Abstract
Purpose
Asthenozoospermia is a major cause of male infertility. However, the molecular mechanisms underlying sperm-motility defects remain largely unknown in the majority of cases. In our previous study, we applied a proteomic approach to identify unknown proteins that were downregulated in spermatozoa with low motility compared to spermatozoa with good motility. Several sperm motility- related proteins have been identified. In this study, 3-hydroxyisobutyrate dehydrogenase (HIBADH), one of the proteins identified using the proteomic tools, is further characterized.
Methods
Reverse-transcription polymerase chain reactions (RT-PCR), western blotting, and immunofluorescence assays (IFA) were preformed to investigate the expression pattern. The enzymatic activity of HIBADH was evaluated in sperm with good (>50 %), moderate (< 50 %) and lower motility (< 20 %).
Results
Using RT-PCR, we found that transcripts of HIBADH are enriched in the cerebellum, heart, skeletal muscle, uterus, placenta, and testes of male humans. In western blotting, it is expressed in the placenta, testes, and spermatozoa. During spermiogenesis, HIBADH is located at the mid-piece (a specialized development from the mitochondria) of elongating, elongated, and mature sperm. The enzymatic activity of HIBADH in sperm with moderate and lower motility were significantly reduced compared with good motility (P < 0.0001 and P < 0.05, respectively).
Conclusions
Our study indicated that HIBADH is involved in the mitochondrial function of spermatozoa, and maintains sperm motility. It may serve as a sperm-motility marker.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-013-9954-8) contains supplementary material, which is available to authorized users.
Keywords: HIBADH, Asthenozoospermia, Sperm motility, Proteomics
Introduction
Approximately 10 % of couples worldwide are affected by reduced fertility, and male infertility accounts for approximately half of infertile couples [1, 2]. Several studies have reported concerns that infertility rates and the sperm quality of men may be increasing worldwide [3, 4]. Common problems of male subfertility or infertility include oligozoospermia, azoospermia, asthenozoospermia, teratozoospermia, and any combination of these problems [5, 6]. Asthenozoospermia is sperm motility < 50 % or progressive motility < 25 % [5]. A large clinical study indicated that more than 80 % of infertile men have sperm-motility defects [7]. The causes of poor sperm motility include structural defects in the sperm tail, functional defects of the epididymis or other accessory sex glands, and abnormal metabolism in the testicular tissue and/or epididymis [8–10].
Several proteins have been implicated in the regulation of sperm motility. For example, sperm motility is regulated by the cAMP-dependent phosphorylation of flagellar proteins in mammals. One of the downstream targets of cAMP is protein-kinase A (PK-A), a serine/threonine kinase [11]. Mutations of one isoform of PK-A disrupt sperm motility [12]. Calcium signaling is also involved in sperm motility. Sperm express various voltage-gated Ca2+ channels [13, 14]. Certain voltage-gated Ca2+ channels have been shown to be critical for sperm motility by knockout experiments in mice [15, 16]. In addition to the cAMP-dependent PK-A pathways and Ca2+ signaling pathways, other signaling pathways are also likely to play roles. For example, changes in pH affect sperm motility [17].
A proteomic approach has been used to identify unknown proteins involved in spermatozoa in differential species [18–20]. This approach has been used specifically to discover differential sperm-related proteins (e.g., sperm-surface proteins and antisperm antibodies) [21–23]. In other studies, calcium-binding proteins and proteins undergoing tyrosine phosphorylation during capacitation were identified using the proteomic approach [24, 25]. A modified proteomic method, difference 2D gel electrophoresis, was developed to analyze post-translational modification of proteins during sperm maturation [26]. Using this powerful approach, several studies, including ours, have revealed a growing list of proteins that may be involved in regulating sperm motility [27–32].
In our previous study, one metabolism-related protein, 3-hydroxyisobutyrate dehydrogenase (HIBADH; EC 1.1.1.31), which is one of the critical enzymes generating glucose by metabolizing amino acids in the gluconeogenesis pathway, was identified [32]. HIBADH belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donors with NAD+ or NADP+ acceptors [33, 34]. The 2 substrates of this enzyme are 3-hydroxy-2-methylpropanoate and NAD+, whereas its 3 products are 2-methyl-3- oxopropanoate, NADH, and H+. HIBADH participates in valine, leucine, and isoleucine degradation and is a central metabolic enzyme in the valine catabolic pathway [35]. HIBADH is primarily expressed in the mitochondria of the liver, kidney, muscle, and cultured neuron cells [33, 34]. In neural cells, HIBADH may use valine imported into the brain for the generation of energy. In previous study, muscle RING-Finger protein-1 (MuRF1), which has been proposed to trigger muscle protein degradation during pathophysiological muscle wastage, interacted with HIBADH to regulate the amino acid metabolism [36].
In this study, we found that HIBADH is downregulated in low-motility sperm, and is enriched expressed during human spermiogenesis. The enzymatic ability of HIBADH was also reduced in lower-motility sperm. Our findings suggest that HIBADH plays an important role in regulating sperm motility and may serve as a novel sperm-motility marker.
