Abstract
Purpose
The objective of this study was to evaluate a panel of three sperm function tests; tests known to assess different aspects of sperm functionality and genomic integrity, the: 1) Sperm DNA Accelerated Decondensation (SDADTM) Test, 2) Sperm DNA Decondensation (SDDTM) Test, and 3) Sperm Penetration Assay (SPA), determining if positive and negative test scores correlated with failed and successful ICSI outcomes, respectfully.
Methods
A prospective, double blinded, cohort study was performed. One study sample (ejaculated semen) was collected by each of the 60 male partners of the 60 couples enrolled in the study; males whose female partners were found to have no major female factor issues. The sperm from each male was analyzed in the SPA, and SDAD and SDD Tests, and used for ICSI (1 ICSI cycle per couple).
Results
The ICSI cycle pregnancy rate for this study was 50 %, with a delivery rate = 40 % (n = 60 ICSI cycles). The SPA and SDD Test scores did not significantly predict ICSI outcome when used as stand-alone tests (p> > 0.05). However, when the SPA and SDD Test scores were used together, ICSI outcomes for a subgroup of 10 (16.7 %) males, were significantly predicted (p = 0.03), with 1 live birth, and 9 negatives where the transferred embryos did not implant. In total, 38.4 % of the couples in this study were found to have a very poor chance for a successful ICSI cycle.
Conclusion
SDAD Test scores alone, and SPA and SDD Test scores used together, significantly predicted failed ICSI outcomes. This indicates that the scores obtained when analyzing patients’ sperm using a panel of sperm function tests; specifically, the SPA, and SDAD and SDD Tests, can be used to identify infertile couples who should not be directed to ICSI.
Keywords: Sperm function testing, DNA integrity testing, Sperm Penetration Assay (SPA), Sperm DNA Accelerated Decondensation (SDAD) Test, Sperm DNA Decondensation (SDD) Test, Intracytoplasmic Sperm Injection (ICSI), Assisted Reproductive Technology (ART)
Background
Couple infertility is estimated to affect 1 in 7 couples (80 million) worldwide [10], with 1 in 4 couples in western countries seeking treatment for their involuntary childlessness (Dunson [20]). Approximately 50 % of couple infertility is predominantly or partially attributed to the male [54].
Male contribution to couple infertility was largely ignored until the late 1970s when the age of assisted reproductive technology (ART) treatment began when Louise Brown, the first in vitro fertilization (IVF) baby was born. As clinics all over the world began using IVF to treat infertile couples, specifically treating tubal infertility, it was noted that when using sperm from males with abnormal semen parameters, that fertilization rates were not predictable, with reported failure rates of 40 % or higher [18]. The evaluation of male fertility based only on sperm concentration, motility and morphology [54] began to be questioned.
When IVF babies became a reality in the late 1970s, and intracytoplasmic sperm injection (ICSI) babies followed in the early 1990s [42]; this stimulated a rapid increase in the use of ART throughout the past decade including use of intrauterine insemination (IUI), in vitro fertilization (IVF), and/or intracytoplasmic sperm injection (ICSI; [8, 20]). However, once again, the use of updated traditional semen parameters described in the 5th edition of the 1987 World Health Organization manual on semen analysis [55], have not been useful predictors of infertility [11, 26], with limited utility for directing couples to appropriate ART including: IUI, IVF-ET, or ICSI [17, 39].
As use of ART has been steadily increasing, there is a need for new tests, and re-evaluation of older tests that evaluate the integrity of the sperm DNA, and the many sperm functions required for the fertilization of the oocyte, and the post-fertilization events that must occur for the fertilized egg to have a live birth outcome; tests with capacity to predict the ‘best’ ART for a couple based on their infertility profile [17, 30, 40, 51]. Of the many DNA integrity tests developed in the past decade, only the sperm chromatin structure assay (SCSATM) first described by [21], has the capacity to predict IUI outcome, but not IVF or ICSI outcome [16, 17].
While DNA integrity tests were being developed and tested for capacity to predict ART outcomes, sperm function tests were also being developed with the same goal. Two such sperm function tests; the Sperm Penetration Assay; SPA [46], and the Sperm DNA Decondensation (SDDTM) Test [13, 14], were developed and tested for capacity to predict ART outcome.
In 1976, Yanagamachi and colleagues demonstrated the ability of human sperm to bind and penetrate the zona-free hamster oocyte [56]. After removal of the zona pellucida, hamster oocytes lose their species specificity with regards to human sperm [57]. Soon thereafter, these findings led to the development of a human male fertility test; the Sperm Penetration Assay (SPA) that was shown to have use in predicting the fertilization potential of human sperm to fertilize human oocytes [46]. The SPA evaluates aspects of the ability of human sperm to complete certain processes necessary to achieve fertilization using zona-free hamster oocytes. These processes include capacitation, proacrosin/acrosin conversion, nuclear (chromatin) decondensation, acrosome reaction and sperm-oocyte fusion [9].
Many studies have found significant correlation between SPA results and pregnancy outcome with IVF or controlled ovarian hyperstimulation [27, 35–37]. However, several investigators have noted that false positive results are a problem in the SPA. A number of sperm samples prepared with chymotrypsin (a proteolytic enzyme) have an increased penetration rate in the SPA. Likewise, when sperm are processed with a gradient as described in Materials and Methods, an increase in penetration rate in the SPA is seen that in effect eliminates approximately 80 % of the false positive results (unpublished data). The true positive SPA appears to correlate with pregnancy outcome with IUI and IVF [35–37].
The Sperm DNA Decondensation (SDDTM) Test is a trademark name for the part of the Human Sperm Activation Assay sperm chromatin decondensation event; one of several sperm activation events that are assessed in vitro by incubating permeabilized human sperm in frog egg extract mimicking the environment the sperm nucleus would enter post-fertilization [13, 14]. The SDD Test assesses the sperms capacity to reformat the DNA after entering an ovum [13, 14]. This event is a critical step in fertilization, during which protamine disulphide bonds are reduced to SH and the polycationic protamines combine with the polyanionic egg protein, nucleoplasmin, thus being stripped from DNA; DNA that then combines with histones, allowing the formation of a pronucleus. As the DNA decondenses, the DNA is reformatted such that upon combining with the female’s DNA during syngamy, and cleavage, resulting in a 2-cell stage embryo and the developmental program set in motion [33].
It is important that the tests chosen for the panel have utility in evaluating the male’s overall reproductive health that can be negatively affected by exposure to a wide variety of agents including: drugs, oxidative stress, cigarette smoking, environmental toxins, and even use of cell phones [1, 2, 4, 6, 7, 50]. Males have a complete turnover of their sperm every 3 months. If the couple’s infertility is male factor related; and identified during the male fertility work-up, many male reproductive challenges are reversible, and can be corrected in 3 months by removing the male from exposure to reproductive toxicants, or by a change in life style, or in the case of oxidative stress given a daily dose of anti-oxidants [3].
The SDD Test has been applied successfully using both human and rat sperm to detect damage by environmental toxicants, specifically, alkylating agents. The SDD Test was used to evaluate effects of alkylating agents on sperm from a fertile male exposed in vitro to alkylating agents as compared to the normal decondensation observed in untreated sperm from the same male. The SDD Test was also used to evaluate effects of cyclophosphamide, an alkylating agent known to adversely affect male fertility, on sperm from untreated and treated rats. Both, in vitro or in vivo exposure of sperm to alkylating agents significantly diminished sperm DNA decondensation [48, 49].
When evaluating if the SDD Test would also have utility in assessing the effects of in vitro exposure of normal sperm from fertile males to oxidative stress (reactive oxygen species; ROS), unlike what was found for alkylating agent exposure, sperm DNA decondensation was found to be significantly accelerated; not diminished [52]. The SDD Test that measures delayed or blocked decondensation was modified as described in the Materials and Methods section to measure accelerated sperm DNA decondensation. This new sperm function test, a novel test for sperm with ROS damage, is called the Sperm DNA Accelerated Decondensation (SDADTM) Test.
In a recent report, data was presented showing a significant increased risk of birth defects in the offspring from couples who had successful ICSI attempts at pregnancy; a risk not found for the offspring from couples with successful IUI, and/or IVF-ET attempts at pregnancy [19]. This supports using clinically relevant sperm function tests pre-ART; tests performed to detect males producing sperm with compromised functionality and/or genomic integrity. Without such pre-ART testing, especially in the cases of IVF and ICSI attempts at pregnancy, the laboratory will be providing the treating physician with what appear to be viable embryos for transfer; embryos that will not develop to term. Unlike the DNA integrity tests, the SPA, and SDD Test tests predict IUI and IVF-ET outcomes [13, 14, 35, 36]. The SDAD and SDD Tests, and the SPA are sperm function tests that analyze the sperm parameters related to overall sperm functionality as well as genomic integrity, in a physiologically relevant environment.
Based on the differences between sperm function and DNA integrity testing, the hypothesis tested in this study was that the SPA, and SDAD and SDD Tests would predict ICSI outcome. The SDAD Test scores were found to significantly predict ICSI outcome. When the SPA and SDD Test were evaluated as stand-alone tests, the SPA and SDD Test scores did not predict ICSI live birth outcome in couples with normal SDAD Test scores. However, when the SPA and SDD Test scores were used together, ICSI outcome for a subgroup of these couples could be predicted. This has far reaching implications for taking a panel approach when determining infertile couples’ treatment paradigms.
