Abstract
Complex systems abound in public health. Complex systems are made up of heterogeneous elements that interact with one another, have emergent properties that are not explained by understanding the individual elements of the system, persist over time and adapt to changing circumstances. Public health is starting to use results from systems science studies to shape practice and policy, for example in preparing for global pandemics. However, systems science study designs and analytic methods remain underutilized and are not widely featured in public health curricula or training. In this review we present an argument for the utility of systems science methods in public health, introduce three important systems science methods (system dynamics, network analysis, and agent-based modeling), and provide three case studies where these methods have been used to answer important public health science questions in the areas of infectious disease, tobacco control, and obesity.
Keywords: complex systems, system dynamics, network analysis, agent-based modeling, computer models, simulation
INTRODUCTION
In 2006 the United States established the Biomedical Advanced Research and Development Authority (BARDA), partly in response to the threat of a global H5N1 pandemic as well as the 9/11 terrorist attacks. Part of BARDA’s mission is to help prepare the national plan to address the threat of emerging infectious diseases like pandemic influenza. As part of this planning, BARDA has utilized the most current public health science to make decisions and recommendations about aspects of pandemic planning such as stockpiling and distribution of vaccines, the timing and targeting of vaccines, and the most effective use of non-medical interventions such as social distancing (i.e., quarantines) (38). The science base for these recommendations relies to a great extent on new types of methods for simulating and modeling complex systems (56). This is a historical moment for public health science. Government, business leaders, and other stakeholders are already using public health systems science to guide national pandemic strategy--this highlights the utility and impact of these methods. However, systems science methods remain underutilized and are not featured prominently in public health curricula or training(137). The purpose of this review is to present an argument for the utility of systems science methods in public health, to introduce three important and relevant systems science methods (system dynamics, network analysis, and agent-based modeling), and to illustrate these methods through three case studies where these methods have been used to answer important public health science questions in the areas of infectious disease, tobacco control, and obesity.
THE NEED FOR NEW METHODS TO STUDY COMPLEX PUBLIC HEALTH SYSTEMS
What are Complex Systems?
Complex systems abound in public health. In fact it has been suggested that most of the interesting processes in nature, society, and the economy derive from complex systems (3; 123). So what is a complex system? Wanting to avoid semantic distractions, Gallagher and Appeneller (55) introduce a special issue of Science by stating that a complex system is “…one whose properties are not fully explained by an understanding of its component parts.” Although formal definitions may vary, there is broad acceptance that complex systems have the following properties (101; 108):
They are made up of a large number of heterogeneous elements;
These elements interact with each other;
The interactions produce an emergent effect that is different from the effects of the individual elements;
This effect persists over time and adapts to changing circumstances.
Consider the example of the national vaccine system, which according to the above criteria we can clearly see as a complex system (133). It is made up of heterogeneous components (individuals, health clinics, public health agencies, pharmaceutical companies) which interact with each other and are organized at different levels. Certain properties of this system such as herd immunity emerge from the interactions of its various components. The vaccination system has existed over a long time period, but it does respond to changing circumstances. For example, vaccination rates across the U.S. have started going down, partially in response to media coverage of the autism-vaccination debate in the general media (129). Because the interesting behavior of systems is emergent, it is necessary to study a system as a whole, rather than to decompose it and study its individual parts (3). This implies that traditional study designs and analytic tools will not suffice to explore complex public health systems.
The Argument from Study Design
In 1968, the sociologist Allen Barton stated that (17):
For the last thirty years, empirical social research has been dominated by the sample survey. But as usually practiced, using random sampling of individuals, the survey is a sociological meatgrinder, tearing the individual from his social context and guaranteeing that nobody in the study interacts with anyone else in it.. . . . If our aim is to understand people’s behavior rather than simply to record it, we want to know about primary groups, neighborhoods, organizations, social circles, and communities; about interaction, communication, role expectations, and social control.
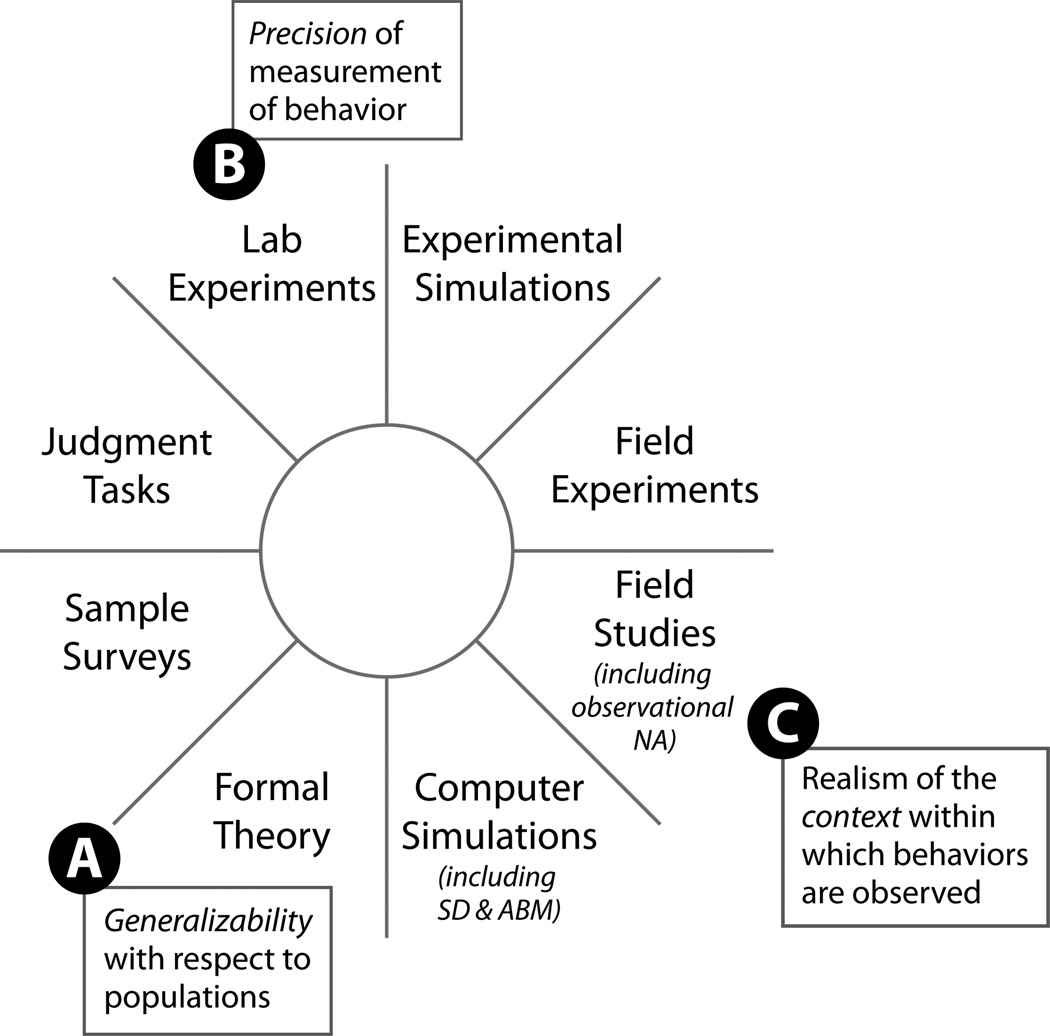
This is as true now for public health as it was a generation ago. Public health science is dominated by randomized control trial (RCT) and epidemiologic risk factor study designs (59; 132). The social psychologist Joseph McGrath provided a framework for understanding the complementary strengths and weaknesses of various types of study designs that can be used to illustrate why traditional RCT and risk factor designs are inappropriate for studying complex public health systems (Figure 1) (100). Essentially, RCTs and other types of quasi-experimental designs are concerned more with internal validity and the ability to precisely measure intervention effects. However, this precision sacrifices external validity and the ability to measure and understand contextual and ecological effects. Systems science study designs (such as computer simulation modeling and network observation studies) appear on the opposite side of McGrath’s figure from experimental designs—thus, these types of studies may sacrifice measurement precision, but they gain external validity and the ability to assess the influence of context on behavior.
Figure 1.
Complementary strengths and weaknesses of various study designs, based on McGrath’s 3-Horned Dilemma (100).
There are two particular aspects of traditional study designs that severely limit their appropriateness for complex systems. First , RCTs and risk-factor studies gain their precision partly through randomization for group assignment and/or sample selection. This randomization ensures that study participants are not typically drawn from naturally existing social or organizational systems. Not only are the behavioral effects of these social systems excluded, but study participants are not allowed to interact with one another as they typically would (132). (In fact, we often label this type of interaction ‘contamination,’ and consider it a study flaw.) Second , experimental and risk-factor studies (e.g., case-control studies) are primarily designed to identify the existence or size of a specific effect or relationship, not the mechanism of the effect (sometimes called the ‘black-box’ problem) (72). As Meehl has pointed out, rejecting a null hypothesis of no effect is a very low epistemological bar, and the social and health sciences are better served by proposing richer predictions based on more sophisticated models of causal mechanisms (102). Thus, if we are to study complex public health systems, we will need to use study designs and methods that allow for interactions among elements of the complex system, and that are able to study, identify, and characterize the mechanisms that drive the behavior of the system (54).
