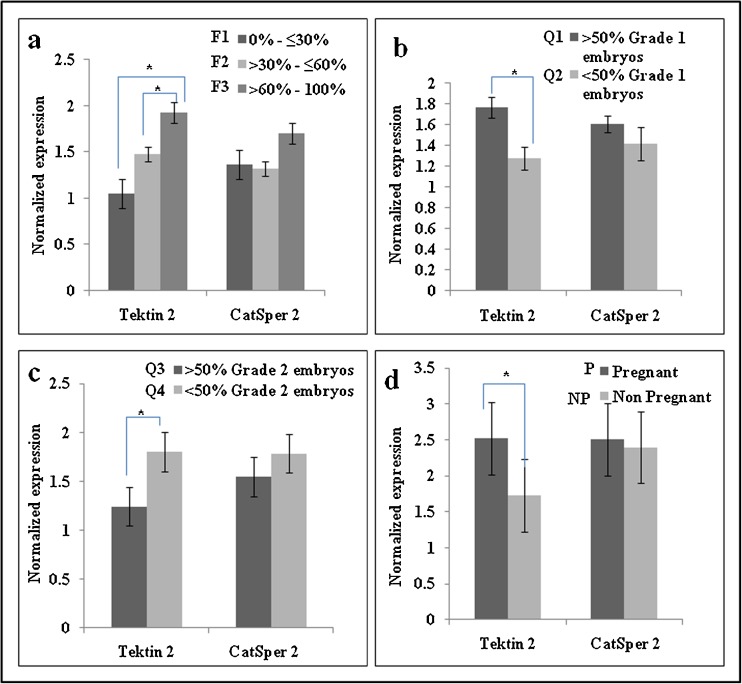
As evident from Fig. 4a, lowest Tektin 2 levels were observed in group 0 % to ≤30 % fertilization rate and the levels were found to be progressively increasing with increase in fertilization rate. Statistically, the levels of Tektin 2 were significantly lower in group 0 % to ≤30 % fertilization rate as compared to groups >30 % to ≤60 % and >60 % to 100 % fertilization rate. In contrast, CatSper 2 levels did not show significant variations in association to fertilization rate.
Fig. 4.
Association of Tektin 2 and CatSper 2 levels with fertilization rate (a), embryo quality (b and c) and pregnancy rate (d). The values on the Y axis are Mean ± SE of the Tektin 2 and CatSper 2 values normalized to β actin. * significant difference between the groups (p < 0.001)
Association of Tektin 2 and CatSper 2 with embryo quality
Quality of the embryos of all 104 couples was assessed 40–44 h after ICSI. Embryo quality was then segregated into two defined groups.
As evident from Fig. 4b, Tektin 2 levels were significantly lower in spermatozoa of group Q2 that had <50 % Grade 1 embryos as compared to group Q1 that had >50 % Grade 1 embryos. CatSper 2 levels did not show any significant difference between the groups. Also as evident from Fig. 4c, Tektin 2 levels were significantly lower in spermatozoa of group Q3 that had >50 % Grade 2 embryos as compared to group Q4 that had <50 % Grade 2 embryos. CatSper 2 levels did not show any significant difference between the groups.
Association of Tektin 2 and CatSper 2 with pregnancy rate
As evident from Fig. 4d, Tektin 2 levels were significantly lower in spermatozoa of men of group NP which included male partner of couples not conceived after ICSI as compared to spermatozoa of men of group P which included male partner of couples conceived after ICSI. The level of Tektin 2 was 32 % lower and CatSper 2 was 8 % lower in spermatozoa of men of group NP as compared to group P.
