Abstract
Purpose
To determine whether the use of Magnetic Activated Cell Sorting (MACS) as a sperm selection technique improves ART success rates in couples undergoing assisted reproduction treatment.
Methods
Systematic review and meta-analysis of prospective randomized trials. Two reviewers conducted study selection and data extraction independently.
Results
Five studies (prospective randomized trials) that comprised 499 patients were included. Sperm selection using MACS resulted in statistically significant differences in pregnancy rates when compared with density gradient centrifugation and swim-up techniques (RR = 1.50, 95 % CI 1.14–1.98). No differences were found between the groups according to the implantation (RR = 1.03, 95 % CI 0.80–1.31) and miscarriage (RR = 2.00, 95 % CI 0.19–20.90) rates.
Conclusions
MACS appears to be a safe and efficient method to select functional sperm with consistently good results. This technique may improve pregnancy rates when used to complement standard sperm selection methods in ART.
Keywords: MACS, Sperm selection, Annexin V, Pregnancy, DNA fragmentation, Meta-analysis
Introduction
Assisted reproductive techniques (ART) are used worldwide with increasing frequency because these techniques greatly benefit couples who have problems trying to conceive. Studies of infertile couples have demonstrated that male factor plays a major role in infertility. Basic semen analyses and standard methods for sperm selection, such as density gradient centrifugation (DGC) and swim-up (SU) techniques, have been used with good results. However, to improve the diagnosis and treatment of male infertility, basic semen analyses should be complemented with tests that provide data on sperm functionality. Sperm DNA fragmentation has recently become the most widely studied complementary test. Studies have demonstrated that sperm with genetic defects are directly associated with infertility [7]. However, there is controversy regarding the role of sperm DNA fragmentation in assisted reproduction techniques because this method detects late apoptosis in sperm, and understanding all of the stages of apoptosis is far more informative.
One of the early markers of apoptosis is the loss of membrane integrity, which leads to phospholipid phosphatidylserine externalization (a molecule with a high affinity for annexin V) [45]. Therefore, annexin V (used as an apoptotic sperm marker) conjugated with magnetic microspheres, which are exposed to a magnetic field in an affinity column, can separate apoptotic from non-apoptotic sperm. This procedure is called magnetic activated cell sorting (MACS). This technique was used in 1995 by Pesce and De Felici to isolate and purify the primordial germ cells (PGCs) from mouse embryos (MiniMACS Magnetic Separation System) [26].
Many studies have recently evaluated the use of MACS as a method to reduce apoptotic sperm and improve sperm and embryo quality. Based on the findings from these studies, other groups studied MACS as a sperm selection method for ART. Recent studies have recommended MACS selection regardless of DNA fragmentation results because apoptotic sperm is not exclusively associated with sperm DNA fragmentation [34].
This systematic review and meta-analysis was performed to summarize the available data regarding MACS as a sperm selection technique and determine the efficacy of the method to improve ART success rates. We hypothesized that there would be higher pregnancy rates in couples who undergo sperm selection using MACS compared with couples who do not.
Materials and methods
Search strategy to identify studies
Study selection was completed by two independent reviewers (M.G. and Y.M.). Discrepancies were resolved by group discussion and a consensus with a third reviewer (V.S.).
The following electronic databases were searched until August 2012: Medline, Embase, the Cochrane Central Registry of Controlled Trials (Central), and the Centre for Reviews and Dissemination databases (DARE HAT and NHS EE). A search strategy was generated based on the following terms: fertilization rate, live birth rate, ongoing pregnancy rate, miscarriage rate AND annexin V, MACS, sperm, DNA fragmentation, sperm selection, IVF, ICSI, AND “randomized controlled trials” OR “randomized controlled trials.” Furthermore, we examined the abstracts from major scientific meetings, e.g., the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine, and relevant fertility journals. Studies were selected in a two-step process. First, two reviewers independently reviewed the titles and the abstracts from the electronic searches. If a citation was likely to meet the selection criteria, the full text was obtained. The final inclusion and exclusion decisions were based on the examination of the entire manuscripts. The search was restricted to Spanish and English languages.
Study selection
We selected prospective randomized trials that examined couples who underwent ART with sperm that was selected using MACS compared with sperm that was selected using standard methods. The main outcome of interest was the pregnancy rate. Secondary outcomes were the implantation and miscarriage rates.