The Argument from Analysis
There is a similar mismatch between the characteristics and assumptions of traditional data analysis approaches in public health, and the characteristics of the data and models that derive from complex systems. Stated most concisely, the types of statistical analyses that we use most often in public health are inappropriate for studying complex systems. Even more sophisticated analytic techniques such as SEM, latent class analysis, etc., that are designed to test more complicated relationships break down in the presence of all-too-common feedback loops, threshold effects, and other types of non-linearity. Critiques of statistical modeling are not new, but a common theme of these discussions is that statistical models are most useful when they are connected to strong study designs, appropriate data, and match the structure of the theoretical predictions.(52; 91)
Table 1 summarizes several reasons why the study of complex systems requires new data analysis techniques. Whereas traditional statistical modeling often assumes linear relationships where changes in dependent variables are proportional to changes in independent variables, complex systems are characterized by non-linearity, threshold events, and chaotic behavior (119). Traditional modeling often assumes normality of variables or residuals—not only are normal distributions poor depictions of reality (105), but complex systems are more often characterized by power laws that lead to scale-free distributions (108; 145). Complex systems are characterized by heterogeneous actors, not only do they not require representativeness in the sampling sense (43), but computational models of complex systems can include actors of fundamentally different types (e.g., people, businesses, and products). Although some linear modeling approaches such as random effects models can be applied to multiple levels of analysis, most traditional statistical models are limited to a single level. Complex systems, on the other hand, are often multilevel (15). Although statistical analysis can of course be applied to longitudinal data, in public health these data are typically ‘discretely’ longitudinal—snapshots taken at well-separated points in time. A fundamental property of complex systems is that they are dynamic, and some of the existing computational modeling tools (especially agent-based modeling, see below) allow for tracking systems as they change in ‘real time’ (43). Although statistical modeling can be used to assess relationships among objects (e.g., cluster analysis; (118)) it more often focuses on correlational relationships between variables. Modeling complex systems, on the other hand, typically focuses on the interactions of the actors within the system. Finally, traditional statistical modeling is inherently reductionist, focusing on individual parameter estimates, specific individual interactions, or individual links in the causal chain. Methods for studying complex systems are by their very nature holistic, examining whole systems or models of systems to help identify the complex mechanisms by which they operate (96).
Table 1.
Comparison of traditional and complex system analytic assumptions.
| Domain | Traditional analytic techniques often assume: |
Complex systems assume: |
|---|---|---|
| Functional form | Linearity | Non-linearity |
| Common distributions | Normality | Non-normality |
| Characteristics of actors | Homogeneity | Heterogeneity |
| Level-of-analysis | Single level | Multiple levels |
| Temporality | Static, or discretely longitudinal | Dynamic, with feedback |
| Fundamental relationships | Among variables | Interaction of actors |
| Perspective | Reductionist | Holistic |
Three Key Methods for Studying Complex Systems
The preceding discussion makes it clear that traditional study design and analytic methods commonly used in public health sciences are not appropriate for studying complex systems. The rest of this review will focus on three methodological approaches that are commonly used to study these types of systems: system dynamics (SD), network analysis (NA), and agent-based modeling (ABM). Although there is some overlap, these three methods each approach the study of complex systems in different ways. Table 2 presents the aspects of complex systems that each method is particularly suited to address. For example, agent-based modeling and network analysis are both more suited for describing how the individual actors in a system interact with one another compared to system dynamics (See also Osgood (114)).
Table 2.
Primary strengths of each systems science method
| System Property | SD | NA | ABM |
|---|---|---|---|
| Model breadth | X | ||
| Feedback loops | X | X | |
| Dynamic systems in real time | X | X | |
| Interactions of individual actors | X | X | |
| Interactions between multiple levels | X | X | |
| Complex relational structures | X | ||
| Heterogeneous actors | X | X | X |
System dynamics, network analysis, and agent-based modeling all have rich, multidisciplinary conceptual and technical histories, have benefited from recent developments in computational and modeling advances, and have been used to study complex systems of many types. Not all studies of complex systems in public health use these methods, but many do. However, despite the importance of these methods for studying complex public health systems, they do not have a prominent place in public health training and education. There are important exceptions, such as the annual NIH-sponsored Institute of Systems Science and Health, which provides training in these three specific methods (http://issh.aed.org/).
SURVEY OF COMPLEX SYSTEMS METHODS
System Dynamics
System dynamics (SD) is based on the premise that complex behaviors of a system (e.g., population prevalence of an infection), result from the interplay of feedback loops, stocks and flows, and delays (130). The method arose originally in management science (50) from the recognition of the need to explicitly model non-linear processes that are characteristic of complex phenomena like policy resistance, the law of unintended consequences, and the often counterintuitive behavior of social systems (131). Simulations are implemented as a series of differential equations that track accumulations of stocks (e.g., people, currency, disease counts, etc,), which are determined by flows (e.g., rate of occurrence), feedback loops (causal loops with either balancing or reinforcing effects) and time delays.
The focus of system dynamics is on building models to represent the dynamic complexity of aggregate, often high-level phenomena such as new product adoption in organizations, or predator-prey relationships over time. Simulation results allow for the examination of the system behavior, which may take on various patterns (e.g., exponential growth, oscillation, s-shaped growth, collapse, etc. (130)) and be compared to hypothesized or expected system behaviors (i.e., reference models). System dynamics models have been used to provide useful illustrative models even absent of strong empirical data, to demonstrate relative impacts of various policies or intervention strategies, particularly when feedback loops may be used to explain patterns of non-linearity or unintended consequences (e.g., the chronic disease prevention model in Homer & Hirsch (68)). Compared to other types of complex system models, SD models tend to have broader boundaries (i.e., include a larger number of relevant explanatory variables) and be more amenable to including variables for which strong empirical data may not be available (68; 116). In the SD field there is a strong emphasis on group model building (142), where models are developed in a participatory process between modeler and practitioners or end users. Thus, the process of developing, testing and refining an SD model is ideally both iterative and participatory.
With a typical focus on aggregate characteristics and broad boundaries, system dynamics modeling has lent itself to an array of public health applications. It has been used to model potential public health outcomes in cases where it is not feasible to test various intervention strategies on real populations, particularly where interventions may involve factors far upstream from health outcomes (64; 67; 74). Models to guide practice in clinical preventive care (127), disaster planning (65), and setting more realistic public health benchmarks (107) provide other examples of the practical utility of SD models in public health. An interesting area for further exploration is its utility in examining strategies to address populations with overlapping epidemics, or “syndemics” (106).
Network Analysis
Network analysis (NA) is a research method and scientific paradigm that focuses on the relationships among sets of actors. The actors can be any type of entity that can have a relationship or tie with other entities: persons, animals, organizations, countries, websites, documents, and even genes. Of the three methods considered in this review, network analysis has the longest history—the roots of network analysis can be traced back to a number of different disciplines, including mathematics (especially graph theory and topology), anthropology (kinship systems), and sociology (social ties and structure) (53). However, what we now recognize as modern network analysis was established in the early 1930s with Jacob Moreno’s invention of the sociogram, a graph that depicts the structure of interpersonal relations in a group (109). With the availability of efficient computer algorithms, the development of specialized network analysis software, and the ‘discovery’ of network analysis by modern physicists and mathematicians (24), interest in network analysis has exploded. The ‘new science of networks’ is being used in almost every area of science (16) to study important questions such as the robustness of terrorist networks (120), the structure of the Internet (14; 26), the functioning of the brain (18), political divisions of modern society (39), and the complex interactions of genes and human disease systems (90).
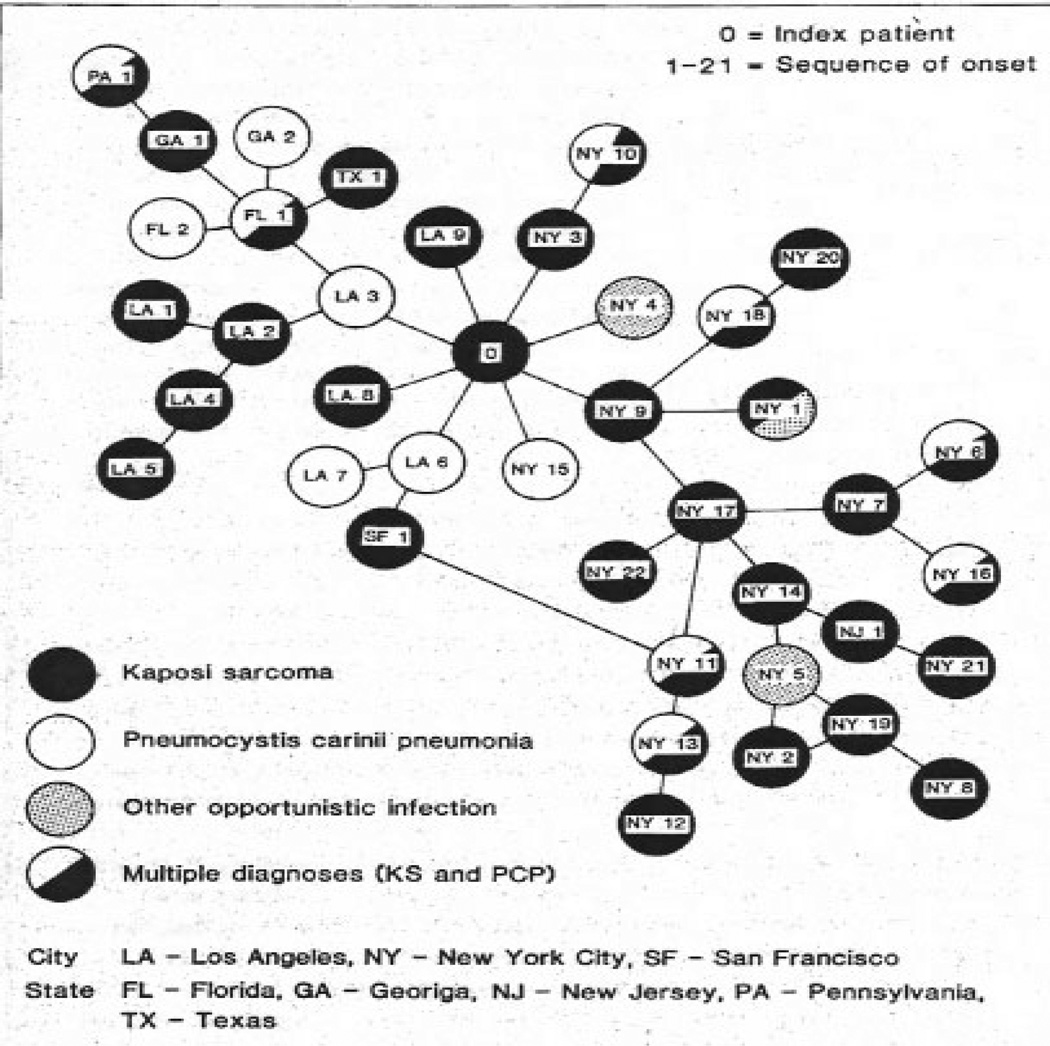
Perhaps because of its longer history, and the ability to quickly analyze real-world data, network analysis has a wider variety of applications and analytic approaches compared to SD and ABM (see Wasserman & Faust (144); Brandes & Erlebach (23)). Despite the analytic variety, almost all network analysis makes use of one or more of three different analytic modes: network visualization, network description, and statistical modeling of networks. One of the attractions of network analysis is the ability to visually examine a given network, especially if it is small to medium-sized. Figure 2for example, shows the first HIV transmission network with ‘Patient 0’ highlighted (9), and highlights both the contagion structure and possible transmission mechanism. Network description makes up the bulk of network analysis, and can be flexibly used to address a wide variety of scientific questions. Figure 3 highlights this diversity.