Materials and methods
Study design: a double blinded prospective study
Infertile Couple Selection
60 infertile couples with no major female factor, who met all inclusion criteria had a semen sample analyzed in the SPA, SDD and SDAD Tests; the same sample that was be used in an ICSI attempt at pregnancy. Patients were recruited for the study under an IRB approved protocol.
Inclusion criteria
Female partner did not have: a) current infection with microorganisms: viral, bacterial, or fungal, known to be associated with female infertility, b) autoimmune disease (lupus, RA, MS, Diabetes, Hashimoto, etc.) determined while interviewing the infertile couple, and c) inflammatory/autoimmune/coagulation blood feature abnormalities assessed by blood work including tests for Lupus anti-coagulant (LAC), Anti cardiolipin Antibodies (ACA), Anti phospholipid antibodies (APA), Natural Killer Cells (NK), Reproductive immunophenotyping (RIP), Anti Microsomal Antibodies (thyroid marker) (AMA) and Factor V (coagulation). Rationale: minimize female factor.
Female age: 25–40.
Male Age 25–45.
Oligo- or normospermic male with >5 million total motile sperm. Rationale: to obtain one ejaculate per patient with the total number of sperm being sufficient to perform the SPA, SDD and SDAD Tests, and 1 ICSI cycle.
Exclusion criteria
Age: Patients/donors younger than 25 y or older than 45 years.
Couples where the female’s blood work identified any of the above female factor conditions.
Female Blood Work-up
Blood was sent to Repromedix, Woburn, MA, up to 6 weeks prior to beginning the female’s cycle for an ICSI attempt at pregnancy. One plasma sample was centrifuged and separated, then sent frozen for the Factor V and LAC tests; 1 heparinized whole blood sample, and 1 test-tube with serum were sent by overnight Fed-Ex, for the cellular immunology -NK and RIP tests, and the ACA, APA and AMA tests, respectively.
Semen Sample Used for the SPA, SDD and SDAD Tests, and the ICSI Attempt at Pregnancy
1 semen sample was collected by masturbation from each of the 60 patients enrolled in the study. Ejaculated semen was liquefied by incubating up to 1 h at room temperature (not less than 22 °C). Specimens were evaluated on-site in the andrology lab for basic parameters, and then prepared for analysis in the SPA and SDAD and SDD Tests, and for ICSI attempts at pregnancy (See Below).
The semen from the 60 patients was split into 3 aliquots: Aliquot A) SPA, ~ 2 million cells with the assays performed at the ART Fertility Program of Alabama; Aliquot B) SDD and SDAD Tests, ~ 2 million cells sent to Repromedix where the tests were performed, and Aliquot(s) C) aliquots that were cryopreserved for future use in ICSI attempts at pregnancy at the ART Fertility Program of Alabama.
Blinding and Unblinding the Study
All samples sent to Repromedix for analysis in the SDD and SDAD Tests, were double blinded at the ART Fertility Program of Alabama clinic. The data collected at the ART Fertility Program of Alabama clinic, and at Repromedix were combined and tabulated on an excel spread sheet for statistical analysis (See Below), after un-blinding patient results 13 weeks after the ICSI attempt at pregnancy. Only the patient’s initials and date of birth information were provided to Repromedix for accessioning samples, tracing, and recording patient results. After un-blinding the study, the patients’ pregnancy outcomes and child assessment results were disclosed to Repromedix as soon as the results were available; results used in the comprehensive statistical analyses described below.
SPA
The SPA was performed as a modified version of the Johnson et al. [27] protocol as described below.
Semen Preparation
Semen was washed and passed through a gradient of ALGradTM 90 %, to separate the human sperm cells from dead sperm, debris, and other sperm cells, following the manufacturer’s instructions (LifeGlobal, an IVF Online company, www.ivfonline.com). The ‘best’ sperm from the gradient were resuspended in sperm wash medium, and diluted 1:1 with Test Yolk Buffer (TYB).
The sperm/TYB mixture was slowly cooled to 2–8 °C and stored at this temperature for 2–3 days. Then, sperm wash medium at 37 °C was added to the cold sperm/TYB mixture, providing a thermal shock. After the sperm/TYB was incubated for 30 min at 37 °C, the mixture was centrifuged for 10 min at 600 g. The supernatant was removed, up to 1.0 mL sperm wash medium added, and the sperm allowed to incubate for 60 min at 37 °C. The sample was then assessed for motility, and the concentration adjusted to 5million total motile sperm/ml.
Controls
For each assay, one negative and one positive (as previously determined) control were run in parallel with the samples from the patients enrolled in the study.
Ova Preparation
Frozen hamster ova were utilized for the SPA. Straws containing ova were thawed at RT for 2 min in a horizontal position. The straws are then shaken at the crimped end to vigorously mix the sucrose column with ova. The straws were then incubated, cotton end down, in a 37 °C water bath for 3 min followed by 3 min at RT, cotton end up. Using a pushrod, the ova were then dispensed into a 35 mm culture dish and washed 2X in sperm wash medium. The ova were transferred to fresh sperm wash medium to rest for 10 min at RT. The ova were then washed 3X in trypsin (1 %) and incubated at RT in the 3rd trypsin drop until the zona was almost depleted (<5 min). The ova were then washed twice in sperm wash medium. The ova were then transferred to fresh sperm wash medium to rest for 5 min at RT.
Sperm/Ova Incubation and Scoring
Six - seven ova were placed into each of two 100 ul drops of sperm wash medium covered with oil containing 250,000 total motile sperm and allowed to incubate for 3.5 h at 37 °C. After incubation, the ova were washed in sperm wash medium to dislodge any loosely bound sperm. The ova were then placed on a microscope slide with cover slip applied so to flatten the ova for penetration assessment. The number of penetrations were counted (a clear zone with discernible tails), and the Sperm Capacitation Index (SCI) calculated by dividing the total number of penetrations by the total number of ova scored.
SDAD and SDD Tests
The SDAD and SDD Tests were performed following the published protocols [13–15].
Semen sample aliquots
were kept in a refrigerator at 2–8 °C, until shipped to Repromedix overnight, non-frozen, using a cold pack shipper no later than 9 days after the sample collection date. The samples were analyzed in the SDAD and SDD Tests within 14 days after sample collection as described below. In previous studies [13, 14]; unpublished QC data), and in this study (see Results: Reproducibility of the SPA, SDAD and SDD Tests), it was determined that fresh samples were not needed for analysis in either the SDAD or SDD Tests, as samples could be analyzed at any time up to 1 month post-sample collection without a significant change in the test results obtained.
Egg Extracts
Female Xenopus Laevis Oocyte positive frogs were maintained at Repromedix and the frog egg extracts prepared every 6 weeks. The extracts were immediately ‘snap’ frozen in liquid N2 (LN) for storage until thawed immediately before performing the sperm DNA decondensation tests.
Performing the SDAD and SDD Tests
For each patient sample, 2 million sperm were washed and then permeablized with lysolecithin. After 4 extensive washes with special buffers, the sperm were treated with dithiotreitol (DTT)-containing buffer. The treated sperm were then incubated with frog egg extract in a sealed 0.5 ml plastic tube at room temperature (22 °C), to induce sperm DNA decondensation.
After a 5 min incubation (SDAD Test), and a 15 min incubation (SDD Test) of permeabilized sperm mixed with frog egg extract, an aliquot of sperm-egg extract mixture was removed from the incubation tube, and placed on a glass slide, and a coverslip was placed on top of the aliquot. During a 5 min window, 50–100 sperm were scored in real time using phase contrast microscopy. The percentage of fully decondensed sperm was determined for sperm from a fertile male negative control sample, and at 5 min (SDAD Test; routinely 70.9 ± 2 % (SD) were fully decondensed), and at 15 min (SDD Test; 95.0 ± 1.42 % (SD) were routinely fully decondensed). This is repeated for each patient sample, and the patient results reported as % of the fertile male negative control sperm that were fully decondensed at 5 min (SDAD Test score), and 15 min (SDD Test score). Note: patients with accelerated DNA decondensation, the % of fully and hyper-decondensed was determined.
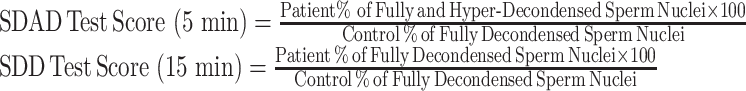 |
Controls
For each test one negative control and one positive control was run in parallel with the patient study samples analyzed during each run. The negative control specimen was from a fertile male, and was stored in a refrigerator for up to 1 month with insignificant variance in both the SDAD and SDD Test scores (See Results). A positive control sample was analyzed for each SDAD and SDD Test run, using a patient sample determined to have a positive SDD Test score, and a normal SDAD Test score in a previous run. These samples were stored in a 4 °C refrigerator for up to 1 month before analyzing in the SDAD and SDD Tests, with no significant difference found between the initial test scores obtained that identified the positive control sample, and the test scores obtained on Weeks 1–4 when used as the positive control before analyzing the patients’ samples (data not shown).
ICSI
Thawed frozen aliquots of the same sperm samples analyzed in the SPA, and SDAD and SDD Tests, were processed using the density gradient described above, and conventional ICSI performed for each of the 60 couples enrolled in the study. The live birth rate obtained using frozen sperm (study samples) was 40 %; the same live birth rate obtained for the clinic’s patients when using fresh sperm, excluding the possibility that the results observed during this study were influenced by the use of sperm that had been frozen before use in ICSI cycles.
