Abstract
High levels of resistance to spinosad, a macrocyclic lactone insecticide, have been reported previously in western flower thrips, Frankliniella occidentalis, an economically important insect pest of vegetables, fruit and ornamental crops. We have cloned the nicotinic acetylcholine receptor (nAChR) α6 subunit from F. occidentalis (Foα6) and compared the nucleotide sequence of Foα6 from susceptible and spinosad-resistant insect populations (MLFOM and R1S respectively). A single nucleotide change has been identified in Foα6, resulting in the replacement of a glycine (G) residue in susceptible insects with a glutamic acid (E) in resistant insects. The resistance-associated mutation (G275E) is predicted to lie at the top of the third α-helical transmembrane domain of Foα6. Although there is no direct evidence identifying the location of the spinosad binding site, the analogous amino acid in the C. elegans glutamate-gated chloride channel lies in close proximity (4.4 Å) to the known binding site of ivermectin, another macrocyclic lactone pesticide. The functional consequences of the resistance-associated mutation have been examined in the human nAChR α7 subunit. Introduction of an analogous (A272E) mutation in α7 abolishes the modulatory effects of spinosad whilst having no significant effect upon activation by acetylcholine, consistent with spinosad having an allosteric mechanism of action.
Keywords: Frankliniella occidentalis, insecticide resistance, nicotinic acetylcholine receptor, spinosad
Spinosad is a macrocyclic lactone, isolated from the microorganism Saccharopolyspora spinosa (Sparks et al. 1998; Thompson et al. 2000). It is a naturally occurring mixture of two components, spinosyn A and spinosyn D (Fig. 1) and was introduced as a commercial insecticide in 1997 (Thompson et al. 2000). Spinosad is used extensively in crop protection to control a wide range of insect pests, including lepidoptera and thysanoptera, but it is also used in animal health applications and to control head lice in humans. Insect toxicity is associated with widespread neuronal excitation in insects (Salgado et al. 1998), because of its action on nicotinic acetylcholine receptors (nAChRs) (Salgado and Saar 2004).
Fig. 1.
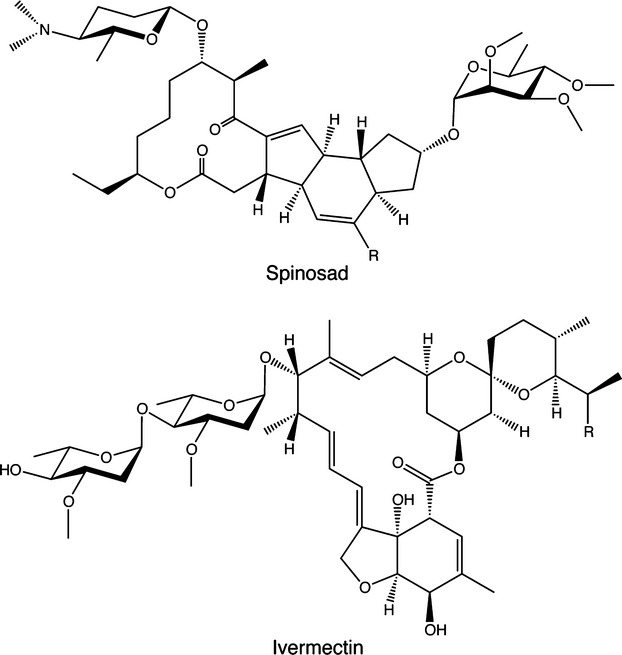
Chemical structure of spinosad and ivermectin. Spinosad is a mixture of spinosin A (in which R = H) and spinosin D (in which R = CH3). Also shown is ivermectin, another macrocyclic lactone pesticide. Ivermectin is a mixture of dihydroavermectin B1a (in which R = CH2CH3) and dihydroavermectin B1b (in which R = CH3).
Nicotinic receptors are members of the Cys-loop family of ligand-gated ion channels (Lester et al. 2004) and are important neurotransmitter receptor subtypes in both vertebrate and invertebrate species (Millar and Denholm 2007; Millar and Gotti 2009; Jones and Sattelle 2010). The Cys-loop family includes both excitatory (cation-selective) receptors, such as nAChRs, and also inhibitory (anion-selective) receptors (Lester et al. 2004). The inhibitory glutamate-gated chloride channel (GluCl), found in several invertebrate species, has close structural similarity to nAChRs and is the target site for ivermectin (Fig. 1), another macrocyclic lactone pesticide (Wolstenholme 2010).
In common with all Cys-loop receptors, nAChRs and GluCls are transmembrane proteins in which five subunits are arranged around a central ion channel pore. Each of the five subunits contains four α-helical transmembrane domains (TM1–TM4), with the second of these domains lining the ion channel pore. The conventional orthosteric agonist binding site is located within the extracellular domain of Cys-loop receptors at the interface between two adjacent subunits (Sine, 2002 #1539). However, several allosteric modulatory sites have also been identified in Cys-loop receptors. In the case of ivermectin, there is clear evidence that it interacts with an allosteric site in the transmembrane domain of GluCls (Hibbs and Gouaux 2011). In addition, ivermectin is an allosteric modulator of nAChRs, and there is evidence that it interacts with nAChRs via the receptor transmembrane region (Krause et al. 1998; Collins and Millar 2010). The binding site of spinosad on nAChRs is less well defined, but there is evidence that it also acts an allosteric ligand (Salgado and Saar 2004) at a site that is distinct from the conventional extracellular agonist binding site (Orr et al. 2009).
In common with most other pesticides, resistance to macrocyclic lactones such as spinosad and ivermectin is an established problem and one that is increasing as a result of intensive pesticide use (Wolstenholme and Kaplan 2012). Resistance to spinosad has been reported in several insect species (Wolstenholme and Kaplan 2012). For example, there have been reports of resistance in Colorado potato beetle Leptinotarsa decemlineata (Mota-Sanchez et al. 2006), house fly Musca domestica (Shono and Scott 2003) and tobacco budworm, Heliothis virescens (Young et al. 2003). In such species, there is evidence of resistance being a result of either enhanced metabolism (Markussen and Kristensen 2011) or a consequence of changes in the target site (Roe et al. 2010). Studies conducted with the model insect species Drosophila melanogaster have implicated the nAChR Dα6 subunit in determining target-site resistance to spinosad (Perry et al. 2007; Watson et al. 2010). For example, a Dα6 knockout strain of D. melanogaster has been shown to confer high levels of resistance to spinosad (Perry et al. 2007). In addition, a variety of chemically-induced mutations within Dα6 (generating either truncated proteins or mis-sense mutations) have been found to confer resistance to spinosad (Watson et al. 2010). Further evidence indicating that resistance to spinosad can arise through changes to its target-site (the nAChR α6 subunit) is provided by studies with the diamondback moth, Plutella xylostella (Baxter et al. 2010; Rinkevich et al. 2010). Resistance to spinosad in P. xylostella has been linked to mis-spliced transcripts of the nAChR α6 subunit resulting in expression of a truncated subunit protein (Baxter et al. 2010) and to point mutations generating premature stop codons (Rinkevich et al. 2010).
High levels of resistance to the insecticide spinosad have been reported in western flower thrips (Frankliniella occidentalis), particularly in areas such as southern Spain, where spinosad has been used intensively to protect greenhouse crops (Bielza et al. 2007a, b; Bielza 2008). In this study, we describe work conducted with a previously reported laboratory-selected strain of F. occidentalis (R1S) displaying high levels of resistance (resistance ratio > 350 000) to spinosad (Bielza et al. 2007b). R1S was selected from a field population of F. occidentalis collected in 2003 (in Almeria, Spain), from greenhouses that had been subjected to intensive treatment with spinosad (Bielza et al. 2007a, b). Resistance to spinosad in strain R1S has been reported to be autosomal, almost completely recessive and controlled by a single locus (Bielza et al. 2007b).