Figure 2.
Sexual and disease status network of 40 men with HIV/AIDS (9).
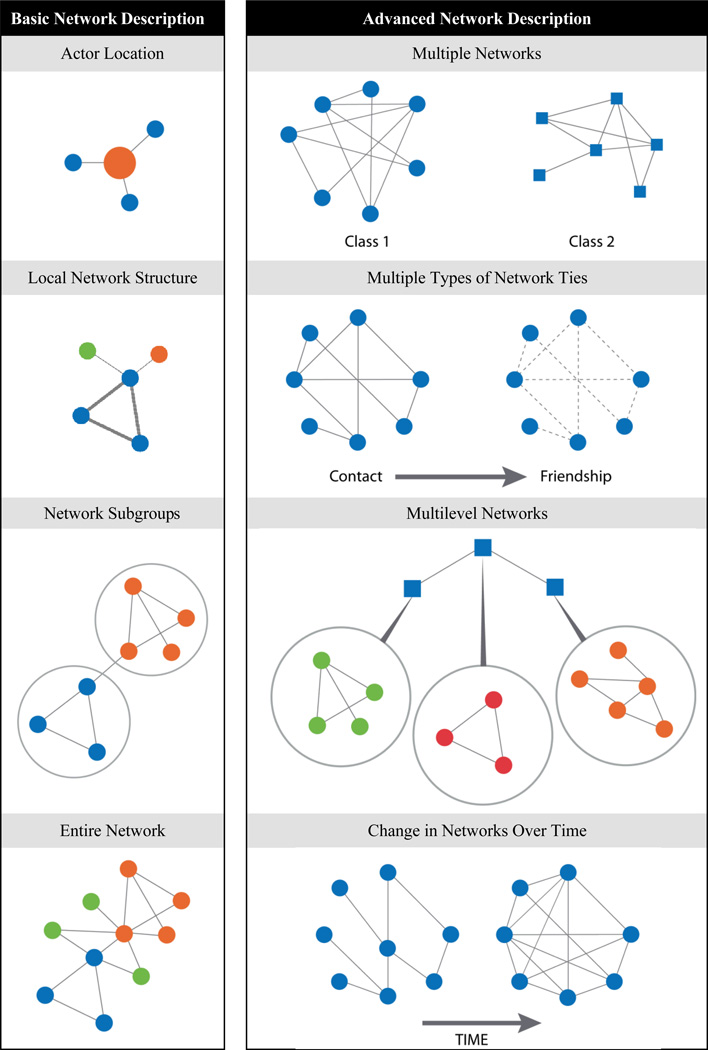
Figure 3.
Analysis approaches for basic and advanced network analysis.
Working down each column in the figure, basic network analysis can focus on the location of individual actors in the network, the structure of local connections and network subgroups, or the entire network. More advanced network analysis can examine multiple networks, the relationships among multiple types of network ties, multilevel networks, or how networks change over time. Finally, relatively new developments in statistical network theory are allowing for the first time the building and testing of statistical models and hypotheses of network processes and structures (57).
Over the past two decades network analysis has become more widely used in public health, especially in the following five areas (92): disease transmission, social support and social capital, network influences on health behavior, public health service and organizational networks, and the social structure of information diffusion. Three examples illustrate how the use of network methods have helped facilitate a greater move towards network and systems theories in public health. First it was during the early years of the HIV epidemic (followed by work on SARS and other infectious diseases) that epidemiologists started to employ network analytic methods as a new way to chart the spread of a disease, and to plan how to counter disease outbreaks (104; 124). This moved the fundamental S-I-R disease model away from a pure population-level model by incorporating local social network information into the basic model (76). The second example is in the area of information diffusion. Early empirical work on Rogers’ Diffusion of Innovations theory emphasized the temporal aspects of the rate of diffusion over time, and the identification of distinctive types of people or organizations involved with diffusion (e.g., opinion leaders) (36). It was not until Valente focused on the network aspects of Diffusion of Innovations, for example by studying network threshold effects on diffusion patterns (138), that diffusion studies started incorporating more relational and structural aspects of communication systems (63; 99). Finally a recent series of studies by Christakis and Fowler have collectively suggested that a wide variety of health behaviors and functioning (including smoking, obesity, and happiness) are ‘socially contagious’ and directly shaped by social networks (30; 31; 51). The methods and conclusions of some of these studies have been challenged (34; 95), but the visibility of this work has helped to highlight the continuing importance of network analysis methods in modern public health science.
Agent-Based Modeling
Agent-based modeling (ABM) uses computer simulation to study complex systems from the ground up, by examining how individual elements of a system (agents) behave as a function of individual properties, their environment, and their interactions with each other. Through these behaviors, emergent properties of the overall system are revealed. Compared to system dynamics, this results in a form of decentralized modeling where there is no formalized definition of global system behavior (i.e., no differential equations that drive the high level processes of the system) (22). ABM is the youngest of these three systems science methods, although its conceptual roots trace back to important 20th Century discoveries in mathematics, philosophy, and computer science, including Von Neumann’s invention of cellular automata, and John Conway’s Game of Life (108). One of the first influential agent-based models that clearly demonstrated how the behavior of complex systems could be described using only simple agent-level rules was Reynolds’ simulation of flocking birds (121). Reynolds ‘boids’ model used only three simple bird-level rules: 1) separation (don’t get too close to any other bird); 2) alignment (match the speed and direction of nearby birds); and 3) cohesion (head for the center of mass of nearby birds). The result of the simulation using these rules was “…the graceful dance-like movement of the flock whose hypnotic rhythm is clearly patterned yet also highly non-linear” ((97) p. 144). ABM has been employed in a great number of disciplines, but has been particularly useful to describe emergent properties of organizational, social, and cultural systems in anthropology, sociology, political science, business and economics (10; 12; 66). More generally, ABM has been shown to be particularly useful for modeling emergent phenomena such as contagion flows, markets, organizational behavior, and diffusion (21); all of which are relevant in public health research.
ABM employs computer simulations that start with characteristics and rules about individual agents, and then generate dynamic ‘histories’ that reveal overall system properties and behavior. Table 3 lists the most important characteristics of agents and agent-based models that collectively distinguish this approach from other modeling approaches such as SD (42). Although specialized software packages and libraries for ABMs exist (e.g., RePast), much of the academic ABM projects are based on hand-written software that utilizes object-oriented programming techniques (117). Given the dynamic nature of ABM models, one of the important attractions of these simulations is the visual nature of the modeling environment. Many ABM simulation environments allow researchers to view the system behavior in ‘real-time’ (see the RePast user interface: http://repast.sourceforge.net/; AnyLogic: http://www.xjtek.com/anylogic; or NetLogo: http://ccl.northwestern.edu/netlogo/). The development of ABM methods has been extremely rapid--exciting recent developments include the integration of GIS and social network information into agent-based models (11; 37), and the ability to use extremely large sets of agents in the simulations, including synthetic populations of entire communities or nations (25).
Table 3.
Core properties that collectively underly most agent-based models
| • Heterogeneous | Agents allowed to differ from one another on important characteristics |
| • Spatial | Agents are located in some explicitly defined space |
| • Interactive | Agents can interact locally with one another and their environment |
| • Bounded Rationality | Agents are assumed to have imperfect knowledge |
| • Dynamic | Models are recursive, allowed to change non-linearly and exhibit non-equilibrium |
The signature success of agent-based modeling in public health is in the study of epidemics and infectious disease dynamics. ABMs have been used to study disease transmission at multiple scales, from individual communities to global pandemics (44). ABMs of epidemics have helped move epidemiology beyond the traditional S-I-R model, and have demonstrated the importance of examining the role of social networks, transportation systems, local geography, and diverse behavioral responses to changing contexts on the spread of disease (45; 46; 148). ABMs have also started to be used to study chronic disease and health behavior, including drinking (58) and smoking (11), as well as complex public health and healthcare systems (75; 128). Conceptually, these models have been useful in suggesting possible mechanisms by which contexts (i.e., neighborhoods, communities, residential environments) influence health and health behavior (8). Finally, much like SD, ABMs promise to provide powerful ‘simulation laboratories’ where different types of public health interventions, programs, and policies can be tested when more traditional outcome studies are not possible (83).
