Abstract
The aim of our study was to evaluate the prognostic significance of blood transfusion on recurrence and survival in patients undergoing curative resections for colorectal cancer. Retrospective analysis of prospectively collected data of patients after elective resections for colorectal cancer between January 2001 and December 2009 was undertaken. The main endpoint was overall survival, disease-free survival, and recurrence rate. These data were evaluated in relation to blood transfusion (group A, no blood transfusion; group B, one to two blood transfusions; group C, three and more blood transfusions). A total of 583 patients met the criteria for inclusion in the study. Of these, 132 (22.6 %) patients received blood transfusion in the perioperative period. There were 83 (14.2 %) patients who received one or two blood transfusions and 49 (8.4 %) patients who required three or more transfusions. Patients with three or more transfusions had a significantly worse 5-year overall survival, disease-free survival, and increased incidence of distant recurrences in comparison with the group without transfusion or the group with one or two transfusions. Multivariate analysis showed that the application of three or more blood transfusions is an independent risk factor for overall survival (P = 0.001; HR 2.158; 95 % CI 1.370–3.398), disease-free survival (P < 0.001; HR 2.514; 95 % CI 1.648–3.836), and the incidence of distant recurrence (P < 0.001; HR 2.902; 95 % CI 1.616–5.212). Application of three or more blood transfusions in patients operated for colorectal carcinoma is an adverse prognostic factor. Indications for blood transfusion should be carefully considered not only with regard to the risk of early complications, but also because of the possibility of compromising long-term results.
Keywords: Colorectal cancer, Blood transfusion, Survival, Recurrence
Introduction
Clinical evidence for the existence of transfusion associated immunomodulation has been available since 1973, when Opelz et al [1] provided evidence that recipients of allogenic blood transfusion had improved renal allograft survival. In 1981, Gantt [2] raised the question whether the immunomodulation induced by blood transfusion might also influence the prognosis of patients treated for malignancy. One year later, Burrows and Tartter [3] confirmed an association between allogenic blood transfusion and the incidence of recurrence in patients with colon cancer. Following these early reports, several other studies have been published in the literature with contradictory results. Some of these studies have shown an adverse effect of transfusion on the prognosis of patients with colorectal carcinoma [4–6]. Conversely, others have found no transfusion-dependent effect on survival and recurrence [7, 8].
The exact mechanisms of the transfusion associated immunomodulation remain unclear. Allogenic white blood cells and inflammatory cytokines have been shown to increase suppressor T-cell activity and inhibit natural killer cell activity, depressed macrophage and monocyte phagocyte activity. Besides the impairment of immune surveillance of cancer cells, the growth factors released during blood storage may also be responsible for tumor recurrence [9–11]. However, most studies evaluating proposed mechanisms have been done in animal models, and these findings may not be applicable to the human immune system.
The aim of our study was to evaluate the prognostic significance of blood transfusion on recurrence and survival in patients undergoing curative resections for colorectal cancer.
Material and Methods
We conducted a retrospective analysis of prospectively collected data of patients after elective resections for colorectal cancer at the surgical clinic of University Hospital Ostrava between January 2001 and December 2009. The main endpoint was overall survival, disease-free survival, and incidence of recurrence. These data were evaluated in relation to blood transfusion (group A, no blood transfusion; group B, one to two blood transfusions; group C, three and more blood transfusions). Overall survival was defined as a time from surgery to death from any cause. Disease-free survival was defined as a time from surgery to a recurrence or death from any cause as the event of interest. Recurrences were classified as distant metastasis or as locoregional (tumor restricted to the region of primary operation). Indications for perioperative blood transfusion were made by the anesthesiologist or surgeon, depending on the hemodynamic status, age, comorbidity, and hemoglobin concentration, respecting principles of effective hemotherapy.
Patients with metastatic disease, previous malignant tumor in the past 5 years, emergency surgery, or lost to follow-up were excluded. Inasmuch as primarily long-term results were analyzed, patients dying within 30 days after surgery were also excluded from the study. Data on survival and recurrences were collected from medical records of the surgical or radiotherapeutic department of University Hospital Ostrava, National Cancer Registry, and eventually from general practitioners.