Photography
All photographs were taken using phase contrast microscopy in real time using a 40X phase contrast objective, and a Nikon Cool Pix 4500 digital camera. The Nikon View Imaging software was used to prepare the microscopic images shown in Figs. 1, 2, 3 and 4. The images were put together as Figs. 1, 2, 3 and 4 using Microsoft Powerpoint software.
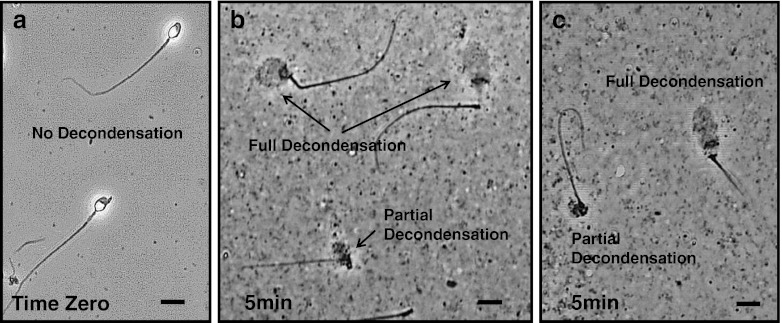
Fig. 1.
Phase contrast microscopic images of permeabilized fertile male sperm (negative control) at Time Zero, and sperm nuclei after a 5 min incubation in egg extract. All images are at the same magnification: Bar = 15 microns. Panel a Permeabilized negative control sperm from a fertile male at Time Zero, before mixing with egg extract. Panels b and c Typical examples of partially and fully decondensed negative control sperm nuclei after a 5 min incubation in egg extract
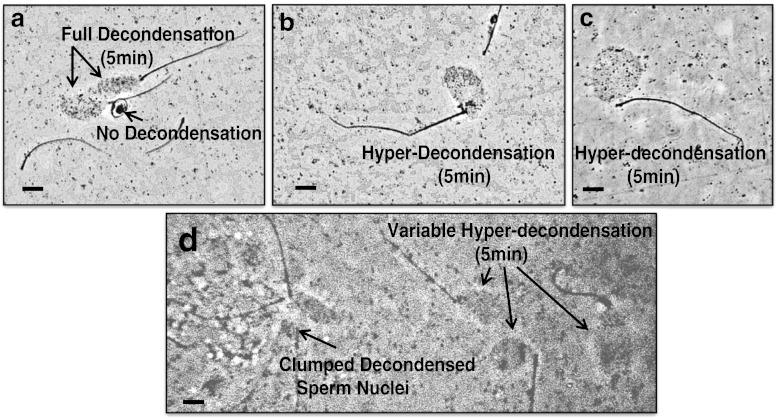
Fig. 2.
(Panels a-d): Microscopic images of a patient’s sperm with a positive (abnormal) SDAD Test score = 124.7 % of the negative control with fully decondensed sperm nuclei after a 5 min incubation in egg extract. All images are at the same magnification: Bar = 15 μm. Panel a: Microscopic images of 2 fully decondensed sperm nuclei by a phase dark sperm with no decondensation, and the sperm’s tail wrapped around the sperm head. Panels b and c: Typical images of hyper-decondensed sperm nuclei. Panel d: Clumped decondensed sperm nuclei, and variable hyper-decondensed sperm nuclei, all in the same field of view
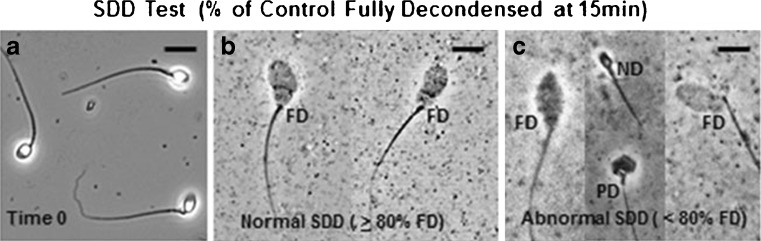
Fig. 3.
Microscopic images of typical sperm nuclear decondensation observed for patients with normal, or abnormal SDD Test scores, after 15 min incubations in egg extract. All images are at the same magnification: Bar = 15 microns. Panel a: Permeabilized negative control sperm from a fertile male at Time 0, before mixing with egg extract. Panel b: Fully decondensed (FD) negative control sperm nuclei after a 15 min incubation in egg extract where 97.9 % of the sperm nuclei were fully decondensed. This is representative of the fully decondensed sperm nuclei observed when scoring patients’ found to have normal SDD Test scores. Panel c: Microscopic images of sperm from a patient with an SDD Test score = 62.0 % of the negative control with fully decondensed sperm nuclei after a 15 min incubation in egg extract. Thirty eight percent of this patient’s sperm nuclei had either no decondensation (ND) or partial decondensation (PD)
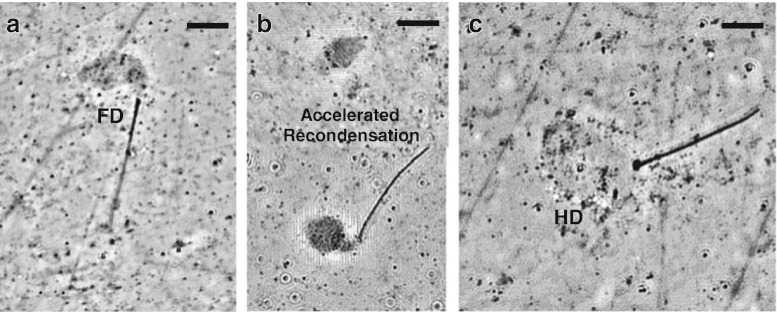
Fig. 4.
Pictorial representation of decondensed sperm nuclei of a patient with an abnormal SDAD Test score ≥ 120 % of the negative control (Fig. 2); with a normal SDD Test score ≥ 80 % of the negative control after a 15 min incubation in egg extract. Panels a and b: Fully decondensed sperm nuclei, and variable recondensation (99.7 % of the negative control). Panel c: Typical very hyper-decondensed sperm nucleus; such sperm nuclei were never observed to recondense. Note: these sperm are always very phase light with little contrast
Statistical Analysis
Data presented in the Results section, were analyzed using SAS (Version 9, SAS Institute, Inc., Cary, NC). Descriptive values were expressed as means and as proportions. The Chi-Square or Fisher’s Exact tests were used in analyzing categorical data evaluating the hypothesis that positive SPA, or SDAD or SDD Test scores, or combined SPA and SDD Test scores had utility for predicting the results from ICSI attempts at pregnancy, specifically live birth outcome. Positive Predictive Values (PPV), Negative Predictive Values (NPV), sensitivity, and specificity were calculated for the SPA and SDD and SDAD Test scores, and for combined SPA, and SDAD and SDD Test scores. The T-Test was used to analyze continuous data for differences between groups. Multiple logistic regression analyses were performed to identify factors that correlated significantly with positive SDAD Test scores found to predict ICSI live birth outcome. P values of < 0.05 were considered statistically significant.
Results
Determining if the SDAD test, SDD test, and/or SPA have utility in predicting ICSI outcome: study overview
A prospective, double blinded, cohort study was performed. A total of 70 couples were enrolled in the study who met all inclusion criteria described in Materials and Methods, with the female blood work-up pending. Both semen from the male, and blood samples from the female, for all 70 couples, was analyzed as described in the Materials and Methods. The female was tested for inflammatory, autoimmune, and coagulation blood abnormalities known to be indicators of female factor related infertility. Two couples withdrew before having an ICSI attempt at pregnancy. Upon receiving the blood results, couples where the female was found to have female factor related infertility were excluded from the study, thus minimizing failed ICSI attempts at pregnancy related to female factor issues. A total of 60 couples met all inclusion criteria; couples with minimal female factor issues.
The sperm from each semen sample from the 60 males in the study, were analyzed in the SPA, and SDAD and SDD Tests, and were also used in one ICSI cycle per couple as described in the Materials and Methods section. Data was collected and evaluated testing the hypothesis: positive and negative SPA, SDAD and SDD Test results significantly correlate with failed or successful ICSI outcomes, respectively.
SPA, and SDAD and SDD test scores: positive (Abnormal) and negative (Normal) cut-off values
For the 60 ICSI cycles performed, the cycles were grouped according to the SCI scores obtained when analyzing gradient purified sperm in the SPA; abnormal (SCI < 14) or normal (SCI ≥ 14). The SDAD Test Scores were grouped as: abnormal (SDAD scores ≥120) or normal (SDAD scores <120). The SDD Test Scores were grouped as: abnormal (SDD scores < 80) or normal (SDD ≥ 80). Both the SPA and SDD Test normal cut-off scores were determined using previous outcome data from cycles other than ICSI, including IVF and IUI [2, 13, 14, 35].
The rationale for the SDAD cut-off value of 120 was based on using a SDAD Test score cut-off value of 118 and 120, where 2 or 0 false positives were found when SDAD Test scores were ≥ 118 or ≥ 120, respectively. The p value for the 118 cut-off value was significant (< 0.05), with a significant positive predictive value (PPV) of 86.7 % (Table 1). The cut-off chosen for this study was 120 where there were no false positives; this being the most stringent cut-off value with a 100 % PPV for ICSI failure (true positives), and a 100 % specificity for live birth outcome (true negatives; Table 1).
Table 1.
