Abstract
Objective
Prior studies have validated the ability of the SART embryo scoring system to correlate with outcomes in cleavage stage embryo transfers. However, this scoring system has not been evaluated in blastocyst transfers. The objective of this study was to estimate the correlation between the simplified SART embryo scoring system and ART cycle outcomes in single blastocyst transfers.
Materials and methods
All fresh, autologous single blastocyst transfers cycles from a large ART center from 2010 were analyzed. Blastocysts were given a single grade of good, fair, or poor based upon SART criteria which combines the grading of the inner cell mass and trophectoderm. Multiple logistic regression assessed the predictive value of the SART grade on embryo implantation and live birth.
Results
Seven hundred seventeen fresh, autologous single blastocyst transfers cycles were included in the analysis. The live birth rate was 52 % and included both elective and non-elective SBT. Chi square analysis showed higher live birth in good grade embryos as compared to fair (p = 0.03) and poor (p = 0.02). Univariate binary logistic regression analysis demonstrated SART embryo grading to be significantly correlated with both implantation and live birth (p < 0.01). This significance persisted when patient age, BMI, and the stage of the blastocyst were controlled for with multiple logistic regression. In five patients with a poor blastocyst score, there were no live births.
Conclusion
These data demonstrate that the SART embryo scoring system is highly correlated to implantation and live birth in single blastocyst transfers. Patients with a good grade embryo are excellent candidates for a single blastocyst transfer.
Keywords: In-vitro fertilization, Single blastocycst transfer, Embryo morphology, Implantation, Live birth
Introduction
Predicting successful ART outcomes is important for physicians when selecting and counseling patients who are most likely to get pregnant as the result of a single blastocyst transfer. Single embryo transfer with resultant singleton pregnancy and live birth is the most desired outcome of an ART cycle. It is well documented that multiple gestation pregnancies are a higher risk pregnancy when compared to singleton gestations. Recent publications have reported similar live birth rate with a significant reduction in multiple gestation with the use of selective single embryo transfer when compared to multiple embryo transfer [2,8,12].
One of the factors to consider when assessing patients for single embryo transfer is embryo quality or morphology. The Society for Assisted Reproductive Technology (SART) has developed a standardized morphological assessment for embryo grading [14]. This standardization of embryo morphological grading and ART outcomes has been established and validated for cleavage stage embryos [13,14,18]. However, we are not aware of any publications validating the SART simplified grading for blastocysts.
The objective of this study was to estimate the correlation between the simplified SART embryo scoring system and ART cycle outcomes in single blastocyst transfers. We hypothesized that the simplified SART scoring system is highly correlated with implantation and live birth.
Materials and methods
Study design
This was a retrospective cohort analysis of all fresh, autologous single blastocyst embryo transfers from January 2010 through December 2010. The study was conducted at Shady Grove Fertility Reproductive Science Center in Rockville, MD. The retrospective review and analysis of data collected during routine clinical care was approved by Schulman Associates Institutional Review Board.
Patients
All patients who underwent fresh, autologous, elective and non-elective single blastocyst embryo transfer during the calendar year 2010 were included in the analysis. Those who underwent donor oocyte or embryo transfer and frozen-thaw embryo transfers were excluded from the study. Day 5 or day 6 transfers of morula stage embryos were also excluded from the analysis.
Stimulation protocol
Ovarian hyperstimulation occurred primarily with mixed FSH/LH protocol under pituitary suppression with either GnRH antagonist or GnRH agonist as previously described [16]. In general, oral contraception was started 3 weeks prior to ovarian stimulation. For GnRH antagonist protocol cycles, an antagonist (Ganirelix) was initiated when the lead follicle measured 14 mm in diameter. For GnRH agonist protocol cycles, leuprolide acetate (Lupron) 20 units was started during the last 3 days of oral contraceptive pills. This dose was decreased to five units after ovarian suppression was confirmed.
A mixed protocol using recombinant FSH and hMG was typically used to achieve ovarian hyperstimulation. Final oocyte maturation was triggered with 10,000 units of HCG or with 40 units of GnRH agonist (in the GnRH antagonist protocols) when the lead follicle(s) were greater than or equal to 18 mm. Oocyte retrieval was performed 36 h later and insemination occurred using conventional IVF or ICSI as clinically indicated. Ultrasound guided single blastocyst transfer using the afterload technique [9] was performed on day 5 after ooctye retrieval by a board certified/board eligible Reproductive Endocrinologist. Serum hCG levels were assessed 2 weeks after oocyte retrieval and ultrasound confirmation of all pregnancies was performed on all patients between five to six weeks estimated gestational age based on embryo transfer day.