Materials and methods
Reverse transcription
A human total RNA panel (Clontech, Palo Alto, CA) was used to study the expression patterns of HIBADH. Reverse transcription-polymerase chain reactions (RT-PCR) for the synthesis of complementary DNA (cDNA), 12 μL aliquots of master mixture containing 100 ng of human testes RNA, 1 μL of 500 ng/μL oligo(dT)12–18 primer (Invetrogen, Grand Island, NY, USA), and 9 μL of diethylpyrocarbonate-treated water were heated to 70 °C for 10 min and put on ice. RT reactions were performed in 20 μL containing master mixture, 4 μL of 5X first-strand synthesis buffer, 0.1 M dithiothreitol, 10 mM of each dNTP, and 200 units of SuperscriptTM II RNase H- reverse transcriptase (Invetrogen, Grand Island, NY, USA). The RT temperature profile used was 42 °C for 1 h, 75 °C for 15 min, and final cooling to 4 °C. Aliquots of the cDNA were stored at −20 °C until use. The PCR conditions, product detections, and control primer were used as described in our previous publication [37]. Primer sequences of HIBADH for PCR were shown as Forward: tgg,cta,agg,atc,tgg,gat,tg and Reverse: gat,gct,tgg,gga,tca,aac,tt.
Western blotting and immunofluorescence assay
Western blotting and immunofluorescence (IF) were performed using an anti-HIBADH antibody (Sigma, Cat NO. HPA021002; http://www.sigmaaldrich.com/catalog/product/sigma/hpa021002?lang=en®ion=TW). Western blot analysis was performed according to the standard protocols [38]. For the immunofluorescence assay (IFA), the slides were treated with 0.1 % Triton X-100 and washed twice with Tris-buffered-saline (TBS), followed by incubation with the anti-HIBADH antibody (1:100) for 60 min at room temperature. Following the washing steps with TBS, sections were exposed to goat-anti-rabbit Alexa Flou 488 (Molecular Probes, Carlsbad, CA, USA) for 60 min at room temperature and washed with TBS. DAPI was used to stain the nucleus.
Separation of the testicular germ cell populations and sperm preparation
Separation of spermatogenic cells was performed by a centrifugal system based on the density of different types of germ cells, as described in our previous study [39]. After de-capsulation and enzyme digestion of testes biopsies from the case treated with testicular sperm extraction (TESE), germ cell suspensions were filtered through 35 μM nylon filters (Falcon; Becton Dickinson, Franklin Lakes, NJ, USA), followed by centrifugation using a Kubota centrifuge 2010. Germ cells at different developmental stages were collected. Mature spermatozoa were collected from the semen samples of both infertile and fertile men. Finally, suspensions were centrifuged with maximal force (2,580 x g, Kubota 2010) for 10 min, spread on a slide, and air-dried. The slides were then subjected to IFA.
Preparation of sperm for expression levels and enzyme activity for HIBADH
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was also approved by the ethics committee of National Cheng Kung University Hospital, and all patients signed informed consent forms approving the use of their semen for research purposes. To assay the activity of HIBADH, sperm samples were obtained from 10 fertile men with normal sperm motility and 20 men with various degrees of asthenozoospermia. The control patients had more than 50 % sperm motility (n = 10). Men with asthenozoospermia had < 50 % of motile sperm (n = 20). The semen parameters of fertile men and patients with asthenozoospermia are shown in Table 1. No significant pyospermia, hemospermia, or hyperviscosity was present in the semen samples. The semen samples were washed 3 times with 1X PBS, followed by centrifugation at 5,000 x g, and the cell pellet was immediately frozen at −70 °C before future use.
Table 1.
Sperm parameters for normal and cases with asthenozoospermia subjected to enzyme assay
| Group | Range of sperm count (×106/mL) | Average of sperm count (×106/mL) | Range of sperm motility in category A (%) | Average of sperm motility in category A (%) |
|---|---|---|---|---|
| Normal | 33–204 a | 101 | 55–92 b | 72 |
| Asthenozoospermia | 27–199 a | 83 | 2–48 b | 31 |
a There is no significant difference for sperm counts between normal and asthenozoospermic men
b Statistically significant difference for sperm motility (p < 0.001 by Student’s t test) between normal and asthenozoospermic men
Enzyme activity assay
Enzyme activity of HIBADH was measured spectrophotometrically, as described [33, 40]. In brief, the enzyme was assayed in 25 mM ammonium chloride/ammonium hydroxide (pH 10.0 at 20 °C), 1 mM NAD+, 1 mM DTT, and 2 mM S-3-hydroxyisobutyrate (Sigma). Production of NADH was measured by absorbance at 340 nm in a Beckman DU spectrophotometer (Beckman, Fullerton, CA, USA). One unit of activity is defined as the product of 1 μmol NADH produced in 1 min.
Statistical analysis
Sperm samples were grouped according to their progressive motility percentage. The differences for sperm motility and sperm counts between groups were analyzed by the unpaired Student t test. Results of enzyme assays were also analyzed by the unpaired Student t test. We used Prism statistical package version 4.0 (GraphPad, San Diego, CA, USA) for statistical analysis.