Statistical analysis
The occurrence of dichotomous events was expressed as a risk ratio (RR) with a 95 % confidence interval (CI) using a fixed effects model. The statistical significance was set at a P value <0.01. The heterogeneity between the studies was evaluated using a chi-square test and the I2 index. All the statistical analyses were conducted using Review Manager 5 software
Results
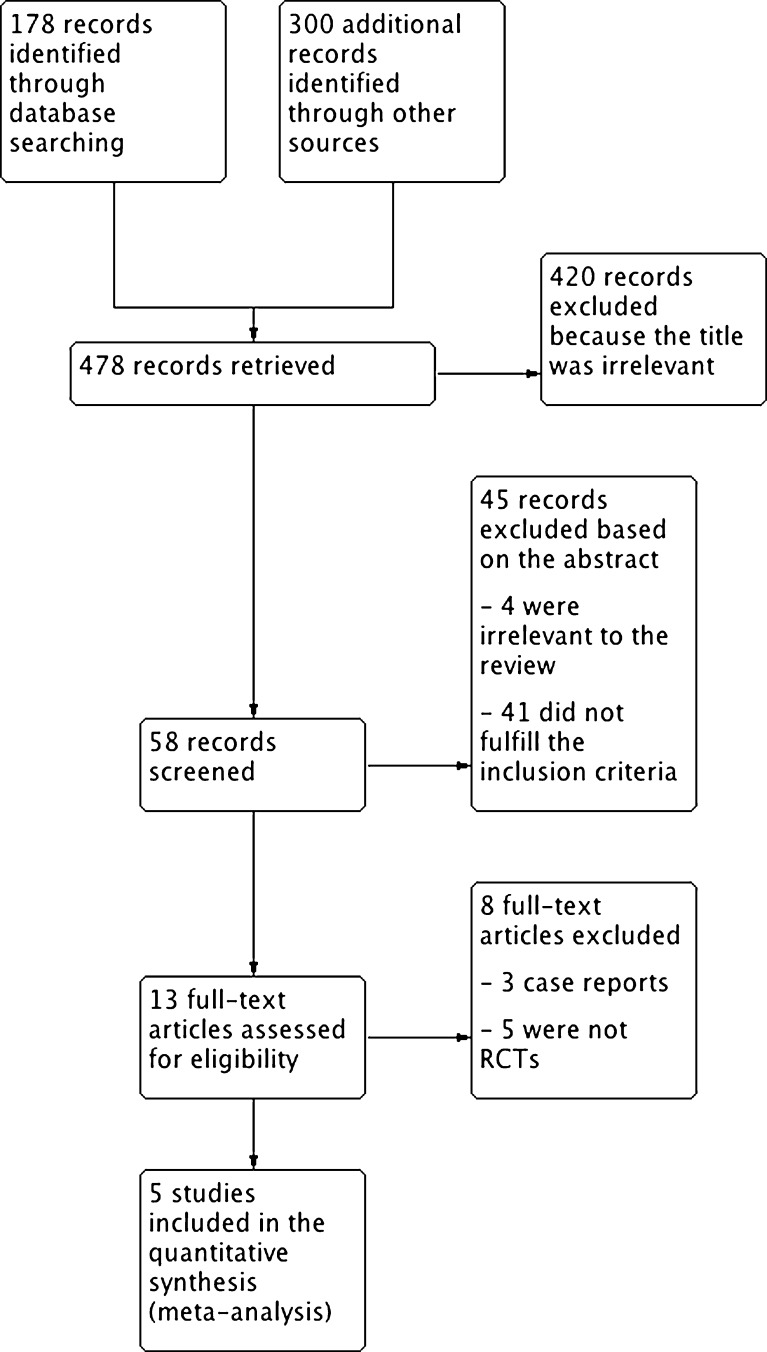
A total of 479 studies related to the topic were identified in the initial electronic search, 59 of which were considered eligible based on the title and the abstract. In a second step of the selection process, 45 studies were excluded based on the abstract. Studies were most commonly excluded because the outcomes of interest were not examined or the studies were not randomized. Of the 14 articles that were selected to be reviewed, five were included in the analysis (Fig. 1).
Fig. 1.