For statistical analysis, continuous variables were categorized and compared using chi-square test. Survival curves were constructed using the Kaplan–Meier method and compared with log-rank test. Analysis of predefined predictive factors of survival was performed. The analyzed variables were sex, age (<66 years, ≥66 years), body mass index (<30 kg/m2 vs. ≥30 kg/m2), anesthesia risk (according to ASA classification—American Society of Anesthesiologists), tumor localization (colon, rectum), pathologic T status, pathologic N status, differentiation of primary tumor (well, moderately, poorly/undifferentiated), surgical approach (open, laparoscopic), operating time (≤150 min, >150 min), blood loss (<500 ml, 501–1000 ml, >1000 ml), postoperative morbidity, and perioperative blood transfusion (no transfusion, 1–2 transfusions, ≥3 transfusions). Any variable reaching a P value of less than 0.10 in the univariate analysis was used for multivariable analysis using a stepwise Cox proportional hazard model. Hazard ratios (HR) and 95 % confidence intervals (CI) were reported. All P values of less than 0.05 were considered to indicate a statistically significant difference. All calculations were performed in Statistica software version 9 (StatSoft Inc., Tulsa, USA) and PASW Statistics version 17.0 (SPSS Inc., Chicago, Illinois, USA).
Results
A total of 583 patients met the criteria for inclusion in the study. Of these, 132 (22.6 %) in the perioperative period received a blood transfusion: 83 (14.2 %) patients received one or two blood transfusions and 49 (8.4 %) patients received three or more transfusions. Table 1 presents the baseline demographic, clinical, operative, and pathological data. Patient and tumor characteristics were not evenly distributed among the groups of patients with different number of transfusions. Patients with three or more transfusions were older, more often polymorbid, had greater perioperative blood loss and an increased rate of postoperative complications. There was no statistically significant difference with regard to tumor stage. The median duration of follow-up was 46 months (range 1–110 months).
Table 1.
Patient and tumor characteristics according to number of blood transfusions
| no transfusion | 1-2 transfusions | > 3 transfusions | p | |
|---|---|---|---|---|
| n = 451 (77.4 %) | n = 83 (14.2 %) | n = 49 (8.4 %) | ||
| Age | 0.015 | |||
| ≤ 66 years | 236 (52.3 %) | 43 (51.8 %) | 15 (30.6 %) | |
| > 66 years | 215 (47.7 %) | 40 (48.2 %) | 34 (69.4 %) | |
| Gender | 0.269 | |||
| Female | 159 (35.3 %) | 37 (44.6 %) | 18 (36.7 %) | |
| Male | 292 (64.7 %) | 46 (55.4 %) | 31 (63.3 %) | |