Initial studies of spinosad-resistant F. occidentalis indicated that resistance might be associated with target-site changes, rather than enhanced metabolism (Bielza et al. 2007a). These findings have prompted us to employ molecular biological techniques to examine the nAChR α6 subunit in F. occidentalis. A nicotinic acetylcholine receptor point mutation (G275E), located in the transmembrane region of the receptor has been identified in spinosad-resistant F. occidentalis. In addition to its identification in a laboratory-selected strain (R1S), we have also identified this resistance-associated mutation in a recently isolated field population of F. occidentalis (Guillén and Bielza 2012). As well as providing evidence for target-site resistance to spinosad in F. occidentalis, work described in this article also provides support for the proposal that spinosad acts as a nAChR allosteric modulator via a transmembrane binding site.
Materials and methods
Insects
The susceptible strain of F. occidentalis (MLFOM) was collected from an organic peach crop from the Murcia region of Spain in 2001 and was maintained subsequently in the laboratory without exposure to insecticide (Bielza et al. 2007b). Another population of F. occidentalis was collected in 2003 (in Almeria, Spain), from greenhouses that had been subjected to intensive treatment with spinosad, and a resistant strain (R1S) was isolated from this field population after several years of laboratory selection with spinosad (Bielza et al. 2007b). A further field population of F. occidentalis (MOJO) was collected in 2011 in Almeria, Spain (Guillén and Bielza 2012). Work with F. occidentalis was conducted in accordance with procedures reviewed by the Spanish Ministry of Science and Technology.
Plasmids
The following plasmid expression constructs used in this study have been described previously: human nAChR α7 subunit cDNA in plasmid expression vector pSP64GL (Broadbent et al. 2006), mouse 5-HT3A subunit in plasmid pcRK5 (Harkness and Millar 2001) and a subunit chimera containing the extracellular domain of the human α7 subunit fused to the transmembrane domain of the mouse 5-HT3A subunit in plasmid pcDNA3 (Craig et al. 2004).
Molecular cloning of Foα6
Messenger RNA was isolated from approximately 100 spinosad-susceptible F. occidentalis (strain MLFOM) using a QuickPrep Micro mRNA purification kit (GE Healthcare, Little Chalfont, UK). Hybrid mRNA/cDNA was synthesized using a First-Strand cDNA Synthesis kit with NotI-d(T)18 primers (GE Healthcare). Degenerate oligonucleotide primers were designed to two conserved regions of nAChR α6 subunits from other insect species (encoding amino acids DVDEKNQ and WTYDGNQ) and used to amplify a cDNA fragment of 341 bp. The cDNA fragment was ligated into the TA cloning vector pCRII and used to transform E. coli One Shot INVαF' competent cells (Invitrogen Life Technologies, Paisley, UK). Individual colonies were grown overnight in LB broth containing ampicillin (50 μg/mL). Plasmid DNA was isolated using GeneJet plasmid miniprep kit (Thermo Fisher Scientific, Waltham, MA, USA) and examined by nucleotide sequencing. Specific oligonucleotide primers were designed to the Foα6 nucleotide sequence and used to isolate longer cDNA fragments by means of 3′ and 5′ rapid amplification of cDNA ends (RACE) using GeneRacer™ kit (Invitrogen Life Technologies). Specific primers were then used to amplify and sequence regions from both susceptible (MLFOM) and resistant (R1S) F. occidentalis. This was done with pools of approximately 100 insects (as described above), and also with individual insects. To amplify cDNA from individual insects total RNA was isolated using TRIzol reagent (Invitrogen Life Technologies) using quantities half of that recommended in the manufacturer's protocol. First-strand cDNA was synthesized, using all of the RNA sample extracted from an individual insect, with SuperScriptIII reverse transcriptase (Invitrogen Life Technologies), primed with oligo(dT). Routine PCR amplifications used DreamTaq™ Green PCR Master Mix (Fermentas) and direct nucleotide sequenced performed with specific primers. EMBL nucleotide sequence database accession number: HE965755.
To identify the intron/exon boundaries adjacent to alternative exons 8a and 8b, PCR amplification was performed on genomic DNA isolated from pooled insect samples using TRIzol reagent (Invitrogen Life Technologies) following the manufacturer's protocol. Gene-specific oligonucleotide primers were designed to exon sequences and PCR amplification performed using Long PCR Enzyme Mix (Fermentas, Life Sciences). Amplified DNA fragments were examined either by direct nucleotide sequencing using gene-specific primers or cloned into pCR2.1 vector (Invitrogen Life Technologies) and sequenced with M13 Forward and Reverse primers. All nucleotide sequencing was performed using the Big Dye Terminator Cycle Sequencing kit and ABI Prism 3100-Avant automated sequencer according to the manufacturer's instructions (Applied Biosystems, Life Technologies, Paisley, UK).
Site-directed mutagenesis and cRNA synthesis
Site-directed mutagenesis was performed with the QuikChange mutagenesis kit (Stratagene, Agilent Technologies, Waldbronn, Germany) with the human nAChR α7 subunit cDNA in pSP64GL (Broadbent et al. 2006). Alanine at position 272 (numbering according to Peng et al. 1994) was mutated to glutamic acid (A272E) to create a mutation at a position analogous to the G275E mutation in Foα6. Mutated cDNA constructs were verified by nucleotide sequencing, as described above. A full-length F. occidentalis nAChR α6 cDNA was amplified from the susceptible (MLFOM) strain using KAPA2G™ Robust HotStart polymerase (KAPA Biosystems, Woburn, MA, USA) and subcloned into pGEMHE. In vitro transcription of cRNA, from plasmids encoding Foα6 and human α7, was carried out using mMESSAGE mMACHINE SP6 and T7 transcription kits (Ambion, Life Technologies, Paisley, UK). SP6 and T7 transcription kits were used for pSP6GL-hα7 and pGEMHE-Foα6 respectively.
Two-electrode voltage-clamp recording
Xenopus laevis oocytes were isolated and defolliculated as described previously (Young et al. 2007) by treatment with collagenase (2 mg/mL; Worthington, Lakewood, NJ, USA) in calcium-free Barth's solution. Oocytes were injected with 12–25 ng cRNA in a volume of 50 nL into the cytoplasm (Foα7 and hα7 cRNA) or with 0.3 ng cDNA in 18 nL into the oocyte nucleus (pcDNA3-hα7/m5HT3 and pRK5-m5HT3A) using a Drummond variable volume microinjector. After injection, oocytes were incubated at 18°C in Barth's solution containing 0.77 mM CaCl2 and supplemented with antibiotics (100 units/mL penicillin, 100 μg/mL streptomycin, 4 μg/mL kanamycin and 50 μg/mL tetracycline). Experiments were performed on oocytes after 3–5 days of incubation. Oocytes were placed in a recording chamber and continuously perfused with a saline solution (115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM Hepes, pH 7.3). Two-electrode voltage-clamp recordings were performed (with the oocyte membrane potential held at −60 mV), as described previously (Young et al. 2007) using a OC-725C amplifier (Warner Instruments, Hamden, CT, USA), PowerLab 8SP and Chart 5 software (AD Instruments, Oxford, UK). Drugs were applied to oocytes using a BPS-8 solenoid valve solution exchange system (ALA Scientific Instruments, Farmingdale, NY, USA). Difficulties were encountered in preparing aqueous solutions of spinosad and in obtaining consistent effects on recombinant nAChRs. Reproducible effects were, however, obtained by preparing, on the day of use, stock solutions (10 mM) of spinosad (Sigma, Poole, UK) in dimethylsulfoxide by sonication for 15 min at 30–40°C. Spinosad was then diluted to its final concentration in saline solution. Consistent effects were observed on α7 nAChRs by pre-incubation with spinosad for 5 min followed by co-application of spinosad with acetylcholine.
Transient expression of nAChRs in mammalian cells
Human kidney tsA201 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen Life Technologies) containing 10% foetal calf serum (Sigma), penicillin (100 U/mL) and streptomycin (100 μg/mL) (Invitrogen Life Technologies). Cells were maintained in a humidified incubator containing 5% CO2 at 37°C. Cells were transfected using the Effectene reagent (Qiagen, Crawley, UK) according to the manufacturer's instructions. After overnight incubation in Effectene, cells were incubated at 37°C for 24–48 h before being assayed for radioligand binding.