Flowchart of the trial identification and selection process
The five selected studies included 499 patients who underwent ART, 278 sperm samples that were treated using MACS (the study group), and 221 samples that were treated with either DGC or SU techniques (the control group). The characteristics of the selected studies are displayed in Table 1. Table 2 shows the methodological data from the clinical trials that were included in the review.
Table 1.
Characteristics of the clinical trials that were included in the review
| Author and year | Country | Description of the study | No. of patients | Inclusion criteria | Intervention | Outcome |
|---|---|---|---|---|---|---|
| San Celestino et al. 2011 | Spain | Prospective randomized study | 55 | Egg donors and frozen sperm samples from patients and donors | Study group: 27 patients with sperm preparation by DGC + MACS. | Pregnancy rate |
| Control group: 28 patients with sperm preparation only by DGC | ||||||
| Khalid and Quereshi 2011a | Pakistan | Prospective randomized study | 60 | Sperm count ≥ 20 million/ml and male age <40 years. Normal baseline early hormonal assays and female age <35 years with no uterine pathology | Study group: 30 patients with sperm preparation by DGC + MACS. | Occurrence of pregnancy (biochemical or clinical) and the miscarriage rate |
| Control group: 30 patients with sperm preparation only by DGC | ||||||
| Romany et al. 2010a | Spain | Prospective randomized double-blind study | 56 | Baseline FSH levels <10 mIU/ml, E2 levels <75 pg/ml, ovulatory menstrual cycles, age <38 years, no uterine abnormalities and >3 million sperm with progressive motility after swim up | Study group: 32 patients with sperm preparation by SU + MACS. | Pregnancy rate and the ongoing pregnancy rate |
| Control group: 24 patients with sperm preparation only by SU | ||||||
| Dirican et al. 2008 | Turkey | Prospective randomized study | 196 | Female: FSH levels <10 mIU/ml, E2 levels <75 pg/ml, ovulatory menstrual cycles, age <35 years, no uterine abnormalities or communicating hydrosalpinx, and no history of low or absent ovarian response during FSH/HMG treatment | Study group: 122 patients with sperm preparation by MACS. | Chemical and clinical pregnancy rates, and implantation rate |
| Male: The presence of oligozoospermia, asthenozoospermia, oligoasthenospermia, and/or teratozoospermia | Control group: 74 patients with sperm preparation only by DGC | |||||
| Romany et al. 2012a | Spain | Prospective randomized study | 132 | Female: 30–45 years of age, BMI <30 Kg/m2, undergoing their first ICSI cycle with oocyte donation. Embryo transfer was always performed 72 h after oocyte retrieval. | Study group: 67 patients with sperm preparation by SU + MACS | Implantation rate |
| Male: The presence of more than 1 million motile spermatozoa per ejaculate after SU | Control group: 65 patients with sperm preparation only by SU |
Table 2.
Methodological data from the clinical trials that were included in the review
| Criterion | Author, year | ||||
|---|---|---|---|---|---|
| San Celestino 2011 | Khalid 2011a | Romany 2010a | Dirican 2008 | Romany 2012a | |
| Explicit eligibility criteria | Yes | Yes | Yes | Yes | Yes |
| Sequence generation | Unclear | Unclear | Unclear | Unclear | Unclear |
| Allocation concealed | Unclear | Unclear | Unclear | Unclear | Unclear |
| Patient blinding | Unclear | Unclear | Unclear | No | Unclear |
| Outcome assessor blinding | Unclear | Unclear | Unclear | No | Unclear |
In all five studies, female factor was not considered. Two studies used semen samples diagnosed with male factor [9, 33], two studies used normal sperm samples [15, 35], and one study analyzed frozen sperm from patients and donors [42]. In two studies, the sperm selection technique for the control group was SU [33, 35], whereas DGC was used in the other three studies [9, 15, 42]. Two studies included couples who underwent intrauterine insemination (IUI) [15, 35], whereas the other three studies performed ICSI [9, 33, 42].
Outcomes of interest
Sperm selection using MACS in ART demonstrated statistically significant differences in the pregnancy rates when the study group was compared with the control group. No differences were found between the groups according to the implantation and miscarriage rates (Table 3).
Table 3.