Determination of the most stringent SDAD Test score cut-off value for predicting ICSI failure
| SDAD (2 False Positives) (N = 60, Critical value = 118) | SDAD (0 False Positives) (N = 60, Critical value = 120) | ||||
|---|---|---|---|---|---|
| Score | N | Failed ICSI | Score | N | Failed ICSI |
| Negative (<118) | 45 | 23 | Negative (<120) | 47 | 23 |
| Positive (≥ 118) | 15 | 13 | Positive (>120) | 13 | 13 |
| P-Value* | P < 0.05 | P-Value* | P < 0.001 | ||
| PPV** Highly Predictive of True Positives | 86.7 % | PPV** Highly Predictive of True Positives | 100 % | ||
| Odds Ratio*** | 1.8 | Odds Ratio | 1.8 | ||
| Sensitivity 41.7 % | Not Predictive of False Negatives | Sensitivity 36.1 % | Not Predictive of False Negatives | ||
| Specificity 91.7 % | Highly Predictive of True Negatives | Specificity 100 % | Highly Predictive of True Negatives | ||
* The difference is statistically significant if P-value < 0.05
** PPV positive predictive value
*** OR Odds Ratio = Odds of ICSI failure when test is positive in ratio to odds of ICSI failure if test is negative
Determination of SPA (SCI) Scores
For each of the SPAs performed during the study, gradient prepared ‘best’ sperm from a positive and negative control sample, and from the study sample were obtained. The ‘best’ positive and negative control sperm were run in parallel with the ‘best’ sperm from males enrolled in the study, and the SCI determined as described in Materials and Methods.
Determination of SDAD (5 min) and SDD (15 min) test scores
Permeabilized sperm from a fertile male, and from each of the 60 patients enrolled in the study, were incubated in egg extract for 5 min (SDAD Test), and 15 min (SDD Test). Aliquots were taken at each time point, wet mounts prepared, and the % of fully decondensed sperm nuclei determined scoring 50–75 sperm nuclei in a 5 min window, using phase contrast microscopy. The SDAD and SDD Test scores were then calculated as described in Materials and Methods.
SDAD Test (5 min incubation in egg extract)
Throughout the course of this study, the % of fully decondensed fertile male sperm nuclei (negative control) was determined in each of the 41 SDAD Tests performed, before analyzing the patient samples. The average SDAD Test score was 70.9 ± 2 % SD of the negative control sperm with fully decondensed nuclei, with 29.1 % of the nuclei partially decondensed. Typical examples of the partially and fully decondensed negative control sperm nuclei scored are shown in Fig. 1.
Typical phase contrast microscopic images of the sperm nuclei scored when determining the SDAD Test score of a patient positive for accelerated DNA decondensation are shown in Fig. 2. The % of fully and hyper-decondensed sperm nuclei was determined for each patient sample after 5 min incubations in egg extract. The SDAD Test score was determined and presented as % of the negative control.
 |
Patients positive for accelerated DNA decondensation had fully and hyper-decondensed sperm nuclei ≥ 120 % of the negative control with fully decondensed sperm nuclei after a 5 min incubation in egg extract.
In this study, 13 of the 60 patients had positive SDAD Test scores ≥ 120 % of the negative control. Accelerated DNA decondensation, hyper-decondensed sperm nuclei, the non-decondensed sperm (Panel a), and the clumped decondensed sperm nuclei (Panel d), are believed to be related to reactive oxygen species (ROS) sperm damage (See Discussion).
Does the SDAD test have utility for predicting ICSI outcome?
SDAD test results (n = 60)
Live birth outcomes of the 13 patients with abnormal SDAD Test scores ≥ 120 % of the negative control (13 of 60 cycles; 129.0 ± 6.3 % SD), as compared to live birth outcomes of the 47 patients with normal SDAD Test scores < 120 % of the negative control (47 of 60 ICSI cycles; 75.9 ± 25.3 % SD) are shown in Table 2. The delivery rate (DR) was 0.0 % when SDAD Test scores were ≥ 120 % of the negative control, vs. 40.0 % when SDAD Test scores were < 120 % of the negative control. The positive predictive value (PPV; true positives) for failed ICSI attempts at pregnancy = 100 % (PPV; p = 0.001); with a negative predictive value (NPV) for false positives = 51.0 %. The sensitivity (false negatives) and specificity (true negatives) was 36.1 % and 100 %, respectively (Table 2).
Table 2.
Treatment outcomes as a function of SDAD Test scores (Pos, Abnormal; Neg, Normal) n = 60 ICSI Cycles
| SDAD Test Scores (% of Negative Control) | ||
|---|---|---|
| ≥120 Positive Score | <120 Negative Score | |
| ICSI Cycles | 13 | 47 |
| Failed ICSIs | 13 | 23 |
| Live Births (Successful ICSI) | 0 | 24 |
| Delivery Rate (DR) | 0 % | 51.1 % |
| Embryos transferred (average) | 1.8a | 1.9a |
| P | <0.001 | |
| Odds Ratio (OR) of failing ICSI when the SDAD Test score is positive | 1.8 | |
| Positive Predictive Value (PPV) | 100 % | |
| Negative Predictive Value (NPV) | 51.0 % | |
| *Sensitivity (Not Predictive of False Negatives) | 36.1 % | |
| Specificity (Highly Predictive of True Negatives) | 100 % | |
aNot Significant
The SDAD Test is:
Highly predictive of true positives (PPV),
Highly predictive of true negatives (specificity),
Not predictive of false positives (sensitivity), or false negatives (NPV).
When weighting sensitivity and NPV, you must consider the overall delivery rate that in this study was 40 %. Both sensitivity and NPV are expected to be low as 36 (60 %) of the 60 ICSI attempts at pregnancy failed, 13 being true positives, with 23 falling within the range of expected failures based on the success rate of the procedure, i.e., a low sensitivity.
SDD Test (15 min incubation in egg extract)
For each of the 41 SDD Test runs performed for this study, before analyzing the patient sample(s), a SDD Test score was determined for a fertile male negative- and patient (previously determined) positive-control sperm sample as described in Materials and Methods. After 15 min incubations in egg extract, 95 ± 1.4 % SD of the negative control sperm nuclei were fully decondensed. Less than 80 % of the positive control sperm nuclei were fully decondensed. Patient samples were analyzed only after determining that the negative and positive control samples had ≥ 92 % and < 80 % fully decondensed sperm nuclei, respectively.
The % of fully decondensed sperm nuclei was determined for each patient sample after 15 min incubations in egg extract. The SDD Test score was determined and presented as % of the negative control.
 |
Typical examples of negative control sperm nuclei that were scored are shown in Fig. 3b. Typical examples of patient sperm nuclei scored as not decondensed (ND), partially decondensed (PD), or fully decondensed (FD) when SDD Test scores were positive (< 80 % of the negative control with fully decondensed sperm nuclei), are shown in Fig. 3c.
Interestingly, when determining the SDD Test score for the patient with an abnormal SDAD Test score of 124.7 % of the negative control with fully and hyper decondensed sperm nuclei (Fig. 2); after a 15 min incubation in egg extract, this patient’s SDD Test score was normal (99.4 % of the negative control). A typical example of a fully decondensed sperm nucleus is shown in Fig. 4a. Patients with accelerated decondensation also had accelerated recondensation (Fig. 4b), and in some cases hyper-decondensed sperm that never recondensed (Fig. 4c), after 15 min incubations in egg extract.
In this study:
Patients with abnormal SDD Test scores < 80 % of the negative control had normal SDAD Test scores < 120 % of the negative control.
Patients with abnormal SDAD Test scores ≥ 120 % of the negative control had normal SDD Test scores ≥ 80 % of the negative control.
Are SPA, or SDD test scores predictive indicators of ICSI outcome?
SDD test results (n = 60)
Outcomes of ICSI cycles using sperm from patients with abnormal SDD Test scores <80 % of the normal control (64.3 ± 7.3 % SD) with fully decondensed sperm nuclei, as compared to ICSI outcomes using sperm from patients with normal SDD Test Scores ≥ 80 % of the normal control (93.0 ± 6.9 % SD) were determined. The delivery rate (DR) was 40.0 % (scores < 80) vs. 40.0 % (scores ≥ 80) with a positive predictive value (PPV; true positives) for failed ICSI attempts at pregnancy = 60 % (PPV; (p = 0.727); with a negative predictive value (NPV) for false positives = 37.8 %. The sensitivity (false negatives; live birth outcomes) and specificity (true negatives; live birth outcomes) were 41.7 % and 58.3 %, respectively (Table 3).
Table 3.