Results
Expression patterns of HIBADH in mature human spermatozoa
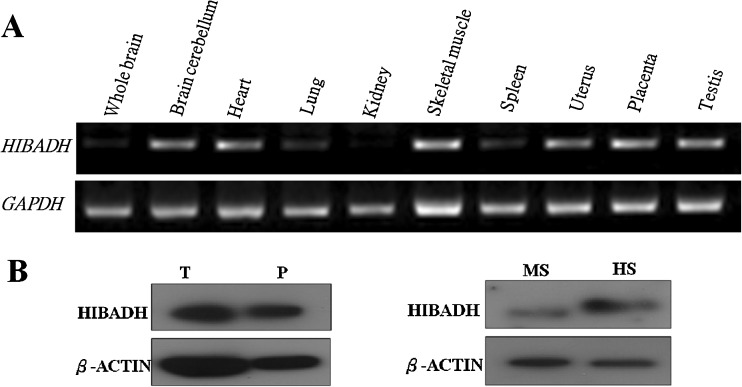
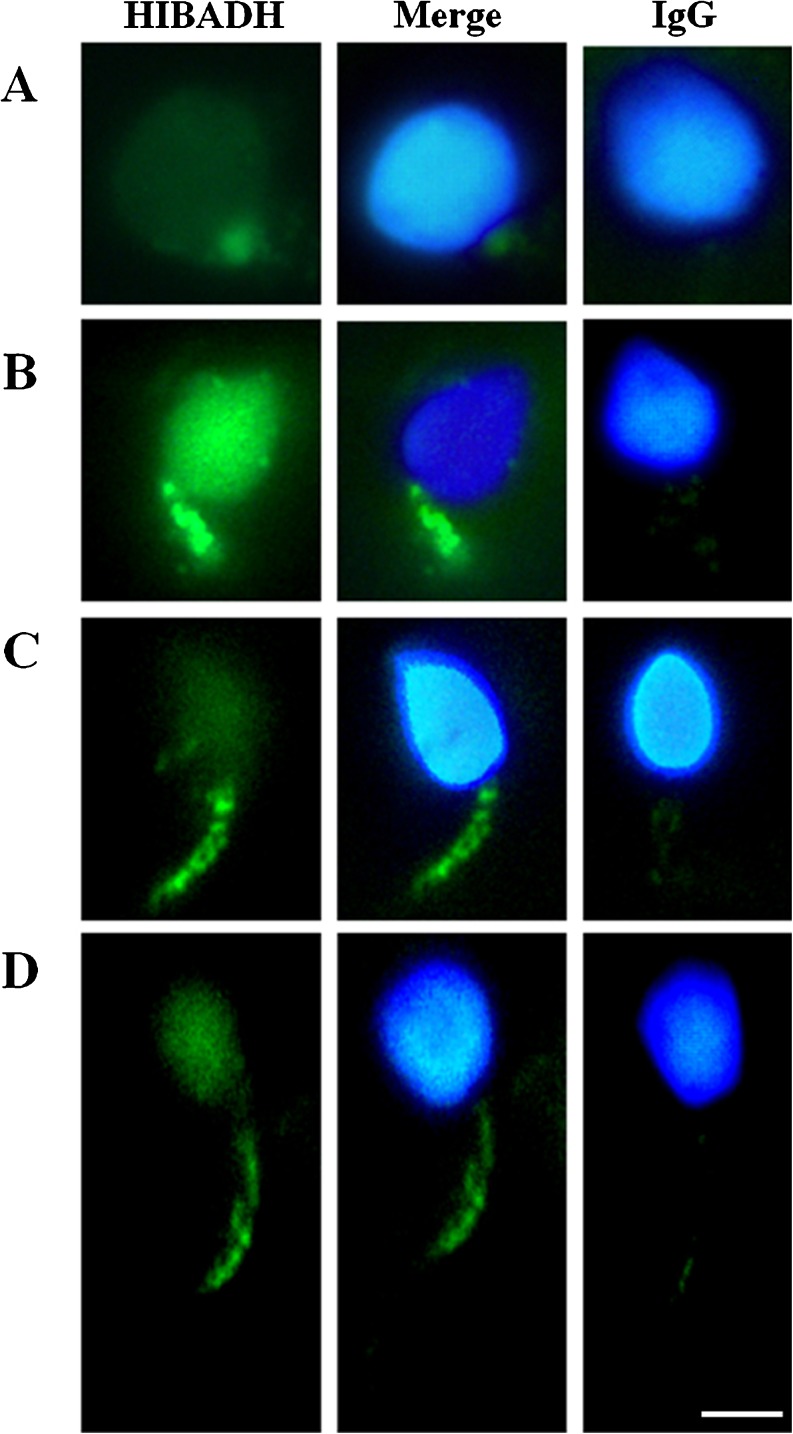
In previous studies, we identified decreasing HIBADH in poor-motility sperm compared to good-motility sperm by using proteomic analysis (Fig. 1) [32]. HIBADH is an NAD+-dependent enzyme that catalyzes the reversible oxidation of 3-hydroxyisobutyrate to methylmalonate semialdehyde in valine catabolism in the gluconeogenesis pathway. HIBADH is expressed in rat liver, heart, muscle, kidney, and cultural neural cells [34, 41]. A larger-scale proteomic study identified approximately 100 proteins in mature human sperm, including HIBADH [18]. However, its detailed expressional patterns in different human organs and developmental stages have not been fully characterized. We learned that transcripts of HIBADH are enriched in the human cerebellum, heart, skeletal muscle, uterus, placenta, and testes by RT-PCR (Fig. 2a). Using western blotting, HIBADH was expressed in human placenta, testis, spermatozoa, and murine spermatozoa (Fig. 2b). It was expressed the most in the neck- and mid-piece, an area that contains the mitochondria in men’s spermatozoa, through IFA (Fig. 3). Because HIBADH is mainly expressed in post-meiotic germ cells, IFA was applied to study its expression patterns in different steps of spermiogenesis using fractionated germ cells. HIBADH was expressed in the neck region of early elongating spermatids (Fig. 4a). In elongating spermatids, the protein was aggregated to form the mid-piece, and the signals were slightly diffused in the head region (Fig. 4b–d). After completion of spermiogenesis, HIBADH is located mainly at the neck- and mid-piece. Dislocalization may cause spermatozoa with low motility. We determined the localization of HIBADH in spermatozoa from asthenospermia cases. However, the expression patterns are similar to spermatoza from the control cases (data not shown, Fig. 3).