| BMI | 0.122 | |||
| < 30 | 326 (72.3 %) | 68 (81.9 %) | 39 (79.6 %) | |
| ≥ 30 | 125 (27.7 %) | 15 (18.1 %) | 10 (20.4 %) | |
| ASA | 0.058 | |||
| I | 51 (11.3 %) | 8 (9.6 %) | 3 (6.1 %) | |
| II | 229 (50.8 %) | 35 (42.2 %) | 16 (32.7 %) | |
| III | 156 (34.6 %) | 36 (43.4 %) | 28 (57.1 %) | |
| IV | 15 (3.3 %) | 4 (4.8 %) | 2(4.1 %) | |
| Tumor localization | 0.263 | |||
| colon | 275 (61.0 %) | 44 (53.0 %) | 26 (53.1 %) | |
| rectum | 176 (39.0 %) | 39 (47.0 %) | 23 (46.9 %) | |
| pT stage | 0.138 | |||
| T1 | 47 (10.4 %) | 4 (4.8 %) | 3 (6.1 %) | |
| T2 | 79 (17.5 %) | 18 (21.7 %) | 4 (8.2 %) | |
| T3 | 267 (59.2 %) | 45 (54.2 %) | 34 (69.4 %) | |
| T4 | 58 (12.9 %) | 16 (19.3 %) | 8 (16.3 %) | |
| pN stage | 0.658 | |||
| N0 | 268 (59.4 %) | 53 (63.9 %) | 25 (51.0 %) | |
| N1 | 125 (27.7 %) | 22 (26.5 %) | 17 (34.7 %) | |
| N2 | 58 (12.9 %) | 8 (9.6 %) | 7 (14.3 %) | |
| Tumor differentiation | 0.361 | |||
| well | 148 (32.8 %) | 24 (28.9 %) | 16 (32.6 %) | |
| moderately | 262 (58.1 %) | 47 (56.6 %) | 31 (63.3 %) | |
| poorly | 41 (9.1 %) | 12 (14.5 %) | 2 (4.1 %) | |
| Surgical approach | 0.715 | |||
| open | 176 (39.0 %) | 32 (38.6 %) | 22 (44.9 %) | |
| laparoscopic | 275 (61.0 %) | 51 (61.4 %) | 27 (55.1 %) | |
| Duration of operation | 0.020 | |||
| ≤ 150 min | 245 (54.3 %) | 32 (38.6 %) | 22 (44.9 %) | |
| > 150 min | 206 (45.7 %) | 51 (61.4 %) | 27 (55.1 %) | |
| Blood loss | <0.001 | |||
| ≤ 500 ml | 418 (92.7 %) | 64 (77.1 %) | 30 (61.2 %) | |
| 501–1000 ml | 32 (7.1 %) | 13 (15.7 %) | 9 (18.4 %) | |
| > 1000 ml | 1 (0.2 %) | 6 (7.2 %) | 10 (20.4 %) | |
| Postoperative morbidity | <0.001 | |||
| no | 363 (80.5 %) | 57 (68.7 %) | 17 (34.7 %) | |
| yes | 88 (19.5 %) | 26 (31.3 %) | 32 (65.3 %) |
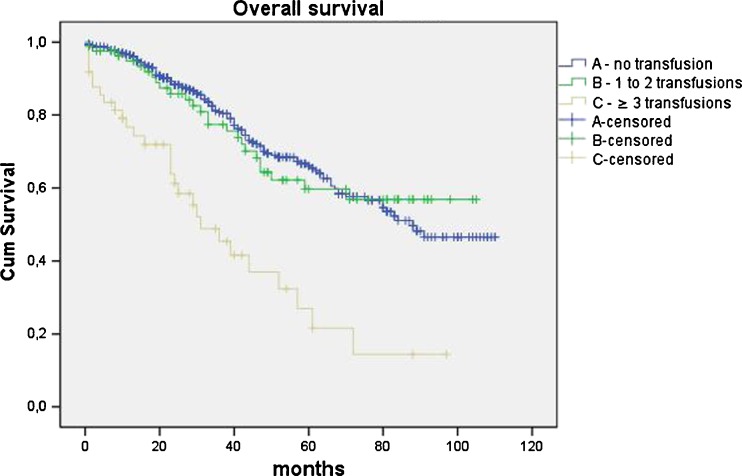
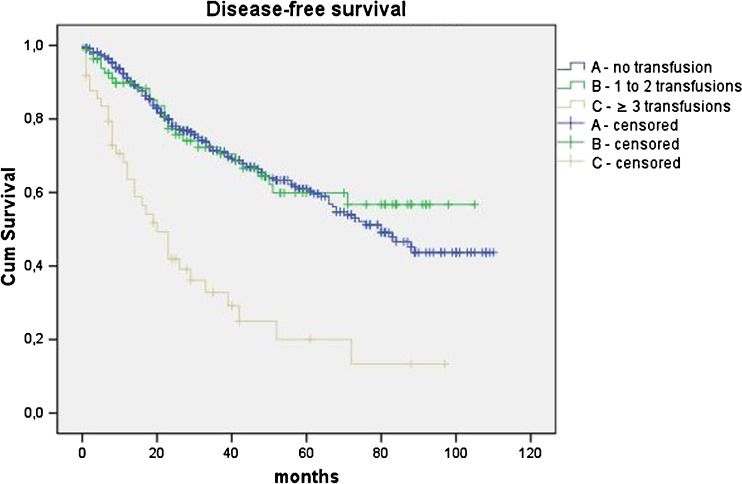
There was no significant difference in 5-year overall survival between patients in no transfusion group and group with one to two transfusions (P = 0.324). However, patients with three or more transfusions had a significantly worse five-year overall survival in comparison with patients with no transfusion (P < 0.001) and with patients with one to two transfused units (P = 0.001). Similarly, a five-year disease-free survival did not differ in patients with no transfusion and or patients with one to two transfusions (P = 0.764), but was significantly worse in patients when three or more units had been transfused (A vs. C P < 0.001; B vs. C P <0.001) (Figs. 1 and 2, Table 2).
Fig. 1.
Kaplan–Meier curves of overall survival
Fig. 2.
Kaplan–Meier curves of disease-free survival
Table 2.