Embryo grading
All blastocysts were evaluated by a member of a team of embryologists using the grading system described by Gardner and Schoolcraft [5]. The laboratory is certified and all embryologists are examined and graded yearly on their performance. All embryo grading is also reviewed in real-time by one of two senior embryologists for verification and consistency. Upon extraction and review of the data the reviewers gave each embryo a grade according to the simplified SART grading system, which is displayed in Table 1. SART grade good was assigned for inner cell mass (ICM) grade A and trophectoderm (TE) grade A or B (AA or AB blastocysts). SART grade fair was assigned for ICM grade B and TE grade A, B or C (BB, BC, or BA blastocysts). SART grade poor was assigned for any ICM grade C (CC or CB blastocysts). Table 2 shows the SART grade given according to the embryology grade assigned. Stage of development (early blast, blast, expanded blast, hatching blast) was assigned to each embryo but not included in the SART grading.
Table 1.
SART grading system
| Growth phase | Overall grade | Stage |
|---|---|---|
| Cleavage | Good, Fair, Poor | Cell #: 1 through >8 |
| Fragmentation: 0 %, <10 %, 11–25 %, >25 % | ||
| Symmetry: Perfect, Moderately, Asymmetric Severely Asymmetric | ||
| Morula | Good, Fair, Poor | Compaction: Complete, Incomplete |
| Fragmentation: 0 %, <10 %, 11–25 %, >25 % | ||
| Blastocyst | Good, Fair, Poor | Expansion: Early, Expanding, Expanded, Hatched |
| Inner cell mass: Good, Fair, Poor | ||
| Trophectoderm: Good, Fair, Poor |
Table 2.
SART grade according to embryology grading system
| SART grade | Embryology grade (ICM/TE) |
|---|---|
| Good | AA or AB |
| Fair | BA, BB, or BC |
| Poor | CB or CC |
Outcomes
The primary outcomes were implantation and live birth. Implantation was defined as a rising serum HCG value and a gestation sac seen in the uterus by transvaginal ultrasound (TVUS). Live birth was defined as the birth of a live infant greater than 23 weeks of gestation.
Statistics
Chi-square analysis was used to assess the proportion of ART outcomes within the three SART morphological grades. Univariate binary logistic regression analysis was used to assess the SART embryo grading with implantation and live birth. Multiple logistic regression was used to assess any confounding with the variables of age, BMI and stage of the blastocyst. Statistical analysis was performed using SPSS (International Business Machines Corp (IBM), New York) software. A p-value of <0.05 was considered statistically significant.
Results
A total of 717 fresh, autologous SBT cycles were identified and included in the analysis. The overall live birth rate was 52 %. The mean age was 32.1 years old and mean BMI 24.8. The twin birth rate was 0.7 % and the remaining live births were singleton gestations.
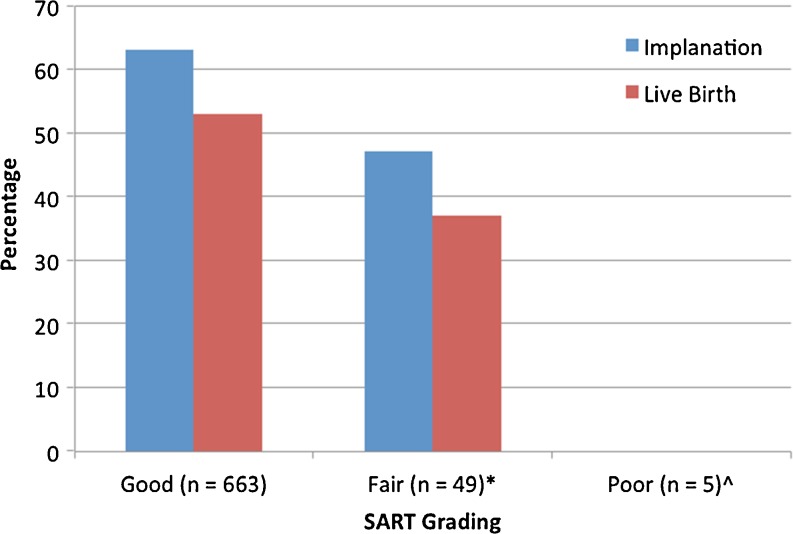
Embryos graded as good (n = 663) had a 63 % implantation and 53 % live birth rate (Fig. 1). Those graded as fair (n = 49) had an implantation and live birth rate of 47 % and 37 % respectively. There were no live births among those embryos graded as poor (n = 5). Chi square analysis demonstrated a higher live birth in good grade embryos as compared to fair (p = 0.03) and poor (p = 0.02) embryos. Chi square analysis did not show a difference between SART grade fair and poor embryos for implantation or live birth.
