Abstract
Purpose
To investigate two of the most studied estrogen receptor alpha polymorphisms (PvuII and XbaI) in combination, in order to evaluate their impact on an ART program outcome.
Methods
203 normally ovulating women who underwent IVF or ICSI treatment were genotyped for PvuII and XbaI polymorphisms in ESR1 intron 1 using Real-Time PCR. The relationship between the presence of polymorphic alleles and the ovulation induction parameters and outcome was examined.
Results
Women were grouped according to the number of polymorphic alleles they carried in two groups (0–2 versus 3–4 polymorphic alleles). The presence of 3 or more polymorphic alleles was associated with significantly lower E2 levels on the day of hCG administration and a significantly lower rate of good quality embryos.
Conclusion
There is an association between ESR1 polymorphisms and some ART parameters such as the level of E2 on the day of hCG administration and the quality of the embryos. These results underline the importance of ESR1 as a candidate gene for the prediction of ovarian response to IVF/ICSI protocols. Future research work concerning several more genes is necessary for a better evaluation of patients before entering an IVF/ICSI program.
Keywords: Estrogen receptor alpha, ESR1 gene polymorphisms, PvuII, XbaI, IVF, ICSI, Estradiol, Embryo quality
Introduction
The development of assisted reproduction technologies (ART) has been a major advance towards the management of infertility. Among ARTs in vitro fertilization (IVF) is the most widely used, and it is estimated that 2–3 % of all pregnancies in developed countries occur after IVF procedures [10].
An IVF protocol is a multistep process, where controlled ovarian stimulation (COS) with exogenous gonadotropin administration is of paramount significance in order to retrieve adequate number of good quality oocytes and therefore maximize the chance of success. Many factors have been examined as prognostic markers related to the ovarian response to gonadotropin administration, including age [37], hormones such as follicle-stimulating hormone (FSH) [29], estradiol (E2) [18], inhibin B [24] and anti-Müllerian hormone [24,25], smoking [9], and sonographic indices of ovarian reserve such as ovarian volume, antral follicle count and ovarian blood flow [16,32,33]. Furthermore, certain predictive models of ovarian response have been developed, but they have modest accuracy and limited clinical use, because of high intra- and inter-individual variability [8] . As a result, the optimal gonadotropin starting dose has not been established and it is chosen empirically, based on clinical judgment and experience, while the response may vary and sometimes results either in hypo-response and cycle cancellation or hyper-response and the potentially life-threatening complication of ovarian hyperstimulation syndrome [6,13].
Hormones like gonadotropins and steroids have a fundamental role and interact with each other during the physiologic process of folliculogenesis. Estrogens are produced by growing follicles according to the two-cell two-gonadotropin model, and their production is promoted by FSH that induces aromatase activity. In turn, estrogens increase FSH receptors as well as their own receptors, promote granulosa cell growth and differentiation and rescue them from apoptosis. Experiments with estrogen receptor (ER) knockout mice have shown that in ERα knockout mice folliculogenesis is arrested after the pre-antral state, while in ERβ knockout mice folliculogenesis is diminished [7,14,28]. Clinically, estrogen levels are used to monitor the ovarian response to stimulation and studies have shown that serum estradiol levels on the day of human chorionic gonadotropin (hCG) administration correlate directly to embryo quality and pregnancy rates [19], although the role of serum estrogen levels in ART is controversial [15]. Pharmacogenetic studies have been conducted in order to evaluate the role of hormonal receptors, including ER in COS. Estrogens mediate their actions through two subtypes of nuclear receptors, ERα and ERβ, which are encoded by ESR1 and ESR2 genes, present on distinct chromosomes (locus 6q25.1 and locus 14q23-24.1, respectively). Both forms of the receptor have been identified in the human ovary [27]. The ESR1 gene is highly polymorphic, and two of the most studied single nucleotide polymorphisms in ESR1 are rs2234693 (T/C, defined by the cleavage site of the restriction enzyme PvuII) and rs9340799 (A/G, defined by the cleavage site of the restriction enzyme XbaI) in intron 1. The T and C allele of the PvuII are referred as the p and P allele, while the A and G allele of the XbaI are commonly referred as the x and X allele, respectively [31]. PvuII was the first ER polymorphism that has been associated with decreased pregnancy rates after IVF [11,30]. XbaI has also been related with IVF parameters, while both PvuII and XbaI polymorphisms have been associated with the risk of infertility [3]. However, when studied alone, no polymorphism seems to be a strong determinant or predictive marker of ovarian response and IVF outcome.