Radioligand binding
[3H]-α-bungarotoxin (56 Ci/mmol; Tocris Bioscience, Bristol, UK) was a gift from Syngenta (Bracknell, UK). Radioligand binding to transiently transfected tsA201 cells was performed essentially as described previously (Lansdell and Millar 2004). Transfected cells were re-suspended in Hank's buffered saline solution (Gibco, Paisley, UK) containing 1% bovine serum albumin and incubated with [3H]-α-bungarotoxin for 2 h at 22°C in a total volume of 300 μL. Non-specific binding was determined in the presence of nicotine (1 mM) and carbamylcholine (1 mM). Competition binding experiments were performed by incubating triplicate samples of transfected cells with [3H]-α-bungarotoxin (typically, 1 nM), together with a range of concentrations of either spinosad or methyllycaconitine (MLA). Radioligand binding was assayed by filtration onto Whatman GF/A filters (pre-soaked in 0.5% polyethylenimine), followed by rapid washing with phosphate-buffered saline (Oxoid, Basingstoke, UK) using a Brandel cell harvester. Bound radioligand was quantified by scintillation counting. Curves for equilibrium binding were fitted using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Statistical analysis
Pair-wise comparisons of statistical significance were performed by Student's unpaired t-tests.
Results
Cloning of the nAChR Foα6 subunit
The complete coding sequence of the nAChR α6 subunit from Foα6 was isolated from the spinosad-susceptible strain MLFOM. As described in the Methods, this was achieved by the use of degenerate oligonucleotide primers to isolate partial cDNA fragments, followed by 3′ and 5′ RACE. The nucleotide sequence revealed an open reading frame of 513 amino acids with features typical of a nAChR subunit (Fig. 2) and most closely resembling other insect nAChR α6 subunits (Fig. 3). As has been reported previously for the nAChR α6 subunit cloned from other insect species (Grauso et al. 2002; Rinkevich and Scott 2009), we have identified two sets of alternative exons (3a/3b and 8a/8b) in Foα6 transcripts. The transcript we isolated as a full-length cDNA contained alternative exons 3b and 8a (Fig. 2). This corresponds to what has been described as isoform II in Drosophila melanogaster (Grauso et al. 2002) and Tribolium casteneum (Rinkevich and Scott 2009). As is illustrated in Fig. 2, exons 3a and 3b each encode 15 amino acids, of which five amino acids differ in the two alternative exons. Exons 8a and 8b each encode 29 amino acids and differ by seven amino acids (Fig. 2b).
Fig. 2.

Nucleotide sequence and predicted amino acid sequence of F. occidentalis nicotinic acetylcholine receptor (nAChR) Foα6 (Nucleotide sequence database accession number HE965755). (a) The predicted transmembrane domains (TM1–TM4) are underlined. Amino acids are numbered based on the predicted signal sequence cleavage site and the position of the resistance-associated mutation (G275E) is indicated by an asterisk. (b) Amino acid sequence alignment of two alternative exons, 3a/3b and 8a/8b.
Fig. 3.
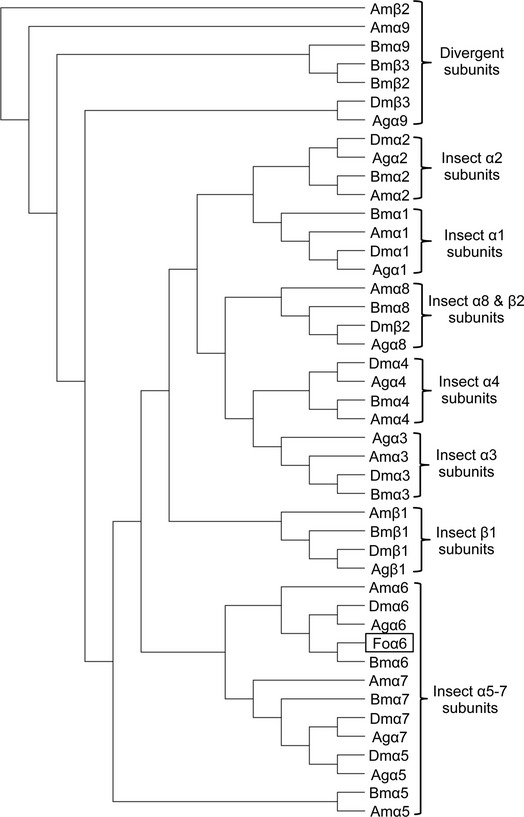
Phylogenetic relationship of insect nicotinic acetylcholine receptor (nAChR) subunits based on predicted amino acid sequence data. The phylogenetic tree was generated in MacVector 12.5.1 using the Best Tree mode and the neighbour-joining analysis method on a ClustalW alignment of selected insect nAChR protein sequences. Species abbreviations are Am: Apis melifera, Bm: Bombyx mori, Dm: Drosophila melanogaster, Ag: Anopheles gambiae and Fo: Frankliniella occidentalis each with their respective nAChR subtype. Foα6 subunit has closest sequence similarity to other insect nAChR α6 subunits from the nAChR α5-α7 group.
Further cDNA clones were isolated from pools of approximately 100 insects or from individual insects from susceptible (MLFOM) and spinosad resistant (R1S) strains. Transcripts were identified in both susceptible and resistant populations that contained alternative exons 3a/3b and 8a/8b with no evidence to indicate that the frequency of alternative splicing was associated with resistance. However, a glycine codon (GGA; nucleotide position 1051–4 in Fig. 2) was found in all transcripts isolated from susceptible insects, whereas glutamic acid codon (GAA) was found in all transcripts from resistant insects.
Our cDNA cloning of F. occidentalis and previously published genomic sequence data from other insect species (Jones and Sattelle 2007; Baxter et al. 2010) indicated that this mutation is located at the boundary of exon 9 and the alternatively spliced exons 8a/8b. To confirm the precise location of the mutation, a series of PCR amplifications were performed. The codon for the mutated amino acid was found to span exon 9 and exons 8a/8b, with the resistance-associated mutation (G-A) being at the start of exon 9. In addition, several polymorphisms were identified in susceptible and resistant individuals, but none of these were associated with a particular phenotype.
G275 is located at conserved position in TM3
Amino acid sequence alignments of Cys-loop ligand-gated ion channel subunits (Table 1) indicated that G275 is located at a conserved position towards the top of the third transmembrane domain (TM3). As explained in the Discussion section, the mutation is predicted to lie four amino acids from the top (the extracellular side) of the α-helical region of the TM3 transmembrane domain. This position is highly conserved, although there are consistent differences between excitatory and inhibitory members of the superfamily. All excitatory (cation-selective) Cys-loop receptors that we have examined contain a glycine (G), alanine (A), isoleucine (I) or serine (S) at this position (Table 1). In contrast, all inhibitory (anion-selective) Cys-loop receptors that we have examined contain an aspartic acid (D). Although the sequences presented in Table 1 are primarily from Drosophila and human receptor subunits, we are unaware of any nAChR subunit from any species that contains glutamic acid (E) at the position identified in spinosad resistant F. occidentalis. Consequently, the occurrence of a glutamic acid at this position in the spinosad-resistant Foα6 subunit (an excitatory receptor subunit) is extremely unusual and is consistent with it being a resistance-associated mutation.
Table 1.