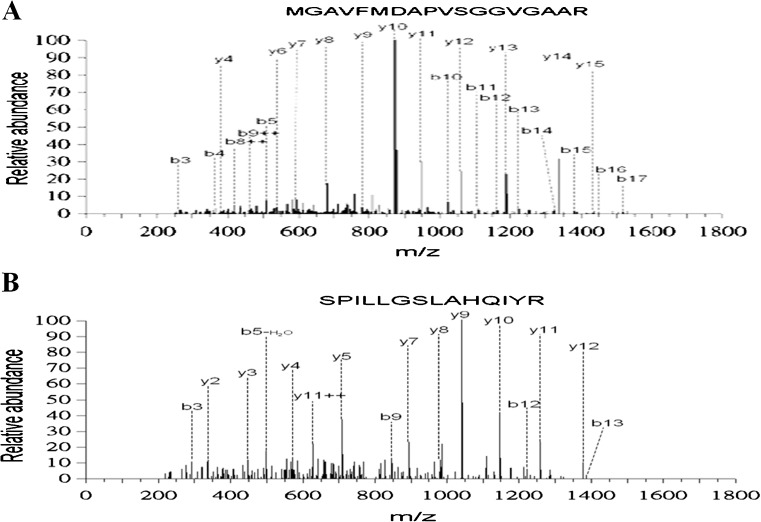
Fig. 1.
Identification of HIBADH from two-dimensional (2-D) gel. a–b Two peptide sequences from 2 D spot match to HIBADH (Product ion scan data against NCBI sequence database)
Fig. 2.
Expression patterns of HIBADH in tissues. a The transcripts of HIBADH are enriched to several human organs. Gapdh was used as an internal control for RT-PCR. b Human testis (T), human placenta (P), human sperm (HS), and mouse sperm (MS). β-actin was used as a loading control
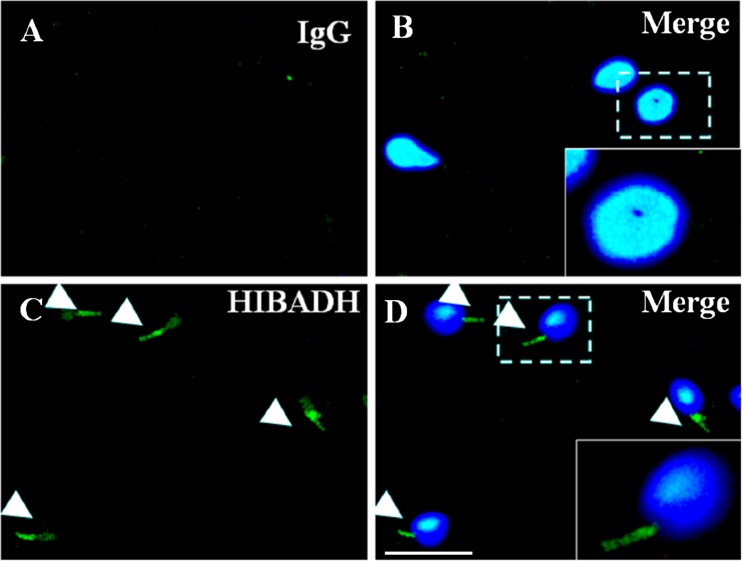
Fig. 3.
HIBADH is expressed at human spermatozoa. Arrow heads indicate that HIBADH proteins are mainly localized at sperm neck and midpiece and to a lesser extent at the sperm head. IgG: negative control. IgG signals (a, green), merge of IgG signals and nuclear DNA signals (b), HIBADH signals (c, green) and merge of HIBADH and nuclear DNA signals (d). Scale bare:10 μm
Fig. 4.