A better insight into genetic control of COS came from the evidence that ovarian stimulation outcome is a polygenic trait, where a number of genes such as ESR1 and FSH-receptor (FSHR) may interact with each other and synergistically control the ovarian response to FSH in humans [5]. Our recent findings further support the polygenic model of controlled ovarian stimulation. By studying three different polymorphisms (ESR1 PvuII, ESR2 RsaI and the Ser680Asn variant of the FSH-receptor) we have found that the TC/SA combination of ESR1 and FSHR gene was related with higher number of pregnancies in poor responders, good responders and in the overall population, while the CC/AA combination was related with a poor responder profile [2]. In the present study we examined the PvuII and XbaI polymorphisms of ESR1 gene in combination in a Greek population of patients entering an IVF/ICSI protocol, and we evaluated their role concerning the ovulation induction factors such as the total FSH dose needed. We also examined the ovulation induction result, such as the number and quality of the retrieved oocytes and embryos, and the pregnancy rate. Our study shows that the presence of the polymorphisms is associated with certain ovulation induction parameters and outcome. These findings further support that these two polymorphisms should be included among the genetic factors that affect COS in IVF/ICSI protocols.
Materials and methods
Patients
A total of 203 normally ovulating female patients (mean age 35 ± 5 years, mean ± SD) who underwent IVF or intracytoplasmic sperm injection (ICSI) at the IVF Unit of the “Alexandra” Maternity Hospital participated in the study. Institutional review board approval was obtained. All patients had to have at least 1 year of infertility before entering the study. Other inclusion criteria were a regular menstrual cycle of 25 to 35 days, age ≤45 years old and the presence of both ovaries. Patients with polycystic ovarian syndrome (as described by Rotterdam criteria) were excluded from the study.
Hormone assays
Basal (day 3) serum FSH, luteinizing hormone (LH) and prolactin levels were measured by electrochemiluminescence immunoassay (Roche Molecular, Biochemicals, Mannheim, Germany) in the cycle just before the ovulation induction. The estradiol level was measured on the 5th day of the controlled ovarian stimulation and every day until the day of hCG administration, using a commercially available chemiluminescent Microparticle Immunoassay (CMIA) kit (Abbott Laboratories, Abbott Park, IL, USA). Prolactin levels were measured for a better evaluation of the patient, since unusually high serum prolactin levels could lead to cycle cancellation and necessitate appropriate treatment prior to the initiation of an IVF cycle.
COS and IVF/ICSI
COS was conducted according to the gonadotropin-releasing hormone (GnRH) agonist protocol, as described previously [21]. Briefly, patients <35 years old began a long stimulation protocol: on day 21 of the previous cycle, a baseline ultrasound scan was performed and buserelin acetate intranasal spray administration began at a dose of 100 μg five times per day. GnRH agonist (GnRHa) administration was maintained until hCG administration began. The extent of ovarian suppression in all patients was evaluated by ultrasound scan and serum E2 levels (≤40 pg/mL) before starting exogenous gonadotropin administration (about 15 days after administering the spray). Having performed a follow up, hCG was given when at least two follicles were larger than 18 mm and serum estrogen levels were rising.
Oocytes were retrieved 36 h after the administration of 10.000 IU hCG. Follicular aspiration and oocyte retrieval were performed by transvaginal ultrasound guided puncture. Approximately 4 h after oocyte collection, the cumulus and corona cells were removed by incubation in Ham’s F-10 medium (Invitrogen Life Technologies, Paisley, UK) with 80 IU/mL hyaluronidase (type VII, 320 IU/mg; Sigma-Aldrich, Dorset, UK) for 30 s. The oocytes were then transferred to fresh medium, and adhering corona cells were further removed by mechanical pipetting. Several microscopic examinations were performed to ensure complete removal of cumulus cells before ICSI took place. ICSI was performed only in mature oocytes which had extruded the first polar body (metaphase II). The ICSI procedure was performed following conventional techniques [19].
Patients ≥35 years old began a short-term protocol with buserelin (500 μg/day intranasal) on cycle day 2. Gonadotrophin administration began on day 3 at a dose of 200 IU of recombinant FSH (rFSH).
Plasma E2 levels were measured daily, starting 5 days after commencing the regimen until the day after hCG administration. The first scan was performed on day 7 and subsequent scans were performed every day until oocyte retrieval.
The dose of rFSH was adjusted according to ovarian response 6 days after the onset of gonadotropin administration. GnRHa administration was continued until 10.000 IU of hCG were injected intramuscularly. At the same time the mean diameter of at least two leading follicles was above 18 mm and serum E2 level was rising.
All the protocols used in these groups of patients have been previously described in detail [20]. All ultrasound scans were performed by the same clinician. A single experienced clinician performed all embryo transfers.
Embryos were scored and chosen for transfer based on rapid cleavage, absence of fragmentation, and size of blastomeres (good quality, A; poor quality, B). Pregnancy was defined as a positive biochemical pregnancy test 18 days after oocyte retrieval and as clinical pregnancy at the presence of a gestational sac on ultrasound at six gestational weeks.
Single nucleotide polymorphism genotyping
Genomic DNA was obtained from peripheral blood leucocytes with the QIAamp DNA Blood Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Patients were genotyped for PvuII (T/C, rs2234693) and XbaI (A/G, rs9340799) polymorphisms in ESR1 intron 1, using Real-Time PCR . After the DNA extraction and before practicing all PCR methods, the DNA concentration of each sample was controlled with photometry (Qubit TM Fluorometer of Invitrogen). The genotyping of ESR1 PvuII and XbaI polymorphisms was performed with Real-Time PCR, using the Light Cycler 480 II (Roche Diagnostics, Germany).