Amino acid sequence surrounding the TM3 4′ position (bold) in cation-selective (upper panel) and anion-selective (bottom panel) Cys-loop receptors. Based on previously published alignments of Cys-loop receptors (Lester et al. 2004; Hibbs and Gouaux 2011)
| F. ocidentalis α6 | DAIPLLGTYFNCI |
| D. melanogaster α1 | LTVPLLGKYLLFT |
| D. melanogaster α2 | LALPLLGKYLLFT |
| D. melanogaster α3 | LVVPLLGKFVLFT |
| D. melanogaster α4 | LVVPLLGKYLIFA |
| D. melanogaster α5 | DAVPLLGTYFNCI |
| D. melanogaster α6 | DAVPLIGVTILLS |
| D. melanogaster α7 | DAVPLLGKYFNCI |
| D. melanogaster β1 | LVLPLIAKYLLFT |
| D. melanogaster β2 | LAVPLLGKYLLFT |
| Human nAChR α1 | SAVPLIGKYMLFT |
| Human nAChR α2 | LVIPLIGEYLLFT |
| Human nAChR α3 | LVIPLIGEYLLFT |
| Human nAChR α4 | LVIPLIGEYLLFT |
| Human nAChR α5 | KVIPLIGEYLVFT |
| Human nAChR α6 | LVVPLVGEYLLFT |
| Human nAChR α7 | DSVPLIAQYFAST |
| Human nAChR α9 | ENVPLIGKYYIAT |
| Human nAChR α10 | ESVPLIGKYYMAT |
| Human nAChR β1 | LSVPIIIKYLMFT |
| Human nAChR β2 | LDVPLVGKYLMFT |
| Human nAChR β3 | KVIPLIGEYLLFI |
| Human nAChR β4 | LDVPLIGKYLMFT |
| Human nAChR γ | QAVPLISKYLTFL |
| Human nAChR δ | MAIPLIGKFLLFG |
| Human nAChR ε | LSVPLLGRFLIFV |
| Human 5-HT3A | IGTPLIGVYFVVC |
| Human 5-HT3B | GSTPLIGHFFTIC |
| C. elegans GluCl | SYIKAIDVWIGAC |
| D. melanogaster RDL | SYVKSIDVYLGTC |
| D. melanogaster GRD | SYPTALDFFVFLS |
| D. melanogaster LCCH3 | SYVKAIDIYLVMC |
| D. melanogaster HisCl | SYLKAVDAFMSVC |
| D. melanogaster GluCl | SYTKAIDVWTGVC |
| Human GABA α1 | AYATAMDWFIAVC |
| Human GABA α2 | AYATAMDWFIAVC |
| Human GABA α3 | AYATAMDWFIAVC |
| Human GABA α4 | SYLTAMDWFIAVC |
| Human GABA α5 | AYATAMDWFIAVC |
| Human GABA α6 | SYATAMDWFIAVC |
| Human GABA β1 | PYVKAIDIYLMGC |
| Human GABA β2 | PYVKAIDMYLMGC |
| Human GABA β3 | PYVKAIDMYLMGC |
| Human GABA δ | SAIKALDVYFWIC |
| Human GABA ρ1 | SYIKAVDIYLWVS |
| Human GABA ρ2 | SYVKAVDIYLWVS |
| Human GylR α1 | SYVKAIDIWMAVC |
| Human GylR α2 | SYVKAIDIWMAVC |
| Human GylR α3 | SYVKAIDIWMAVC |
| Human GylR β | SYVKALDVWLIAC |
Comparison to the ivermectin-binding site in GluCl
Although the site at which spinosad interacts with nAChRs remains unknown with certainty, it seems plausible that it might interact in a manner similar to that by which other macrocyclic lactones interact with Cys-loop neurotransmitter-gated ion channels. A high resolution X-ray structure has been determined recently for the glutamate-gated chloride channel (GluCl) from Caenorhabditis elegans, co-crystallized with ivermectin (Hibbs and Gouaux 2011). Ivermectin, like spinosad, is a macrocyclic lactone (Fig. 1) that is widely used as a pesticide (Raymond and Sattelle 2002; Wolstenholme 2010). From the GluCl structure, it is apparent that ivermectin interacts at a binding site located at the periphery of the transmembrane region towards the extracellular side of the lipid bilayer (Hibbs and Gouaux 2011) (Fig. 4). It makes close contact (by hydrogen-bonding and van der Waals interactions) with the TM1, TM2 and TM3 (Hibbs and Gouaux 2011). As a result of the sequence similarity between nAChRs and GluCls (Hibbs and Gouaux 2011), it is possible to identify the position in the GluCl structure that is analogous to the resistance-associated mutation in the nAChR Foα6 subunit. As is illustrated in Fig. 4, the analogous amino acid in the C. elegans GluCl is located at the top of the TM3 transmembrane helix and is in close proximity to the bound ivermectin. The aspartic acid side chain of GluCl is within 4.4 Å of the tetrahydrofuran ring of ivermectin. It is also one of small number of amino acids in GluCl that have been identified as making van der Waals interactions with ivermectin (Hibbs and Gouaux 2011).
Fig. 4.
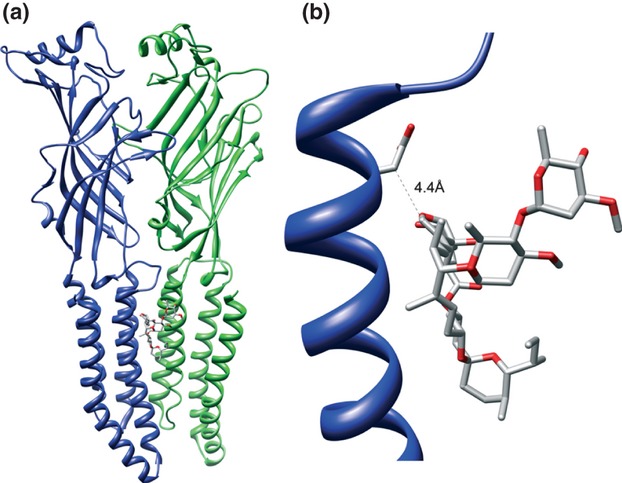
The location of bound ivermectin in the C. elegans glutamate-gated chloride channel (GluCl) crystal structure. (a) For clarity, only two of the five subunits are illustrated (in blue and green). Also shown is ivermectin, bound to the GluCl transmembrane domain. (b) The upper portion of the M3 transmembrane helix of GluCl is shown, together with the position of bound ivermectin. Also illustrated is the side chain of the amino acid (aspartic acid; D) located at a position analogous to the resistance-associated mutation (G275E) in Foα6. The side chain of the aspartic acid residue in GluCl is within 4.4 Å of the bound ivermectin.
Functional characterization of TM3 mutation in α7 nAChRs
The full-length Foα6 cDNA was expressed in Xenopus oocytes, but did not generate functional nAChRs, either when expressed alone or with other nAChR subunits. In addition, co-expression of Foα6 with the nAChR chaperone RIC-3 (Lansdell et al. 2008; Millar 2008) failed to facilitate functional expression. To some extent, this was not unexpected, given the widely acknowledged difficulties in expressing insect nAChRs in heterologous expression systems (Millar 1999; Millar and Lansdell 2010). However, we were able to detect functional homomeric nAChRs routinely, when the closely related human α7 subunit was expressed in oocytes, as has been described previously (Couturier et al. 1990). Consequently, the effect of the analogous mutation (A272E) was examined in the human nAChR α7 subunit (the human α7 subunit contains an alanine, rather than a glycine at this position; Fig. 2).
The A272E mutation was introduced into the human α7 subunit by site-directed mutagenesis and the mutated cDNA expressed in Xenopus oocytes and examined by two-electrode voltage-clamp recording. Acetylcholine dose-response curves were determined for both wild-type receptors and α7 containing the A272E mutation (Fig. 5a). The A272E mutation had no significant effect on either EC50 values for acetylcholine (104 ± 21 μM for wild-type and 134 ± 47 μM for the mutant; n = 3) or on Hill coefficient (1.1 ± 0.2 for wild-type and 1.1 ± 0.3 for the mutant; n = 3). In contrast, the A272E mutation had a dramatic effect on modulation of α7 nAChRs by spinosad (Fig. 5b). Spinosad was found to be an inhibitor of human α7 nAChRs, causing a substantial reduction in acetylcholine-evoked responses (Fig. 5b). Spinosad (30 μM) had no significant effect on the EC50 value for acetylcholine, but caused a reduction in the maximum acetylcholine response (Fig. 5b). Responses to a maximal concentration of acetylcholine (100 μM) were significantly reduced in the presence of spinosad (68.1 ± 6.4% compared with responses in the absence of spinosad; n = 21; p < 0.001; Fig. 5c). In contrast, spinosad had no significant effect on α7 nAChRs containing the A272E mutation (Fig. 5c).
Fig. 5.