ICSI outcome in normozoospermic and oligoasthenozoospermic men
As evident from Table 1, out of 104 couples, 102 had an embryo transfer. Fertilization rate was significantly lower i.e. 62.5 % in Group II as compared to 80 % in Group I. Also Grade I embryos were significantly lower i.e. 53 % in Group II as compared to 77 % in Group I. Grade II embryos were significantly higher i.e. 47 % in Group II as compared to 23 % in Group I. Twenty pregnancies were achieved in Group I as compared to eleven in Group II out of which two resulted in clinical abortion. Only nine pregnancies were achieved in this group. The ongoing pregnancy rate was 38.5 % in Group I and 17.3 % in Group II. All the pregnancies have resulted in live birth of a baby.
Table 1.
Clinical characteristics of normozoospermic and oligoasthenozoospermic men included in the study
| Normozoospermic men | Oligoasthenozoospermic men | |
|---|---|---|
| Group I | Group II | |
| Total number of patients (n) | 52 | 52 |
| Total number of cycles | 52 | 52 |
| Number of oocytes retrieved | 584 | 624 |
| Number of oocytes injected | 504 | 505 |
| Fertilization rate | 404 (80 %) | 316 (62.5 %)a |
| Embryo Quality | Grade 1–310 (77 %) | Grade 1–167 (53 %)a |
| Grade 2–94 (23 %) | Grade 2–149 (47 %)a | |
| Number of embryos transferred | 170 | 151 |
| Pregnancy rate | 20/52 (38.5 %) | 11/52 (21.1 %)a |
| Ongoing pregnancy rate | 20/52 (38.5 %) | 9/52 (17.3 %)a |
| Number of live births | 20 | 9 |
p value < 0.001 is considered significant
aSignificant difference as compared to normozoospermic men
Table 2 includes a general overview of the clinical data of normozoospermic and oligoasthenozoospermic men attending the infertility clinic in the last 5 years. As evident, there is an overall reduction in the ART outcome in the oligoasthenozoospermic group of men as compared to the normozoospermic group of men. Fertilization rate was significantly lower i.e. 39.2 % in OA group as compared to 75.71 % in NZ group. Also Grade I embryos were significantly lower i.e. 42 % in OA group as compared to 86 % in NZ group. Grade II embryos were significantly higher i.e. 55.9 % in OA group as compared to 12.4 % in NZ group. One hundred and forty five pregnancies were achieved in NZ group as compared to forty in OA group out of which five resulted in clinical abortion. Only thirty five pregnancies were achieved in this group. All the pregnancies have resulted in live birth of a baby.
Discussion
The results of the present study demonstrate that Tektin 2 and CatSper 2 which are expressed in the principal piece of human spermatozoa are reduced in immotile sperm and also in spermatozoa obtained from oligoasthenozoospermic males. The levels of Tektin 2 and CatSper 2 correlate positively with spermatozoa motility. Tektin 2 levels are also associated with fertilization rate, embryo quality and pregnancy rates.
Several proteins have been identified in the sperm tails which have been implicated in regulation of motility and belonging to diverse classes including ion channels, cytoskeletal proteins, cell signaling proteins and glycolytic enzymes [43]. Amongst the several spermatozoa proteins, gene targeting studies have demonstrated an indispensible role of Tektin 2 and CatSper 2. Not surprisingly, as observed in the present study and reported earlier, both Tektin 2 and CatSper 2 are localized on the spermatozoa principal piece [34–36, 41].