The conditions of the Real-Time PCR for the ESR1 PvuII polymorphism are described elsewhere [12]. For the ESR1 XbaI polymorphism, the PCR reaction was performed using as primer pairs primer GGGTTATGTGGCAATGACGT and primer AGACTTAATGTTTTTGCAGGAAT and as detection probes sensor-5′-AGACCCTGAGTGTGGTCTAGAGTT-FL and anchor-5′-LC670-GGATGAGCATTGGTCTCTAATGGTTC-PH, (TIB MOLBIOL, Berlin, Germany). The Real-Time PCR was performed in a final volume of 20 μL, containing 1 μg DNA, 1 × Light Cycler 480 Genotyping Master Mix (Roche), 1.5 mM MgCl2 of Light Cycler 480 Genotyping Master (Roche), 0.5 μM of primer F, 2 μM of primer R, 0.2 μM of each detection probe. The PCR amplification conditions were an initial denaturation step of 95 °C for 10 min, followed by 40 cycles of 95 °C for 5 s, 53 °C for 20 s and 72 °C for 10 s.
Statistical analysis
Statistical analysis was performed with Statistics Package for Social Sciences (SPSS), version 15, Minitab 12, while the Sasieni algorithm (1997) and Hardy-Weinberg equilibrium were performed with the on line calculator which is available at http://ihg.gsf.de.
The statistical methods used for the control of statistical hypothesis were: two independent samples t-test, 2-proportion test (normal approximation) and parametric one way Analysis of Variance. For qualitative data used the chi-square test (Fisher exact test and Monte Carlo procedure). The non-parametric tests Mann-Whitney U and Kruskal-Wallis test were used when needed.
A P-value less than 0.05 was regarded as statistically significant. Values are presented as mean ± SD, unless otherwise stated.
Results
Patient characteristics and characteristics of COS outcome
A total of 203 women were included in this study. The mean age was 35 ± 5 years old. For 121 patients, this was their first IVF/ICSI treatment cycle, 50 patients had one previous unsuccessful cycle, and 32 patients had already two or more unsuccessful cycles. Their indications for IVF/ICSI were as follows: tubal factor infertility (53.2 %, n = 108), male factor infertility (41.8 %, n = 85), endometriosis (1 %, n = 2) and unexplained infertility (2 %, n = 4). Few of these women had a double etiology for infertility (2 %, n = 4). 44 % of the patients underwent ICSI, 56 % underwent IVF, while there were two patients that produced 2 follicles after COS, but no oocytes were retrieved afterwards (cycle cancellation).
The basal serum FSH and LH levels were 7.7 ± 3.2 mIU/L and 5.4 ± 2.6 mIU/L, respectively. The mean prolactin level was 15.3 ± 28.1 ng/ml. The mean stimulation days were 10 ± 1, and an average of 3,523 ± 1,315 IU of FSH was used during ovarian stimulation. The mean E2 on the day of hCG administration was 1,806 ± 1,153 pg/ml. The mean number of follicles and oocytes per patient were 7 ± 3 and 6 ± 3, respectively. The maturation rate was 0.69 ± 0.17. The mean number of fertilized oocytes was 4 ± 2 and the fertilization rate was 0.67 ± 0.17. The mean number of grade A embryos was 4 ± 2, while the mean number of grade B embryos was 3 ± 1. The total number of Grade A embryos was 422, while the total number of Grade B embryos was 244. The total pregnancy rate was 22.2 % (number of pregnancies = 45).
Associations of gene polymorphisms with COS and pregnancy outcome of IVF
The distribution of ESR1 PvuII genotypes among IVF patients was as follows: 25.1 % (51/203) were homozygous for pp, 55.2 % (112/203) were pP and 19.7 % (40/203) were homozygous for PP, with p and P allele frequencies of 52.7 and 47.3 %, respectively. The ESR1 XbaI genotypes distributed as follows: 33.5 % (68/203) were xx, 53.2 % (108/203) were xX and 13.3 % (27/203) were XX, which gave the x and X allele frequencies of 60.1 and 39.9 %, respectively.
When studying the two polymorphisms in combination, there exist 9 possible combinations: ppxx, ppXx, pPxx, pPxX, ppXX, PPxx, pPXX, PPxX and PPXX. These combinations can be further divided in categories, according to how many polymorphic alleles they present: 0 (ppxx), 1 (ppXx, pPxx), 2 (pPxX, ppXX, PPxx), 3 (pPXX, PPxX) and 4 (PPXX). The distribution of patients according to the number of the polymorphic alleles that they carry is shown in Table 1. There was no woman carrying the ppXX, pPXX and PPxx combinations. This is not a surprising result, knowing that these two polymorphisms are in linkage disequilibrium. The XbaI polymorphism in an homozygous state appeared always with the PvuII polymorphism in an homozygous state in our sample.