Summary of outcomes
| Outcome | No. of participants (trials) | MACS | Control | Risk ratio (95 % CI) | P value |
|---|---|---|---|---|---|
| Pregnancy rate | 367 (4) | 96/211 (45.49 %) | 47/156 (30.12 %) | 1.50 (1.14–1.98) | 0.004 |
| Implantation rate | 834 (2) | 122/510 (22.18 %) | 80/324 (24.69 %) | 1.03 (0.80–1.31) | 0.82 |
| Miscarriage rate | 60 (1) | 2/30 (6.66 %) | 1/30 (3.33 %) | 2.00 (0.19–20.90) | 0.56 |
Pregnancy rate
The results from four trials (367 events) that reported pregnancy rate outcomes indicated a significantly higher pregnancy rate in patients who underwent sperm selection using MACS compared with those who received treatment without MACS (RR = 1.50, 95 % CI 1.14–1.98). The I2 value was 4 %, which indicates low heterogeneity between the studies (Fig. 2).
Fig. 2.
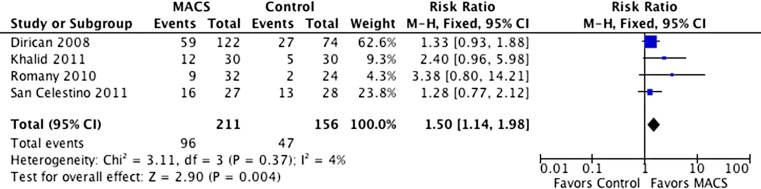
Pregnancy rates with and without sperm selection using MACS
Implantation rate
The results from two trials (834 events) that reported implantation rate outcomes indicated no statistically significant differences between the groups (RR = 1.03, 95 % CI 0.80–1.31). The I2 value was 43 %, which indicates low heterogeneity between the studies (Fig. 3).
Fig. 3.
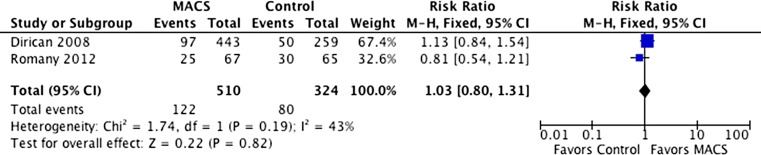
Implantation rates with and without sperm selection using MACS
Miscarriage rate
Only one trial that was included (60 events) reported miscarriage rates. The outcomes did not indicate any statistically significant differences between the groups (RR = 2.00, 95 % CI 0.19–20.90). Heterogeneity was not applicable to this outcome (Fig. 4).
Fig. 4.
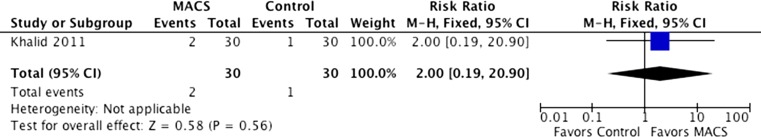
Miscarriage rates with and without sperm selection using MACS
Discussion
This systematic review and meta-analysis included the most recent prospective randomized trials that compared pregnancy implantation and miscarriage rates between MACS and conventional sperm selection techniques (SU and DGC). The results of this meta-analysis suggest that there was an improvement in the pregnancy rates when sperm was selected using MACS.
Our results are in agreement with those from other studies that reported an improvement in pregnancy rates using MACS [1, 6, 9]. Several authors did not include a control group in their studies; however, they reported good pregnancy rates (single and multiple ongoing pregnancies with normal cardiac activity) and healthy infants who were born with normal neonatal assessments [1, 27, 29, 31, 44, 46, 48]. This technique selects the best sperm; however, no evidence of a decline in miscarriage rates was found in our results. These findings are in contrast to those reported by Robinson et al. [32], which indicated a direct relationship between miscarriage rates and DNA damage.
MACS is an efficient method that can avoid apoptotic sperm during selection. MACS efficiently reduces sperm DNA fragmentation levels [13, 19, 29, 30, 40, 47, 48] and effectively separates apoptotic from non-apoptotic spermatozoa [6, 8, 11, 14, 17–20, 28, 29, 34, 37–39, 44]. This selection leads to an improvement in sperm quality and functionality [14, 19, 23] because MACS positively affects sperm motility [5, 8, 29, 34, 38, 41] and morphology as determined by the sperm deformity index [2–4, 38, 39, 41]. Several authors reported an improvement in fertilization rates [12, 36] and embryo quality [1, 9, 40] because the best sperms were selected using MACS compared with standard selection methods. Other authors found no differences in fertilization rates [1, 6, 9, 40].