THREE CASE STUDIES
As the above methods review suggests, system dynamics, network analysis, and agent-based modeling have been used in a wide variety of public health research situations. In this section, we present three short case studies that highlight how these systems science methods have been used in particular public health research programs to answer critical scientific and policy questions that would be difficult, if not impossible, to answer using more traditional research designs and analytic tools.
Infectious Disease
The study of Infectious disease has been the earliest and most important testing ground for systems science methods in public health. Scientists have long understood that the course of disease transmission in a population is the result of the complex interplay between biology, environment and society (6). Systems science methods have been critical in moving theories of disease transmission from simplistic temporal models that assume random mixing, to sophisticated models which recognize the importance of geography, social connections, travel patterns, and non-rational behavior (78; 115). Collaborative modeling networks, such as MIDAS (111), offer an example of the types of shared investigative efforts that can approach infectious disease models from various angles to advance innovations in methodological development and utility of the models.
The importance of social ties in infectious disease underlies the relevance of methods able to capture the complexities of social interactions. HIV transmission provides an illustrative example, spreading through a heterogeneous set of contact types including sexual and intravenous drug use (77), and largely determined by interactions between the structure of social networks and their interaction with population level characteristics (125). Specific characteristics of network structure that are predictive of infection can lead to improved understanding of transmission processes, as illustrated by the work of Rothenberg and colleagues, who identified relevant “microstructures” in syphilis transmission (126) and Christley et al. (33), who identified measures of network structure that are predictive of novel infections in a previously uninfected population. Christakis et al. (32) utilize a novel approach to studying friend networks and flu spread, based on previous knowledge of the centrality of individuals in a social network who are randomly selected as friends of initial contacts. Described as the “friendship paradox” (your friends have more friends than you do), they show that this faster way of sampling and assessing social networks may be useful in improving response time to a broad array of infections.
Applications of system dynamics in infectious disease range from early studies that emphasized describing dynamics of the spread of disease, to recent work more strongly oriented toward testing potential impacts of infectious control strategies. Early examples of SD in infectious disease as applied to the AIDS epidemic focused on describing the dynamics of the disease transmission process and characteristics of the HIV virus, like incubation period (70; 122). Models have offered particularly powerful results when data exist to provide a test of model validity. A classic example of the use of SD in studying the dynamic of unintended consequences is provided by Homer and colleagues (69), who developed and tested a model to study the development of antibiotic resistant in pneumonia using existing population-based data from various countries. Vickers et al. (143) employed SD modeling to test various assumptions for the rebound in chlamydia rates, and used surveillance data to choose the most parsimonious model whose behavior mirrored that of the surveillance data, which pointed to increased testing and not to any real increases in occurrence as the reason behind the uptick in chlamydia rates. Thompson (135) helped frame the debate about polio programs oriented toward long-term eradication vs. short-term controls, clarifying the economic costs and benefits for policymakers. The usefulness of broad model boundaries in SD is illustrated by studies of overlapping epidemics. The co-occurrence of HIV and multi-drug resistant tuberculosis provide a strong example of this, with the accompanying complex menu of potential policy options that public health decision makers struggle with to address various treatment and control approaches in affected populations (7; 81).
Agent-based (and hybrid system dynamic/agent-based) modeling is currently at the forefront of the modern science of infectious disease (44), with its ability to address the complex interplay between individual behavior and social connections on a large scale. Results from the MIDAS modeling network provide some good examples of the multiple ways of testing ABM models in simulating outcomes of potential infectious disease policy and practice decisions. Lee et al. (84) modeled vaccination allocation policies in the face of an H1N1 epidemic to examine priority recommendations around high-risk individuals versus highly-infective children when vaccines are in short supply, and comparisons among outcomes such as attack rate, hospitalizations and overall cost. Members of the same group (82) identified problems with school closing strategies for controlling influenza outbreaks, and found that short closures were counter-productive and that only longer closures would provide the needed lag time for implementation of long-term effective vaccination programs. In another study (83) utilized the influenza models to examine the impact of strategies for workplace H1N1 vaccination and found that those aimed at larger firms were more efficient and effective than those that were spread across a larger number of smaller workplaces.
Studies of infectious disease continue to be at the forefront of the development of combined approaches that join elements across system methods to model the interaction between individual agent behavior with social networks (29; 46; 79), and with system dynamics of epidemics (45).
Tobacco Control
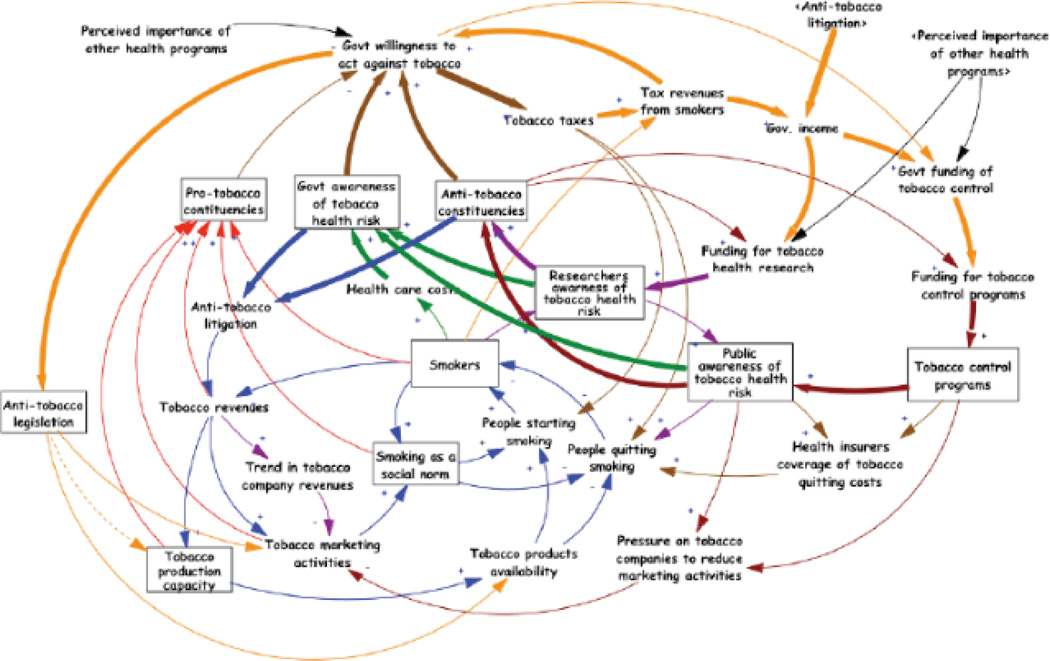
Tobacco control is at once the biggest challenge for public health (tobacco use is still the number one preventable cause of death in much of the world) and one of its biggest success stories (27). Starting in the mid-20th century, epidemiologists and clinical scientists were able to identify the links in the causal chain between tobacco use and death and disability (mainly via cancer and heart disease), using fairly traditional research methods. However, scientists are now realizing that systems science methods may be critical tools for understanding the complex factors shaping tobacco use and addiction at the individual level; and similarly for understanding the complex interactions of the various organizational actors in the tobacco control public health system (98). Tobacco control science reaches from ‘cells to society,’ and is clearly a classic example of a complex system: it has interacting, heterogeneous actors and the system as a whole adapts and changes over time. This is reflected in Figure 4which is a causal map for a systems dynamic model for tobacco control, developed as part of NCI’s Initiative on the Study and Implementation of Systems (113). This causal map illustrates some of the complex feedback loops (more than 1,900!) that exist between the various actors that include individual smokers, tobacco growers, government regulators, public health scientists, and the tobacco industry. Similarly, public health scientists are increasingly aware that changes in tobacco control and tobacco use are likely to have complicated and sometimes unintended consequences in the larger health, economic, and political systems, including changes in healthcare costs, worker displacement and employment, philanthropy, state and local budgets, and health disparities (19).
Figure 4.
ISIS System Dynamics Model for Tobacco Control (113).
System dynamics and network analysis have both been used more widely in tobacco control science compared to agent-based modeling (68). Dynamic systems simulations and modeling have been particularly useful for forecasting population-level trends, such as smoking initiation and prevalence. For example, dynamic modeling work done by Mendez and colleagues (103) has charted smoking initiation and cessation rates over time in the U.S. to determine the likelihood of achieving Healthy People goals of 10% smoking prevalence by 2025. These dynamic models have suggested that under a variety of conditions, smoking rates will decline over time, but achieving a 10% prevalence rate is unlikely (73). System dynamics have also been used to explore the additive and interactive effects of multiple policies and interventions on smoking rates. This is a particularly attractive use of SD, given that in the real world, single policies are never implemented in isolation. The most well-known example of this approach is the SimSmoke models of Levy and colleagues, which have been used to explore the potential effects of tobacco control policies on smoking in the U.S. and other countries (86; 87). Similar dynamic systems approaches have been used to explore tobacco control educational programs (134) and governmental investment in cessation services (136). The cumulative lesson learned from these dynamic modeling studies is that multiple, evidence-based policies need to be implemented in a comprehensive strategy to continue to lower smoking rates (88).