Previous studies have demonstrated that the levels of CatSper 1, 2, 3, 4 mRNA are lower in immotile sperm fractions as compared to motile sperm fraction [22, 23]; the testicular expression of CatSper is lower in biopsies derived from men with deficient sperm motility as compared to controls [29]. Tektin 1 has been reported to be down regulated in spermatozoa of asthenozoospermic men as compared to normozoospermic men [38]. Keeping with these observations, in the present study, we found reduced expression of CatSper 2 and Tektin 2 in immotile fraction as compared to motile fraction of spermatozoa from normozoospermic men. The levels are also lower in spermatozoa derived from oligoasthenozoospermic individuals as compared to normozoospermic men. These observations indicate that reduced spermatozoa motility may be caused by low amounts of both the proteins. It will be of interest to determine if levels of other proteins involved in spermatozoa motility are also altered in spermatozoa of oligoasthenozoospermic men. In this context it is of interest to note that the expression of Centrin which is involved in spermatozoa motility has been found to be reduced in spermatozoa of oligoasthenozoospermic men as compared to fertile controls and this is associated with pregnancy rate of 25 % in oligoasthenozoospermic men as compared to 50 % in normozoospermic men [13]. Beyond Tektin 2, CatSper 2 and Centrin; proteomic analysis of normozoospermic and asthenozoospermic samples have identified several cytoskeletal proteins, metabolic enzymes and folding/stress response proteins to be differentially expressed in the motile and immotile sperm [6, 8, 11, 38, 49].
Since the expression of both Tektin 2 and CatSper 2 were found to be reduced in spermatozoa of oligoasthenozoospermic men as compared to controls, we hypothesize that the levels of these proteins may correlate with their motility. Indeed, the levels of Tektin 2 and CatSper 2 were found to be the highest in the semen samples that had total motility >60 % whereas the levels were lowest in semen samples that have total motility <30 %. Intriguingly, the levels of Tektin 2 differed more dramatically within the groups as compared to CatSper 2. Tektin 2 levels were almost half in semen samples that had <30 % motile spermatozoa as compared to those having >60 % motile spermatozoa. CatSper 2 levels did not show such dramatic alterations. Reduced Tektin 2 but not CatSper 2 levels were also associated with low sperm counts. These observations indicate that Tektin 2 might have dominant roles in regulation of spermatozoa motility as compared to CatSper 2. Not surprisingly, in Tektin 2 and 4 knockout mice, the spermatozoa show major defects associated with motility which includes severe reduction in forward progressive motility and alteration in flagellar beat patterns [36, 41], whereas only reduced spermatozoa velocity and failure of its hyper activation is observed in CatSper 2 knockout mice [7, 35, 36, 41].
Amongst the various causes of male factor infertility, asthenozoospermia comprises of 24 % of infertile men and may be a significant factor in another 55 % of patients with combined defects in spermatozoa density, motility and morphology [24]. Infertility in such individuals can be circumvented by use of ART. Both IVF and ICSI have been successfully used in couples where the male partner has asthenozoospermia and not unexpectedly the success rates of ICSI are higher over IVF [10, 31, 46]. However, retrospective analysis of the ART outcomes have demonstrated that the fertilization rates, embryo qualities and pregnancy rates are poor when spermatozoa with low motility have been used for fertilization as compared to those with normal motility [14, 17, 28]. Surprisingly, the fertilization rate and embryo quality could only be marginally improved even after the use of ICSI [28]. These findings are further reconfirmed in our retrospective analysis where couples with oligoasthenozoospermia has poor ART outcome despite the use of ICSI (Table 2). These clinical observations led to the hypothesis that the spermatozoa derived from asthenozoospermic men are not just defective from the perspective of motility but might also harbor defects which may be responsible for the poor fertilization capacity, poor embryonic development and low pregnancy rate. Since the expression of Tektin 2 and CatSper 2, were found to be associated with spermatozoa motility and the levels are lower in spermatozoa of oligoasthenozoospermic men prompted us to investigate, if their levels are also associated with fertilization rate, embryo quality and pregnancy rate. To our surprise, we observed that the levels of Tektin 2 were significantly lower when the fertilization rate was less than 30 % as compared to fertilization rate more than 30 %. Such differences were not noted in case of CatSper 2. It is important to note that in this study the fertilization rates were calculated only with good quality mature oocytes that were in Metaphase II and had the first polar body extruded. These observations indicate that the spermatozoa derived Tektin 2 but not CatSper 2 associate with fertilization rates and it is tempting to propose that sperm Tektin 2 levels may serve as a marker to determine fertilization.