Table 1.
Distribution of patients according to the number of the polymorphic alleles that they carry
| Number of polymorphic alleles | Allele combination | N | % |
|---|---|---|---|
| 0 | ppxx | 47 | 23.2 |
| 1 | pPxx/ppxX | 25 | 12.3 |
| 2 | pPxX | 91 | 44.8 |
| 3 | PPxX | 13 | 6.4 |
| 4 | PPXX | 27 | 13.3 |
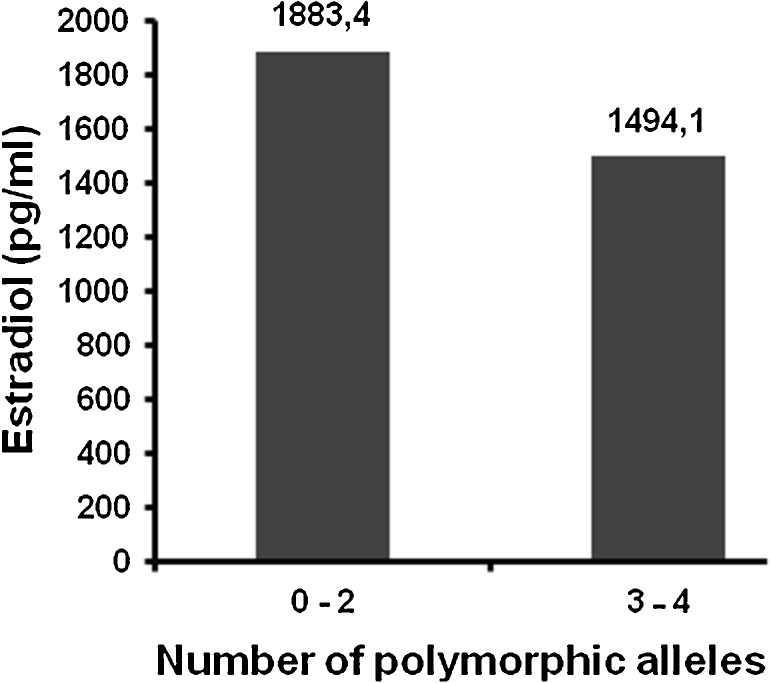
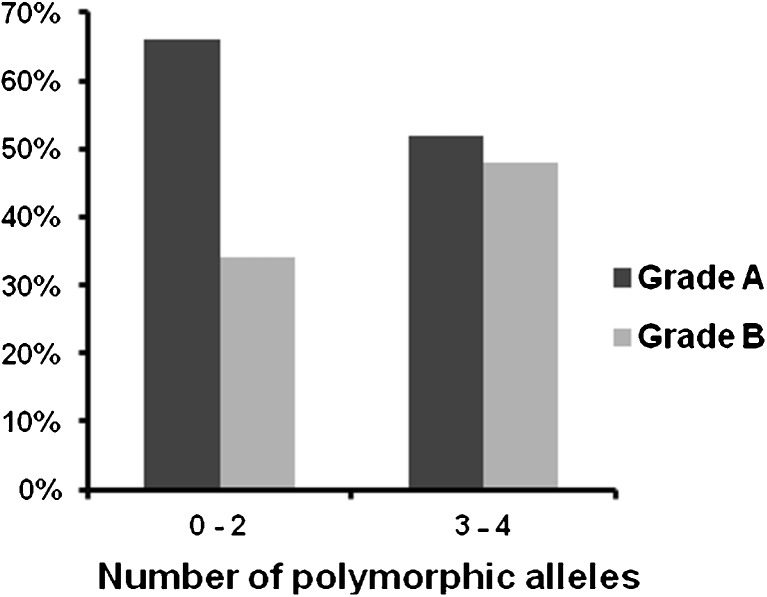
Taking this under consideration, the results of the research were analyzed by dividing the women in two categories, according to the total number of polymorphic alleles that they carry for the PvuII and XbaI polymorphisms: women who presented from 0 to 2 polymorphic alleles, and women who presented 3–4 polymorphic alleles. The two groups of patients were analyzed on the basis of the clinical, biochemical and ovarian stimulation factors and concerning the ovarian stimulation and pregnancy outcomes. The results are presented in Table 2. The mean age of the women and the basal biochemical parameters (FSH, LH) were not significantly different among the two groups. Women that presented 3–4 polymorphic alleles appeared to have lower E2 on the day of hCG administration (1494.1 versus 1883.4 pg/ml, p-value = 0.017) in comparison with women that presented from 0 to 2 polymorphic alleles, as shown in Fig. 1. Furthermore, an interesting finding is the total number and percentage of good quality (Grade A) and poor quality (Grade B) embryos in the two groups, which differed in a statistically significant way (p-value = 0.004). Women carrying 0–2 polymorphic alleles produced better quality embryos than women carrying 3–4 polymorphic alleles (66 % versus 52 % the percentages of grade A embryos, respectively and 34 % versus 48 % the percentages of grade B embryos, respectively). These results are also presented in Fig. 2. There was no significant difference in pregnancy rates among the two groups (22.1 versus 22.5 %, p-value = 0.955).