Although high-level, aggregate models (as found in many SD models) have been useful for forecasting long-term population-level trends, they are by their very nature less useful in identifying important mechanisms or relational structures that drive tobacco use. Network analysis has proven to be more successful in this regard, and has been used to primarily address two broad sets of tobacco control questions: how social networks influence individual tobacco use, and how community, state, national and international tobacco control systems are structured. Although it has been known for some time that there are strong peer and family influences on smoking behavior, network analysis can be used to identify what types of ties and network structures are most associated with smoking. Ennett & Bauman (41) were among the first to do this, showing that adolescents who were most isolated from their peers were most likely to smoke. Subsequent network studies have expanded on this basic effect, showing the buffering effects of friendship groups (5), and the interaction of network ties with school environments (4). Current network studies of smoking are starting to establish more specific causal mechanisms—for example, Lakon and colleagues suggest that networks influence smoking by structuring flows of emotional support (80).
Network analysis has also been frequently used to describe and explore the complex structures of tobacco control systems from state-level to global. Harris, Luke, and colleagues (62) analyzed the contact and collaboration networks of eight state tobacco control programs to identify a common star-shaped pattern of connections between the lead agency and four other types of organizational partners. At the national level, network analysis has been used to map the structure of tobacco control leadership across agencies in the Department of Health and Human Services (85) and to develop models of collaboration among five national tobacco control networks (93). At the international level, Wipfli and colleagues (146) used network analysis to show that engagement with an online network of international tobacco control advocates was positively associated with the likelihood of formal adoption of the Framework Convention on Tobacco Control.
At this point in time, agent-based modeling has not been applied to tobacco control research in any comprehensive way, although ABM has been used to study other addictive behaviors (58; 60). The agent-based modeling group at the Brookings Institution have started developing agent-based models of smoking behavior and policies (11), but these methods have yet to be widely adopted by tobacco control scientists. This is expected to change, however, as ABMs are ideal approaches for studying the effects of different policies when traditional experimental designs are not possible. For example, ABM models could be built to test the dynamic effects of tobacco retailer density reduction through distinct policy approaches such as attrition, increased licensing fees, or buffer zones around schools (94).
Obesity
Like tobacco control, obesity is a growing global public health challenge. In the past two decades alone there has been an increase in weight in the US such that over 2/3 of the population is now overweight or obese (48). Causal factors in obesity range from individual metabolic components to society at large, resulting in causal models that describe the various levels and sectors of society that offer potential points of public health intervention (e.g., policy, built environment, social networks) (71). Complex systems models offer a set of analytical methods that can account for this complexity (61), and build upon previous simulation studies (89) to further develop models that examine the interplay between cells, individuals and society. Large-scale, team modeling efforts now exist, such as COMNet, CompMod, and Foresight (1), that have constructed models to simulate the complex web of causation in obesity prevention. See Vandenbroeck et al, (141) for an impressive example of the complex systems map of obesity. These models can be further utilized to examine the impact of interventions applied as various inputs and modifications to the models.
Social network analysis is perhaps the best-known systems method approach in obesity and offers an illustrative example of more broadly applicable considerations in interpreting SNA results. In their influential paper, Christakis and Fowler (30) describe the spread of obesity through social networks. The authors investigated a large network of individuals in a population-based cohort of adults over a period of 32 years, and found that subjects’, or “egos”, weight gain was a function of weight gain in persons to whom they were socially connected, or “alters”. Using generalized estimating equations (GEE) to examine the impact of other factors, such as smoking behavior and geographic distance, on the relationship between relatedness and weight gain and found little mediating effect, which the authors interpreted as providing support for perception of social norms as the driving force over behavioral imitation, the former being less a function of frequency of contact than the latter. However, Cohen-Cole et al. (35) challenge these findings, and suggest that if a more comprehensive set of contextual factors are taken into account, it is the shared environment that drives social network patterns in obesity, though they concede that the evidence for tightly woven network ties in obesity still suggest the usefulness of intervention approaches aimed at social networks. Other NA work on the spread of obesity in adolescent social networks (49; 139) further underscores the applicability of this analytical approach. Greater understanding of the types and directionality of friendship and other social and even geographic (20; 28) ties can improve efforts to develop more effective intervention approaches based on specific network targets, social norms, and broad population vs. high-risk group strategies (13), despite some of the challenges in attributing causality between network structures and obesity (35; 40).
Agent-based and system dynamics models have also been employed in obesity research to examine the impact of dynamic interactions among multiple causal components. Recent work in ABM has explored the dynamics of determinants of walking behaviors, and neighborhood vs. environmental determinants of SES differentials in obesity. Auchincloss and colleagues (8) used ABM simulations to explore income differentials in nutrition as a function of both food prices and preference, and discuss the utility of computational models in developing a stronger set of evidence on which to base public health policy, particularly where strong empirical data are not available. Yang et al. (149) used ABM to study the role of the social and built environments on SES differentials in walking behavior, incorporating feedback mechanisms such that, for example, individual walking behavior is enforced as the number of other walkers increases. A subset of the system dynamic literature in obesity is focused on individual weight loss models (2; 47; 112). However, large scale, multi-group modeling initiatives, such as the CompMod and ComNet modeling networks of the Envision project in the National Collaborative on Childhood Obesity Research (1), are supporting broader model boundaries that represent higher-level societal sectors that are likely to play a meaningful role in designing better interventions with population-level impact.
Taken as a whole, while the literature is still relatively young, these studies have helped shift the paradigms of etiology and intervention in obesity, and perhaps chronic disease in general, to include mechanisms akin to communicable disease, and that while much of the current emphasis is rightly placed on the built environment, the social environment may be another important driver in creating opportunities for weight loss and healthy weight maintenance.
MOVING FORWARD WITH SYSTEMS SCIENCE METHODS IN PUBLIC HEALTH
In early 2000, Stephen Hawking said “I think the next century will be the century of complexity.” As we have suggested in this review, public health is well on its way to fulfilling this prediction by using an array of systems science methods to study complex public health problems.
Complex system methods challenge traditional study design and data analysis approaches in public health research. The picture that emerges from reviewing existing work is that system methods are inherently translational, with real world applications often explicit in the models. With a greater push for translational research in complex, real-world settings (147), we expect a growing demand for methods able to account for complexity. The field of complex system methods appears to be moving toward greater integration among the systems science methods to account for the interaction between social networks, broad system boundaries, and individual behavior, in order to improve the utility of models for policy and practice decision-making that span multiple levels of influence (114).
Despite the promise of systems science methods for public health, they remain underutilized and lack visibility (137; 140). Although there are a number of institutions and settings around the country that train and support systems scientists (e.g., University of Michigan’s Center for the Study of Complex Systems, the Santa Fe Institute, NIH’s Institute on Systems Science and Health), schools of public health are only now starting to think about developing their own curriculum and degree programs. While a brief review of course offerings in the top 20 schools of public health in the US found that about half offered at least one course that addressed a complex system method, it is not clear that such coursework is well-integrated into the methods curricula. It has been suggested that computational modeling and systems science is a ‘3rd Way’ that moves beyond traditional quantitative and qualitative research design dichotomies (110). If this is so, then we need to start producing more public health scientists who are comfortable and skilled using concepts and tools that focus on dynamics, agents, and networks; and ensure that the public is aware of the benefits of this approach to public health science.
ACKNOWLEDGMENTS
We would like to thank Bobby Milstein and the anonymous reviewer for their input and very helpful suggestions. In addition, we would like to recognize Laura Brossart’s extremely competent help in figure production and reference management.
GLOSSARY
- Systems Science
Systems science is an interdisciplinary field of science focusing on complex natural and social systems. Complex systems are characterized by: heterogeneous elements that interact with each other; non-linearity; and effects that are emergent (i.e., different from the effect of individual elements), persist over time and adapt to changing circumstances.
- System Dynamics
System dynamics (SD) uses computer simulation to model non-linear processes, such as stocks and flows, feedback loops, and time delays, based on a series of differential equations.
- Network Analysis
Network analysis (NA) is a research method and scientific paradigm that focuses on the relationships among sets of actors; these can be any type of entity that can have a relationship or tie with other entities: persons, animals, organizations, countries, websites, documents, and even genes.
- Agent-Based Modeling
Agent-based modeling (ABM) uses computer simulation to study complex systems from the ground up, by examining how individual elements of a system (agents) behave as a function of individual properties, their environment, and their interactions with each other, to reveal emergent properties of the overall system.
Literature Cited
- 1.National Collaborative on Childhood Obesity Research, Envision projects. http://nccororg/envision/indexhtml.