Immuno-fluorescence microscopy showed multiple localizations of HIBADH signals during human spermiogenesis. Early elongating spermatids (a), middle elongating spermatid (b), late elongating spermatids (c) and mature spermatozoa (d). a–d from left to right: HIBADH (green signal), merge of HIBADH and nucleus signal (blue) and IgG control (merge of IgG and nucleus signals). Scale bare:5 μm
Enzyme activity of HIBADH in spermatozoa from patients with asthenozoospermia
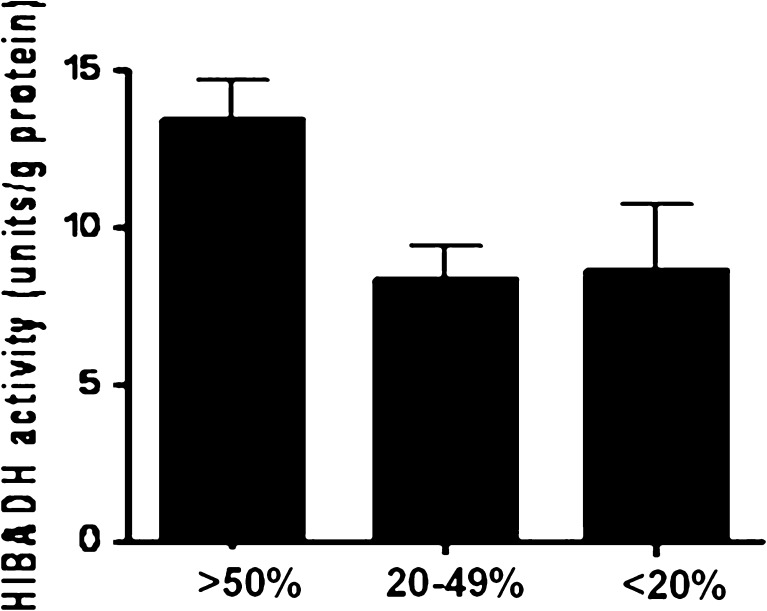
According to the proteomic data and expression pattern in sperm, we propose that HIBAD is involved in regulating sperm motility, and could be used as a sperm-motility marker. Enzyme activity of HIBADH was measured in the spermatozoa of 10 fertile controls and 20 infertile men with asthenozoospermia (<50 % of motile sperm) (Table 1). An enzyme assay showed decreased HIBADH enzyme activity in sperm samples of infertile men with asthenozoospermia compared with the sperm samples of fertile men (P < 0.05 by the Student t test, Fig. 5). Together, our findings show reduced expression and enzymatic activity of HIBADH in poor-motility sperm.
Fig. 5.
Enzyme activity for 3-hydroxyisobutyrate dehydrogenase (HIBADH) in human sperm with different motility. Sample collected from normal (n = 10) and asthenozoospermic men (n = 20). Significant differences were noted between the two groups with P-value of <0.0001 and 0.0186, respectively (unpaired Student’s t-test)
Discussion
The major indices of semen parameters are sperm count, motility, and morphology [42]. Sperm motility is the critical attribute that allows sperm to travel along the uterus to the fallopian tubes prior to penetrating the surface of the ovum and fertilizing the egg. Thus, adequate sperm motility is vital for successful fertilization [43]. Our previous study focused on proteomic profiles of spoor-motility spermatozoa [32]. Approximately 300 areas were resolved in a 2D map in pH values ranging from 4 to 7. Four areas were found to have reduced intensity in at least quintuplicate runs of 2D electrophoresis. In this study, the expression pattern of 1 of these 4 genes (HIBADH) was characterized, and may be a novel sperm-motility marker.
In mammalian spermatozoa, large quantities of adenosine triphosphate (ATP) are required along the full length of the motile flagellum [44]. Sperm mitochondria are located in the mid-piece and produce ATP through aerobic respiration. Several ATP production-related proteins have been identified in the mitochondria of spermatozoa. For example, both sperm-specific isoforms of lactate dehydrogenase catalyze the interconversion of pyruvate and lactate with concomitant interconversion of NADH and NAD+; hexokinase catalyzes the conversion of glucose to glucose-6-phosphate in the initial step of glycolysis and is expressed at the mid-piece of mouse sperm [45, 46]. HIBADH is an NAD+-dependent mitochondrial enzyme that catalyzes oxidation of 3-hydroxyisobutyrate, an intermediate of valine catabolism, to methylmalonate semialdehyde [33, 34]. In this study, we found that both the protein-expressional level and enzymatic activity of HIBADH are reduced in poor-motility spermatozoa. Considering this enzyme is concentrated in the sperm neck and mid-piece, it may be involved in regulating sperm motility.
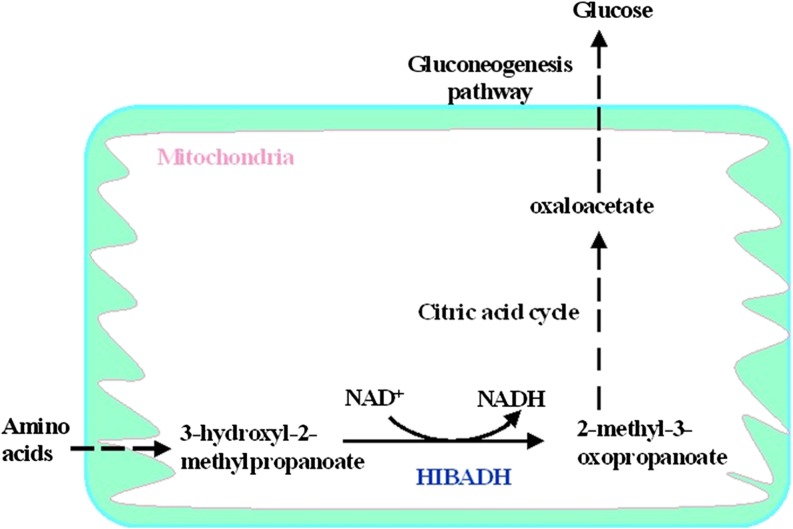
HIBADH is an NAD+-dependent enzyme that catalyzes the reversible oxidation of 3-hydroxyisobutyrate to methylmalonate semialdehyde in valine [47] . This mitochondrial enzyme has been characterized from the liver, kidney, and muscle [33, 34]. Radovan et al. showed that HIBADH is expressed in several types of cultured neural cells [41]. In spermatozoa, amino acids can be readily converted to glucose, primarily through degradation pathways that generate citric acid cycle intermediates, which are subsequently converted to oxaloacteate. Oxaloacteate is used for gluconeogenesis (Fig. 6, modified from Mathews et al.) [33, 47, 48]. Several studies have also shown that glycogen is present in dog sperm, suggesting that gluconeogenesis is important to maintain motility and to achieve in vitro capacitation [49, 50]. Considering that glycogen is stored in human sperm, and sperm from numerous species can remain motile in glucose-free media, it is highly likely that human sperm might be capable of gluconeogenesis [51, 52]. If this is correct, HIBADH is involved in gluconeogenic pathways and is important for the regulation of sperm motility.