Table 2.
Women who present from 0 to 2 polymorphic alleles and women who present 3–4 polymorphic alleles, analyzed on the basis of the clinical, biochemical and ovarian stimulation factors and concerning the ovarian stimulation and pregnancy outcomes (aT-test, bx2 test). The results are shown as Mean (SE), unless otherwise stated
| 0–2 polymorphic alleles (n = 163) | 3–4 polymorphic alleles (n = 40) | p-valuea,b | |
|---|---|---|---|
| Mean (SE) | Mean (SE) | ||
| Age (years) | 35.5 (0.41) | 34.6 (0.77) | 0.349a |
| Number of previous trials | 1.59 (0.07) | 1.95 (0.22) | 0.116a |
| FSH (mIU/L) | 7.55 (0.25) | 8.42 (0.48) | 0.124a |
| LH (mIU/L) | 5.25 (0.2) | 5.79 (0.43) | 0.238a |
| Number of days of stimulation | 10.44 (0.1) | 10.78 (0.25) | 0.182a |
| Total FSH dose (IU) | 3456.1 (95.8) | 3794.3 (256.7) | 0.223a |
| E2 (day of hCG) (pg/ml) | 1883.4 (95.1) | 1494.1 (129.3) | 0.017a |
| No of follicles | 7.12 (0.24) | 6.75 (0.47) | 0.494a |
| No of oocytes | 6.39 (0.24) | 6 (0.48) | 0.471a |
| Maturation rate (%) | 70 (1) | 66 (4) | 0.264a |
| Fertilized oocytes | 4.17 (0.15) | 3.98 (0.32) | 0.577a |
| Fertilization rate (%) | 68 (1) | 67 (3) | 0.974a |
| Total number of Grade A embryos-Number (%) | 356 (66 %) | 66 (52 %) | 0.004b |
| Total number of Grade B embryos-Number (%) | 183 (34 %) | 61 (48 %) | |
| Endometrium thickness (mm) | 9.92 (0.18) | 9.70 (0.34) | 0.570a |
| Pregnancy N (%) | 36 (22.1 %) | 9 (22.5 %) | 0.955 b |
The statistically significant differences are marked in bold characters (p-value <0.05)
Fig. 1.
E2 levels on the day of hCG administration. Women that present 3–4 polymorphic alleles appear to have lower estradiol (E2) on the day of human chorionic gonadotropin (hCG) administration (1494.1 versus 1883.4 pg/ml, p-value = 0.017, t-test) in comparison with women that present from 0 to 2 polymorphic alleles
Fig. 2.
The quality of embryos according to the number of polymorphic alleles. Women that present 0–2 polymorphic alleles present higher percentage of Grade A embryos (66 versus 52 %) and lower percentage of Grade B embryos (34 versus 48 %) in comparison with women that present from 3 to 4 polymorphic alleles (p-value = 0.004, x2 test)
Discussion
In the present study, we have analyzed two polymorphisms of the ESR1 gene, PvuII and XbaI, in 203 patients undergoing ovarian stimulation for IVF/ICSI and embryo transfer, in order to evaluate the role of these polymorphisms concerning the ovarian stimulation factors, as well as the ovarian stimulation and pregnancy outcome. Some studies report different incidence of the PvuII and XbaI polymorphisms between fertile women and infertile women undergoing IVF [3], while others report no differences between IVF patients and controls [11,30]. The frequencies of the polymorphisms in our sample are comparable with those previously observed in another IVF population [1], despite the different ethnic groups among the two studies. The new findings of the study are the differences in important factors of the ovarian stimulation, as the levels of E2 on the day of hCG administration and parameters concerning the ovarian stimulation outcome, as the embryo quality, among patients with different genotype profiles. The presence of 3 or more polymorphic alleles of PvuII and XbaI polymorphisms in combination leads to lower E2 on the day of hCG administration and a greater number of poor rather than good quality embryos, in a statistically significant way, without differences concerning the maturation, fertilization and pregnancy rates.
Estrogens affect the oocyte maturation, providing an optimal oocyte cytoplasm and oolemma maturation and stand as an important factor that determines the quality of oocyte [3]. Regarding the infertility management of women undergoing IVF/ICSI, peak E2 on the day of hCG administration is a marker of embryo quality [19]. Equally important is the role of estrogen receptors, which mediate the estrogen signal onwards. The hypothesis that genes involved in the reproductive mechanism, like ESR1, harbour several polymorphisms and can affect to an extend the IVF outcome, is well documented by several studies, which focused on the subgroup of women with infertility problems that are recruited in ovarian stimulation protocols.