Treatment outcomes as a function of SDD Test and SPA scores (Pos, Abnormal; Neg, Normal) in a group of 60 cycles
| SDD Test Score % of Negative Control | SPA Score (%) (Post-gradient SCI) | |||
|---|---|---|---|---|
| <80 Pos Score | ≥80 Neg Score | <14 Pos Score | ≥14 Neg Score | |
| ICSI cycles | 25 | 35 | 23 | 37 |
| Failed ICSIs | 15 | 21 | 13 | 23 |
| Live Births (Successful ICSI) | 10 | 14 | 10 | 14 |
| Delivery Rate (DR) | 40.0 % | 40.0 % | 43.4 % | 37.8 % |
| Embryos transferred (average) | 1.7a | 2.0a | 1.8a | 1.9a |
| Odds Ratio (OR) Odds of failed ICSI when SDD or SPA scores are Pos ratio to failed ICSI with neg scores | 1.4 | 1.8 | ||
| Positive Predictive Value (PPV) for a failed ICSI | 60.0 % | 56.5 % | ||
| Negative Predictive Value (NPV) for a live birth outcome | 40.0 % | 37.8 % | ||
| P | 0.727a | 0.614a | ||
| Sensitivity | 41.7 % | 36.1 % | ||
| Specificity | 58.3 % | 58.3 % | ||
aNot Significant
SPA results (n = 60)
Outcomes of ICSI cycles using sperm from patients with abnormal SPA (SCI) scores <14 (7.8 ± 4.1 SD), and normal scores of ≥ 14 (21.24 ± 3.21 SD) were determined. The DR was 43.5 % (scores < 14) vs. 37.8 % (scores ≥ 14) with a positive predictive value (PPV; true positives) for failed ICSI attempts at pregnancy = 56.5 % (p = 0.614); with a negative predictive value (NPV) for false positives = 58.3 %. The sensitivity (false negatives; live birth outcomes) and specificity (true negatives; live birth outcomes) were 36.1 % and 58.3 %, respectively (Table 3). Outcomes of ICSI cycles using sperm from patients with abnormal SDD Test scores <80 % of the normal control equaled (64.3 ± 7.3 % SD) with fully decondensed sperm nuclei, as compared to ICSI outcomes using sperm from patients with normal SDD Test Scores ≥ 80 % of the normal control (93.0 ± 6.9 % SD) were calculated. The DR was 40.0 % (scores <80) vs. 40.0 % (scores ≥ 80) with a positive predictive value (PPV; true positives) for failed ICSI attempts at pregnancy = 60 % (PPV; (p = 0.727); with a negative predictive value (NPV) for false positives = 37.8 %. The sensitivity (false negatives; live birth outcomes) and specificity (true negatives; live birth outcomes) were 41.7 % and 58.3 %, respectively (Table 3).
Conclusion: the SPA or SDD Test have limited to no utility as stand-alone tests in predicting ICSI failure or success (Table 3).
Do combined SPA and SDD test scores have utility for predicting ICSI outcome?
The SDD Test and SPA scores were not predictive of failed ICSIs when used as stand-alone tests. This was an unexpected outcome of this study. To explore this further, a new hypothesis was tested: the combined use of SDD Test and SPA scores will predict ICSI outcomes for subgroup(s) of couples once separated from the population of patients for whom ICSI outcomes cannot be predicted. In order to test the hypothesis, the combined data was organized and analyzed as 4 separate groups; Group 1) SDD < 80, SPA < 14; Group 2) SDD ≥ 80, SPA <14; Group 3) SDD < 80, SPA ≥ 14; and Group 4) SDD ≥ 80, SPA ≥ 14. The results are presented in Table 4.
Table 4.
Combined Use of SDD Test and SPA Scores in Predicting ICSI Outcome (n = 60)
| Category 1 SDD < 80 SPA < 14 | Category 2 SDD ≥ 80 SPA < 14 | Category 3 SDD < 80 SPA ≥ 14 | Category 4 SDD ≥ 80 SPA ≥ 14 | |
|---|---|---|---|---|
| ICSI cycles | 15 | 8 | 10 | 27 |
| Delivered pregnancies | 9 | 1 | 1 | 13 |
| Mono (M) | 5 | 0 | 0 | 2 |
| Mono (F) | 3 | 1 | 0 | 3 |
| Twins (MM) | 0 | 0 | 0 | 2 |
| Twins (MF) | 1 | 0 | 0 | 5 |
| Twins (FF) | 0 | 0 | 1 | 1 |
| Total Failed ICSIs | 6 | 7 | 9 | 14 |
| *No ET | 2 | 0 | 2 | 0 |
| *Negative (Implantation) | 3 | 4 | 7 | 9 |
| *Biochemical | 0 | 1 | 0 | 2 |
| *SAB (First Trimester) | 1 | 2 | 0 | 3 |
| aEmbryos Transferred (ave) | 1.6 | 2.1 | 1.9 | 2 |
| bTable Probability (P) | 0.05 | 0.08 | 0.03b | 0.11 |
| Positive Predictive Value (PPV) | 40.0 % | 87.5 % | 90.0 % | 51.9 % |
| Negative Predictive Value (NPV) | 42.9 % | 44.2 % | 46.0 % | 33.3 % |
| Sensitivity | 16.7 % | 19.4 % | 25.0 % | 38.9 % |
| Specificity | 62.5 % | 95.8 % | 95.8 % | 45.8 % |
aNot Significant by Chi-Square Analysis (p < 0.05)
bSignificant by Fisher’s Exact Test (p < 0.05)
When combined SPA and SDD Test scores were used to group patients into the 4 categories shown in Table 4, only patients in Category 3 (SPA ≥ 14, SDD < 80) had scores that were:
Highly predictive of ICSI cycles that will fail (PPV = 90 %; p = 0.03),
Highly predictive of true negatives (Specificity), and
Not predictive of false negatives (Sensitivity), or false positives.
Patients in Categories 1 had combined scores with a borderline significance for predicting outcomes. Patients in Categories 2 and 4 had combined SPA and SDD Test scores that were not significant predictors of ICSI outcome (p = 0.08, and p = 0.11, respectively).
Reproducibility of the SPA, and SDAD and SDD tests
A critical parameter that must be demonstrated when evaluating any fertility test, is the reproducibility of the test. The 3 tests evaluated in this study all provided significant reproducible results.
SPA reproducibility
A quality control (QC) system has been in place since the early 1990s for the optimized sperm penetration assay; developed to provide highly reproducible results that met the strict criteria required for clinical laboratory [28]. This same QC system was in effect throughout the course of the study.
SDAD and SDD test reproducibility
When analyzing study samples in the SDAD and SDD Tests, the established protocol used for the study was same protocol and QC system in place for 4 years for the commercial analysis of patient samples that like the SPA met the criteria for highly reproducible test scores required for obtaining clinical laboratory certification. During the course of the study, a control fertile male sample was collected once a month, and stored at 4 °C in a refrigerator for use in the SDAD and SDD Tests when study samples were received and analyzed, and also used as the control sample for the commercial samples being analyzed weekly in the SDD Test. Fresh sperm is not required for performing the SDD Test [13, 14] Throughout the course of the study, the same QC system standards were in place when scoring the 5 min time point (SDAD Test) that were in place for the 15 min time point (SDD Test) as described in Materials and Methods. During the study, the SDAD and SDD Tests were run 41 times using control fertile male samples obtained from 5 different fertile males.
For this study, the fertile male control samples used in determining the SDAD and SDD Test scores were used 1–4 weeks post-collection. The average SDAD and SDD Test scores for the fertile male control samples used throughout the course of this study were 70.9 ± 2 % (SD), and 95.0 ± 1.42 % (SD), respectively (n = 41 test runs).
The commercial SDAD and SDD Tests are now performed weekly (AndroJek Male Reproductive Health Laboratory, Fort Lauderdale, FL). To demonstrate the reproducibility of SDAD and SDD Test scores, and that there is not a significant difference in test scores when using the fertile male control sample for up to 1 month post-collection, test scores for both tests were determined for 12 semen samples from 3 different fertile males, analyzing either fresh sample, or the same sample stored 2–4 weeks at 4 °C, in the SDAD and SDD Tests (Table 5).
Table 5.
Reproducibility of the SDAD and SDD Test Scores: Fresh sample used Week 1; The same sample stored at 4 °C, used Weeks 2–4 (Fertile Male 1, n = 2; Fertile Male 2, n = 2; Fertile Male 3, n = 8)
| *SDAD (n = 48) 4 Weeks | **SDD (n = 48) 4 Weeks | ||
| Min | 66.70 | Min | 92.90 |
| Max | 74.50 | Max | 97.10 |
| Ave (mean) | 70.25 | Ave (Mean) | 94.99 |
| SD | 1.69 | SD | 0.89 |
| *SDAD (n = 12) Fresh | **SDD (n = 12) Fresh | ||
| Min | 67.90 | Min | 94.30 |
| Max | 73.40 | Max | 96.20 |
| Ave (mean) | 71.06 | Ave (mean) | 94.84 |
| SD | 1.88 | SD | 0.56 |
| *SDAD (n = 36) Weeks 2–4 | **SDD (n = 36) Weeks 2–4 | ||
| Min | 66.70 | Min | 92.90 |
| Max | 74.50 | Max | 97.10 |
| Ave (mean) | 70.26 | Ave (mean) | 95.04 |
| SD | 1.64 | SD | 0.98 |
Standard Deviation (SD)
No Significant Difference in:
*SDAD or **SDD Test scores (p < 0.05)
This demonstrates the reproducibility of the SDAD and SDD Test scores when analyzing different semen samples from the 3 different fertile males, whether fresh or stored for 2–4 weeks in a 4 °C refrigerator. There was no significant difference between the SDAD or SDD Test scores obtained during the study (n = 41 Test runs), and the SDAD and SDD Test scores obtained when analyzing commercial patient samples on a weekly basis (n = 48 Test runs. This is important for 2 reasons:
All study and patient sample SDAD and SDD Test scores are presented as % of the fertile male control, and
Fresh sperm samples are not required for obtaining reproducible results when analyzing sperm in the SDAD and SDD Tests.
ICSI cycle failures
Failed ICSI attempts at pregnancy were grouped according to types of failure when SPA, SDD and SDAD Test scores were either positive or negative (Table 6).
Table 6.