Fig. 6.
3-hydroxyisobutyrate dehydrogenase (HIBADH) as a key enzyme in the gluconeogenesis pathway (modified from Mathews et. al, 2000)
In summary, HIBADH was identified as a novel sperm-motility marker in this study. Because HIBADH is abundantly expressed in the mid-piece, it may be involved in regulating the amino acid metabolism and energy supply. Further studies are required to investigate the physiological roles of amino acid metabolism in human spermatozoa. Whether gluconeogenesis is essential for sperm motility must also be investigated. Our results may provide valuable knowledge in developing preventive or therapeutic strategies for asthenozoospermia.
Electronic supplementary material
Supplemental Figure. HIBADH is expressed at human spermatozoa with asthenozoospermia. Human spermatozoa from control (A, B and C) and astherozoospermia case (D, E, and F). A and D: HIBADH signals (red); B and E: mitrotrack (green); C merge of A and B; F merge of D and E. Scale bare:10 μm (JPEG 227 kb)
Acknowledgments
The authors thank National Cheng-Kung University Proteomics Research Core Laboratory for the assistance in 2D gel electrophoresis and mass spectrometry analysis.
Footnotes
Capsule
HIBADH is involved in the mitochondrial function of spermatozoa and maintains sperm motility. It may serve as a sperm-motility marker.
Pao-Lin Kuo and Ying-Hung Lin contributed equally to co-corresponding author.
This study was supported by grants from the National Science Council of the Republic of China (97-2622-B-006-002-CC1; NSC 98-2622-B-006-004-CC1; 99-2628-B-006 -027 -MY3; 101-2320-B-030-012-MY2) and Chi-Mei Medical Center (CMFJ10005).
References
- 1.Hughes V. Geneticists crack the code of infertility. Nat Med. 2008;14:1174. doi: 10.1038/nm1108-1174. [DOI] [PubMed] [Google Scholar]
- 2.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–12. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 3.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukhopadhyay D, Varghese AC, Pal M, Banerjee SK, Bhattacharyya AK, Sharma RK, Agarwal A. Semen quality and age-specific changes: a study between two decades on 3,729 male partners of couples with normal sperm count and attending an andrology laboratory for infertility-related problems in an Indian city. Fertil Steril. 2010;93:2247–54. doi: 10.1016/j.fertnstert.2009.01.135. [DOI] [PubMed] [Google Scholar]
- 5.WHO. World Health Organization Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 1999.
- 6.Boyle CA, Khoury MJ, Katz DF, Annest JL, Kresnow MJ, DeStefano F, Schrader SM. The relation of computer-based measures of sperm morphology and motility to male infertility. Epidemiology. 1992;3:239–46. doi: 10.1097/00001648-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Curi SM, Ariagno JI, Chenlo PH, Mendeluk GR, Pugliese MN, Sardi Segovia LM, Repetto HE, Blanco AM. Asthenozoospermia: analysis of a large population. Arch Androl. 2003;49:343–9. doi: 10.1080/01485010390219656. [DOI] [PubMed] [Google Scholar]
- 8.Ryder TA, Mobberley MA, Hughes L, Hendry WF. A survey of the ultrastructural defects associated with absent or impaired human sperm motility. Fertil Steril. 1990;53:556–60. doi: 10.1016/s0015-0282(16)53357-3. [DOI] [PubMed] [Google Scholar]
- 9.Comhaire FH, Mahmoud AM, Depuydt CE, Zalata AA, Christophe AB. Mechanisms and effects of male genital tract infection on sperm quality and fertilizing potential: the andrologist’s viewpoint. Hum Reprod Updat. 1999;5:393–8. doi: 10.1093/humupd/5.5.393. [DOI] [PubMed] [Google Scholar]
- 10.Infante JP, Huszagh VA. Synthesis of highly unsaturated phosphatidylcholines in the development of sperm motility: a role for epididymal glycerol-3-phosphorylcholine. Mol Cell Biochem. 1985;69:3–6. doi: 10.1007/BF00225921. [DOI] [PubMed] [Google Scholar]
- 11.Visconti PE, Johnson LR, Oyaski M, Fornes M, Moss SB, Gerton GL, Kopf GS. Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev Biol. 1997;192:351–63. doi: 10.1006/dbio.1997.8768. [DOI] [PubMed] [Google Scholar]
- 12.Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci U S A. 2004;101:13483–8. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White DR, Aitken RJ. Relationship between calcium, cyclic AMP, ATP, and intracellular pH and the capacity of hamster spermatozoa to express hyperactivated motility. Gamete Res. 1989;22:163–77. doi: 10.1002/mrd.1120220205. [DOI] [PubMed] [Google Scholar]
- 14.Ho HC, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev Biol. 2002;250:208–17. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- 15.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–9. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakata Y, Saegusa H, Zong S, Osanai M, Murakoshi T, Shimizu Y, Noda T, Aso T, Tanabe T. Ca(v)2.3 (alpha1E) Ca2+ channel participates in the control of sperm function. FEBS Lett. 2002;516:229–33. doi: 10.1016/S0014-5793(02)02529-2. [DOI] [PubMed] [Google Scholar]