Survival and recurrence data according to number of blood transfusions
| no transfusion | 1-2 transfusions | ≥ 3 transfusions | p | p | |
|---|---|---|---|---|---|
| A | B | C | A vs.B | A vs.C | |
| 5 year overall survival | 66.0 ± 3.1 % | 59.7 ± 6.6 % | 27.0 ± 8.8 % | 0.324 | < 0.001 |
| 5 year disease-free survival | 61.0 ± 3.0 % | 59.9 ± 6.5 % | 20.0 ± 7.5 % | 0.764 | < 0.001 |
| 5 year recurrence rate | |||||
| total | 25.6 ± 2.7 % | 26.0 ± 6.1 % | 60.1 ± 10.5 % | 0.992 | < 0.001 |
| local | 6.7 ± 1.7 % | 5.6 ± 2.7 % | 12.6 ± 7.3 % | 0.909 | 0.204 |
| distant | 19.7 ± 2.4 % | 20.0 ± 5.9 % | 54.5 ± 11.6 % | 0.630 | 0.001 |
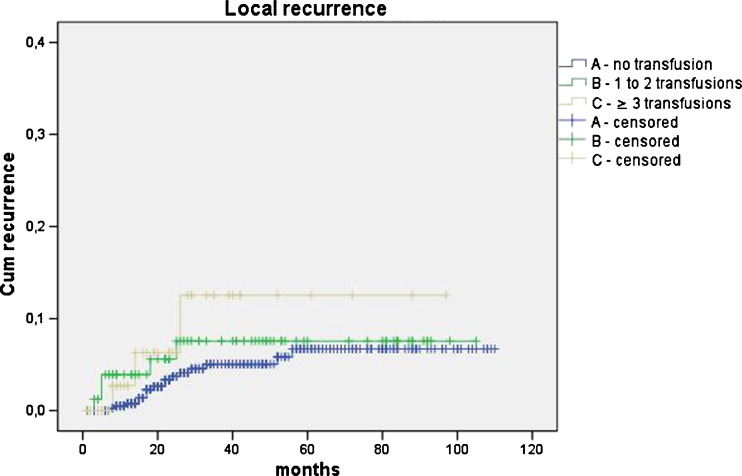
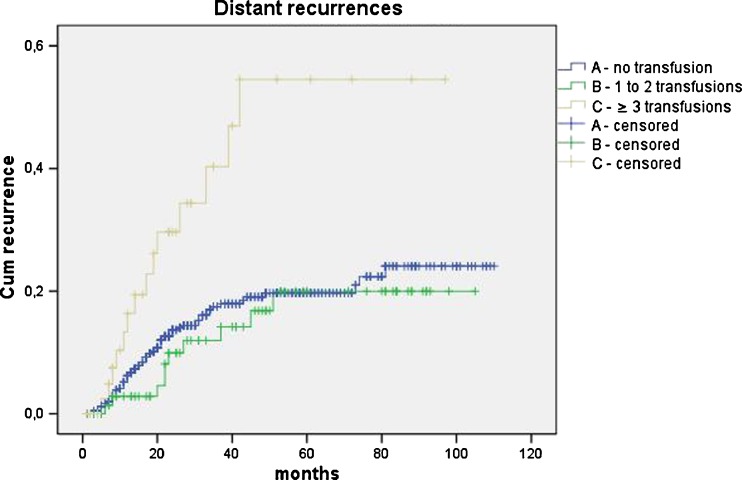
The five-year total cancer recurrence rate was significantly higher in patients with three or more transfusions in comparison with non-transfused and patients with one to two transfusions (A vs. C P < 0.001; B vs. C P = 0.002). When recurrence was specified according to the site, the local recurrence rate was higher in patients with three or more transfusions; however, this difference did not reach statistical significance (A vs. C P = 0.204; B vs. C P = 0.714). The occurrence of distant metastases increased significantly when three or more units of blood had been transfused (A vs. C P = 0.001; B vs. C P = 0.002). Transfusion of one to two blood units was not associated with increased distant recurrence rate (A vs. B P = 0.630) (Figs. 3 and 4 and Table 2).
Fig. 3.
Kaplan–Meier curves of local recurrences
Fig. 4.
Kaplan–Meier curves of distant recurrences
Results of univariate analysis of predictive factors for survival and recurrence are presented in Table 3. In the multivariate analysis, age more than 66 years (P < 0.001; HR 2.589; CI 1.861–3.603), lymph node involvement (N1 P < 0.001; HR 2.041; CI 1.440–2.893; N2 P < 0.001; HR 3.855; CI 2.500–5.945), postoperative morbidity (P < 0.001; HR 1.923; CI 1.362–2.716), and perioperative transfusion of three and more blood units (p = 0.001; HR 2.158; CI 1.370–3.398) were significantly and independently associated with overall survival. The independent risk factors for disease-free survival were age more than 66 years (P < 0.001; HR 2.046; CI 1.517–2.760), lymph node involvement (N1 P < 0.001; HR 2.288; CI 1.668–3.138; N2 P < 0.001; HR 3.785; CI 2.534–5.654), postoperative morbidity (P = 0.046; HR 1.410; CI 1.006–1.976), and transfusion of three and more blood units (P < 0.001; HR 2.514; CI 1.648–3.836). The risk factors independently associated with local recurrence were tumor localization in the rectum (P = 0.001; HR 4.919; CI 1.948–12.422) and poorly differentiated tumor (P = 0.014; HR 3.993; CI 1.325–12.039). Finally, independent risk factors for distant recurrence were three and more transfusions (p < 0.001; HR 2.902; CI 1.616–5.212) and lymph node involvement (N1 P < 0.001; HR 3.281; CI 2.010–5.355; N2 P < 0.001; HR 5.332; CI 3.042–9.347).