Summary of ICSI Failures (n = 36) and Live Births (n = 24) when SDD Test, SPA, and SDAD Test Scores were Abnormal (AB) or Normal (N)
| AB | N | AB | N | AB | N | |
|---|---|---|---|---|---|---|
| SDD < 80 25 ICSI Cycles | SDD ≥ 80 35 ICSI Cycles | SPA < 14 23 ICSI Cycles | SPA ≥ 14 37 ICSI Cycles | SDAD ≥120 13 ICSI Cycles | SDAD <120 47 ICSI Cycles | |
| Poor embryo development No ET | 4 | 0 | 2 | 2 | 0 | 4 |
| SAB (1st trimester) | 1 | 5 | 3 | 3 | 5 | 1 |
| Negative | 10 | 13 | 7 | 16 | 7 | 16 |
| Biochemical | 0 | 3 | 1 | 2 | 1 | 2 |
| Pregnancies | 11 | 19 | 13 | 17 | 5 | 25 |
| Live births | 10 | 14 | 10 | 14 | 0 | 24 |
All but 1 of the patients with abnormal SDAD Test scores ≥ 120 had normal SDD Test scores and were included in Patient Categories 2 and 4 (Table 4). The patient, whose sperm had an abnormal SDAD Test score of 127.9, and an abnormal SDD Test score of 71.7, was the first and only patient in the study identified to have accelerated sperm DNA decondensation with an abnormal SDD Test score. This patient is believed to have a false positive SDD Test score resulting from scoring some of the sperm pronuclei with very recondensed DNA, as partially decondensed sperm pronuclei. Early on in the study, it became clear that patients with abnormal SDAD scores had both accelerated sperm DNA decondensation, and recondensation (Figs. 2 and 4). In the other 12 patients in this study with abnormal SDAD Test scores, and in over 70 patients with abnormal SDAD Test scores identified to date, since finishing this study (unpublished data), all of these patients had normal SDD Test scores.
It is interesting to note that when female factor is minimized as described in the Materials and Methods section, 5 of the 13 patients with abnormal SDAD Test scores had pregnancies from their ICSI cycles. However, all of the pregnancies ended as SABs within the first 12 weeks of pregnancy (range 6–11 weeks).
What patient parameters had a significant correlation with the SDAD test’s utility in predicting ICSI outcome?
Multiple logistic regression analyses were performed to identify factors that correlated significantly with SDAD Test score ranges found to predict ICSI outcome. The male and female average age, and standard andrology test results were evaluated, and shown in Table 7.
Table 7.
Patient Characteristics (N = 60) Critical Cut-off Value = 120 (All data presented as Mean ± SD)
| SDAD negative (<120) | SDAD positive (≥120) | % Difference | |
|---|---|---|---|
| ICSI cycles | N = 47 | N = 13 | |
| Female age | 32.0 ± 4.5 | 30.5 ± 3.0 | NS |
| Male age | 33.6 ± 4.0 | 31.8 ± 4.2 | NS |
| Sperm counts (106) | 32.5 ± 19.1 | 59.3 ± 37.0 | 66%a, p < 0.02 |
| Sperm morphology | 32.7 ± 11.7 | 31.8 ± 14.3 | 34 %, NS |
| Motility | 53.7 ± 13.2 | 61.9 ± 7.1 | NS |
| Volume | 3.0 ± 1.2 | 3.5 ± 2.7 | 17 %, NS |
aSignificant if equality of variance (p < 0.05)
Not Significant (NS)
Other parameters were also evaluated including: normal and abnormal SPA scores using gradient prepared sperm, normal and abnormal SDD Test scores, total number of eggs obtained, fertilization rate, grade or quality of the fertilized eggs (Day 5 embryos), and # of embryos transferred, for each of the 60 ICSI cycles. The only significant correlation between results obtained for abnormal vs. normal SDAD Test scores (p < 0.05), was related to sperm count (Table 7).
Discussion
Rationale for using a panel of tests to evaluate male infertility pre-ART
There is an increasing demand for more advanced and sophisticated diagnostic tests to examine the behavior and quality of human sperm in a variety of conditions so as to elucidate abnormalities in the most critical aspects of sperm structure and function related to male infertility. A panel of tests will likely be required to evaluate the multiple critical processes that must occur initially during fertilization, as well as what must occur both pre- and post-fertilization in order to produce an embryo that will result in a live birth approximately 9 months post-conception [58].
Tests of DNA integrity are going to be an important part of the overall evaluation of male infertility as use of sperm with damaged DNA in ART has been linked with adverse clinical outcomes, including an increased risk of miscarriage and morbidity in the offspring, including childhood cancer [6, 7]. However, DNA integrity test scores must be critically evaluated for utility in directing infertile couples to the ‘best’ ART keeping in mind that there is a significant increased risk of birth defects in the offspring from couples who had successful ICSI attempts at pregnancy; a risk not found for the offspring from couples with successful IUI, and/or IVF-ET attempts at pregnancy [19]. The increased risk of birth defects is believed to be a result of the invasive ICSI procedure where a sperm is mechanically introduced into an oocyte.
As panels of male infertility tests are developed, both the new, and older established male fertility tests that will be included in the panel, need to be evaluated for utility in directing infertile couples to treatment(s) with the highest chance of a live birth outcome, with the lowest risk of birth defects. This is very important if a DNA integrity test will be included in the panel as DNA integrity testing has recently been challenged as not having clinical relevance when used to evaluate male infertility [59]. The need to reevaluate old test scores is best illustrated by how a reevaluation of the use of the SCSA DNA fragmentation index (DFI) scores has dramatically changed how the test results are used to direct patients to appropriate ART [16, 17]. The SCSA is the only DNA integrity test with substantial scientific literature supporting the credibility of this test for use in diagnosing male infertility, and up until 2007, patients whose sperm had a DNA fragmentation index (DFI) ≥ 30 were told that their sperm had minimal to no chance for successful ART attempts at pregnancy [22, 31, 32]. However, as the number of clinics using ICSI in treating their infertile couples, this DFI cut-off began to be questioned as many groups using sperm with DFIs of 30 or higher were having excellent live birth outcome rates [23, 43, 60]. The reason for what many interpreted to be a loss of the SCSA’s utility to predict ART outcome was not understood until the SCSA was evaluated for utility in predicting live birth outcome in each of the commonly used ART treatments, analyzing IUI, IVF, and ICSI live birth outcome as separate data sets. When this was done, it was found that the DFI significantly predicted live birth outcome in IUI cycles, but had no utility in predicting IVF or ICSI live birth outcome [16]. At present, the suggested course of treatment for patients with abnormal SCSA scores is that they be directed to ICSI, as this procedure has a higher live birth outcome rate than IVF. However, based on what is now known about the increased risk of birth defects when using ICSI [19], it may be prudent to direct infertile couples where the male has an abnormal DFI score, to IVF, rather than ICSI.
It is important to have pre-ART tests that have utility in detecting males producing sperm with compromised sperm structural and genomic integrity. The results from this study indicate that 56 of the 60 ICSI cycles resulted in good quality embryos for transfer, with only 4 of the 60 ICSI cycles having poor embryo development, with no embryos transferred (Table 6). This indicates that sperm can have compromised structural and/or genomic integrity that when used in IVF or ICSI attempts at pregnancy will result in normal looking embryos that are transferred, and in many cases will result in a pregnancy. However, without such pre-ART testing the treating physician will be using sperm in IUI, and transferring embryos 5 days post-IVF, and/or –ICSI, that will result in pregnancies, but these pregnancies will in most cases miscarriage during the first trimester as occurred in this study.
Some abnormal SDD Test responses are believed to be a result of exposure to reproductive toxicants that directly affect the chromatin such that there is either a delay, or an enhancement of in vitro decondensation, depending upon the type of exposure. For example, exposure to alkylating agents causes a delayed decondensation [48, 49].
On the other hand, in vitro exposure to reactive oxygen species (ROS) resulted in membrane damage [52] that is linked with an increase in ROS induced DNA oxidation that renders the sperm unable to fertilize the oocyte or produce a viable pregnancy [24, 29, 38, 41, 53]. The resulting increase in the kinetics of decondensation (accelerated decondensation), as well as an increase in the recondensation kinetics shown in Figs. 2 and 4, may be a result in altered quantities, or enzyme activity of activation factors related to the decondensation/recondensation processes, again due to the damaged membrane [12–14, 34, 44, 45, 47]. This supports the study hypothesis that the 5 min time point (SDAD Test) could be used to identify a new subgroup of infertile males with ROS damaged sperm observed to have accelerated DNA decondensation that would have normal SDD Test scores. During the course of this study, the 5 min time point was shown to be a novel male fertility test; the sperm DNA accelerated decondensation (SDAD) Test, and was the second test making up the panel that was evaluated for utility in predicting ICSI cycle outcomes.
The SPA was chosen as the third test for this study to evaluate utility in predicting ICSI cycle outcomes; a test already known to predict IUI and IVF outcomes [27, 35, 36]. This test is also a physiologically relevant sperm function test performed in vivo (live sperm fertilize live oocytes), that focuses on the events required for a sperm to penetrate the oocyte.
Can the SDAD test, SDD test, and/or SPA predict ICSI outcome as stand-alone sperm function tests?
Upon un-blinding the study as described in Materials and Methods, of the 60 ICSIs performed, 40 % resulted in live births (negatives) with 60 % failing ICSI (positives) post-conception (Table 6). The average age of the 60 women enrolled in this study was 33.6 ± 3.8 SD. The ICSI study, and clinic overall delivery rate for women in this age group was 40 %. The average delivery rate for women in the study, and SART member US clinics, in the age groups: 37–35, and < 35, was 37.9 % (www.sart.org, sortcors/online).