Another benefit of MACS, which may improve ART results, is the efficient removal of the caspases that are present in human spermatozoa, which represent the main pathway of apoptosis [22]. Their removal enhances human sperm motility and cryosurvival rates following cryopreservation [10, 11, 20, 24, 25, 38, 40, 41]. An ideal sperm preparation method should select the best sperm from the ejaculate. Most studies demonstrated that double density gradient centrifugation (DGC) combined with MACS was the more advantageous sperm selection method [1–5, 7–9, 11–14, 16–18, 21, 25, 27, 29, 34, 36–40, 43, 46, 48].
To date, one of the most promising results of MACS was observed in the outcomes of couples with previous assisted reproduction failure. Studies that included IUI in couples with unexplained infertility [17, 18] and ICSI in patients with high sperm DNA fragmentation [27, 44, 48] concluded that the use of MACS would improve the results for couples with repeated assisted reproduction failure.
Even though there were no significant discrepancies between the results in these studies, and none of them considered the female factor; these studies did demonstrate considerable variability. This variability and the relatively small sample size in our study may be a limitation. Further research that includes live birth rates and a greater sample of patients is needed to determine the usefulness of the MACS technique as a clinically beneficial sperm selection method in assisted reproduction. In addition to well-designed prospective studies, we suggest that researchers should use a uniform study design under controlled conditions. The implementation of these recommendations should lead to better evidence regarding the role of MACS in ART.
This analysis is the first study of its kind to report pregnancy rates as a parameter of the efficacy of MACS in selecting non-apoptotic sperm.
In conclusion, the implantation and miscarriage rates did not vary between MACS or standard sperm selection methods; however, we did observe an improvement in pregnancy rates.
Footnotes
Capsule Sperm selection using magnetic activated cell sorting (MACS) is an efficient method to select functional sperm and improves pregnancy rates when used to complement standard sperm selection methods in ART.
References
- 1.Alvares Sedó C, Uriondo H, Lavolpe M, Noblia F, Papier S, Nodar F. Clinical outcome using non-apoptotic sperm selection for ICSI procedures: report of 1 year experience. Fertil Steril. 2010;94(Suppl 4):S232. doi: 10.1016/j.fertnstert.2010.07.902. [DOI] [Google Scholar]
- 2.Aziz N, Said T, Paasch U, Agarwal A. The relationship between human sperm apoptosis, morphology and the sperm deformity index. Hum Reprod. 2007;22(5):1413–1419. doi: 10.1093/humrep/dem016. [DOI] [PubMed] [Google Scholar]
- 3.Aziz N, Said TM, Paasch U, Grunewald S, Glander HJ, Agarwal A. Impact of apoptosis on sperm morphology indices. Fertil Steril. 2005;84(Suppl 1):S407. doi: 10.1016/j.fertnstert.2005.07.1064. [DOI] [Google Scholar]
- 4.Aziz N, Said TM, Paasch U, Grunewald S, Sharma RK, Agarwal A. The cause why apoptotic sperm have poor morphology profile as assessed by the sperm deformity index (SDI): a prospective study. Fertil Steril. 2006;86(Issue 3, Suppl 2):S506. doi: 10.1016/j.fertnstert.2006.07.1400. [DOI] [Google Scholar]
- 5.Bochev I, Gavrilov P, Kyurkchiev S, Shterev A, 27th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE) Sperm motility improvement after magnetic-activated cell sorting in men with astheno/oligo-asthenozoospermia. Hum Reprod. 2011;26(suppl 1):i133. [Google Scholar]