- 2.Abdel-Hamid TK. Modeling the dynamics of human energy regulation and its implications for obesity treatment. System Dynamics Review. 2002;18:431–471. [Google Scholar]
- 3.Ahmed E, Elgazzar A, Hegazi A. An overview of complex adaptive systems. ArXiv Nonlinear Sciences. 2005 [Google Scholar]
- 4.Alexander C, Piazza M, Mekos D, Valente T. Peers, schools, and adolescent cigarette smoking. Journal of Adolescent Health. 2001;29:22–30. doi: 10.1016/s1054-139x(01)00210-5. [DOI] [PubMed] [Google Scholar]
- 5.Aloise-Young PA, Graham JW, Hansen WB. Peer influence on smoking initiation during early adolescence: a comparison of group members and group outsiders. J Appl Psychol. 1994;79:281–287. doi: 10.1037/0021-9010.79.2.281. [DOI] [PubMed] [Google Scholar]
- 6.Anderson RM. The Population dynamics of infectious diseases : theory and applications. London ; New York: Chapman and Hall; 1982. [Google Scholar]
- 7.Atun RA, Lebcir RM, McKee M, Habicht J, Coker RJ. Impact of joined-up HIV harm reduction and multidrug resistant tuberculosis control programmes in Estonia: System dynamics simulation model. Health Policy. 2007;81:207–217. doi: 10.1016/j.healthpol.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Auchincloss AH, Riolo RL, Brown DG, Cook J, Diez-Roux AV. An agent-based model of income inequalities in diet in the context of residential segregation. Am J Prev Med. 2011;40:303–311. doi: 10.1016/j.amepre.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auerbach DM, Jaffe HW, Curran JW, Darrow WW. Cluster of cases of the Acquired Immune-Deficiency Syndrome - Patients linked by sexual contact. American Journal of Medicine. 1984;76:487–492. doi: 10.1016/0002-9343(84)90668-5. [DOI] [PubMed] [Google Scholar]
- 10.Axelrod R. Agent-based modeling as a bridge between disciplines. In: Tesfatsion L, Judd KL, editors. Handbook of Computational Economics. Vol. 2. Elsevier; 2006. pp. 1565–1584. Number of 1565-84 pp. [Google Scholar]
- 11.Axtell RL, Durlauf S, Epstein JM, Hammond R, Klemens B, et al. Social influences and smoking behavior. Brookings Institution; 2006. [Google Scholar]
- 12.Axtell RL, Epstein JM, Dean JS, Gumerman GJ, Swedlund AC, et al. Population growth and collapse in a multiagent model of the Kayenta Anasazi in Long House Valley. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7275–7279. doi: 10.1073/pnas.092080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahr DB, Browning RC, Wyatt HR, Hill JO. Exploiting social networks to mitigate the obesity epidemic. Obesity. 2009;17:723–728. doi: 10.1038/oby.2008.615. [DOI] [PubMed] [Google Scholar]
- 14.Balthrop J, Forrest S, Newman MEJ, Williamson MM. Technological networks and the spread of computer viruses. Science. 2004;304:527–529. doi: 10.1126/science.1095845. [DOI] [PubMed] [Google Scholar]
- 15.Bar-Yam Y. Complex systems and thinking on multiple scales. Lisbon, Portugal: Technical University of Lisbon; 1999. [Google Scholar]
- 16.Barabási AL. Linked : the new science of networks. Cambridge, MA: Perseus Pub.; 2002. [Google Scholar]
- 17.Barton A. Bringing society back in: Survey research and macro-methodology. American Behavioral Scientist. 1968;12:1–9. [Google Scholar]
- 18.Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- 19.Bearman PS, Neckerman KM, Wright L. After tobacco : what would happen if Americans stopped smoking? New York: Columbia University Press; 2011. pp. vii–446. [Google Scholar]
- 20.Bejleri I, Steiner RL, Fischman A, Schmucker JM. Using GIS to analyze the role of barriers and facilitators to walking in children's travel to school. Urban Design International. 2011;16:51–62. [Google Scholar]
- 21.Bonabeau E. Agent-based modeling: methods and techniques for simulating human systems. Proc Natl Acad Sci U S A. 2002;99(Suppl 3):7280–7287. doi: 10.1073/pnas.082080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borshchev A, Filippov A. From system dynamics and discrete event to practical agent based modeling: Reasons, techniques, tools. Proc. The 22nd International Conference of the System Dynamics Society; Oxford, England. 2004. [Google Scholar]
- 23.Brandes U, Erlebach T. Network analysis : methodological foundations. New York: Springer; 2005. [Google Scholar]
- 24.Buchanan M. Nexus : Small worlds and the groundbreaking science of networks. New York: W.W. Norton; 2002. [Google Scholar]
- 25.Cajka JC, Cooley PC, Wheaton WD. Attribute assignment to a synthetic population in support of agent-based disease modeling, RTI International. 2010 doi: 10.3768/rtipress.2010.mr.0019.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calvert KI, Doar MB, Zegura EW. Modeling internet topology. Communications Magazine, IEEE. 1997;35:160–163. [Google Scholar]
- 27.CDC. Ten great public health achievements--United States: 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60:619–623. [PubMed] [Google Scholar]
- 28.Charreire H, Casey R, Salze P, Simon C, Chaix B, et al. Measuring the food environment using geographical information systems: a methodological review. Public Health Nutrition. 2010;13:1773–1785. doi: 10.1017/S1368980010000753. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Marathe A, Marathe MV. Coevolution of epidemics, social networks, and individual behavior: a case study. Lecture Notes in Computer Science. 2010;6007:218–227. [Google Scholar]
- 30.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 31.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med. 2008;358:2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christakis NA, Fowler JH. Social network sensors for early detection of contagious outbreaks. PLoS One. 2010;5:e12948. doi: 10.1371/journal.pone.0012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christley RM, Pinchbeck GL, Bowers RG, Clancy D, French NP, et al. Infection in social networks: using network analysis to identify high-risk individuals. Am J Epidemiol. 2005;162:1024–1031. doi: 10.1093/aje/kwi308. [DOI] [PubMed] [Google Scholar]
- 34.Cohen-Cole E, Fletcher JM. Detecting implausible social network effects in acne, height, and headaches: longitudinal analysis. BMJ. 2008;337:a2533. doi: 10.1136/bmj.a2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen-Cole E, Fletcher JM. Is obesity contagious? Social networks vs. environmental factors in the obesity epidemic. Journal of Health Economics. 2008;27:1382–1387. doi: 10.1016/j.jhealeco.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Dearing JW. Evolution of diffusion and dissemination theory. J Public Health Manag Pract. 2008;14:99–108. doi: 10.1097/01.PHH.0000311886.98627.b7. [DOI] [PubMed] [Google Scholar]
- 37.Delre SA, Jager W, Bijmolt THA, Janssen MA. Will it spread or not? The effects of social influences and network topology on innovation diffusion. Journal of Product Innovation Management. 2010;27:267–282. [Google Scholar]
- 38.Dimitrov NB, Goll S, Hupert N, Pourbohloul B, Meyers LA. Optimizing tactics for use of the U.S. antiviral strategic national stockpile for pandemic influenza. PLoS One. 2011;6:e16094. doi: 10.1371/journal.pone.0016094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiPrete TA, Gelman A, McCormick T, Teitler J, Zheng T. Segregation in social networks based on acquaintanceship and trust. AJS. 2011;116:1234–1283. doi: 10.1086/659100. [DOI] [PubMed] [Google Scholar]
- 40.Ellen JM. Social networks research and challenges to causal inference. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2009;45:109–110. doi: 10.1016/j.jadohealth.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Ennett ST, Bauman KE. Peer group structure and adolescent cigarette smoking: a social network analysis. Journal of Health and Social Behavior. 1993:226–236. [PubMed] [Google Scholar]
- 42.Epstein JM. Agent-based computational models and generative social science. Complexity. 1999;4:41–57. [Google Scholar]
- 43.Epstein JM. Generative social science : studies in agent-based computational modeling. Princeton: Princeton University Press; 2006. [Google Scholar]
- 44.Epstein JM. Modelling to contain pandemics. Nature. 2009;460:687. doi: 10.1038/460687a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Epstein JM, Parker J, Cummings D, Hammond RA. Coupled contagion dynamics of fear and disease: mathematical and computational explorations. PLoS One. 2008;3:e3955. doi: 10.1371/journal.pone.0003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eubank S, Guclu H, Anil Kumar V, Marathe MV, Srinivasan A, et al. Modelling disease outbreaks in realistic urban social networks. Nature. 2004;429:180–184. doi: 10.1038/nature02541. [DOI] [PubMed] [Google Scholar]
- 47.Flatt JP. Carbohydrate-fat interactions and obesity examined by a two-compartment computer model. Obesity Research. 2004;12:2013–2022. doi: 10.1038/oby.2004.252. [DOI] [PubMed] [Google Scholar]
- 48.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults: 1999–2000. JAMA. 2002;288:1723. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 49.Fletcher A, Bonell C, Sorhaindo A. You are what your friends eat: systematic review of social network analyses of young people's eating behaviours and bodyweight. J Epidemiol Community Health. 2011;65:548–555. doi: 10.1136/jech.2010.113936. [DOI] [PubMed] [Google Scholar]
- 50.Forrester J. Industrial dynamics. Cambridge: The MIT Press; 1961. [Google Scholar]
- 51.Fowler JH, Christakis NA. Dynamic spread of happiness in a large social network: longitudinal analysis over 20 years in the Framingham Heart Study. BMJ. 2008;337:a2338. doi: 10.1136/bmj.a2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freedman DA. Statistical models and shoe leather. Sociological methodology. 1991;21:291–313. [Google Scholar]
- 53.Freeman LC. The development of social network analysis : a study in the sociology of science. Vancouver, BC: Empirical Press; 2004. [Google Scholar]
- 54.Galea S, Riddle M, Kaplan GA. Causal thinking and complex system approaches in epidemiology. Int J Epidemiol. 2010;39:97–106. doi: 10.1093/ije/dyp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallagher R, Appenzeller T. Beyond reductionism. Science. 1999;284:79. [Google Scholar]
- 56.Glasser JW, Hupert N, McCauley MM, Hatchett R. Modeling and public health emergency responses: lessons from SARS. Epidemics. 2011;3:32–37. doi: 10.1016/j.epidem.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodreau SM. Advances in exponential random graph (p*) models applied to a large social network. Social Networks. 2007;29:231–248. doi: 10.1016/j.socnet.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorman DM, Mezic J, Mezic I, Gruenewald PJ. Agent-based modeling of drinking behavior: a preliminary model and potential applications to theory and practice. Am J Public Health. 2006;96:2055–2060. doi: 10.2105/AJPH.2005.063289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green LW. Public health asks of systems science: to advance our evidence-based practice, can you help us get more practice-based evidence? Am J Public Health. 2006;96:406–409. doi: 10.2105/AJPH.2005.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gruenewald PJ. The spatial ecology of alcohol problems: niche theory and assortative drinking. Addiction. 2007;102:870–878. doi: 10.1111/j.1360-0443.2007.01856.x. [DOI] [PubMed] [Google Scholar]