Fig. 1.
Implantation and live births among the SART grading categories. Chi Square analysis performed on live birth between the different grading categories. p value <0.05 considered significant. *Good vs. Fair. p = 0.03. ^Good vs. Poor. p = 0.02
There were 112 non-elective single embryo transfers in which the patients had no additional blastocysts available for transfer or cryopreservation. The live birth rate was 31 % in this group compared to 55 % in patients having an elective single embryo transfer (p < 0.0001). In those patients with cryopreserved embryos, the number of cryopreserved embryos was predictive of live birth. In multivariate regression, patient age (p < 0.01), SART grade (p < 0.05) and the number of cryopreserved blastocysts (p < 0.01) were all significantly correlated with live birth. Even when controlling for cryopreserved embryos and age, SART grading remained predictive of live birth.
Stage of embryo development was assigned to each embryo but was not included in the SART grading. Embryo stage was controlled for in the multivariate regression analysis. While embryo expansion was correlated with live birth in univariate regression analysis, embryo expansion was not significantly correlated with live birth in the multivariate regression analysis (p = 0.18). Subgroup analysis was also performed to analyze the differences in live birth by ethnicity and SART grade. Results are shown in Tables 2 and 3. There was no statistical difference between the ethnicity subgroups, largely due to the small number of patients in these comparisons. However, those of Asian and African American ethnicity didn’t have lower live birth rates with “good” embryos compared to Whites, which is consistent with previous literature examining ethnicity related IVF outcomes [3,4,7,11,15].
Table 3.
Live birth by ethnicity and SART grade
| Ethnicity | Patients (n) | Good | Fair | Poor |
|---|---|---|---|---|
| White | 433 | 58.2 % | 40 % | 0 % |
| Asian | 109 | 47 % | 42.8 % | 0 % |
| African American | 67 | 44.6 % | 0 % | 0 % |
| Hispanic | 24 | 57.1 % | 0 % | 0 % |
Univariate binary logistic regression analysis demonstrated SART embryo grading to be significantly correlated with both implantation (OR 2.33, 95 % CI 1.30–4.10) and live birth (OR 2.07, 95 % CI 1.14–3.76). The significance of the SART grade persisted when patient age, BMI, and the stage of the blastocyst were controlled for with multiple logistic regression. In the multivariate regression analysis, the OR for implantation was 2.18 (95 % CI 1.20–3.96) and the OR for live birth was 1.87 (95 % CI 1.01–3.48). The only other significant predictor of outcomes in the multivariate analysis was patient age (p < 0.05).
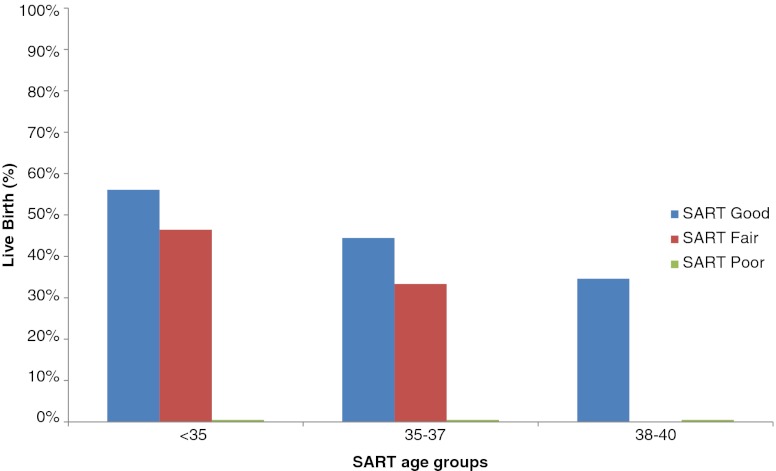
The live birth rate was further stratified by SART age category and SART grade (Fig. 2). There was a decrease in live birth for patients with SART grade good blastocysts as age increased, from 56 % in patients under 35, to 44 % for patients age 35–37, and 35 % for patients aged 38–40. Similarly, there was a decrease in live birth for patients with SART grade fair blastocysts as age increased, from 46 % in patients under 35, to 33 % for patients age 35–37, and 0 % for patients aged 38–40. Patients under the age of 35 had statistically similar live birth rates with SART grade good and fair blastocysts (56 % versus 46 %, p = 0.30), likely due to the small number of young patients with only a fair grade embryo (n = 28).