How Tektin 2 might regulate fertilization is a matter of investigation. It is possible that the spermatozoon which contains lower amounts of Tektin 2 might also have reduced expression of molecules required for early fertilization events, which may be responsible for the poor fertilization rate observed in oligoasthenozoospermic men. Analysis of the living oocytes after IVF or ICSI have demonstrated that the sperm tail gets penetrated into the oocytes at fertilization and is likely to direct the intra oocyte movement of the male pronucleus and is critical for the relative success or failure of embryogenesis [4]. It is possible that the sperm tails with low amounts of Tektin 2 may not have the ability to direct the male pronucleus within the oocyte for fertilization. Indeed, in the group of patients analyzed herein, two individuals that had negligible amounts of Tektin 2, in one of them failure of fertilization was observed in almost 70 % of oocytes (unpublished data). These results suggest that the sperm derived Tektin 2 may regulate the process of pronuclear fusion for a successful fertilization.
Another critical determinant for the success rate of ART is the embryo quality. While poor egg quality is associated with defective embryonic development, observational studies have demonstrated that despite the use of good quality eggs, the Grade 2 and 3 embryos were more in couples where the male partner has defective sperm motility, even after ICSI [25, 28]. These observations together with the result that low Tektin 2 levels are associated with poor fertilization rates prompted us to investigate if Tektin 2 levels are also associated with embryo quality. Interestingly when Tektin 2 and CatSper 2 levels were compared between the Grade 1 and Grade 2 embryos, we observed that the Tektin 2 levels were significantly low in group Q2 that had <50 % grade 1 embryos as compared to group Q1 that had >50 % grade 1 embryos. Also Tektin 2 levels were significantly low in group Q3 that had >50 % grade 2 embryos as compared to group Q4 that had <50 % grade 1 embryos. CatSper 2 did not show any significant difference. These observations imply that the levels of Tektin 2 are not only associated with sperm motility and the success of fertilization but also with embryo quality.
Post fertilization the sperm tail is retained in a single blastomere upto the 12-cell stage and this tail contributes to the development of mitotic spindles and microtubule nucleation during cleavage stages [4]. While the centriolar protein, Centrin is known to play a critical role in this process, Tektin is also found in the centrosome and is proposed to be associated during the process of mitotic spindle formation [13, 39]. It is possible that the sperm with low Tektin 2 levels may be able to fertilize the egg but it may not be able to direct the movement of male pronucleus for fusion, leading to failed fertilization or it may not be able to orient the first mitotic spindle and permit microtubular nucleation for the early cleavages. Interestingly, in the two individuals that had very low levels of Tektin 2 and CatSper 2, almost all the eggs that showed pronuclear fusion, demonstrated abnormal cleavages, ultimately leading to degenerating embryos (unpublished data). These observations tempt us to hypothesize that Tektin 2 may not only contribute to sperm motility but also may regulate the process of fertilization and direct embryonic cleavages for the development of normal embryo. It will be necessary to investigate the levels of these proteins in spermatozoa of the male partners where a large number of poor quality embryos are obtained to prove this hypothesis. It will be of interest to identify the associating protein partners of Tektin 2 and CatSper 2 in the fertilized eggs and the early embryos to investigate their roles in early embryonic development.
Beyond fertilization rate and embryo quality Tektin 2 levels seem to be associated with pregnancy rate. The level of Tektin 2 were 32 % lower and CatSper 2 were 8 % lower in group NP which included male partners of couples not conceived after ICSI as compared to group P which included male partners of couples conceived after ICSI. It is possible that low levels of the protein leads to poor embryonic development in the uterus ultimately leading to an abortion.