Table 3.
Univariate analysis of predictive factors for survival and recurrence
| overall survival | disease-free survival | distant metastasis | local recurrence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95 % CI | p | HR | 95 % CI | p | HR | 95 % CI | p | HR | 95 % CI | p | |
| Age | ||||||||||||
| < 66 years | 1 | 1 | 1 | 1 | ||||||||
| ≥ 66 years | 2.209 | 1.616–3.019 | <0.001 | 1.777 | 1.339–2.357 | <0.001 | 1.046 | 0.691–1.584 | 0.830 | 0.793 | 0.356–1.766 | 0.570 |
| Gender | ||||||||||||
| female | 1 | 1 | 1 | 1 | ||||||||
| male | 1.175 | 0.854–1.615 | 0.321 | 1.018 | 0.764–1.355 | 0.905 | 0.726 | 0.480–1.100 | 0.131 | 1.301 | 0.561–3.015 | 0.540 |
| BMI | ||||||||||||
| < 30 | 1 | 1 | 1 | 1 | ||||||||
| ≥ 30 | 1.05 | 0,740–1,489 | 0.784 | 0.869 | 0,624–1,212 | 0.408 | 0.799 | 0,482–1,326 | 0.385 | 1.162 | 0,485–2,782 | 0.737 |
| ASA | ||||||||||||
| I | 1 | 1 | 1 | 1 | ||||||||
| II | 2.384 | 1.191–4.772 | 0.014 | 1.960 | 1.093–3.513 | 0.024 | 2.019 | 0.860–4.741 | 0.107 | 1.887 | 0.429–8.313 | 0.401 |
| III | 3.504 | 1.757–6.989 | <0.001 | 2.789 | 1.559–4.988 | 0.001 | 2.401 | 1.015–5.675 | 0.046 | 1.632 | 0.352–7.560 | 0.531 |
| IV | 4.415 | 1.638–11.896 | 0.003 | 2.829 | 1.125–7.104 | 0.027 | 0.740 | 0.089–6.150 | 0.780 | x | x | x |
| Tumor localization | ||||||||||||
| colon | 1 | 1 | 1 | 1 | ||||||||
| rectum | 1.014 | 0.745–1.378 | 0.931 | 1.190 | 0.899–1.574 | 0.223 | 1.391 | 0.919–2.104 | 0.119 | 5.053 | 2.018–12.655 | 0.001 |
| pT stage | ||||||||||||
| 1 | 1 | 1 | 1 | 1 | ||||||||
| 2 | 1.169 | 0.500–2.731 | 0.719 | 1.686 | 0.754–3.770 | 0.204 | 5.597 | 0.716–43.729 | 0.101 | 1.135 | 0.103–12.524 | 1.135 |
| 3 | 2.092 | 1.020–4.294 | 0.044 | 2.572 | 1.258–5.256 | 0.010 | 7.666 | 1.200–62.589 | 0.032 | 2.764 | 0.369–20.712 | 0.323 |
| 4 | 2.398 | 1.116–5.151 | 0.025 | 3.058 | 1.435–6.514 | 0.004 | 12.487 | 1.683–92.645 | 0.014 | 2.328 | 0.260–20.835 | 0.450 |
| pN stage | ||||||||||||
| 0 | 1 | 1 | 1 | 1 | ||||||||
| 1 | 1.918 | 1.365–2.696 | <0.001 | 2.160 | 1.582–2.948 | <0.001 | 3.424 | 2,101–5,580 | <0.001 | 1.874 | 0,739–4,751 | 0.186 |
| 2 | 3.121 | 2.076–4.690 | <0.001 | 3.304 | 2.266–4.818 | <0.001 | 5.222 | 2.989–9.124 | <0.001 | 4.210 | 1.599–11.087 | 0.004 |
| Tumor differentiation | ||||||||||||
| well | 1 | 1 | 1 | 1 | ||||||||
| moderately | 0.991 | 0.711–1.381 | 0.957 | 0.967 | 0.713–1.313 | 0.831 | 1.194 | 0.747–1.907 | 0.459 | 1.373 | 0.514–3.670 | 0.527 |
| moderately | 1.909 | 1.200–3.036 | 0.006 | 1.937 | 1.263–2.971 | 0.002 | 2.140 | 1.123–4.078 | 0.021 | 4.952 | 1.654–14.824 | 0.004 |
| Surgical approach | ||||||||||||
| open | 1 | 1 | 1 | 1 | ||||||||
| laparoscopic | 0.786 | 0.580–1.067 | 0.123 | 0.894 | 0.676–1.182 | 0.432 | 1.390 | 0.901–2.142 | 0.136 | 0.699 | 0.319–1.534 | 0.372 |
| Duration of operation | ||||||||||||
| ≤ 150 minut | 1 | 1 | 1 | 1 | ||||||||
| > 150 minut | 1.136 | 0.838–1.539 | 0.411 | 1.258 | 0.953–1.661 | 0.106 | 1.357 | 0.897–2.053 | 0.148 | 1.652 | 0.749–3.644 | 0.214 |
| Blood loss | ||||||||||||
| ≤ 500 ml | 1 | 1 | 1 | 1 | ||||||||