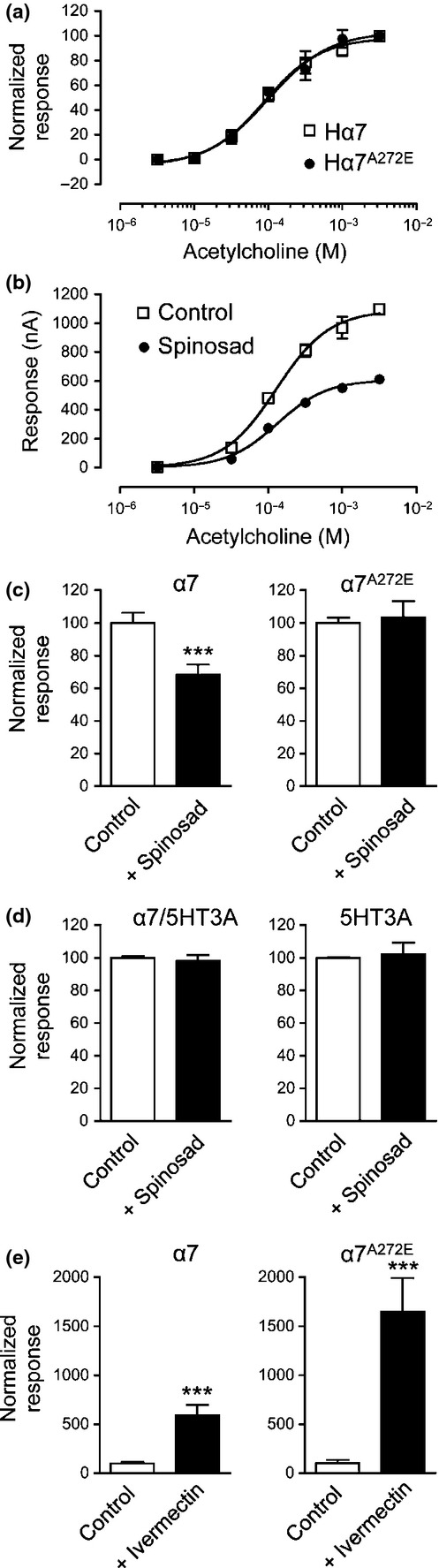
Functional characterization of wild-type and mutated human α7 nicotinic acetylcholine receptors (nAChRs) expressed in Xenopus oocytes. (a) Acetylcholine dose-response curves for wild-type and mutant (A272E) human nAChR α7 subunit. Data points are means (± SEM) of three separate determinations. (b) Dose-response data from a single oocyte (but representative of three independent experiments from different oocytes) in which the effect of spinosad was examined on responses to a range acetylcholine concentrations. Data points are means of two to three responses from a single oocyte. (c) Bar graphs indicating the effect of spinosad on acetylcholine-induced responses on wild-type α7 nAChRs (n = 21) and mutated (A272E) α7 nAChRs (n = 16). (d) Bar graphs indicating the effect of spinosad on agonist (acetylcholine or 5-hydroxytryptamine)-induced responses on α7/5HT3A subunit chimera (n = 6) and the 5-HT3A receptor (n = 10). (e) Bar graphs indicating the effect of ivermectin on acetylcholine-induced responses on wild-type α7 nAChRs (n = 11) and mutated (A272E) α7 nAChRs (n = 10). In all experiments (c–e), modulators (spinosad or ivermectin; 30 μM) were pre-applied for 5 min and then co-applied with agonist. Data (c–e) obtained with spinosad or ivermectin (filled bars) are normalized to responses to acetylcholine (100 μM) or 5-hydroxytryptamine (100 μM) in the absence of modulator (open bars) and are means ± SEM from 6 to 21 independent paired experiments. ***p < 0.001.
Whereas, spinosad is an antagonist of α7 nAChRs, ivermectin (another macrocyclic lactone; Fig. 1) has been shown previously to be a positive allosteric modulator of this receptor (Krause et al. 1998; Collins and Millar 2010). We therefore examined whether the α7 nAChR A272E mutation also had an effect on modulation by ivermectin of acetylcholine responses. On wild-type α7 nAChRs, ivermectin (30 μM) potentiated responses to acetylcholine (100 μM) by 5.9 ± 1.0 fold (n = 11), but it caused a significantly larger potentiation (16.5 ± 3.6 fold; n = 10; p < 0.005) of α7 nAChRs containing the A272E mutation (Fig. 5e).
In contrast to the effects of spinosad on wild-type α7 nAChRs, spinosad had no significant effect on the amplitude of agonist responses with 5-HT3A receptors or with a previously described subunit chimera (Craig et al. 2004) that contains the extracellular domain of the α7 subunit fused to the transmembrane domain of the 5-HT3A subunit (Fig. 5d). These findings are consistent with the hypothesis that spinosad is an allosteric antagonist of human α7 nAChRs and interacts non-competitively at a site other than the conventional extracellular orthosteric binding site, perhaps similar to the transmembrane binding site of ivermectin (Collins and Millar 2010; Hibbs and Gouaux 2011).
Competition radioligand binding
To test the hypothesis that spinosad is a non-competitive (allosteric) antagonist of human α7 nAChRs, we examined whether it is able to displace the binding of [3H]-α-bungarotoxin to the receptor. No significant displacement of [3H]-α-bungarotoxin binding was observed, even at the maximum concentration of spinosad tested (100 μM; Fig. 6). This suggests that spinosad does not bind competitively at the orthosteric nicotinic ligand-binding site, a result that is consistent with our functional data. In contrast to spinosad, the competitive antagonist MLA caused complete displacement [3H]-α-bungarotoxin from α7 nAChRs (Fig. 6). The calculated Ki value for MLA was 4.2 ± 0.2 nM (n = 3), similar to previous estimates of the affinity of MLA for α7 nAChRs (Davies et al. 1999).
Fig. 6.
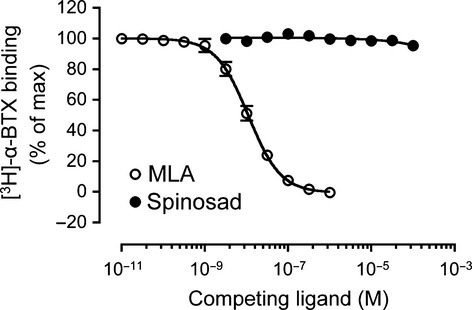
Competition radioligand binding on tsA201 cells transiently expressing with human α7 nicotinic acetylcholine receptors (nAChRs). Equilibrium radioligand binding was performed with [3H]-α-bungarotoxin (1 nM). Spinosad caused no significant displacement of [3H]-α-bungarotoxin binding, whereas MLA caused complete displacement of specific radioligand binding (Ki = 4.2 ± 0.2 nM). Data points are means of triplicate samples (± SEM) from a single experiment, and data are typical of three independent experiments.
Identification of the G275E mutation in a field population of F. occidentalis
As described, we have identified a resistance-associated mutation (G275E) in a laboratory-selected strain of F. occidentalis (R1S). As has been described previously, R1S was selected from a field population (R1) that, itself, had high levels of resistance to spinosad (Bielza et al. 2007a). This would suggest that the G275E mutation may have been present in the original population (collected in 2003). However, to examine whether this mutation is present in field populations, RT-PCR was performed on individual F. occidentalis from a field population (MOJO) collected in 2011 from Almeria, Spain (Guillén and Bielza 2012). This is the same location that the original resistant population was collected and a region in which severe problems of resistance to spinosad have been reported. Initial studies have revealed that this field population (MOJO) is a mixture of homozygous wild-type (glycine at position 275), mutant (G275E) and heterozygous individuals. Further work will be required to determine the frequency of this mutation within this population and also the geographical spread of the mutation. Nevertheless, this provides clear evidence that the G275E mutation is present within field populations of F. occidentalis in southern Spain.
Discussion
There is extensive evidence to indicate that insecticide resistance can arise as a consequence of enhanced metabolic detoxification (Scott 1999). However, in recent years, there has been increasing evidence that resistance can also occur as a consequence of mutations in the insecticide target site. The phenomenon of target-site resistance is well established for several insecticides, including organophosphates, acting on acetylcholine esterase and pyrethroids, acting on voltage-gated sodium channels. In contrast, it is only relatively recently that target-site resistance has been reported for insecticides acting on nAChRs (Millar and Denholm 2007; Wolstenholme and Kaplan 2012). For example, point mutations altering single amino acids in nAChR α or β subunits have been described that are associated with resistance to neonicotinoid insecticides (Liu et al. 2005; Bass et al. 2011).