From the above findings it appears that that Tektin 2 associates with ICSI outcome, in terms of fertilization rate, embryo quality and pregnancy rate, whereas CatSper 2 levels have an association with motility. Evidence indicates that CatSper proteins are needed only for sperm hyperactivated motility and no other developmental landmarks. CatSper null spermatozoa are sluggish and display less directed movements. Also CatSper is required for pentration of egg outer layers i.e. zona pellucida, but not for egg activation and fertilization [7, 35]. As against this, Tektin present in the axonemal microtubules and centrioles of the sperm tail, may play a role in the intra oocyte movement of the male pronucleus and contributes to the development of mitotic spindles and microtubule nucleation during cleavage stages. Both these functions are critical for the oocyte fertilization and embryo development [4, 39]. It will be necessary to expand this study in a larger group of infertile individuals to
In conclusion, the results of the present study demonstrate the expression of sperm tail proteins Tektin 2 and CatSper 2 are lower in immotile spermatozoa and spermatozoa of oligoasthenozoospermic men. In this limited study of a cross-sectional nature, the reduced levels of these proteins appear to associate with reduced fertilization rates, poor embryo quality and low pregnancy rate. It will be necessary to conduct large scale studies and determine if the levels of these proteins can be used as predictors of fertilization rates, embryo quality and pregnancy rate and hence serve as markers for predicting the success rate of ART in infertile men. Eventually poor spermatozoa motility may be treatable or even cured by, for example, by stimulating relevant signaling pathways or by genetic therapy, rather than bypassing it with ICSI. Viewed from a different angle knowledge of the molecules that are required to assemble and regulate a functional flagellum may allow us to intentionally disrupt the normal function of crucial, spermatozoa specific proteins and may result in the development of safe, effective male contraceptive.
Acknowledgment
We thank Dr Veena Bangera, Sister Benny Castellino, Sheetal Shah and the entire staff of INKUS INF CENTRE, for providing semen samples and also for their assistance and advice without which this work was impossible. We are also grateful to ICMR and the PhD students of Molecular and Cellular Biology Laboratory at NIRRH, Parel for excellent technical assistance.
Funding
We are extremely thankful to Jaslok Hospital for funding the project (Research project number 448).
Footnotes
Capsule
Sperm tail proteins Tektin 2 and CatSper 2 are lower in immotile spermatozoa and in the spermatozoa of oligoasthenozoospermic men. Reduced levels of these proteins appear to associate with reduced fertilization rates, poor embryo quality and low pregnancy rate in oligoasthenozoospermic men as compared to normozoospermic men. Large scale studies need to be conducted to determine if Tektin 2 and CatSper 2 can serve as markers for predicting the success rate of ART in infertile men.
References
- 1.Abid S, Gokral J, Maitra A, Meherji P, Kadam S, Pires E, et al. Altered expression of progesterone receptors in testis of infertile men. RBM Online. 2008;17:175–84. doi: 10.1016/s1472-6483(10)60192-7. [DOI] [PubMed] [Google Scholar]