| 501–1000 ml | 1.748 | 1.132–2.699 | 0.012 | 1.541 | 1.004–2.364 | 0.048 | 1.797 | 0.976–3.308 | 0.060 | 2.232 | 0.763–6.533 | 0.143 |
| > 1000 ml | 2.364 | 1.202–4.650 | 0.013 | 1.799 | 0.919–3.519 | 0.086 | 1.788 | 0.654–4.892 | 0.258 | 1.673 | 0.224–12.474 | 0.616 |
| Morbidity | ||||||||||||
| no | 1 | 1 | 1 | 1 | ||||||||
| yes | 2.139 | 1.554–2.944 | <0.001 | 1.756 | 1.302–2.369 | <0.001 | 1.422 | 0.896–2.258 | 0.135 | 1.160 | 0.463–2.908 | 0.752 |
| No. of transfusions | ||||||||||||
| 0 | 1 | 1 | 1 | 1 | ||||||||
| 1–2 | 1.020 | 0.662–1.573 | 0.928 | 0.924 | 0.608–1.405 | 0.712 | 0.793 | 0.407–1.543 | 0.495 | 1.584 | 0.584–4.293 | 0.366 |
| ≥ 3 | 3.409 | 2.250–5.164 | <0.001 | 3.404 | 2.324–4.984 | <0.001 | 2.941 | 1.649–5.246 | <0.001 | 2.403 | 0.702–8.224 | 0.162 |
Discussion
The question concerning the influence of perioperative blood transfusions on survival and incidence of recurrences of malignant diseases has not been clearly answered. Even though numerous studies have evaluated this subject, the results are contradictory in many respects. In our study, we found an independent and significant association between perioperative transfusion of three or more blood units and poor long-term prognosis in colorectal cancer patients.
The exact mechanism leading to this detrimental effect is far from being elucidated. Allogenic leukocytes and inflammatory mediators released during blood storage are thought to play a key role in the transfusion-associated immunomodulation [9, 10]. However, neither leukocyte depletion nor transfusion of autologous blood favorably affects long-term oncological results. In a multicentric randomized trial, Lange et al [12] compared the use of leukocyte-depleted and buffy-coat-depleted blood and found no improvement in five-year overall survival, disease-free survival, and tumor recurrences in patients with gastrointestinal cancer. Similarly, van de Watering [13] and Skånberg [14] in other prospective randomized trials, designed specifically for colorectal cancer, showed no significant effect of leukocyte depletion on overall five-year survival and cancer recurrence. In all these studies, the prognosis of patients who received blood transfusion, regardless of their type, was significantly worse compared with non-transfused patients. Jagoditsch [7] in a retrospective analysis and Busch [15] in a multicentric randomized trial compared the effect of autologous-versus-allogenic transfusions on survival and incidence of relapses. The use of autologous blood transfusion was not associated with improved oncological results in comparison with red blood cells without buffy coat. In our study the type of blood transfusion was not evaluated. The reason for this was that in majority of patients allogenic transfusions were used. Autologous blood transfusions have rarely been possible in patients with colorectal cancer because they are often anemic preoperatively. Leucodepleted blood transfusions were indicated in selected patients especially to prevent serious transfusion reactions and reduce the risk of infection transmission and alloimmunization.