- 6.Buzzi J, Valcarcel A, Lombardi E, Oses R, Rawe V, Young E, 26th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE), Magnetic activated cell sorting (MACS) improves oocyte donation results associated to severe male factor infertility. Hum Reprod. 2010;25(suppl 1):i118–i152. [Google Scholar]
- 7.Cortés-Gutiérrez E, Dávila-Rodríiguez M, Lóopez-Fernández C, Fernández J, Gosáalvez J. Evaluación del daño en el DNA espermático. Actas Urológicas Españolas. 2007;31(2):120–131. doi: 10.1016/S0210-4806(07)73609-4. [DOI] [PubMed] [Google Scholar]
- 8.De Vantéry Arrighi C, Hervé L, Chardonnens D, Agostini A. Removal of spermatozoa with externalized phosphatidylserine from sperm preparation in human assisted medical procreation: effects on viability, motility and mitochondrial membrane potential. Reprod Biol Endocrinol. 2009;7(1):1–12. doi: 10.1186/1477-7827-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dirican EK, Özgün OD, Akarsu S, Okhan A, Özge E, Mukaddes U, et al. Clinical outcome of magnetic activated cell sorting of non-apoptotic spermatozoa before density gradient centrifugation for assisted reproduction. J Assist Reprod Genet. 2008;25:375–381. doi: 10.1007/s10815-008-9250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duru NK, Morshedi MS, Schuffner A, Oehninger S. Cryopreservation-thawing of fractionated human spermatozoa is associated with membrane phosphatidylserine externalization and not DNA fragmention. J Androl. 2001;22(4):646–651. [PubMed] [Google Scholar]
- 11.Grunewald S, Paasch U, Glander HJ. Enrichment of non-apoptotic human spermatozoa after cryopreservation by immunomagnetic cell sorting. Cell Tissue Bank. 2001;2:127–133. doi: 10.1023/A:1020188913551. [DOI] [PubMed] [Google Scholar]
- 12.Grunewald S, Reinhardt M, Blumenauer V, Said TM, Agarwal A, Hmeidan FA, Glander HJ, Paasch U. Increased sperm chromatin decondensation in selected nonapoptotic spermatozoa of patients with male infertility. Fertil Steril. 2009;92(2):572–577. doi: 10.1016/j.fertnstert.2008.07.1705. [DOI] [PubMed] [Google Scholar]
- 13.Herrero MB, Delbes G, Troueng E, Holzer H, Chan PTK, 28th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE) Differential enrichment of sperm with no DNA strand breaks using magnetic activated cell sorting (MACS) in men with various categories of semen parameters. Hum Reprod. 2012;27(suppl 2):ii121–ii150. doi: 10.1093/humrep/27.s2.73. [DOI] [Google Scholar]
- 14.Huang C, Lee T, Chen C, Wu G, Lee C, Lee M, 25th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE), Sperm preparation by magnetic-activated cell sorting improve the sperm-zona pellucida binding capacity and reduces apoptotic sperm. Hum Reprod. 2009;24(suppl 1):2147–i218. [Google Scholar]
- 15.Khalid SN, Qureshi IZ. Pregnancy rate improves in couples with unexplaines infertility following intrauterine insemination (IUI) with magnetically selected non-apoptotic sperms. Fertil Steril. 2011;96(3):S25. doi: 10.1016/j.fertnstert.2011.07.104. [DOI] [Google Scholar]
- 16.Khalid SN, Qureshi IZ. Effect of magnetic selected sperm on fertilization and embryo development: an animal model study. Fertil Steril. 2011;96(3):S169. doi: 10.1016/j.fertnstert.2011.07.659. [DOI] [Google Scholar]
- 17.Lee H, Liu C, Lee M. Sperm preparation by magnetic. Activated Cell Sorting (MACS) reduced apoptotic sperm and improved acrosome reaction for unexplained infertility. Fertil Steril. 2009;S143.