- 17.Makler A, David R, Blumenfeld Z, Better OS. Factors affecting sperm motility. VII. Sperm viability as affected by change of pH and osmolarity of semen and urine specimens. Fertil Steril. 1981;36:507–11. doi: 10.1016/s0015-0282(16)45802-4. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Heredia J, Estanyol JM, Ballesca JL, Oliva R. Proteomic identification of human sperm proteins. Proteomics. 2006;6:4356–69. doi: 10.1002/pmic.200600094. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Shen J, Xia Z, Zhang R, Zhang P, Zhao C, Xing J, Chen L, Chen W, Lin M, Huo R, Su B. Proteomic analysis of proteins involved in spermiogenesis in mouse. J Proteome Res. 2010;9:1246–56. doi: 10.1021/pr900735k. [DOI] [PubMed] [Google Scholar]
- 20.Baker MA. The ’omics revolution and our understanding of sperm cell biology. Asian J Androl. 2011;13:6–10. doi: 10.1038/aja.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shetty J, Diekman AB, Jayes FC, Sherman NE, Naaby-Hansen S, Flickinger CJ, Herr JC. Differential extraction and enrichment of human sperm surface proteins in a proteome: identification of immunocontraceptive candidates. Electrophoresis. 2001;22:3053–66. doi: 10.1002/1522-2683(200108)22:14<3053::AID-ELPS3053>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 22.Shetty J, Naaby-Hansen S, Shibahara H, Bronson R, Flickinger CJ, Herr JC. Human sperm proteome: immunodominant sperm surface antigens identified with sera from infertile men and women. Biol Reprod. 1999;61:61–9. doi: 10.1095/biolreprod61.1.61. [DOI] [PubMed] [Google Scholar]
- 23.Shibahara H, Sato I, Shetty J, Naaby-Hansen S, Herr JC, Wakimoto E, Koyama K. Two-dimensional electrophoretic analysis of sperm antigens recognized by sperm immobilizing antibodies detected in infertile women. J Reprod Immunol. 2002;53:1–12. doi: 10.1016/S0165-0378(01)00092-4. [DOI] [PubMed] [Google Scholar]
- 24.Naaby-Hansen S, Mandal A, Wolkowicz MJ, Sen B, Westbrook VA, Shetty J, Coonrod SA, Klotz KL, Kim YH, Bush LA, Flickinger CJ, Herr JC. CABYR, a novel calcium-binding tyrosine phosphorylation-regulated fibrous sheath protein involved in capacitation. Dev Biol. 2002;242:236–54. doi: 10.1006/dbio.2001.0527. [DOI] [PubMed] [Google Scholar]
- 25.Ficarro S, Chertihin O, Westbrook VA, White F, Jayes F, Kalab P, Marto JA, Shabanowitz J, Herr JC, Hunt DF, Visconti PE. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J Biol Chem. 2003;278:11579–89. doi: 10.1074/jbc.M202325200. [DOI] [PubMed] [Google Scholar]
- 26.Baker MA, Witherdin R, Hetherington L, Cunningham-Smith K, Aitken RJ. Identification of post-translational modifications that occur during sperm maturation using difference in two-dimensional gel electrophoresis. Proteomics. 2005;5:1003–12. doi: 10.1002/pmic.200401100. [DOI] [PubMed] [Google Scholar]
- 27.Zhao C, Huo R, Wang FQ, Lin M, Zhou ZM, Sha JH. Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fertil Steril. 2007;87:436–8. doi: 10.1016/j.fertnstert.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Heredia J, de Mateo S, Vidal-Taboada JM, Ballesca JL, Oliva R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod. 2008;23:783–91. doi: 10.1093/humrep/den024. [DOI] [PubMed] [Google Scholar]
- 29.Chan CC, Shui HA, Wu CH, Wang CY, Sun GH, Chen HM, Wu GJ. Motility and protein phosphorylation in healthy and asthenozoospermic sperm. J Proteome Res. 2009;8:5382–6. doi: 10.1021/pr9003932. [DOI] [PubMed] [Google Scholar]
- 30.Siva AB, Kameshwari DB, Singh V, Pavani K, Sundaram CS, Rangaraj N, Deenadayal M, Shivaji S. Proteomics-based study on asthenozoospermia: differential expression of proteasome alpha complex. Mol Hum Reprod. 2010;16:452–62. doi: 10.1093/molehr/gaq009. [DOI] [PubMed] [Google Scholar]
- 31.Parte PP, Rao P, Redij S, Lobo V, D’Souza SJ, Gajbhiye R, Kulkarni V. Sperm phosphoproteome profiling by ultra performance liquid chromatography followed by data independent analysis (LC-MS(E)) reveals altered proteomic signatures in asthenozoospermia. J Proteomics 2012. [DOI] [PubMed]
- 32.Chao HC, Chung CL, Pan HA, Liao PC, Kuo PL, Hsu CC. Protein tyrosine phosphatase non-receptor type 14 is a novel sperm-motility biomarker. J Assist Reprod Genet. 2011;28:851–61. doi: 10.1007/s10815-011-9602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rougraff PM, Paxton R, Kuntz MJ, Crabb DW, Harris RA. Purification and characterization of 3-hydroxyisobutyrate dehydrogenase from rabbit liver. J Biol Chem. 1988;263:327–31. [PubMed] [Google Scholar]