In a meta-analysis, Amato and Pescatori [4] showed that recurrences were more likely in transfused patients, regardless of their timing and the type of blood product used. This review estimated that increased risks were observed with increased number of transfused units: one to two units showed an odd ratio of 1.48, which became 1.72 for three to four units and 1.92 for five and more units. In 2006, these same authors presented an updated meta-analysis with similar results [16]. However, conclusions of presented meta-analyses are limited due to significant heterogeneity of the included studies. In our study, cutoff value of two blood units was used for the analysis of dose-related effect. This cutoff was defined according to median number of blood units used in transfused patients. Transfusion of one to two blood units was proved to be oncologically safe, neither compromising survival nor occurrence of recurrences.
In addition to the amount of transfused blood units, long-term prognosis may be affected by circumstances under which they are applied. Miki et al [17] reported that preoperative blood transfusion for correcting anemia was not associated with survival, whereas intraoperative blood transfusion due to excessive blood loss affected long-term prognosis. Blood transfusion under intense surgical stress synergistically exaggerates postoperative systemic inflammatory response, which may have effect on minimal residual disease in resected cancer patients. Similarly, development of postoperative infectious complications is also associated with enhanced systemic induction of inflammatory cytokines. Mynster et al [18] shows that a combination of perioperative blood transfusion and subsequent development of postoperative infectious complications may be associated with a poor long-term prognosis in patients after potentially curative resections for colorectal cancer.
Blood storage time may be another significant factor associated with impaired survival and higher cancer recurrence rate. Even in this regard, results of published studies have been contradictory. Evidence has suggested that bioactive substances are accumulated extracellularly during storage of blood, in particular angiogenesis-stimulating factors, such as vascular endothelial growth factor (VEGF) [19]. Patel et al [11] demonstrated a significant posttransfusion increase in the proangiogenic VEGF and a significant decrease in the antiangiogenic factor endostatin. This angiogenic factor imbalance was associated with enhanced in vitro angiogenesis. However, Edna and Bjerkeset [6] failed to find any relationship between the time of blood storage and long-term prognosis. On the other hand, Mynster et al [20] showed that buffy-coat-depleted red cells stored for less than 21 day could be an independent risk factor for cancer recurrence, while blood stored more than 21 days had insignificant effect on the risk of recurrence. However, the results of these studies are difficult to compare because of the significant differences in their methodology. The main difference lies in how the blood storage time was determined in patients who received more than one transfusion. While the first of these studies used mean and median storage time, in the second study, the blood unit with the longest duration of storage was selected as the time representing blood storage.
From a surgical point of view, an effort to minimize perioperative blood loss represents principal measure to reduce the need for blood transfusion. Rates of perioperative blood transfusions ranging from 20 to 70 % have been reported in patients undergoing colorectal resection for cancer [7]. In our study, the overall transfusion rate was 22.6 %. This relatively low transfusion rate may be associated with a high proportion (60.5 %) of surgeries performed laparoscopically. Surgical blood loss is one of the most significant perioperative predictors of patient outcome, but represents a rather neglected quality indicator [21]. Adequate surgical technique can reduce the need for transfusions. In this regard, minimally invasive approach, that is generally associated with lower perioperative blood loss, represents the method of choice.
Conclusions
Our study showed that transfusion of three and more units of blood in patients undergoing surgical treatment of colorectal cancer was significantly associated with poor long-term outcome. At present, the exact mechanisms of this deleterious effect are not known, but both immunomodulation and growth-regulating, leukocyte-derived bioactive substances accumulated extracellularly during storage of blood may play certain roles. Indications for blood transfusions should be carefully considered with regard to the possibility of compromising long-term results. Perioperative approaches to minimize blood loss are of paramount importance.