The research teams of Georgiou [11] and Sundarrajan [30] were the first to investigate the possible impact of the genotype concerning PvuII polymorphism on ESR1 gene in women undergoing IVF. More recently, Altmäe et al. evaluated the impacts of ESR1 PvuII, XbaI and (TA)n genotypes on the etiology of female infertility, as well as their contributions to the COS and pregnancy outcome of IVF in 159 infertile women undergoing IVF-ET [1] . They concluded that ESR1 variants predict the chance for clinical pregnancy rate per COS rather than per single embryo transfer. Contrary to findings in this study, Georgiou et al. and Sundarrajan et al. had shown that there was a relationship between some ESR1 variants and clinical pregnancy rate per embryo transfer. A more recent study concerning both PvuII and XbaI polymorphisms was conducted on a Turkish population and involved both fertile and infertile women. PvuII and XbaI polymorphisms conferred risk for infertility in a simple dominant manner in which a significant relationship was observed between infertile and control women. ESR1 genotypes were compared concerning maturation, fertilization, pregnancy rates and embryo quality. Although no difference was found in terms of pregnancy rates, maturation and fertilization rates were significantly smaller in pp and xx genotypes. Furthermore, pp genotypes had significantly lower number of good quality embryos [3].
In our study, PvuII and XbaI polymorphisms were studied together in combination, and not by a single marker approach, because they are located in intron 1 of ESR1 gene very close, only about 50 bp apart from each other, a fact that also explains the linkage disequilibrium between them, and reinforces their close relation. Contrary to the findings in Ayvaz’s study [3], our study supports that the presence of ≥3 polymorphic alleles in both polymorphisms leads to significantly lower number of good quality (Grade A) embryos. This finding is further on supported by the lower levels of E2 on the day of hCG administration in the group of women that present 3 or 4 polymorphic alleles. The absence of a statistically significant association between maturation rate and polymorphisms in our study may be explained by the fact that the outcome of COS is multifactorial and no single gene is per se a strong determinant of outcome. However, the different rates of Grade A embryos indicate the effect of the polymorphisms on oocyte quality and further embryonic development. The results of our study are supported by a previous study of Corbo et al., which examined the association of ESR1 PvuII and XbaI polymorphisms with fertility in two populations with different reproductive patterns, a sample of healthy Italian men and women and a sample of healthy African-Ecuadorian women [4] . Corbo et al. did not study an infertile population, but they investigated the two polymorphisms in combination, like the present study, and presented results that support that the non-presence of the polymorphic alleles results to an increased reproductive efficiency. Specifically, ESR1 xx and ppxx genotypes among the Italian men were found to be associated with an above-median number of children. ESR1 pp genotype among the Italian women showed a tendency to be associated with a lower number of abortions, whereas ESR1 pp and ppxx genotypes among African-Ecuadorian women were associated with a higher number of children. Recently M’ Rabet et al. have associated PvuII polymorphism with infertility and found a statistically significant higher prevalence of the PP allele among infertile women with ongoing menstrual cycles [23]. Taken together, the above results indicate that according to some studies the presence of certain ESR1 polymorphisms is related with an increased reproductive efficiency that is expressed with different phenotypic patterns (e.g. increased fertility and higher parity, lower number of abortions or favourable ovulation induction outcome), depending on the different reproductive circumstances in each occasion.
Introns may affect gene expression at many different levels [17,26]. However it remains unknown whether and by which mechanism the two studied polymorphisms may affect ESR expression. In vitro studies have demonstrated that enhancer activity differs, although not significantly, among ESR1 haplotypes, the highest being associated with ESR1 xp haplotype and ESR1*x allele [22] . This difference suggested that the expression of ESR1 could be regulated depending on the ESR1 genotype. In patients with schizophrenia, the presence of the CC (PP) genotype has been associated with decreased ESR1 mRNA levels at the frontal cortex compared to patients homozygous for TT (pp) [35]. According to present data, it could be hypothesized that the presence of ESR1 x and p alleles, increasing the ESR1 function, may affect estrogen biological action. A possible synergistic effect of two different mechanisms favoured by the two different polymorphisms may also be hypothesized. A better interaction and regulation at the level of the estrogen receptor may offer the explanation to the higher levels of E2 on the day of hCG administration and the existence of more good quality embryos in the group of women that present 0–2 polymorphic allele. Furthermore, although neither PvuII nor XbaI polymorphism cause amino acid substitutions they may be in linkage disequilibrium with other ESR1 mutations which may affect both the estrogen receptor gene expression and function [36] .
Our study examined the two most studied ESR1 polymorphisms related with ovulation induction outcome and fertility parameters. The response to ovarian stimulation is a multigenic trait and the first genome-wide analysis was unable to identify single nucleotide polymorphisms significantly associated with parameters of oocyte yield, ovarian sensitivity to stimulation and oocyte quality [34]. However we consider that according to our results ESR1 PvuII and XbaI polymorphisms are two polymorphisms that have an important role in the development of a patient-tailored approach to ovarian stimulation.
Conclusion
In conclusion, this study showed an association between ESR1 genotypes and some IVF parameters, such as the levels of levels of E2 on the day of hCG administration. This study underlines that ESR1 gene should be listed as a candidate gene for the prediction of ovarian response to IVF/ICSI protocols. Research work that will combine several candidate genes is necessary to evaluate which of them play a major role to the fertility mechanism, and will allow further application to the evaluation of a patient, before entering an IVF/ICSI program.