SDAD test
Of the three tests evaluated, the SDAD Test was the only test highly significant utility for predicting ICSI outcome as a stand-alone test, with a PPV (true positives) = 100 %, and a specificity (true negatives) = 100 % (p = 0.001). The SDAD Test had limited capacity to predict false positives (sensitivity = 36.1 %) and negatives (NPV = 51.0). When evaluating a test like the SDAD Test for capacity to predict a treatment’s outcome, the sensitivity for this test is not an accurate measure of the predictive capacity of the test. The PPV and specificity are the key indicators of predictive capacity if the p value is less than 0.05.
Treatment option
In an ongoing multi-site study (n = 10), 249 males have had their sperm evaluated in the SDAD Test before starting the males on a daily dose of anti-oxidants that includes Acetyl-L-Carnitine, Vitamins C (200 mg) and E(400 IU). Twenty eight (11.2 %) of these males were found to have abnormal SDAD Scores ≥ 120. To date, 10 of these males have had repeat samples analyzed after 2–6 months of anti-oxidant therapy (AOT) with 8 of the 10 male’s having their scores significantly lowered to normal scores (<120; t-test, p = 0.01). A retrospective study of this group of men is planned to determine if the significantly improved SDAD scores translates to a significant increase in live birth outcome [15]; unpublished data). In another study, pregnancy rates were significantly increased in males given daily doses of 1 g amounts of Vitamins C and E for 2 months [25]. Based on these results, the suggested therapy for males with abnormal SDAD Test scores is AOT for 2–3 months followed by a repeat SDAD Test. If the score is negative (<120), the couple should be scheduled for an ART cycle.
SPA and SDD test
The SPA or SDD Test have limited to no utility as stand-alone tests in predicting ICSI failure or success (Table 3). However, both tests predict IUI and IVF as stand-alone tests [13, 14, 35, 36].
Treatment option
If only the SPA or SDD Test scores are used in determining treatment options, 23 of the 60 (38.3 %) of the couples with male factor infertility (SPA scores < 14 or SDD Test scores < 80), that in this study was 38 % or 42 %, respectively; such couples will not benefit from IUI, or IVF. Because the ICSI delivery rate is essentially the same whether the male had positive or negative SPA or SDD Test scores, these couples should be scheduled for an ICSI cycle.
Combined SPA and SDD test scores (≥ 14 and < 80, respectively) have utility for predicting ICSI outcome in a sub-group of infertile couples: how and why
When grouping the 60 study couples into 4 Categories based on the male’s SPA and SDD Test Scores (Table 4), only the Category 3 group was found to have utility in predicting ICSI outcomes. In this group, Ten of the males (17 %) had negative SPA and positive SDD Test scores, with a PPV (true positives) = 90 %; a specificity (true negatives) = 95.8 % (p = 0.03 %; Table 4). This was the only category of patients with no abnormal SDAD scores (true positives Only 1 ICSI cycle resulted in a live birth (Table 4).
Treatment options for category 3 couples (n = 10)
The 1 couple with a successful ICSI cycle requires no treatment.
The 9 patients with failed ICSI cycles should be evaluated for exposure to agents known to negatively affect male reproductive health by having these patients review a list of the common agents known to negatively affect fertility including, medications, recreational drugs, alcohol, oxidative stress, cigarette smoking, environmental toxins, and even use of cell phones [1, 2, 4–7, 50]. If possible, these patients should be removed from agents that they identify may be causing their infertility for 3 months, and then retested to see if their SDD Test score has improved. If improvement is seen, another ART cycle should be scheduled for the couple.
Category 1, 2, and 4 couples
The other 3 categories of the patients include the males with abnormal SDAD Test scores; the 13 couples with a 100 % failure rate. Patients in these 3 groups have NO significant utility in predicting ICSI outcomes based on p values ≥ 0.05, PPV, NPV, sensitivity and/or specificity values < 80 % (Tables 4 and 6). The parameters identified to correlate with the SDAD Test’s utility in significantly predicting ICSI failure apply to couples in Categories 2 and 4. Apparently the patients in Categories 1, 2, and 4 have the patients with false positive and negative SPA and SDD scores that explain why the stand-alone use of either the SPA or SDD Test scores have no utility in predicting ICSI outcome (Table 3).
Treatment options for category 1, 2, and 4 couples (n = 50)
Any couple where the male has an abnormal SDAD Test score should be given anti-oxidants for 3 months as described above for the 21.7 % of the males in this study had abnormal SDAD Test scores. When such males’ repeat SDAD Test score is normal (<120), the couple should resume ART attempts at pregnancy that can include all ART.
Category 1 couples (n = 15), 25 % of the 60 couples enrolled in this study, had males with a significant number of false positives and negatives (low sensitivity and NPV percentages, respectively), and a significant utility in predicting ICSI (p < 0.05), should be fast-tracked to ICSI.
Category 2 and 4 couples with normal SDAD and SDD Test scores (36.7 % of the couples in this study) can be directed to IUI, IVF, and/or ICSI with the chance for a successful ART attempt at pregnancy that will be equal to the fertility clinic’s reported delivery rates dependent upon which ART is used.
Summary
The results from this study support using a panel of male fertility tests that includes physiologically relevant sperm function tests that will evaluate overall sperm functionality and genomic integrity. Ideally, the panel test scores will give the treating physician options for their patients that maximize successful ART live birth outcomes while minimizing an increased risk of birth defects, higher than what is observed in offspring conceived naturally.
Acknowledgments
The authors wish to acknowledge Olivera Vragovic, MBA (Research Manager, Dept. of Ob/Gyn, Boston University School of Medicine), for her invaluable help with the statistical analyses performed during the course of this study.
Footnotes
Capsule
A Panel of physiologically relevant sperm function tests was shown to have significant clinical utility when used to predict ICSI outcome.
David B. Brown and Deborah C. Merryman contributed equally to this work.
References
- 1.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9:331–345. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A. Current and future perspectives on intracytoplasmic sperm injection: a critical commentary. Reprod BioMed Online. 2007a;15:719–727. doi: 10.1016/S1472-6483(10)60540-8. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Prabakaran S. Clinical relevance of oxidative stress in patients with male factor infertility; evidence-based analysis. Am Urol Assoc Updat Ser. 2007b;26:1–11. [Google Scholar]
- 4.Agarwal A, Deepinder F, Sharma RK, Ranga G, Li J. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril. 2008;89:124–128. doi: 10.1016/j.fertnstert.2007.01.166. [DOI] [PubMed] [Google Scholar]
- 5.Aitken RL, Buckingham D, West K, Wu FC, Zikopoulos K, Richardson DW. Differential contribution of leucocytes and spermatozoa to the high levels of reactive oxygen species recorded in the ejaculates of oligozoospermic patients. J Reprod Fertil. 1992;94:451–462. [DOI] [PubMed]
- 6.Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, et al. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod. 1998;59:1037–1046. doi: 10.1095/biolreprod59.5.1037. [DOI] [PubMed] [Google Scholar]
- 7.Aitken RJ. Whither must spermatozoa wander? The future of laboratory seminology. Asian J Androl. 2010;12:99–103. doi: 10.1038/aja.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson AN, Goossens V, Ferraretti AP, et al. Assisted reproductive technology in Europe. Hum Reprod. 2008;23:1158–1176. doi: 10.1093/humrep/den252. [DOI] [PubMed] [Google Scholar]
- 9.Bar-Charma N, Lamb DJ. Evaluation of sperm function. What is available in the modern andrology laboratory. Urol Clin N Am. 1994;21:433–446. [PubMed] [Google Scholar]
- 10.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 11.Bonde JPE, Ernst E, Jensen TK, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–1177. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- 12.Brown DB, Blake E, Wolgemuth D, Gordon K, Ruddle F. Chromatin decondensation and DNA synthesis in human sperm activated in vitro by using Xenopus laevis egg extracts. J Exp Zool. 1987;242:215–231. doi: 10.1002/jez.1402420213. [DOI] [PubMed] [Google Scholar]
- 13.Brown DB, Nagamani M. Use of Xenopus laevis frog egg extract in diagnosing human male unexplained infertility. Yale J Biol Med. 1992;65:29–38. [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DB, Hayes EJ, Uchida T, Nagamani M. Some cases of human male infertility are explained by abnormal in vitro human sperm activation. Fertil Steril. 1995;64:612–622. doi: 10.1016/s0015-0282(16)57801-7. [DOI] [PubMed] [Google Scholar]
- 15.Brown DB, Gelman KM, Whitman-Elia GF, Witt MA, Kordus RJ, Roseff SJ. Comparing the sperm decondensation (SDDTM) and sperm DNA accelerated decondensation (SDADTM) tests’ capacity for identifying infertile males likely to benefit from anti-oxidant treatment. Fertil Steril. 2010;94(Suppl 4):S238. doi: 10.1016/j.fertnstert.2010.07.922. [DOI] [Google Scholar]