- 2.Avenarius MR, Hildebrand MS, Zhang Y, Meyer NC, Smith Luke LH, Kahrizi K, et al. Human male infertility caused by mutations in the Catsper1 Channel Protein. Am J Hum Genet. 2009;84:505–10. doi: 10.1016/j.ajhg.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avidan N, Tamary H, Dgany O, Cattan D, Pariente AE, Thulliez M, et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur J Hum Genet. 2003;11:497–502. doi: 10.1038/sj.ejhg.5200991. [DOI] [PubMed] [Google Scholar]
- 4.Blerkom JV, Davis P, Merriam J, Sinclair J. Nuclear and cytoplasmic dynamics of sperm penetration, pronuclear formation and microtubule organization during fertilization and early preimplantation development in the human. Hum Reprod Updat. 1995;1:429–61. doi: 10.1093/humupd/1.5.429. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Cai ZM, Gui YT, Guo X, Yu J, Guo LD, Zhang LB, et al. Low expression of glycoprotein subunit 130 in ejaculated spermatozoa from asthenozoospermic men. J Androl. 2006;27:645–52. doi: 10.2164/jandrol.106.000562. [DOI] [PubMed] [Google Scholar]
- 7.Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, et al. CatSper 1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci. 2003;100:14864–8. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan CC, Shui HA, Wu CH, Wang CY, Sun GH, Chen HM, et al. Motility and protein phosphorylation in healthy and asthenozoospermic sperm. J Proteome Res. 2009;8:5382–6. doi: 10.1021/pr9003932. [DOI] [PubMed] [Google Scholar]
- 9.Chang XJ, Piperno G. Cross reactivity of antibodies specific for flagellar Tektin and intermediate filament subunits. J Cell Biol. 1987;104:1563–8. doi: 10.1083/jcb.104.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chetrit AB, Senoz S, Greenblatt EM, Casper RF. In vitro fertilization outcome in the presence of severe male factor infertility. Fertil Steril. 1995;63:1032–7. doi: 10.1016/s0015-0282(16)57543-8. [DOI] [PubMed] [Google Scholar]
- 11.Heredia JM, de Mateo S, Vidal-Taboada JM, Ballescà JL, Oliva R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod. 2008;23:783–91. doi: 10.1093/humrep/den024. [DOI] [PubMed] [Google Scholar]
- 12.Hinduja IN, Mehta RH, Gopalkrishnan K, Anandkumar TC. Manual for human in vitro fertilization – Embryo transfer and Gamete Intra-Fallopian Transfer, 1st ed. ICMR; 1991 pp 24–28.
- 13.Hinduja I, Zaveri K, Baliga N. Human sperm centrin levels & outcome of intracytoplasmic sperm injection (ICSI) - A pilot study. Indian J Med Res. 2008;128:606–10. [PubMed] [Google Scholar]
- 14.Hoshi K, Katayose H, Yanagida K, Sato A, Yazawa H. Intra cytoplasmic sperm injection using immobilizes or motile human spermatozoon. Fertil Steril. 1995;63:1241–5. doi: 10.1016/s0015-0282(16)57604-3. [DOI] [PubMed] [Google Scholar]
- 15.Iguchi N, Tanaka H, Nakamura Y, Nozaki M, Fujiwara T, Nishimune Y. Cloning and characterization of the human tektin-t gene. Mol Hum Reprod. 2002;8:525–30. doi: 10.1093/molehr/8.6.525. [DOI] [PubMed] [Google Scholar]
- 16.Jin JL, O’doherty AM, Wang S, Zheng H, Sanders KM, Yan W. Catsper 3 and Catsper 4 encode two cation channel like proteins exclusively expressed in the testis. Biol Reprod. 2005;73:1235–42. doi: 10.1095/biolreprod.105.045468. [DOI] [PubMed] [Google Scholar]
- 17.Kahraman S, Tasdemir M, Polat I, Islk AZ, Biberoglu K, Vanderzwalmen P, et al. Pregnancies achieved with testicular and ejaculated spermatozoa in combination with intracytoplasmic sperm injection in men with totally or initially immotile spermatozoa in the ejaculate. Hum Reprod. 1996;11:1343–6. doi: 10.1093/oxfordjournals.humrep.a019384. [DOI] [PubMed] [Google Scholar]
- 18.Kovalski N, Lamirande R, Gagnon C. Reactive oxygen species generated by human neutrophils inhibit sperm motility, protective effect of seminal plasma and scavengers. Fertil Steril. 1992;58:809–16. [PubMed] [Google Scholar]