Evidence has also accumulated in recent years to indicate that resistance to spinosad can occur as a result of changes in its target site, the nAChR (Wolstenholme and Kaplan 2012). Disruption of the Dα6 subunit in Drosophila has been reported to confer resistance to spinosad (Perry et al. 2007). In addition, mis-spliced α6 transcripts and truncated α6 subunits in Plutella xyostella are associated with resistance to spinosad (Baxter et al. 2010; Rinkevich et al. 2010). There is also evidence for resistance to spinosad in Drosophila because of chemically-induced mutations resulting in truncated or non-functional Dα6 subunits (Watson et al. 2010). In contrast, this study has identified a resistance-associated mutation located at a position close to a plausible binding site for spinosad. In addition, when this mutation is introduced into a closely related vertebrate nAChR, it generated a functional receptor with reduced sensitivity to spinosad, but no apparent effect on the potency of the endogenous agonist acetylcholine. In this respect, the spinosad resistance-associated mutation identified in Foα6 resembles a previously characterized nAChR mutation that is associated with resistance to neonicotinoid insecticides (Liu et al. 2005), which has a profound effect on agonist activation by neonicotinoids but only minimal effects on agonist activation by acetylcholine (Liu et al. 2006).
The best structural data available for the transmembrane region of a native nAChR are that generated by electron diffraction studies conducted with receptors purified from Torpedo electric organ (Unwin 2005). However, on the basis of higher-resolution X-ray diffraction data from bacterial ligand-gated ion channels and GluCl, it has been proposed that the assignment of amino acids in the TM3 domain of the Torpedo nAChR is of register by four residues, equivalent to about one turn of the α helix (Corringer et al. 2010; Hibbs and Gouaux 2011). On the basis of this information, we have assigned the position of the amino acid that is mutated in spinosad-resistant F. occidentalis as being the fourth amino acid from the top of the TM3 helix.
As is illustrated in Fig. 2, the position of the G275E mutation in Foα6 is at a position analogous to an aspartic acid (D) in GluCl. Not only is this aspartic acid residue in very close proximity (4.4 Å) to bound ivermectin in the GluCl crystal structure, it is also one of the amino acids that is involved in forming a van der Waals interaction with ivermectin (Hibbs and Gouaux 2011). Given the known location of the ivermectin biding site in GluCl, it seems plausible that the G275E mutation might be in close proximity to the spinosad binding site on nAChRs. This is consistent with our data indicating that the A272E mutation in human α7 nAChRs has an effect on the modulation of agonist responses by both spinosad and ivermectin (Fig. 5). The finding that spinosad does not modulate agonist responses in a subunit chimera containing the extracellular domain of the nAChR α7 subunit but the transmembrane domain of the 5-HT3A subunit (Fig. 5d), is also consistent with spinosad binding at a transmembrane location, similar to the known binding site of ivermectin on GluCl (Hibbs and Gouaux 2011). Furthermore, competition binding data (Fig. 6) provide further support for the conclusion that spinosad binds at a site other than the conventional orthosteric nicotinic binding site and is in agreement with previous evidence indicating that spinosad modulates nAChRs by interacting with a site distinct from the conventional agonist binding site (Orr et al. 2009). There are reports that spinosad acts as an agonist on some insect nAChRs (Salgado and Saar 2004; Watson et al. 2010). This is entirely consistent with spinosad acting via an allosteric transmembrane site, given the recent evidence indicating that nAChRs can be activated by allosteric agonists binding to a transmembrane site (Gill et al. 2011, 2012).
Further evidence that the TM3 domain of Cys-loop receptors is important in the binding of macrocyclic lactones comes from studies conducted with an insect GluCl channel and a vertebrate glycine receptor (GlyR). Both studies have examined mutations influencing ivermectin and both have identified an amino acid in TM3 that is predicted to lie four amino acids below that of the G275E mutation in Foα6. Significantly, as four amino acids corresponds to one turn of an α-helix, the residue identified in this study and that identified in the insect GluCl and vertebrate GlyR are predicted to have side chains pointing in the same approximate orientation. A study investigating resistance to ivermectin identified a resistance-associated point mutation (G323D) in the TM3 domain of a GluCl subunit from the two-spotted spider mite Tetranychus urticae (Kwon et al. 2010). Interestingly, like the mutation that we have identified in Foα6, the mutation identified in the GluCl also corresponds to a change from a glycine to an acidic residue (Kwon et al. 2010). In addition, studies with the vertebrate GlyR have demonstrated that the amino acid in GlyR α1 subunit equivalent to G323 the GluCl in (A228) can confer either enhanced sensitivity (A288G) or reduced sensitivity (A288T) to ivermectin (Lynagh and Lynch 2010; Lynagh et al. 2011).
Considerable problems have been encountered in expressing insect nAChRs in heterologous expression systems (Millar 1999; Millar and Lansdell 2010). Indeed, such problems have been reported in connection with α6 subunits cloned from other insect species (Lansdell and Millar 2004). In situations where functional expression has been achieved with α6-containing nAChRs, it has been reported to be inconsistent and often unsuccessful (Watson et al. 2010). Attempts were made to express the cloned Foα6 subunit in Xenopus oocytes, but these were unsuccessful. Because of the relative ease with which the vertebrate nAChR α7 subunit can be expressed as a functional homomeric receptor, it has been used extensively as a model for investigating mutations affecting neonicotinoid insecticides (Matsuda et al. 2000; Shimomura et al. 2002, 2003; Amiri et al. 2008). By comparing the functional properties of the vertebrate α7 nAChR containing a TM3 A272E mutation, it has been possible to demonstrate that this mutation has no significant effect on acetylcholine potency, as might be expected for a mutation located far from the extracellular binding site for acetylcholine. The absence of an effect on acetylcholine agonist potency is similar to the effects that have been described previously for a target-site mutation associated with resistance to neonicotinoid insecticides (Liu et al. 2006). Significantly, the resistance-associated mutation identified in Foα6 has also been found to abolish modulation of human α7 nAChRs by spinosad. Although spinosad appears to act as an agonist of insect nAChRs (Salgado and Saar 2004; Watson et al. 2010), with features similar to that of an allosteric agonist (Gill et al. 2011), we have found that spinosad is an antagonist of human nAChRs. This difference in the influence of spinosad on two different nAChRs is not unexpected. The chemically related macrocyclic lactone ivermectin is a positive allosteric modulator of human α7 nAChRs (Krause et al. 1998), but a single point mutation in the transmembrane region can convert it from a positive to a negative allosteric modulator (Collins and Millar 2010). Consequently, it is plausible that spinosad might interact at a similar transmembrane site in insect and human nAChRs but have opposing effects. What is significant is that a A272E mutation introduced into the human α7 nAChR abolishes the modulatory effects of spinosad, perhaps through a direct action on its binding and, consequently, this mutation might reasonably be expected to have a similar effect on the interaction of spinosad with insect nAChRs. It seems likely that both spinosad and ivermectin modulate Cys-loop receptors by interacting with an allosteric transmembrane site. Furthermore, it appears that both of these macrocyclic lactones, depending on the receptor they are acting upon, can exert a range of allosteric modulatory effects. These include positive allosteric modulation (potentiation), negative allosteric modulation (non-competitive antagonism) and allosteric agonist activation (activation in the absence of a conventional orthosteric agonist). As has been demonstrated recently for allosteric modulators of vertebrate nAChRs, it appears that all of these effects can potentially occur through transmembrane allosteric binding sites (Young et al. 2008; Collins et al. 2011; Gill et al. 2011).
In summary, we have identified a resistance-associated point mutation in the transmembrane domain of the Foα6 subunit, in a position analogous to the known binding site for ivermectin in a related Cys-loop receptor. Studies with the vertebrate nAChR α7 subunit provide evidence to suggest that the TM3 G275E mutation identified in Foα6 may be responsible for conferring target-site resistance to spinosad by exerting a selective effect on modulation by spinosad at its presumed allosteric binding site, together with a negligible effect on acetylcholine acting at its extracellular orthosteric binding site.