- 16.Bungum M, Spano M, Humaidan P, Eleuteri P, Rescia M, Giwercman A. Sperm chromatin structure assay parameters measured after density gradient centrifugation are not predictive for the outcome of ART. Hum Reprod. 2008;23:4–10. doi: 10.1093/humrep/dem353. [DOI] [PubMed] [Google Scholar]
- 17.Bungum M (2012) Sperm DNA integrity assessment: a new tool in diagnosis and treatment of fertility. Obstet Gynecol Int doi:10.1155/2012/531042. [DOI] [PMC free article] [PubMed]
- 18.Cohen J, Edwards RG, Fehilly CB, Fishel SB, Hewitt J, Rowland G, et al. Treatment of male infertility by in vitro fertilization: factors affecting fertilizations and pregnancy. Acta Eur Fertil. 1984;15:455–465. [PubMed] [Google Scholar]
- 19.Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- 20.Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol. 2004;103:51–56. doi: 10.1097/01.AOG.0000100153.24061.45. [DOI] [PubMed] [Google Scholar]
- 21.Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science. 1980;210:1131–1133. doi: 10.1126/science.7444440. [DOI] [PubMed] [Google Scholar]
- 22.Evenson DP, Jost LK, Marshall D, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14:1039–1049. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 23.Gandlnl L, Lombardo F, Paoli D, et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod. 2004;19:1409–1417. doi: 10.1093/humrep/deh233. [DOI] [PubMed] [Google Scholar]
- 24.Gomez E, Irvine DS, Aitken RJ. Evaluation of a spectrophotometric assay for the measurement of malondialdehyde and 4-hydroxyalkenals in human spermatozoa: relationships with semen quality and sperm function. Int J Androl. 1998;21:81–94. doi: 10.1046/j.1365-2605.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- 25.Greco E., Lacobelli M, Ferrero S, Baroni E, Minasi MG, Ubaldi F, et al. ICSI in cases of sperm DNA damage: beneficial effect of oral anti-oxidant treatment. J Androl. 2005;20:2590–2594. [DOI] [PubMed]
- 26.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. Sperm morphology, motility and concentration in infertile and fertile men. N Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 27.Johnson AR, Bassham B, Lipshultz LI, Lamb DJ. Methodology for the optimized sperm penetration assay. In: Keel B, Webster B, editors. Handbook of the laboratory diagnosis and treatment of infertility. Boca Raton: CRC Press; 1990. pp. 135–147. [Google Scholar]
- 28.Johnson A, Bassham B, Lipshultz LI, Lamb DJ. A quality control system for the optimized sperm penetration assay. Fertil Steril. 1995;64:832–837. doi: 10.1016/s0015-0282(16)57862-5. [DOI] [PubMed] [Google Scholar]
- 29.Kemal Duru N, Morshedi M, Oehninger S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril. 2000;74:287–291. doi: 10.1016/S0015-0282(00)01591-0. [DOI] [PubMed] [Google Scholar]
- 30.Lamb DJ. Semen analysis in 21st century medicine: the need for sperm function testing. Asian J Androl. 2010;12:64–70. doi: 10.1038/aja.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum Reprod. 2000;15:1717–1722. doi: 10.1093/humrep/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 32.Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET, Evenson DP. Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril. 2003;80:895–902. doi: 10.1016/S0015-0282(03)01116-6. [DOI] [PubMed] [Google Scholar]
- 33.Longo FJ, Kunkle M. Transformation of sperm nuclei upon insemination. Curr Top Dev Biol. 1978;12:149–184. doi: 10.1016/S0070-2153(08)60596-7. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto K, Nagata K, Miyaji-Yamaguchi M, Kikuchi A, Tsujimoto M. Sperm chromatin decondensation by template activating factor I through direct interaction with basic proteins. Mol Cell Biol. 1999;19:6940–6952. doi: 10.1128/mcb.19.10.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merryman DC, Stringfellow SE, Yancey CA, Houserman VL, Long CA, Honea KL. Does in vitro fertilization (IVF) with intracytoplasmic sperm injection compensate for impaired sperm function as predicted by the sperm penetration assay (SPA)? Fert Steril. 2001;76(Suppl 1):S215. doi: 10.1016/S0015-0282(01)02641-3. [DOI] [Google Scholar]
- 36.Merryman DC, Stringfellow SE, Dalton KE, Houserman VL, Long CA, Honea KL. Sperm capacitation index (SCI) predicts pregnancy outcome with controlled ovarian hyperstimulation (COH) + intrauterine insemination (IUI) Fertil Steril. 2007;88(Suppl 1):S118–S119. [Google Scholar]
- 37.Merryman DC, Rivnay B, Honea KL, Brown DB. Sperm DNA decondensation and sperm penetration assay with gradient preparation are not predictive of pregnancy outcome in in vitro fertilization cycles with intracytoplasmic sperm injection. Fertil Steril. 2007;88(Suppl 1):S380–S381. doi: 10.1016/j.fertnstert.2007.07.1266. [DOI] [Google Scholar]
- 38.Misro MM, Choudbury L, Upreti K, Gautam D, Chaki SP, Mahaian AS, et al. Use of hydrogen peroxide to assess the sperm susceptibility to oxidative stress in subjects presenting a normal semen profile. Int J Androl. 2004;27:82–87. doi: 10.1046/j.0105-6263.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 39.Muratori M, Marchiani S, et al. Markers of human sperm functions in the ICSI era. Front Biosci. 2011;16:1344–1363. doi: 10.2741/3793. [DOI] [PubMed] [Google Scholar]
- 40.Natali A, Turek PJ. An assessment of new sperm tests for male infertility. Urology. 2011;77:1027–1034. doi: 10.1016/j.urology.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Ochsendorft FR. Infections in the male genital tract and reactive oxygen species. Hum Reprod. 1999;5:399–420. doi: 10.1093/humupd/5.5.399. [DOI] [PubMed] [Google Scholar]
- 42.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 43.Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, Walmer DK. Redefining the relationship between sperm deoxyribonucleic acid fragmentations as measured by the sperm chromatin structure assay and outcomes of assisted reproductive tehniques. Fertil Steril. 2005;84:356–364. doi: 10.1016/j.fertnstert.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 44.Philpott A, Leno GH, Laskey RA. Sperm decondensation in Xenopus egg cytoplasm is mediated by nucleoplasmin. Cell. 1991;65:569–578. doi: 10.1016/0092-8674(91)90089-H. [DOI] [PubMed] [Google Scholar]
- 45.Philpott A, Leno GH. Nucleoplasmin remodels sperm chromatin in Xenopus egg extracts. Cell. 1992;69:759–767. doi: 10.1016/0092-8674(92)90288-N. [DOI] [PubMed] [Google Scholar]
- 46.Rogers BJ, Van Campen H, Ueno M, Lambert H, Bronson R, Hanson RW. Analysis of human spermatozoa fertilizing ability using zona-free ova. Fertil Steril. 1979;32:664–670. doi: 10.1016/s0015-0282(16)44416-x. [DOI] [PubMed] [Google Scholar]
- 47.Sawyer DE, Brown DB. Diminished decondensation and DNA synthesis in activated sperm from rats treated with cyclophosphamide. Toxicol Lett. 2000;114:19–26. doi: 10.1016/S0378-4274(99)00189-7. [DOI] [PubMed] [Google Scholar]
- 48.Sawyer DE, Brown DB. The use of a in vivo sperm activation assay to detect chemically-induced damage of human sperm nuclei. Reprod Toxicol. 1995;9:351–357. doi: 10.1016/0890-6238(95)00021-2. [DOI] [PubMed] [Google Scholar]
- 49.Sawyer DE, Hillman GR, Uchida T, Brown DB. Altered nuclar activation parametersof Rat sperm treated in vitro with chromatin damaging agents. Toxicol Sci. 1998;44:52–62. doi: 10.1006/toxs.1998.2466. [DOI] [PubMed] [Google Scholar]
- 50.Sigman M. Medications that impair male fertility. Sex Reprod Menopause. 2007;5:11–16. [Google Scholar]
- 51.Sigman M, Zini A. Semen analysis and sperm function assays: what do they mean? Semin Reprod Med. 2009;27:115–123. doi: 10.1055/s-0029-1202300. [DOI] [PubMed] [Google Scholar]
- 52.Tirado EE, Sharma R, Sawyer DE, Awasthi YC, Brown DB. Effects of oxidative stress on human sperm activation. Fertil Steril. 2003;80(Suppl 3):S240. doi: 10.1016/S0015-0282(03)01581-4. [DOI] [Google Scholar]
- 53.Turkyilmaz Z, Gulen S, Sonmez K, Karabulut R, Dincer S, Can Basaklar A, et al. Increased nitric oxide is accompanied by lipid oxidation in adolescent varicocele. Int J Androl. 2004;27:183–187. doi: 10.1111/j.1365-2605.2004.00474.x. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization Towards more objectivity in diagnosis and management of male infertility. Int J Androl. 1987;7:1–53. [Google Scholar]
- 55.WHO laboratory manual for the examination and processing of human semen. 5. Geneva: WHO Press; 2010. [PubMed] [Google Scholar]
- 56.Yanagimachi R, Yanagimachi H, Rogers BJ. The use of zona-free animal ova as a test system for the assessment of the fertilizing capacity of human spermatozoa. Biol Reprod. 1976;15:471–476. doi: 10.1095/biolreprod15.4.471. [DOI] [PubMed] [Google Scholar]
- 57.Yanagimachi R. Mechanisms of fertilization in mammals. In: Mastrioni, Biggers J, editors. Fertilization and embryonic development. New York: Plenum Press; 1981. pp. 81–187. [Google Scholar]
- 58.Yanagimachi R. Problems of sperm fertility: A reproductive biologist’s view. Systems Boil Reprod Med doi:10.3109/19396368.2010.507860. [DOI] [PubMed]
- 59.The Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013;99:673–677. [DOI] [PubMed]
- 60.Zini A, Meriano J, Kader K, Jarvi K, Laskin CA, Cadesky K. Potential adverse effect of sperm DNA damage on embryo quality after ICSI. Hum Reprod. 2005;20:3476–3480. doi: 10.1093/humrep/dei266. [DOI] [PubMed] [Google Scholar]