Fig. 2.
Live birth stratified by SART blastocyst grade (good, fair, and poor) and SART age groups (<35, 35–57, and 38–40). No patients in the study were in the SART age group 41–42
The miscarriage rate was 15.7 % in the SART grade good patients, 18.8 % in the grade fair patients, and there were no pregnancies in the grade poor patients. By chi-square analysis, there was no difference in miscarriage rate between the SART grade good and fair blastocysts (p = 0.99). In univariate regression analysis, SART grade was not correlated with miscarriage (OR 1.34, 95%CI 0.4–4.5). In multivariate regression analysis controlling for age, BMI, SART grade, and the stage of the embryo, only embryo stage was significantly correlated with miscarriage (OR 0.53, 95 % CI 0.30–0.95). In this analysis age was not statistically correlated with miscarriage (p = 0.15), likely because of the overall young, good prognosis group these patients represented.
Discussion
There is movement in the field of ART toward transfer of a single blastocyst on day 5 in order to minimize multiple gestations. Multiple gestation places the fetuses and mother at higher risk when compared to singleton pregnancies. The maternal risks of multiple gestations include an increase in gestational hypertension, pre-eclampsia, gestational diabetes, cesarean section and maternal hospital admission [10]. Neonatal risks include low birth weight, prematurity, neonatal intensive care admission and increase morbidity and mortality [10].
Embryo morphology is one criteria used when evaluating and counseling patients for single blastocyst transfer. The ability to predict successful ART outcomes based on embryo morphology is key. Numerous studies have evaluated systems devised to grade embryo morphology [1,6]. There are simple systems used to grade cleavage stage embryo based on appearance, which take into account cell symmetry and fragmentation [17].
For blastocyst embryos, grading is based on the morphology of the inner cell mass (ICM) and trophectoderm (TE), as well as the expansion of the blastocyst cavity [6]. These grading systems of the ICM and TE can be difficult to understand by patients when discussing their embryo quality prior to an embryo transfer. The simplified SART grading system is much easier for patients to comprehend and understand.
In 2010, SART issued a report on the standardization of grading embryo morphology. This report gives embryo grades in the categories of good, fair and poor [14]. This grading system was evaluated and validated as predictive of live birth rate in cleavage stage transfers [13,18]. No studies have been published validating the SART scoring system for blastocysts. This study validates the embryo grading system employed my SART is predictive of ART outcomes. These data demonstrate that embryos graded as good are correlated with better implantation and live birth when compared to embryos graded as either fair or poor.
The strengths of this study include the fact that this is the first study of its kind in the literature to evaluate the simplified SART embryo scoring system for blastocyst transfers. The use of single embryo transfer makes the assessment of each morphologic grade with ART outcomes more simple and straightforward. The large number of patients, the use of live birth as an outcome measure and the use of univariate and multiple logistic regression to control for confounders further adds to the strengths of this study.
The weaknesses of this study include its retrospective nature. There also were limited numbers of embryos given a fair or poor morphological grade. Unless there is only one poor grade embryo to transfer, patients with poor grade embryos are more likely to receive a double embryo transfer instead of a single blastocyst. This may lead to bias in the results; however, chi-square analysis demonstrated significantly different results even between the good and fair categories. These data suggest that patients with only poor grade embryos are not good candidates for single blastocyst transfer.
In conclusion, the results of this study demonstrate that the simplified SART embryo morphology grading system is highly correlated with implantation and live birth in those patients undergoing single blastocyst transfer. This information can be used to better counsel patients on the number of embryos to be transferred and further strengthen recommendations for single blastocyst transfer.
Footnotes
Capsule
The simplified SART morphological grading system is highly correlated to implantation and live birth in single blastocyst transfers.
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense or the U.S. Government.
This research was supported, in part, by the intramural research program of the Program in Reproductive and Adult Endocrinology, NICHD, NIH.