Acknowledgments
Work was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) Industrial Partnership Award [BB/G009392/1] in partnership with Syngenta and by a grant from the Wellcome Trust [WT085141]. Toby Collins was supported by a BBSRC doctoral training account PhD studentship. Financial support was provided to Universidad Politécnica de Cartagena by the Spanish Ministry of Economy and Competitiveness [AGL2011-25164]. The authors declare that they have no conflicts of interest.
Glossary
- GluCl
glutamate-gated chloride channel
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
References
- Amiri S, Shimomura M, Vijayan R, Nishiwaki H, Akamatsu M, Matsuda K, Jones AK, Sansom MSP, Biggin PC, Sattelle DB. A role for Leu118 of loop E in agonist binding to the α7 nicotinic acetylcholine receptor. Mol. Pharmacol. 2008;73:1659–1667. doi: 10.1124/mol.107.041590. [DOI] [PubMed] [Google Scholar]
- Bass C, Puinean AM, Andrews M, et al. Mutation of a nicotinic acetylcholine receptor β subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. BMC Neurosci. 2011;12:51. doi: 10.1186/1471-2202-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter SW, Chen M, Dawson A, Zhao J-Z, Vogel H, Shelton AM, Heckel DG, Jiggins CD. Mis-spliced transcripts of nicotinic acetylcholine receptor α6 are associated with field evolved spinosad resistance in Plutella xylostells (L.) PLoS Genet. 2010;6:e1000802. doi: 10.1371/journal.pgen.1000802. doi: 10.1371/journal.pgen.1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielza P. Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis. Pest Manag. Sci. 2008;64:1131–1138. doi: 10.1002/ps.1620. [DOI] [PubMed] [Google Scholar]
- Bielza P, Quinto V, Contreras J, Torné M, Martín A, Espinosa PJ. Resistance to spinosad in the western flower thrips, Frankliniella occidentalis (Pergande), in greenhouses of south-eastern Spain. Pest Manag. Sci. 2007a;63:682–687. doi: 10.1002/ps.1388. [DOI] [PubMed] [Google Scholar]
- Bielza P, Quinto V, Fernández E, Grávalos C, Contreras J. Genetics of spinosad resistance in Frankliniella occidentalis (Thysanoptera: Thripidae) J. Econ. Entomol. 2007b;100:916–920. doi: 10.1603/0022-0493(2007)100[916:gosrif]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Broadbent S, Groot-Kormelink PJ, Krashia PA, Harkness PC, Millar NS, Beato M, Sivilotti LG. Incorporation of the β3 subunit has a dominant-negative effect on the function of recombinant central-type neuronal nicotinic receptors. Mol. Pharmacol. 2006;70:1350–1356. doi: 10.1124/mol.106.026682. [DOI] [PubMed] [Google Scholar]
- Collins T, Millar NS. Nicotinic acetylcholine receptor transmembrane mutations convert ivermectin from a positive to a negative allosteric modulator. Mol. Pharmacol. 2010;78:198–204. doi: 10.1124/mol.110.064295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Young GT, Millar NS. Competitive binding at a nicotinic receptor transmembrane site of two α7-selective positive allosteric modulators with differing effects on agonist-evoked desensitization. Neuropharmacology. 2011;61:1306–1313. doi: 10.1016/j.neuropharm.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer P-J, Baaden M, Bocquet N, Delarue M, Dufresne V, Nury H, Prevost M, Van Renterghem C. Atomic structure and dynamics of pentameric ligand-gated ion channels: new insight from bacterial homologues. J. Physiol. 2010;588:565–572. doi: 10.1113/jphysiol.2009.183160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Craig PJ, Bose S, Zwart R, et al. Stable expression and characterisation of a human α7 nicotinic subunit chimera: a tool for functional high-throughput screening. Eur. J. Pharmacol. 2004;502:31–40. doi: 10.1016/j.ejphar.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Davies ARL, Hardick DJ, Blagbrough IS, Potter BVL, Wolstenholme AJ, Wonnacott S. Characterisation of the binding of [3H]methyllycaconitine: a new radioligand for α7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology. 1999;38:679–690. doi: 10.1016/s0028-3908(98)00221-4. [DOI] [PubMed] [Google Scholar]
- Gill JK, Savolainen M, Young GT, Zwart R, Sher E, Millar NS. Agonist activation of α7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc. Natl Acad. Sci. USA. 2011;108:5867–5872. doi: 10.1073/pnas.1017975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JK, Dhankher P, Sheppard TD, Sher E, Millar NS. A series of α7 nicotinic acetylcholine receptor allosteric modulators with close chemical similarity but diverse pharmacological properties. Mol. Pharmacol. 2012;81:710–718. doi: 10.1124/mol.111.076026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauso M, Reenan RA, Culetto E, Sattelle DB. Novel putative nicotinic acetylcholine receptor subunit genes, Dα5, Dα6 and Dα7, in Drosophila melanogaster identify a new and highly conserved target of adenosine deaminase acting on RNA-mediated A-to-I pre-mRNA editing. Genetics. 2002;160:1519–1533. doi: 10.1093/genetics/160.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén J, Bielza P. Thiamethoxam acts as a target-site synergist of spinosad in resistant strains of Frankliniella occidentalis. Pest Manag. Sci. 2012;68 doi: 10.1002/ps.3372. [published on-line]. doi: 10.1002/ps.3372. [DOI] [PubMed] [Google Scholar]
- Harkness PC, Millar NS. Inefficient cell-surface expression of hybrid complexes formed by the co-assembly of neuronal nicotinic acetylcholine receptor and serotonin receptor subunits. Neuropharmacology. 2001;41:79–87. doi: 10.1016/s0028-3908(01)00042-9. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Sattelle DB. The cys-loop ligand-gated ion channel gene superfamily of the red flour beetle, Tribolium castaneum. BMC Genomics. 2007;8:327. doi: 10.1186/1471-2164-8-327. doi:310.1186/1471-2164-1188-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Sattelle DB. Diversity of insect nicotinic acetylcholine receptor subunits. Adv. Exp. Med. Biol. 2010;683:25–43. doi: 10.1007/978-1-4419-6445-8_3. [DOI] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer P-J, Galzi J-L, Changeux J-P, Bertrand D. Ivermectin: a positive allosteric effector of the α7 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Kwon DH, Yoon KS, Clark JM, Lee SH. A point mutation in a glutamate-gated chloride channel confers abamectin resistance in the two-spotted spider mite, Tetranychus urticae Koch. Insect Mol. Biol. 2010;19:583–591. doi: 10.1111/j.1365-2583.2010.01017.x. [DOI] [PubMed] [Google Scholar]
- Lansdell SJ, Millar NS. Molecular characterisation of Dα6 and Dα7 nicotinic acetylcholine receptor subunits from Drosophila: formation of a high-affinity α-bungarotoxin binding site revealed by expression of subunit chimeras. J. Neurochem. 2004;90:479–489. doi: 10.1111/j.1471-4159.2004.02499.x. [DOI] [PubMed] [Google Scholar]
- Lansdell SJ, Collins T, Yabe A, Gee VJ, Gibb AJ, Millar NS. Host-cell specific effects of the nicotinic acetylcholine receptor chaperone RIC-3 revealed by a comparison of human and Drosophila RIC-3 homologues. J. Neurochem. 2008;105:1573–1581. doi: 10.1111/j.1471-4159.2008.05235.x. [DOI] [PubMed] [Google Scholar]
- Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: new twists and turns. Trends Neurosci. 2004;27:329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Liu Z, Williamson MS, Lansdell SJ, Denholm I, Han Z, Millar NS. A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper) Proc. Natl Acad. Sci. USA. 2005;102:8420–8425. doi: 10.1073/pnas.0502901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Williamson MS, Lansdell SJ, Han Z, Denholm I, Millar NS. A nicotinic acetylcholine receptor mutation (Y151S) causes reduced agonist potency to a range of neonicotinoid insecticides. J. Neurochem. 2006;99:1273–1281. doi: 10.1111/j.1471-4159.2006.04167.x. [DOI] [PubMed] [Google Scholar]