Acknowledgments
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5:253–259. [PubMed] [Google Scholar]
- 2.Gantt CL. Red blood cells for cancer patients. Lancet. 1981;15:363. doi: 10.1016/S0140-6736(81)90673-5. [DOI] [PubMed] [Google Scholar]
- 3.Burrows L, Tartter P. Effect of blood transfusions on colonic malignancy recurrent rate. Lancet. 1982;18:662. doi: 10.1016/S0140-6736(82)92764-7. [DOI] [PubMed] [Google Scholar]
- 4.Amato AC, Pescatori M. Effect of perioperative blood transfusions on recurrence of colorectal cancer: meta-analysis stratified on risk factors. Dis Colon Rectum. 1998;41:570–885. doi: 10.1007/BF02235262. [DOI] [PubMed] [Google Scholar]
- 5.Chiarugi M, Buccianti P, Disarli M, Galatioto C, Cavina E. Effect of blood transfusions on disease-free interval after rectal cancer surgery. Hepatogastroenterology. 2000;47:1002–1005. [PubMed] [Google Scholar]
- 6.Edna TH, Bjerkeset T. Perioperative blood transfusions reduce long-term survival following surgery for colorectal cancer. Dis Colon Rectum. 1998;41:451–459. doi: 10.1007/BF02235758. [DOI] [PubMed] [Google Scholar]
- 7.Jagoditsch M, Pozgainer P, Klingler A, Tschmelitsch J. Impact of blood transfusions on recurrence and survival after rectal cancer surgery. Dis Colon Rectum. 2006;49:1116–1130. doi: 10.1007/s10350-006-0573-7. [DOI] [PubMed] [Google Scholar]
- 8.McAlister FA, Clark HD, Wells PS, Laupacis A. Perioperative allogeneic blood transfusion does not cause adverse sequelae in patients with cancer: a meta-analysis of unconfounded studies. Br J Surg. 1998;85:171–178. doi: 10.1046/j.1365-2168.1998.00698.x. [DOI] [PubMed] [Google Scholar]
- 9.Dionigi G, Rovera F, Boni L, et al. The impact of perioperative blood transfusion on clinical outcomes in colorectal surgery. Surg Oncol. 2007;16(Suppl 1):S177–182. doi: 10.1016/j.suronc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: fact or fiction? Blood. 2001;97:1180–1195. doi: 10.1182/blood.V97.5.1180. [DOI] [PubMed] [Google Scholar]
- 11.Patel HB, Nasir FA, Nash GF, Scully MF, Kakkar AK. Enhanced angiogenesis following allogeneic blood transfusion. Clin Lab Haematol. 2004;26:129–135. doi: 10.1111/j.1365-2257.2004.00589.x. [DOI] [PubMed] [Google Scholar]
- 12.Lange MM, van Hilten JA, van de Watering LM, Cooperative clinical investigators of the Cancer Recurrence And Blood Transfusion (CRAB) study and the Transfusion Associated Complications = Transfusion Induced Complications? (TACTIC) study et al. Leucocyte depletion of perioperative blood transfusion does not affect long-term survival and recurrence in patients with gastrointestinal cancer. Br J Surg. 2009;96:734–740. doi: 10.1002/bjs.6636. [DOI] [PubMed] [Google Scholar]
- 13.van de Watering LM, Brand A, Houbiers JG, Klein Kranenbarg WM, Hermans J, van de Velde C, Cancer Recurrence and Blood transfusion study group Perioperative blood transfusions, with or without allogeneic leucocytes, relate to survival, not to cancer recurrence. Br J Surg. 2001;88:267–272. doi: 10.1046/j.1365-2168.2001.01674.x. [DOI] [PubMed] [Google Scholar]
- 14.Skånberg J, Lundholm K, Haglind E. Effects of blood transfusion with leucocyte depletion on length of hospital stay, respiratory assistance and survival after curative surgery for colorectal cancer. Acta Oncol. 2007;46:1123–1130. doi: 10.1080/02841860701441830. [DOI] [PubMed] [Google Scholar]
- 15.Busch OR, Hop WC, Marquet RL, Jeekel J. Blood transfusions and local tumor recurrence in colorectal cancer. Evidence of a noncausal relationship. Ann Surg. 1994;220:791–797. doi: 10.1097/00000658-199412000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;25:CD005033. doi: 10.1002/14651858.CD005033.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miki C, Hiro J, Ojima E, Inoue Y, Mohri Y, Kusunoki M. Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol (R Coll Radiol) 2006;18:60–66. doi: 10.1016/j.clon.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Mynster T, Christensen IJ, Moesgaard F, Nielsen HJ. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Danish RANX05 Colorectal Cancer Study Group. Br J Surg. 2000;87:1553–1562. doi: 10.1046/j.1365-2168.2000.01570.x. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen HJ, Werther K, Mynster T, Brünner N. Soluble vascular endothelial growth factor in various blood transfusion components. Transfusion. 1999;39:1078–1083. doi: 10.1046/j.1537-2995.1999.39101078.x. [DOI] [PubMed] [Google Scholar]
- 20.Mynster T, Nielsen HJ, Danish RANX05 Colorectal Cancer Study Group Storage time of transfused blood and disease recurrence after colorectal cancer surgery. Dis Colon Rectum. 2001;44:955–964. doi: 10.1007/BF02235483. [DOI] [PubMed] [Google Scholar]
- 21.Dixon E, Datta I, Sutherland FR, Vauthey JN. Blood loss in surgical oncology: neglected quality indicator? J Surg Oncol. 2009;99:508–512. doi: 10.1002/jso.21187. [DOI] [PubMed] [Google Scholar]