- 61.Hammond RA. Complex systems modeling for obesity research. Prev Chronic Dis. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 62.Harris JK, Luke DA, Burke RC, Mueller NB. Seeing the forest and the trees: using network analysis to develop an organizational blueprint of state tobacco control systems. Social Science & Medicine. 2008;67:1669–1678. doi: 10.1016/j.socscimed.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 63.Harris JK, Luke DA, Zuckerman RB, Shelton SC. Forty years of secondhand smoke research: the gap between discovery and delivery. Am J Prev Med. 2009;36:538–548. doi: 10.1016/j.amepre.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 64.Hirsch G, Homer J, Evans E, Zielinski A. A system dynamics model for planning cardiovascular disease interventions. Am J Public Health. 2010;100:616–622. doi: 10.2105/AJPH.2009.159434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoard M, Homer J, Manley W, Furbee P, Haque A, Helmkamp J. Systems modeling in support of evidence-based disaster planning for rural areas. International Journal of Hygiene and Environmental Health. 2005;208:117–125. doi: 10.1016/j.ijheh.2005.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holland JH, Miller JH. Artificial adaptive agents in economic theory. The American Economic Review. 1991;81:365–370. [Google Scholar]
- 67.Homer J, Milstein B, Wile K, Pratibhu P, Farris R, Orenstein DR. Modeling the local dynamics of cardiovascular health: risk factors, context, and capacity. Prev Chronic Dis. 2008;5:A63. [PMC free article] [PubMed] [Google Scholar]
- 68.Homer JB, Hirsch GB. System dynamics modeling for public health: background and opportunities. Am J Public Health. 2006;96:452–458. doi: 10.2105/AJPH.2005.062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Homer JB, Ritchie-Dunham J, Rabbino H, Puente LM, Jorgensen J, Hendricks K. Toward a dynamic theory of antibiotic resistance. System Dynamics Review. 2000;16:287–319. [Google Scholar]
- 70.Homer JB, St. Clair CL. A model of HIV transmission through needle sharing. Interfaces. 1991;21:26–49. [Google Scholar]
- 71.Huang TT, Drewnowski A, Kumanyika SK, Glass TA. A systems-oriented multilevel framework for addressing obesity in the 21st century. Prev Chronic Dis. 2009;6:A97. [PMC free article] [PubMed] [Google Scholar]
- 72.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 73.Institute of Medicine. Ending the tobacco problem : a blueprint for the nation. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 74.Jones AP, Homer JB, Murphy DL, Essien JDK, Milstein B, Seville DA. Understanding diabetes population dynamics through simulation modeling and experimentation. Am J Public Health. 2006;96:488–494. doi: 10.2105/AJPH.2005.063529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanagarajah AK, Lindsay P, Miller A, Parker D. An exploration into the uses of agent-based modeling to improve quality of health care. Proc. Proceedings of the 6th International Conference on Complex Systems; Boston, MA. 2006. [Google Scholar]
- 76.Keeling MJ, Eames KT. Networks and epidemic models. Journal of the Royal Society, Interface. 2005;2:295–307. doi: 10.1098/rsif.2005.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klovdahl AS. Social networks and the spread of infectious diseases: the AIDS example. Social Science & Medicine. 1985;21:1203–1216. doi: 10.1016/0277-9536(85)90269-2. [DOI] [PubMed] [Google Scholar]
- 78.Koopman JS. Modeling infection transmission. Annu Rev Public Health. 2004;25:303–326. doi: 10.1146/annurev.publhealth.25.102802.124353. [DOI] [PubMed] [Google Scholar]
- 79.Koopman JS, Chick SE, Riolo CS, Adams AL, Wilson ML, Becker MP. Modeling contact networks and infection transmission in geographic and social space using GERMS. Sex Transm Dis. 2000;27:617–626. doi: 10.1097/00007435-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 80.Lakon CM, Hipp JR, Timberlake DS. The social context of adolescent smoking: a systems perspective. Am J Public Health. 2010;100:1218–1228. doi: 10.2105/AJPH.2009.167973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lebcir RM, Atun RA, Coker RJ. System Dynamic simulation of treatment policies to address colliding epidemics of tuberculosis, drug resistant tuberculosis and injecting drug users driven HIV in Russia. Journal of the Operational Research Society. 2009;61:1238–1248. [Google Scholar]
- 82.Lee BY, Brown ST, Cooley P, Potter MA, Wheaton WD, et al. Simulating school closure strategies to mitigate an influenza epidemic. Journal of Public Health Management & Practice. 2010;16:252–261. doi: 10.1097/PHH.0b013e3181ce594e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee BY, Brown ST, Cooley PC, Zimmerman RK, Wheaton WD, et al. A computer simulation of employee vaccination to mitigate an influenza epidemic. Am J Prev Med. 2010;38:247–257. doi: 10.1016/j.amepre.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee BY, Brown ST, Korch GW, Cooley PC, Zimmerman RK, et al. A computer simulation of vaccine prioritization, allocation, and rationing during the 2009 H1N1 influenza pandemic. Vaccine. 2010;28:4875–4879. doi: 10.1016/j.vaccine.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leischow SJ, Luke DA, Mueller N, Harris JK, Ponder P, et al. Mapping U.S. government tobacco control leadership: networked for success? Nicotine & Tobacco Research. 2010;12:888–894. doi: 10.1093/ntr/ntq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levy DT, Bales S, Lam NT, Nikolayev L. The role of public policies in reducing smoking and deaths caused by smoking in Vietnam: results from the Vietnam tobacco policy simulation model. Social Science & Medicine. 2006;62:1819–1830. doi: 10.1016/j.socscimed.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 87.Levy DT, Bauer JE, Lee HR. Simulation modeling and tobacco control: creating more robust public health policies. Am J Public Health. 2006;96:494–498. doi: 10.2105/AJPH.2005.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levy DT, Mabry PL, Graham AL, Orleans CT, Abrams DB. Exploring scenarios to dramatically reduce smoking prevalence: A simulation model of the three-part cessation process. Am J Public Health. 2010;100:1253–1259. doi: 10.2105/AJPH.2009.166785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levy DT, Mabry PL, Wang YC, Gortmaker S, Huang TTK, et al. Simulation models of obesity: a review of the literature and implications for research and policy. Obesity Reviews. 2011;12:378–394. doi: 10.1111/j.1467-789X.2010.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loscalzo J, Kohane I, Barabasi AL. Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol Syst Biol. 2007;3:124. doi: 10.1038/msb4100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luke DA. Getting the big picture in community science: methods that capture context. Am J Community Psychol. 2005;35:185–200. doi: 10.1007/s10464-005-3397-z. [DOI] [PubMed] [Google Scholar]
- 92.Luke DA, Harris JK. Network analysis in public health: history, methods, and applications. Annu Rev Public Health. 2007;28:69–93. doi: 10.1146/annurev.publhealth.28.021406.144132. [DOI] [PubMed] [Google Scholar]
- 93.Luke DA, Harris JK, Shelton S, Allen P, Carothers BJ, Mueller NB. Systems analysis of collaboration in 5 national tobacco control networks. Am J Public Health. 2010;100:1290–1297. doi: 10.2105/AJPH.2009.184358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luke DA, Ribisl KM, Smith C, Sorg AA. Family Smoking Prevention And Tobacco Control Act: banning outdoor tobacco advertising near schools and playgrounds. Am J Prev Med. 2011;40:295–302. doi: 10.1016/j.amepre.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 95.Lyons R. The spread of evidence-poor medicine via flawed social-network analysis. Statistics, Politics, and Policy. 2010;2 [Google Scholar]
- 96.Mabry PL, Marcus SE, Clark PI, Leischow SJ, Mendez D. Systems science: a revolution in public health policy research. Am J Public Health. 2010;100:1161–1163. doi: 10.2105/AJPH.2010.198176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Macy MW, Willer R. From factors to actors: Computational sociology and agent-based modeling. Annual Review of Sociology. 2002;28:143–166. [Google Scholar]
- 98.Marcus SE, Leischow SJ, Mabry PL, Clark PI. Lessons learned from the application of systems science to tobacco control at the National Cancer Institute. Am J Public Health. 2010;100:1163–1165. doi: 10.2105/AJPH.2010.198721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McAneney H, McCann JF, Prior L, Wilde J, Kee F. Translating evidence into practice: a shared priority in public health? Social Science & Medicine. 2010;70:1492–1500. doi: 10.1016/j.socscimed.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 100.McGrath JE, Martin J, Kulka RA. Judgment calls in research. Beverly Hills: Sage Publications; 1982. [Google Scholar]
- 101.Meadows DH, Wright D. Thinking in systems : a primer. White River Junction, Vt.: Chelsea Green Pub.; 2008. [Google Scholar]
- 102.Meehl PE. Theoretical risks and tabular asterisks - Sir Karl, Sir Ronald, and the slow progress of soft psychology. Journal of Consulting and Clinical Psychology. 1978;46:806–834. [Google Scholar]
- 103.Mendez D, Warner KE. Smoking prevalence in 2010: Why the Healthy People goal is unattainable. Am J Public Health. 2000;90:401–403. doi: 10.2105/ajph.90.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meyers LA, Pourbohloul B, Newman ME, Skowronski DM, Brunham RC. Network theory and SARS: predicting outbreak diversity. J Theor Biol. 2005;232:71–81. doi: 10.1016/j.jtbi.2004.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Micceri T. The unicorn, the normal curve, and other improbable creatures. Psychological Bulletin. 1989;105:156. [Google Scholar]
- 106.Milstein B. Hygeia's constellation: navigating health futures in a dynamic and democratic world. Atlanta, GA: Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 107.Milstein B, Jones A, Homer JB, Murphy D, Essien J, Seville D. Charting plausible futures for diabetes prevalence in the United States: a role for system dynamics simulation modeling. Prev Chronic Dis. 2007;4:A52. [PMC free article] [PubMed] [Google Scholar]
- 108.Mitchell M. Complexity : a guided tour. New York: Oxford University Press; 2009. [Google Scholar]
- 109.Moreno JL. Sociometry in relation to other social sciences. Sociometry. 1937;1:206–219. [Google Scholar]
- 110.Moss S. Relevance, realism and rigour: A third way for social and economic research. Manchester: Centre for Policy Modelling, Manchester Metropolitan University; 1999. [Google Scholar]
- 111.National Institute of General Medical Sciences. Models of Infectious Disease Agent Study (MIDAS) https://www.epimodels.org/midas/home.do.