- 18.Lee TH, Liu CH, Shih YT, Tsao HM, Huang CC, Chen HH, Lee MS. Magnetic-activated cell sorting for sperm preparation reduces spermatozoa with apoptotic markers and improves the acrosome reaction in couples with unexplained infertility. Hum Reprod. 2010;25(4):839–846. doi: 10.1093/humrep/deq009. [DOI] [PubMed] [Google Scholar]
- 19.Losada C, Ortega I, Pacheco A, Bronet F, 28th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE) Can MACS as a sperm preparation technique improve clinic results in patients with Kartagener syndrome? Hum Reprod. 2012;27(suppl 2):ii162–ii205. doi: 10.1093/humrep/27.s2.77. [DOI] [Google Scholar]
- 20.Makker K, Agarwal A, Sharma RK. Magnetic activated cell sorting (MACS) Utility in assisted reproduction. Indian J Exp Biol. 2008;46:491–497. [PubMed] [Google Scholar]
- 21.Nadalini M, Tarozzi N, Di Santo M, Borini A, 27th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE), Annexin V magnetic-activated cell sorting versus swim-up for the selection of human sperm in ART: is the new approach better then the traditional one? Hum Reprod. 2011;26(suppl 1):i130. doi: 10.1007/s10815-014-0267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paasch U, Grunewald S, Agarwal A, Glander HJ. Activation pattern of caspases in human. Fertil Steril. 2004;81(Suppl 1):802–809. doi: 10.1016/j.fertnstert.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 23.Paasch U, Grunewald S, Fitzl G, Glander HJ. Deterioration of plasma membrane is associated with activated caspases in human spermatozoa. J Androl. 2003;24(2):246–252. doi: 10.1002/j.1939-4640.2003.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 24.Paasch U, Grunewald S, Wuendrich K, Jope T, Glander HJ. Immunomagnetic removal of cryo-damaged human spermatozoa. Asian J Androl. 2005;7(1):61–69. doi: 10.1111/j.1745-7262.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- 25.Paasch U, Sharma RK, Gupta AK, Grunewald S, Mascha EJ, Thomas A, Glander HJ, Agarwal A. Cryopreservation and thawing is associated with varying extent of activation of apoptotic machinery in subsets of ejaculated human spermatozoa. Biol Reprod. 2004;71:1828–1837. doi: 10.1095/biolreprod.103.025627. [DOI] [PubMed] [Google Scholar]
- 26.Pesce M, De Felici M. Purification of mouse primordial germ cells by MiniMACS magnetic separation system. Dev Biol. 1995;170:722–725. doi: 10.1006/dbio.1995.1250. [DOI] [PubMed] [Google Scholar]
- 27.Polak De Fried E, Denaday F. Single and twin ongoing pregnancies in two cases of previous ART failure after ICSI performed with sperm sorted using annexin V microbeads. Fertility and Sterility. 2010. doi:10.1016/j.fertnstert.2009.12.037. Elsevier Ltd. [DOI] [PubMed]
- 28.Rawe V. Columnas de anexina como metodología de selección de espermatozoides no apoptóticos durante lCSl. Reproducción Humana. 2009;6(1).
- 29.Rawe V, Alvares Sedó C, Uriondo H, Papier S, Miasnik S, Nodar F. ICSI outcome using annexin V columns to select non-apoptotic spermatozoa. Fertil Steril. 2009;92(Suppl 3):S73–S74. doi: 10.1016/j.fertnstert.2009.07.284. [DOI] [Google Scholar]
- 30.Rawe V, Álvarez G, Uriondo H, Papier S, Miasnik S, Nodar F. Separación magnética por columnas de anexinas V: “filtrado molecular” para la selección de espermatozoides no apoptóticos. Reproducción. 2009;24:104–114. [Google Scholar]
- 31.Rawe V, Uriondo Boudri H, Alvares Sedó C, Carro M, Papier S, Nodar F. Healthy baby born after reduction of sperm DNA fragmentation using cell sorting before ICSI a. Reproductive BioMedicine Online. 2010;20:320–323. doi: 10.1016/j.rbmo.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, Kirkman-Brown J, Coomarasamy A. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27(10):2908–2917. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 33.Romany L, Garrido N, De Los Santos MJ, Aparicio B, Pellicer A, Meseguer M, 9th Biennial Conference of ALPHA Scientists in Reproductive Medicine Effect of sperm selection by anexin-V sorting prior to ICSI in ovum donation program. Preliminary data. Reproductive BioMedicine Online. 2012;24(Suppl1):S2. doi: 10.1016/S1472-6483(12)60128-X. [DOI] [Google Scholar]