Footnotes
Capsule
Study of ESR1 PvuII and XbaI gene polymorphisms in women enrolled in IVF/ICSI protocols.
References
- 1.Altmäe S, Haller K, Peters M, et al. Allelic estrogen receptor 1 (ESR1) gene variants predict the outcome of ovarian stimulation in in vitro fertilization. Mol Hum Reprod. 2007;13:521–6. doi: 10.1093/molehr/gam035. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostou E, Mavrogianni D, Theofanakis C, et al. ESR1, ESR2 and FSH receptor gene polymorphisms in combination: a useful genetic tool for the prediction of poor responders. Curr Pharm Biotechnol. 2012;13:426–34. doi: 10.2174/138920112799361891. [DOI] [PubMed] [Google Scholar]
- 3.Ayvaz OU, Ekmekçi A, Baltaci V, Onen HI, Unsal E. Evaluation of in vitro fertilization parameters and estrogen receptor alpha gene polymorphisms for women with unexplained infertility. J Assist Reprod Genet. 2009;26:503–10. doi: 10.1007/s10815-009-9354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbo RM, Ulizzi L, Piombo L, et al. Estrogen receptor alpha polymorphisms and fertility in populations with different reproductive patterns. Mol Hum Reprod. 2007;13:537–40. doi: 10.1093/molehr/gam041. [DOI] [PubMed] [Google Scholar]
- 5.de Castro F, Moron FJ, Montoro L, et al. Human controlled ovarian hyperstimulation outcome is a polygenic trait. Pharmacogenetics. 2004;14:285–93. doi: 10.1097/00008571-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002;8:559–77. doi: 10.1093/humupd/8.6.559. [DOI] [PubMed] [Google Scholar]
- 7.Drummond AE. The role of steroids in follicular growth. Reprod Biol Endocrinol. 2006;4:16. doi: 10.1186/1477-7827-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fauser BCJM, Diedrich K, Devroey P, Evian Annual Reproduction Workshop Group 2007 Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update. 2008;14:1–14. doi: 10.1093/humupd/dmm034. [DOI] [PubMed] [Google Scholar]
- 9.Freour T, Masson D, Mirallie S, et al. Active smoking compromises IVF outcome and affects ovarian reserve. Reprod Biomed Online. 2008;16:96–102. doi: 10.1016/S1472-6483(10)60561-5. [DOI] [PubMed] [Google Scholar]
- 10.Gearhart J, Coutifaris C. In vitro fertilization, the Nobel Prize, and human embryonic stem cells. Cell Stem Cell. 2011;8:12–5. doi: 10.1016/j.stem.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Georgiou I, Konstantelli M, Syrrou M, Messinis IE, Lolis DE. Oestrogen receptor gene polymorphisms and ovarian stimulation for in-vitro fertilization. Hum Reprod. 1997;12:1430–3. doi: 10.1093/humrep/12.7.1430. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Gomez F, Vergara F, Fernandez A, et al. Detection of pvull polymorphism within intron 1 of ESR1 gene by real-time PCR. Clin Chem Lab Med. 2003;41:392–3. doi: 10.1515/CCLM.2003.060. [DOI] [PubMed] [Google Scholar]
- 13.Kligman I, Rosenwaks Z. Differentiating clinical profiles: predicting good responders, poor responders, and hyperresponders. Fertil Steril. 2001;76:1185–90. doi: 10.1016/S0015-0282(01)02893-X. [DOI] [PubMed] [Google Scholar]
- 14.Kolibianakis EM, Papanikolaou EG, Fatemi HM, Devroey P. Estrogen and folliculogenesis: is one necessary for the other? Curr Opin Obstet Gynecol. 2005;17:249–53. doi: 10.1097/01.gco.0000169101.83342.96. [DOI] [PubMed] [Google Scholar]
- 15.Kolibianakis EM, Venetis CA, Tarlatzis BC. Role of the endocrine profile for the achievement of pregnancy with IVF. Reprod Biomed Online. 2009;18(Suppl 2):37–43. doi: 10.1016/S1472-6483(10)60447-6. [DOI] [PubMed] [Google Scholar]
- 16.Kupesic S, Kurjak A. Predictors of IVF outcome by three-dimensional ultrasound. Hum Reprod. 2002;17:950–5. doi: 10.1093/humrep/17.4.950. [DOI] [PubMed] [Google Scholar]
- 17.Le Hir H, Nott A, Moore MJ. How introns influence and enhance eukaryotic gene expression. Trends Biochem Sci. 2003;28:215–20. doi: 10.1016/S0968-0004(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 18.Licciardi FL, Liu HC, Rosenwaks Z. Day 3 estradiol serum concentrations as prognosticators of ovarian stimulation response and pregnancy outcome in patients undergoing in vitro fertilization. Fertil Steril. 1995;64:991–4. doi: 10.1016/s0015-0282(16)57916-3. [DOI] [PubMed] [Google Scholar]