- 112.Navarro-Barrientos JE, Rivera DE, Collins LM. A dynamical model for describing behavioural interventions for weight loss and body composition change. Mathematical and Computer Modelling of Dynamical Systems. 2010;17:183–203. doi: 10.1080/13873954.2010.520409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.NCI. Greater than the sum: Systems thinking in tobacco control. Bethesda, MD: National Cancer Institute, U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health; 2007. [Google Scholar]
- 114.Osgood N. Using traditional and agent based toolset for system dynamics: Present tradeoffs and future evolution. Proc. 25th International Conference of the System Dynamics Society; Boston, MA. 2007. [Google Scholar]
- 115.Parkes MW, Bienen L, Breilh J, Hsu LN, McDonald M, et al. All hands on deck: transdisciplinary approaches to emerging infectious disease. EcoHealth. 2005;2:258–272. [Google Scholar]
- 116.Rahmandad H, Sterman J. Heterogeneity and network structure in the dynamics of diffusion: Comparing agent-based and differential equation models. Management Science. 2008;54:998–1014. [Google Scholar]
- 117.Railsback SF, Lytinen SL, Jackson SK. Agent-based simulation platforms: Review and development recommendations. Simulation. 2006;82:609–623. [Google Scholar]
- 118.Rapkin BD, Luke DA. Cluster analysis in community research: Epistemology and practice. Am J Community Psychol. 1993;21:247–277. [Google Scholar]
- 119.Resnicow K, Page SE. Embracing chaos and complexity: a quantum change for public health. Am J Public Health. 2008;98:1382–1389. doi: 10.2105/AJPH.2007.129460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ressler S. Social network analysis as an approach to combat terrorism: Past, present, and future research. Homeland Security Affairs. 2006;2:1–10. [Google Scholar]
- 121.Reynolds CW. Flocks, herds and schools: A distributed behavioral model. ACM SIGGRAPH Computer Graphics. 1987;21:25–34. [Google Scholar]
- 122.Roberts C, Dangerfield B. Modelling the epidemiological consequences of HIV infection and AIDS: A contribution from operational research. The Journal of the Operational Research Society. 1990;41:273–289. [Google Scholar]
- 123.Rocha LM. Complex systems modeling: Using metaphors from nature in simulation and scientific models. BITS: Computer and Communications News. 1999 [Google Scholar]
- 124.Rothenberg RB, Narramore J. The relevance of social network concepts to sexually transmitted disease control. Sex Transm Dis. 1996;23:24–29. doi: 10.1097/00007435-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 125.Rothenberg RB, Potterat JJ, Woodhouse DE, Muth SQ, Darrow WW, Klovdahl AS. Social network dynamics and HIV transmission. AIDS. 1998;12:1529–1536. doi: 10.1097/00002030-199812000-00016. [DOI] [PubMed] [Google Scholar]
- 126.Rothenberg RB, Sterk C, Toomey KE, Potterat JJ, Johnson D, et al. Using social network and ethnographic tools to evaluate syphilis transmission. Sex Transm Dis. 1998;25:154–160. doi: 10.1097/00007435-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 127.Royston G, Dost A, Townshend J, Turner H. Using system dynamics to help develop and implement policies and programmes in health care in England. System Dynamics Review. 1999;15:293–313. [Google Scholar]
- 128.Sibbel R, Urban C. Agent-based modeling and simulation for hospital management. In: Saam NJ, Schmidt B, editors. Cooperative agents: applications in the social sciences. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. pp. 183–199. Number of 183-99 pp. [Google Scholar]
- 129.Smith MJ, Ellenberg SS, Bell LM, Rubin DM. Media coverage of the measles-mumps-rubella vaccine and autism controversy and its relationship to MMR immunization rates in the United States. Pediatrics. 2008;121:e836–e43. doi: 10.1542/peds.2007-1760. [DOI] [PubMed] [Google Scholar]
- 130.Sterman J. Business dynamics : Systems thinking and modeling for a complex world. Boston: Irwin/McGraw-Hill; 2000. [Google Scholar]
- 131.Sterman JD. Learning from evidence in a complex world. Am J Public Health. 2006;96:505–514. doi: 10.2105/AJPH.2005.066043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Susser M. Does risk factor epidemiology put epidemiology at risk? Peering into the future. J Epidemiol Community Health. 1998;52:608–611. doi: 10.1136/jech.52.10.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tan J, Wen HJ, Awad N. Health care and services delivery systems as complex adaptive systems. Communications of the ACM. 2005;48:36–44. [Google Scholar]
- 134.Tengs TO, Osgood ND, Chen LL. The cost-effectiveness of intensive national school-based anti-tobacco education: results from the tobacco policy model. Prev Med. 2001;33:558–570. doi: 10.1006/pmed.2001.0922. [DOI] [PubMed] [Google Scholar]
- 135.Thompson KM, Tebbens RJ. Eradication versus control for poliomyelitis: an economic analysis. Lancet. 2007;369:1363–1371. doi: 10.1016/S0140-6736(07)60532-7. [DOI] [PubMed] [Google Scholar]
- 136.Tobias MI, Cavana RY, Bloomfield A. Application of a system dynamics model to inform investment in smoking cessation services in New Zealand. Am J Public Health. 2010;100:1274–1281. doi: 10.2105/AJPH.2009.171165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Trochim WM, Cabrera DA, Milstein B, Gallagher RS, Leischow SJ. Practical challenges of systems thinking and modeling in public health. Am J Public Health. 2006;96:538–546. doi: 10.2105/AJPH.2005.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Valente TW. Network models of the diffusion of innovations. Cresskill, NJ: Hampton Press; 1995. [Google Scholar]
- 139.Valente TW, Fujimoto K, Chou CP, Spruijt-Metz D. Adolescent affiliations and adiposity: A social network analysis of friendships and obesity. Journal of Adolescent Health. 2009;45:202–204. doi: 10.1016/j.jadohealth.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Van Wave TW, Scutchfield FD, Honore PA. Recent Advances in Public Health Systems Research in the United States. Annual Review of Public Health. 2010;Vol 31:283–295. doi: 10.1146/annurev.publhealth.012809.103550. 31: Number of 283-95 pp. [DOI] [PubMed] [Google Scholar]
- 141.Vandenbroeck P, Goossens J, Clemens M. Foresight, Tackling Obesities: Future Choices–Building the Obesity System Map. London: Government Office for Science; 2007. [Google Scholar]
- 142.Vennix J. Group model building: Facilitating team learning using system dynamics. Chichester: John Wiley & Sons; 1996. [Google Scholar]
- 143.Vickers DM, Osgood ND. Current crisis or artifact of surveillance: insights into rebound chlamydia rates from dynamic modelling. BMC Infectious Diseases. 2010;10:70. doi: 10.1186/1471-2334-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wasserman S, Faust K. Social network analysis : Methods and applications. New York: Cambridge University Press; 1994. [Google Scholar]
- 145.Willinger W, Alderson D, Doyle JC, Li L. More "normal" than normal: scaling distributions and complex systems. Proc. 36 th Winter Simulation Conference, Washington, D.C; Winter Simulation Conference; 2004. pp. 130–141. [Google Scholar]
- 146.Wipfli HL, Fujimoto K, Valente TW. Global tobacco control diffusion: the case of the framework convention on tobacco control. Am J Public Health. 2010;100:1260–1266. doi: 10.2105/AJPH.2009.167833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299:211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 148.Yang Y, Atkinson P, Ettema D. Individual space-time activity-based modelling of infectious disease transmission within a city. Journal of the Royal Society, Interface. 2008;5:759–772. doi: 10.1098/rsif.2007.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang Y, Roux AVD, Auchincloss AH, Rodriguez DA, Brown DG. A spatial agent-based model for the simulation of adults' daily walking within a city. Am J Prev Med. 2011;40:353–361. doi: 10.1016/j.amepre.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]