References
- 1.Boiso I, Veiga A, Edwards RG. Fundamentals of human embryonic growth in vitro and the selection of high-quality embryos for transfer. Reprod Biomed Online. 2002;5(3):328–50. doi: 10.1016/S1472-6483(10)61841-X. [DOI] [PubMed] [Google Scholar]
- 2.Csokmay JM, Hill MJ, Chason RJ, et al. Experience with a patient-friendly, mandatory, single-blastocyst transfer policy: the power of one. Fertil Steril. 2011;96(3):580–84. doi: 10.1016/j.fertnstert.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feinberg EC, Larsen FW, Catherino WH, et al. Comparison of assisted reproductive technology utilization and outcomes between Caucasian and African American patients in an equal-access-to-care setting. Fertil Steril. 2006;85:888–894. doi: 10.1016/j.fertnstert.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Fujimoto VY, Luke B, Brown MB, et al. Racial and ethnic disparities in assisted reproductive technology outcomes in the United States. Fertil Steril. 2010;93:382–390. doi: 10.1016/j.fertnstert.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner DK, Schoolcraft WB. In-vitro culture of human blastocyst. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: fertility and genetics beyond. Carnforth: Parthenon Publishing; 1999. pp. 378–88. [Google Scholar]
- 6.Gardner DK, Stevens J, Sheldon CB, Schoolcraft W. Analysis of blastocyst morphology. In: Elder K, Cohen J, editors. Human preimplantation embryo selection. London: Informa Healthcare; 2007. pp. 79–87. [Google Scholar]
- 7.Huddleston HG, Cedars MI, Sohn SH, et al. Racial and ethnic disparities in reproductive endocrinology and infertility. Am J Obstet Gynecol. 2010;202(5):413–19. [DOI] [PubMed]
- 8.Kresowik JD, Stegmann BJ, Sparks AE, et al. Five-years of a mandatory single-embryo transfer (mSET) policy dramatically reduces twinning rate without lowering pregnancy rates. Fertil Steril. 2011;96(6):1367–69. doi: 10.1016/j.fertnstert.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Neithardt AM, Segars JH, Hennessy S, et al. Embryo afterloading: a refinement in embryo transfer technique that may increase clinical pregnancy. Fertil Steril. 2005;83(3):710–14. doi: 10.1016/j.fertnstert.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinborg A. IVF/ICSI twin pregnancies: risks and prevention. Hum Reprod Update. 2005;11:575–93. doi: 10.1093/humupd/dmi027. [DOI] [PubMed] [Google Scholar]
- 11.Purcell K, Schembri M, Frazier LM, et al. Asian ethnicity is associated with reduced pregnancy outcomes after assisted reproductive technology. Fertil Steril. 2007;87:297–302. doi: 10.1016/j.fertnstert.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Ryan GL, Sparks AE, Sipe CS, et al. A mandatory single blastocyst transfer policy with educational campaign in a United States IVF program reduces multiple gestation rates without sacrificing pregnancy rates. Fertil Steril. 2007;88(2):354–60. doi: 10.1016/j.fertnstert.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Racowsky C, Stern JE, Gibbons WE, et al. National collection of embryo morphology data into SARTCORS: associations among cell number, fragmentation and blastomere asymmetry on day 3 with live birth rate. Fertil Steril. 2009;92(3, suppl):S82. doi: 10.1016/j.fertnstert.2009.07.316. [DOI] [PubMed] [Google Scholar]
- 14.Racowsky C, Vernon M, Mayer J, et al. Standardization of grading embryo morphology. J Assist Reprod Genet. 2010;27:437–39. doi: 10.1007/s10815-010-9443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharara FI, McClamrock HD. Differences in in-vitro fertilization (IVF) outcome between white and black women in an inner-city, university-based IVF program. Fertil Steril. 2000;73:1170–1173. doi: 10.1016/S0015-0282(00)00524-0. [DOI] [PubMed] [Google Scholar]
- 16.Stillman RJ, Richter KS, Banks NK, Graham JR. Elective single embryo transfer: A 6-year progressive implementation of 784 single blastocyst transfers and the influence of payment method on patient choice. Fertil Steril. 2009;92(6):1895–906. doi: 10.1016/j.fertnstert.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Veeck L. An atlas of human gametes and conceptuses. Carnforth: Parthenon Publishing; 1999. [Google Scholar]
- 18.Vernon M, Stern JE, Ball GD, et al. Utility of the national embryo morphology data collection by SART: correlation between morphologic grade and live birth rate. Fertil Steril. 2009;92(3, suppl):S164. doi: 10.1016/j.fertnstert.2009.07.1305. [DOI] [PubMed] [Google Scholar]