- 34.Romany L, Garrido N, Fernandez JL, Pellicer A, Meseguer M, 28th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE) Assisted reproduction results improvements due to magnetic activated cell sorting (MACS) depletion of apoptotic sperm is not mediated by sperm DNA fragmentation decrease. Hum Reprod. 2012;27(suppl 2):ii121–ii150. doi: 10.1093/humrep/27.s2.73. [DOI] [Google Scholar]
- 35.Romany L, Meseguer M, Garcia-Herrero S, Pellicer A, Garrido N. Magentic activated sorting selection (MACS) of nonapoptotic sperm (NAS) improves pregnancy rates in homologous intrauterine insemination (IUI). Preliminar data. Fertil Steril. 2010;94(4):S14. doi: 10.1016/j.fertnstert.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 36.Romany L, Meseguer M, García-Herrero S, Romero JL, Pellicer A, Garrido N, 26th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE) Magnetic activated sorting of non-apoptotic sperm result in improved embryo quality in ovum donation cycles with intracytoplasmic sperm injection. Hum Reprod. 2010;25(suppl 1):i8. [Google Scholar]
- 37.Said T, Agarwal A, Grunewald S, Rasch M, Baumann T, Kriegel C, Li L, Glander HJ, Thomas A, Paasch U. Selection of Nonapoptotic Spermatozoa As a New Tool for Enhancing Assisted Reproduction Outcomes: An In Vitro Model. Biol Reprod. 2006;74:530–537. doi: 10.1095/biolreprod.105.046607. [DOI] [PubMed] [Google Scholar]
- 38.Said TM, Agarwal A, Zborowski M, Grunewald S, Glander HJ, Paasch U. Utility of Magnetic Cell Separation as a Molecular Sperm Prepartion Technique. J Androl. 2008;29(2):134–142. doi: 10.2164/jandrol.107.003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Said TM, Aziz N, Paasch U, Grunewald S, Glander HJ, Agarwal A. Elimination of Apoptotic Sperm as a Measure for Enhancing Morphological Quality as Assessed by the Sperm Deformity (SDI) Fertil Steril. 2005;84(Suppl 1):448–449. doi: 10.1016/j.fertnstert.2005.07.1175. [DOI] [Google Scholar]
- 40.Said TM, Grunewald S, Paasch U, Rasch M, Agarwal A, Glander HJ. Effects of magnetic-activated cell sorting on sperm motility and cryosurvival rates. Fertil Steril. 2005;83(5):1442–1446. doi: 10.1016/j.fertnstert.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 41.Said TM, Land JA. Effects of advanced selection methods on sperm quality and ART outcome: a systematic review. Hum Reprod Updat. 2011;17(6):719–733. doi: 10.1093/humupd/dmr032. [DOI] [PubMed] [Google Scholar]
- 42.San Celestino M, Agudo D, Alonso M, Sanjurjo P, Becerra D, Bronet F, Garcia-Velasco JA, Pacheco A, 27th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE) Improved pregnancy rate after sperm magnetic separation technique in egg donation cycles using frozen sperm samples. Hum Reprod. 2011;26(suppl 1):i123–i148. [Google Scholar]
- 43.Tavalee M, Deemeh M, Arbabian M, Nasr-Esfahani M. Density gradient centrifugation before or after magnetic-activated cell sorting: which technique is more useful for clinical sperm selection? J Assit Reprod Genet. 2012;29:31–38. doi: 10.1007/s10815-011-9686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Thillo G, Guidobono M, Young E, Ruiz Jorro MR, Vila M, Rawe V. Biological safety and live births after selection of non-apoptotic spermatozoa during ICSI. Fertil Steril. 2011;96(Suppl 3):S160–S161. doi: 10.1016/j.fertnstert.2011.07.629. [DOI] [Google Scholar]
- 45.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger CP. A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression of early apoptotic cells using fluorescein labeled Annexin V. J Immunol Methods. 2005;184(1):39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- 46.Vilela M, Tiveron M, Lombardi C, Viglierchio MI, Marconi G, Rawe V, 27th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE) Implantation potential after vitrification of embryos obtained using magnetic active sperm sorting combined with Annexin V during ICSI. Hum Reprod. 2011;26(suppl 1):i200. [Google Scholar]
- 47.Winkle T, Ditzel N, Gagsteiger F. Possibilities of spremisolation by the MACS-System. Fertil Steril. 2006;86(Suppl 2):130–131.v. doi: 10.1016/j.fertnstert.2006.07.347. [DOI] [Google Scholar]
- 48.Young E, De Caro R, Marconi G, Lombardi E, Young E, Tiveron M, Valcarcel A, 26th Annual Meeting of the European Society on Human Reproduction and Embryology (ESHRE) Reproductive outcome using Annexin V columns for non-apoptotic sperm selection. Hum Reprod. 2010;25(suppl 1):i6–i9. [Google Scholar]