- 19.Kruger TF, Menkveld R, Stander FSH, Lombard CJ, Van der Merwe JP, Zyl JA, et al. Sperm morphologic factors as a prognostic factor in in-vitro fertilization. Fertil Steril. 1986;46:118–1123. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lambard S, Galeraud-Denis I, Bouraima H, Bourguiba S, Chocat A, Carreau S. Expression of aromatase in human ejaculated spermatozoa: a putative marker of motility. Mol Hum Reprod. 2003;9:117–24. doi: 10.1093/molehr/gag020. [DOI] [PubMed] [Google Scholar]
- 22.Li H-G, Ding X-F, Liao A-H, Kong X-B, Xiong C-L. Expression of CatSper family transcripts in the mouse testis during post-natal development and human ejaculated spermatozoa: relationship to sperm motility. Mol Hum Reprod. 2007;13:299–306. doi: 10.1093/molehr/gam009. [DOI] [PubMed] [Google Scholar]
- 23.Li H-G, Ding X-F, Liao A-H, Zhou H, Xiong C-L. The expression and significance of Catsper1 in human testis and ejaculated spermatozoa. Asian J Androl. 2006;8:301–6. doi: 10.1111/j.1745-7262.2006.00132.x. [DOI] [PubMed] [Google Scholar]
- 24.Lipshultz L. Subfertility. In: Kaufman J, editor. Current urology therapy. Philadelphia: WB Saunders; 1980. [Google Scholar]
- 25.Liu J, Nagy Z, Joris H, Tournaye H, Smitz J, Camus M, et al. Analysis of 76 total fertilization failure cycles out 2732 ICSI cycle. Hum Reprod. 1995;10:2630–6. [PubMed] [Google Scholar]
- 26.Nagvenkar P, Zaveri K, Hinduja I. Comparison of the sperm aneuploidy rate in severe oligozoospermic and oligozoospermic men and its relation to intracytoplasmic sperm injection outcome. Fertil Steril. 2005;8:925–31. doi: 10.1016/j.fertnstert.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 27.Nagy ZP, Liu J, Joris H, Verheyen G, Tournaye H, Camus M, et al. The result of intracytoplasmic sperm injection is not related to any of the three basic sperm parameters. Hum Reprod. 1995;10:1123–9. doi: 10.1093/oxfordjournals.humrep.a136104. [DOI] [PubMed] [Google Scholar]
- 28.Nijs M, Vanderzwalmen P, Vandamme B, G-Bertin S, Lejeune B, Segal L, et al. Fertilizing ability of immotile spermatozoa after intracytoplasmic sperm injection. Hum Reprod. 1996;11:2180–5. doi: 10.1093/oxfordjournals.humrep.a019073. [DOI] [PubMed] [Google Scholar]
- 29.Nikpoor P, Mowla SJ, Movahedin M, Ziaee SA-M, Tiraihi T. Catsper gene expression in postnatal development of mouse testis and in subfertile men with deficient sperm motility. Hum Reprod. 2004;19:124–8. doi: 10.1093/humrep/deh043. [DOI] [PubMed] [Google Scholar]
- 30.Overstreet JW, Gould JE, Katz DF, Hanson FW. In vitro capacitation of human spermatozoa after passage through a column of cervical mucus. Fertil Steril. 1980;34:604–6. doi: 10.1016/s0015-0282(16)45204-0. [DOI] [PubMed] [Google Scholar]
- 31.Palermo GD, Adler A, Cohen J, Rosenwake Z, Alikani M. Intracytoplasmic sperm injection: a novel treatment for all forms of male factor infertility. Fertil Steril. 1995;63:1231–40. doi: 10.1016/s0015-0282(16)57603-1. [DOI] [PubMed] [Google Scholar]
- 32.Pirner MA, Linck RW. Tektins are heterodimeric polymers in flagellar microtubules with axial periodicities matching the tubulin lattice. J Biol Chem. 1994;269:31800–6. [PubMed]
- 33.Quill T, Ren D, Clapham DE, Garbers DL. A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci. 2001;98:12527–31. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quill TA, Sugden SA, Rossi KL, Doolittle LK, Hammer RE, Garbers DL. Hyperactivated sperm motility driven by CatSper 2 is required for fertilization. Proc Natl Acad Sci U S A. 2003;100:14869–74. [DOI] [PMC free article] [PubMed]