- 19.Loutradis D, Drakakis P, Kallianidis K, et al. Oocyte morphology correlates with embryo quality and pregnancy rate after intracytoplasmic sperm injection. Fertil Steril. 1999;72:240–4. doi: 10.1016/S0015-0282(99)00233-2. [DOI] [PubMed] [Google Scholar]
- 20.Loutradis D, Drakakis P, Milingos S, Stefanidis K, Michalas S. Alternative approaches in the management of poor response in controlled ovarian hyperstimulation (COH) Ann N Y Acad Sci. 2003;997:112–9. doi: 10.1196/annals.1290.013. [DOI] [PubMed] [Google Scholar]
- 21.Loutradis D, Patsoula E, Minas V, et al. FSH receptor gene polymorphisms have a role for different ovarian response to stimulation in patients entering IVF/ICSI-ET programs. J Assist Reprod Genet. 2006;23:177–84. doi: 10.1007/s10815-005-9015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama H, Toji H, Harrington CR, et al. Lack of an association of estrogen receptor alpha gene polymorphisms and transcriptional activity with Alzheimer disease. Arch Neurol. 2000;57:236–40. doi: 10.1001/archneur.57.2.236. [DOI] [PubMed] [Google Scholar]
- 23.M'Rabet N, Moffat R, Helbling S, et al. The CC-allele of the PvuII polymorphic variant in intron 1 of the alpha-estrogen receptor gene is significantly more prevalent among infertile women at risk of premature ovarian aging. Fertil Steril. 2012;98:965–972.e1-5. doi: 10.1016/j.fertnstert.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 24.Muttukrishna S, McGarrigle H, Wakim R, et al. Antral follicle count, anti-mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG. 2005;112:1384–90. doi: 10.1111/j.1471-0528.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 25.Nardo LG, Gelbaya TA, Wilkinson H, et al. Circulating basal anti-Mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92:1586–93. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 26.Nott A, Meislin SH, Moore MJ. A quantitative analysis of intron effects on mammalian gene expression. RNA. 2003;9:607–17. doi: 10.1261/rna.5250403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J Clin Endocrinol Metab. 2000;85:4835–40. doi: 10.1210/jc.85.12.4835. [DOI] [PubMed] [Google Scholar]
- 28.Salha O, Abusheikha N, Sharma V. Dynamics of human follicular growth and in-vitro oocyte maturation. Hum Reprod Update. 1998;4:816–32. doi: 10.1093/humupd/4.6.816. [DOI] [PubMed] [Google Scholar]
- 29.Scott RT, Toner JP, Muasher SJ, et al. Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651–4. doi: 10.1016/s0015-0282(16)60615-5. [DOI] [PubMed] [Google Scholar]
- 30.Sundarrajan C, Liao W, Roy AC, Ng SC. Association of oestrogen receptor gene polymorphisms with outcome of ovarian stimulation in patients undergoing IVF. Mol Hum Reprod. 1999;5:797–802. doi: 10.1093/molehr/5.9.797. [DOI] [PubMed] [Google Scholar]
- 31.Sundermann EE, Maki PM, Bishop JR. A review of estrogen receptor alpha gene (ESR1) polymorphisms, mood, and cognition. Menopause. 2010;17:874–86. doi: 10.1097/gme.0b013e3181df4a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syrop CH, Dawson JD, Husman KJ, Sparks AE, Van Voorhis BJ. Ovarian volume may predict assisted reproductive outcomes better than follicle stimulating hormone concentration on day 3. Hum Reprod. 1999;14:1752–6. doi: 10.1093/humrep/14.7.1752. [DOI] [PubMed] [Google Scholar]
- 33.Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod. 1997;12:220–3. doi: 10.1093/humrep/12.2.220. [DOI] [PubMed] [Google Scholar]
- 34.van Disseldorp J, Franke L, Eijkemans R, et al. Genome-wide analysis shows no genomic predictors of ovarian response to stimulation by exogenous FSH for IVF. Reprod Biomed Online. 2011;22:382–8. doi: 10.1016/j.rbmo.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Weickert CS, Miranda-Angulo AL, Wong J, et al. Variants in the estrogen receptor alpha gene and its mRNA contribute to risk for schizophrenia. Hum Mol Genet. 2008;17:2293–309. doi: 10.1093/hmg/ddn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaich L, Dupont WD, Cavener DR, Parl FF. Analysis of the PvuII restriction fragment-length polymorphism and exon structure of the estrogen receptor gene in breast cancer and peripheral blood. Cancer Res. 1992;52:77–83. [PubMed] [Google Scholar]
- 37.Ziebe S, Loft A, Petersen JH, et al. Embryo quality and developmental potential is compromised by age. Acta Obstet Gynecol Scand. 2001;80:169–74. doi: 10.1034/j.1600-0412.2001.080002169.x. [DOI] [PubMed] [Google Scholar]