- Lynagh T, Lynch JW. An improved ivermectin-activated chloride channel receptor for inhibiting electrical activity in defined neuronal populations. J. Biol. Chem. 2010;285:14890–14897. doi: 10.1074/jbc.M110.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynagh T, Webb T, Dixon CL, Cromer BA, Lynch JW. Molecular determinants of ivermectin sensitivity at the glycine receptor chloride channel. J. Biol. Chem. 2011;286:43913–43924. doi: 10.1074/jbc.M111.262634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markussen MDK, Kristensen M. Spinosad resistance in female Musca domestica L. from a field-derived population. Pest Manag. Sci. 2011;68:75–82. doi: 10.1002/ps.2223. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Shimomura M, Kondo Y, et al. Role of loop D of the α7 nicotinic acetylcholine receptor in its interaction with the insecticide imidacloprid and related neonicotinoids. Br. J. Pharmacol. 2000;130:981–986. doi: 10.1038/sj.bjp.0703374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar NS. Heterologous expression of mammalian and insect neuronal nicotinic acetylcholine receptors in cultured cell lines. Biochem. Soc. Trans. 1999;27:944–950. doi: 10.1042/bst0270944. [DOI] [PubMed] [Google Scholar]
- Millar NS. RIC-3: a nicotinic acetylcholine receptor chaperone. Br. J. Pharmacol. 2008;153:S177–S183. doi: 10.1038/sj.bjp.0707661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar NS, Denholm I. Nicotinic acetylcholine receptors: targets for commercially important insecticides. Invert. Neurosci. 2007;7:53–66. doi: 10.1007/s10158-006-0040-0. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Millar NS, Lansdell SJ. Characterisation of insect nicotinic acetylcholine receptors by heterologous expression. Adv. Exp. Med. Biol. 2010;683:65–73. doi: 10.1007/978-1-4419-6445-8_6. [DOI] [PubMed] [Google Scholar]
- Mota-Sanchez D, Hollingworth RM, Grafius EJ, Moyer DD. Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae) Pest Manag. Sci. 2006;62:30–37. doi: 10.1002/ps.1120. [DOI] [PubMed] [Google Scholar]
- Orr N, Shaffner AJ, Richey K, Crouse GD. Novel mode of action of spinosad: receptor binding studies demonstrating lack of interaction with known insecticidal targets. Pest. Biochem. Physiol. 2009;95:1–5. [Google Scholar]
- Peng X, Katz M, Gerzanich V, Anand R, Lindstrom J. Human α7 acetylcholine receptor: cloning of the α7 subunit from the SH-SY5Y cell line and determination of pharmacological properties of native receptors and functional α7 homomers in Xenopus oocytes. Mol. Pharmacol. 1994;45:546–554. [PubMed] [Google Scholar]
- Perry T, McKenzie JA, Batterham P. A Dα6 knockout strain of Drosophila melanogaster confers a high level of resistance to spinosad. Insect Biochem. Mol. Biol. 2007;37:184–188. doi: 10.1016/j.ibmb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Raymond V, Sattelle DB. Novel animal-health drug targets from ligand-gated chloride channels. Nat. Rev. Drug Discov. 2002;1:427–436. doi: 10.1038/nrd821. [DOI] [PubMed] [Google Scholar]
- Rinkevich FD, Scott JG. Transcriptional diversity and allelic variation in nicotinic acetylcholine receptor subunits of the red flour beetle, Tribolium castaneum. Insect Mol. Biol. 2009;18:233–242. doi: 10.1111/j.1365-2583.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- Rinkevich FD, Chen M, Shelton AM, Scott JG. Transcripts of the nicotinic acetylcholine receptor subunit gene Pxylα6 with premature stop codons are associated with spinosad resistance in diamondback moth, Plutella xylostella. Invert Neurosci. 2010;10:25–33. doi: 10.1007/s10158-010-0102-1. [DOI] [PubMed] [Google Scholar]
- Roe RM, Young HP, Iwasa T, Wyss CF, Stumpf CF, Sparks TC, Watson GB, Sheets JJ, Thompson GD. Mechanism of resistance to spinosyn in the tobacco budworm, Heliothis virescens. Pest. Biochem. Physiol. 2010;96:8–13. [Google Scholar]
- Salgado VL, Saar R. Desensitizing and non-desensitizing subtypes of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in cockroach neurons. J. Insect Physiol. 2004;50:867–879. doi: 10.1016/j.jinsphys.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Salgado VL, Sheets JJ, Watson GB, Schmidt AL. Studies on the mode of action of spinosad: the internal effective concentration and the concentration dependence of neural excitation. Pest. Biochem. Physiol. 1998;60:103–110. [Google Scholar]
- Scott JG. Cytochrome P450 and insecticide resistance. Insect Biochem. Mol. Biol. 1999;29:757–777. doi: 10.1016/s0965-1748(99)00038-7. [DOI] [PubMed] [Google Scholar]
- Shimomura M, Okuda H, Matsuda K, Komai K, Akamatsu M, Sattelle DB. Effects of mutations of a glutamine residue in loop D of the α7 nicotinic acetylcholine receptor on agonist profiles for neonicotinoid insecticides and related ligands. Br. J. Pharmacol. 2002;137:162–169. doi: 10.1038/sj.bjp.0704848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura M, Yokota M, Okumura M, Matsuda K, Akamatsu M, Sattelle DB, Komai K. Combinatorial mutations in loops D and F strongly influence responses of the α7 nicotinic acetylcholine receptor to imidacloprid. Brain Res. 2003;991:71–77. doi: 10.1016/j.brainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Shono T, Scott JG. Spinosad resistance in the housefly, Musca domestica, is due to a recessive factor on autosome 1. Pest. Biochem. Physiol. 2003;75:1–7. [Google Scholar]
- Sparks TC, Thompson GD, Kirst HA, Hertlein MB, Larson LL, Worden TV, Thibault ST. Biological activity of the spinosyns, new fermentation derived insect control agents, on tobacco budworm (Lepidoptera: Noctuidae) Larvae. J. Econ. Entomol. 1998;91:1277–1283. [Google Scholar]
- Thompson GD, Dutton R, Sparks TC. Spinosad - a case study: an example from a natural products discovery programme. Pest Manag. Sci. 2000;56:696–702. [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J. Mol. Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Watson GB, Chouinard SW, Cook KR, et al. A spinosyn-sensitive Drosophila melanogaster nicotinic acetylcholine receptor identified through chemically induced target site resistance, resistance gene identification, and heterologous expression. Insect Biochem. Mol. Biol. 2010;40:376–384. doi: 10.1016/j.ibmb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Wolstenholme AJ. Recent progress in understanding the interaction between avermectins and ligand-gated ion channels: putting the pests to sleep. Invert. Neurosci. 2010;10:5–10. doi: 10.1007/s10158-010-0105-y. [DOI] [PubMed] [Google Scholar]
- Wolstenholme AJ, Kaplan RM. Resistance to macrocyclic lactones. Curr. Pharm. Biotechnol. 2012;13:873–887. doi: 10.2174/138920112800399239. [DOI] [PubMed] [Google Scholar]
- Young HP, Bailey WD, Roe RM. Spinosad selection of a laboratory strain of the tobacco budworm, Heliothis virescens (Lepidoptera: Noctuidae), and characterization of resistance. Crop Protection. 2003;22:265–273. [Google Scholar]
- Young GT, Broad LM, Zwart R, Astles PC, Bodkin M, Sher E, Millar NS. Species selectivity of a nicotinic acetylcholine receptor agonist is conferred by two adjacent extracellular β4 amino acids that are implicated in the coupling of binding to channel gating. Mol. Pharmacol. 2007;71:389–397. doi: 10.1124/mol.106.030809. [DOI] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS. Potentiation of α7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc. Natl Acad. Sci. USA. 2008;105:14686–14691. doi: 10.1073/pnas.0804372105. [DOI] [PMC free article] [PubMed] [Google Scholar]