- 35.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–9. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy A, Lin YN, Agno JE, DeMayo FJ, Matzuk MM. Absence of tektin 4 causes asthenozoospermia and subfertility in male mice. FASEB. 2007;21:1013–25. doi: 10.1096/fj.06-7035com. [DOI] [PubMed] [Google Scholar]
- 37.Shah C, Modi D, Sachdeva G, Gadkar S, D’Souza S, Puri C. N-terminal region of progesterone receptor B isoform in human spermatozoa. Int J Androl. 2005;28:360–71. doi: 10.1111/j.1365-2605.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- 38.Siva AB, Kameshwari DB, Singh V, Pavani K, Sundaram CS, Rangaraj N, et al. Proteomics-based study on asthenozoospermia: differential ex-pression of proteasome alpha complex. Mol Hum Reprod. 2010;16:452–62. doi: 10.1093/molehr/gaq009. [DOI] [PubMed] [Google Scholar]
- 39.Steffen W, Linck RW. Evidence for tektins in centrioles and axonemal microtubules. Proc Natl Acad Sci. 1988;85:2643–7. doi: 10.1073/pnas.85.8.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takuya M, Yoshiko H, Masamichi D, Hiroshi I. Molecular Cloning of a now member of TEKTIN family, Tektin 4, located to the flagella of rat spermatozoa. Mol Reprod Dev. 2005;72:120–8. doi: 10.1002/mrd.20331. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka H, Iguchi N, Toyama Y, Kitamura K, Takahashi T, Kaseda K, et al. Mice deficient in the axonemal protein tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol Cell Biol. 2004;24:7958–64. doi: 10.1128/MCB.24.18.7958-7964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turhan N, Pekel A, Ayrim A, Bayrak O. ICSI outcome in severely oligoasthenozoospermic patients and its relationship to prewash progressive sperm motility. Turk J Med Sci. 2011;4:995–9. [Google Scholar]
- 43.Turner RM. Moving to the beat: a review of mammalian sperm motility regulation. Reprod Fertil Dev. 2006;18:25–38. doi: 10.1071/RD05120. [DOI] [PubMed] [Google Scholar]
- 44.Veeck LL. Pre embryo grading. Atlas of Human Oocytes and early conceptus, Vol 2. USA: Williams and Wilkins; 1991. pp. 121–44. [Google Scholar]
- 45.Vendrell JM, Arán B, Veiga A, García F, Coroleu B, Egozcue S, et al. Spermatogenic patterns and early embryo development after intracytoplasmic sperm injection in severeoligoasthenozoospermia. J Assist Reprod Genet. 2003;20:106–12. doi: 10.1023/A:1022626823328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verheyen G, Tournaye H, Staessen C, De Vos A, Vandervorst M, Steirteghem AV. Controlled comparison of conventional in-vitro fertilization and intracytoplasmic sperm injection in patients with asthenozoospermia. Hum Reprod. 1999;14:2313–9. doi: 10.1093/humrep/14.9.2313. [DOI] [PubMed] [Google Scholar]
- 47.WHO Laboratory manual for the examination of Human Semen and sperm-cervical mucus Interaction, World Health Organization, 4th ed. Cambridge University Press; 1999 pp 6–33 and 104–105.
- 48.Xia P. Intra cytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997;12:1750–5. doi: 10.1093/humrep/12.8.1750. [DOI] [PubMed] [Google Scholar]
- 49.Zhao C, Huo R, Wang FQ, Lin M, Zhou ZM, Sha JH. Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fertil Steril. 2007;87:436–8. doi: 10.1016/j.fertnstert.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 50.Zuccarello D, Ferlin A, Cazzadore C, Pepe A, Garolla A, Moretti A, et al. Mutations in dynein genes in patients affected by isolated non-syndromic asthenozoospermia. Hum Reprod. 2008;23:1957–62. doi: 10.1093/humrep/den193. [DOI] [PubMed] [Google Scholar]