- 34.Rougraff PM, Zhang B, Kuntz MJ, Harris RA, Crabb DW. Cloning and sequence analysis of a cDNA for 3-hydroxyisobutyrate dehydrogenase. Evidence for its evolutionary relationship to other pyridine nucleotide-dependent dehydrogenases. J Biol Chem. 1989;264:5899–903. [PubMed] [Google Scholar]
- 35.Marshall VD, Sokatch JR. Regulation of valine catabolism in Pseudomonas putida. J Bacteriol. 1972;110:1073–81. doi: 10.1128/jb.110.3.1073-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koyama S, Hata S, Witt CC, Ono Y, Lerche S, Ojima K, Chiba T, Doi N, Kitamura F, Tanaka K, Abe K, Witt SH. Muscle RING-finger protein-1 (MuRF1) as a connector of muscle energy metabolism and protein synthesis. J Mol Biol. 2008;376:1224–36. doi: 10.1016/j.jmb.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 37.Lin YH, Lin YM, Teng YN, Hsieh TY, Lin YS, Kuo PL. Identification of ten novel genes involved in human spermatogenesis by microarray analysis of testicular tissue. Fertil Steril. 2006;86:1650–8. doi: 10.1016/j.fertnstert.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 38.Cheng YS, Kuo PL, Teng YN, Kuo TY, Chung CL, Lin YH, Liao RW, Lin JS, Lin YM. Association of spermatogenic failure with decreased CDC25A expression in infertile men. Hum Reprod. 2006;21:2346–52. doi: 10.1093/humrep/del163. [DOI] [PubMed] [Google Scholar]
- 39.Yeh YC, Yang VC, Huang SC, Lo NW. Stage-dependent expression of extra-embryonic tissue-spermatogenesis-homeobox gene 1 (ESX1) protein, a candidate marker for X chromosome-bearing sperm. Reprod Fertil Dev. 2005;17:447–55. doi: 10.1071/RD04077. [DOI] [PubMed] [Google Scholar]
- 40.Hawes JW, Crabb DW, Chan RM, Rougraff PM, Harris RA. Chemical modification and site-directed mutagenesis studies of rat 3-hydroxyisobutyrate dehydrogenase. Biochemistry. 1995;34:4231–7. doi: 10.1021/bi00013a012. [DOI] [PubMed] [Google Scholar]
- 41.Murin R, Schaer A, Kowtharapu BS, Verleysdonk S, Hamprecht B. Expression of 3-hydroxyisobutyrate dehydrogenase in cultured neural cells. J Neurochem. 2008;105:1176–86. doi: 10.1111/j.1471-4159.2008.05298.x. [DOI] [PubMed] [Google Scholar]
- 42.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237–48. doi: 10.1016/S0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 43.Olds-Clarke P. How does poor motility alter sperm fertilizing ability? J Androl. 1996;17:183–6. [PubMed] [Google Scholar]
- 44.Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–7. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 45.Burgos C, Maldonado C. Gerez de Burgos NM, Aoki A, Blanco A. Intracellular localization of the testicular and sperm-specific lactate dehydrogenase isozyme C4 in mice. Biol Reprod. 1995;53:84–92. doi: 10.1095/biolreprod53.1.84. [DOI] [PubMed] [Google Scholar]
- 46.Travis AJ, Foster JA, Rosenbaum NA, Visconti PE, Gerton GL, Kopf GS, Moss SB. Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa. Mol Biol Cell. 1998;9:263–76. doi: 10.1091/mbc.9.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Worrall EB, Gassain S, Cox DJ, Sugden MC, Palmer TN. 3-Hydroxyisobutyrate dehydrogenase, an impurity in commercial 3-hydroxybutyrate dehydrogenase. Biochem J. 1987;241:297–300. doi: 10.1042/bj2410297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathews CK vHKaAK. Biochemistry. San Francisco,USA: Addison Wesley Longman Press; 2000.
- 49.Albarracin JL, Mogas T, Palomo MJ, Pena A, Rigau T, Rodriguez-Gil JE. In vitro capacitation and acrosome reaction of dog spermatozoa can be feasibly attained in a defined medium without glucose. Reprod Domest Anim. 2004;39:129–35. doi: 10.1111/j.1439-0531.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 50.Ballester J, Fernandez-Novell JM, Rutllant J, Garcia-Rocha M, Jesus Palomo M, Mogas T, Pena A, Rigau T, Guinovart JJ, Rodriguez-Gil JE. Evidence for a functional glycogen metabolism in mature mammalian spermatozoa. Mol Reprod Dev. 2000;56:207–19. doi: 10.1002/(SICI)1098-2795(200006)56:2<207::AID-MRD12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 51.Williams AC, Ford WC. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl. 2001;22:680–95. [PubMed] [Google Scholar]
- 52.Galantino-Homer HL, Florman HM, Storey BT, Dobrinski I, Kopf GS. Bovine sperm capacitation: assessment of phosphodiesterase activity and intracellular alkalinization on capacitation-associated protein tyrosine phosphorylation. Mol Reprod Dev. 2004;67:487–500. doi: 10.1002/mrd.20034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. HIBADH is expressed at human spermatozoa with asthenozoospermia. Human spermatozoa from control (A, B and C) and astherozoospermia case (D, E, and F). A and D: HIBADH signals (red); B and E: mitrotrack (green); C merge of A and B; F merge of D and E. Scale bare:10 μm (JPEG 227 kb)