Abstract
Older adults who undergo cataract extraction have roughly half the rate of motor vehicle collision (MVC) involvement per mile driven compared to cataract patients who do not elect cataract surgery. Currently in the U.S., most insurers do not allow payment for cataract surgery based upon the findings of a vision exam unless accompanied by an individual’s complaint of visual difficulties that seriously interfere with driving or other daily activities and individuals themselves may be slow or reluctant to complain and seek relief. As a consequence, surgery tends to occur after significant vision problems have emerged. We hypothesize that a proactive policy encouraging cataract surgery earlier for a lesser level of complaint would significantly reduce MVCs among older drivers. We used a Monte Carlo model to simulate the MVC experience of the U.S. population from age 60 to 89 under alternative protocols for the timing of cataract surgery which we call “Current Practice” (CP) and “Earlier Surgery” (ES). Our base model finds, from a societal perspective with undiscounted 2010 dollars, that switching to ES from CP reduces by about 21% the average number of MVCs, fatalities, and MVC cost per person. The net effect on total cost – all MVC costs plus cataract surgery expenditures -- is a reduction of about 16%. Quality Adjusted Life Years would increase by about 5%. From the perspective of payers for healthcare, the switch would increase cataract surgery expenditure for ages 65+ by about 8% and for ages 60 to 64 by about 47% but these expenditures are substantially offset after age 65 by reductions in the medical and emergency services component of MVC cost. Similar results occur with discounting at 3% and with various sensitivity analyses. We conclude that a policy of ES would significantly reduce MVCs and their associated consequences.
Keywords: Motor Vehicle Collision, Cataract Surgery, Monte Carlo Simulation, Elderly, Cost Effectiveness, Medicare, Insurance
1.0 Introduction
Older adults who undergo cataract extraction have roughly half the rate of motor vehicle collision (MVC) involvement per mile driven compared to cataract patients who do not elect cataract surgery (Owsley et al. 2002). Currently in the U.S., most insurers do not allow payment for cataract surgery based upon the findings of a comprehensive eye examination unless accompanied by an individual’s complaint of visual difficulties that seriously interfere with driving or other daily activities. (Corcoran and Johnson 2005). Furthermore, even if insurer regulations are not rigorously enforced, individuals may be slow to seek relief for cataract symptoms due to cognitive deficits, age, insurance coverage, income or simple procrastination (Gerald McGwin et al. 2006) (Hall et al. 2005) (Escarce 1993). As a consequence, surgery tends to occur after significant vision problems have emerged. In the meantime, some drivers who are at risk for crashes may voluntarily modify their driving behavior by avoiding exposure to challenging driving situations (e.g. driving alone, turning left across traffic, driving at night) (Ball et al. 1998). Voluntary restriction, however, is an imperfect tactic because the individual may misjudge their capabilities and risk. For example, Friedman et al. found that a subset of the elderly population have a substantial discrepancy between self -reported reading difficulty and measured reading speed (Friedman et al. 1999). Eby and Molnar also observe that “ Many older individuals in the early stages of dementia can and do drive safely...[but they] are likely to lack the insight needed to make appropriate decisions about stopping or restricting their driving in response to declines in driving-related abilities” (Eby and Molnar 2010). We hypothesize that replacing the current operate-upon-serious-complaint rule with a rule that permits earlier cataract surgery for a less serious complaint of visual difficulty has the potential to appreciably reduce MVC rates for older drivers. In this paper we report the results of a simulation model, based upon data from the Impact of Cataract on Mobility (ICOM) study, described below, (Owsley et al. 2002) and related literature, that compares the number of MVCs under current standards with those under a more relaxed standard.
Research to date has established several important facts about the risk for MVCs among older people with cataracts. First, cataract is the leading cause of vision impairment in older adults in the United States (Centers for Disease Control and Prevention 2011). Population based studies show that approximately 50% of white adults and 60% of African Americans age 65 to 74 years have cataracts (Klein et al. 1992, West et al. 1998). Cataracts cause deficits in visual acuity and contrast sensitivity as well as increased disability glare (Rubin et al. 1997). Studies assessing the Quality Adjusted Life Years (QALYs) that individuals assign to various health conditions have found that mild, moderate, or severe vision impairment due to cataract respectively decrease QALYs to 0.59, 0.45 or 0.29 compared to a rating of 1.0 assigned to perfect health. (Tengs and Wallace 2000). Additionally, older drivers with cataract are more likely to have a history of crash involvement compared with older drivers who are cataract free (Owsley et al. 2001). For all older drivers, the risk of a crash rises with age as does the risk of serious injury or death in a crash (U.S. Dept of Transportation and National Highway Traffic Safety Administration 1993) (Evans 1988). Among various measures of vision, contrast sensitivity has been found to be the best predictor of crash risk among persons who have cataracts after controlling for other vision characteristics, cognitive status, and miles driven (Owsley et al. 2001). Cataract surgery, fortunately, has been found to be successful at slowing the rate of increase in crash risk. In one study of persons with cataracts, those who elected surgery had roughly half the MVC rate compared to those that did not (Owsley et al. 2002). Although some older drivers tend to restrict their driving in response to deteriorating vision, they generally do not increase their driving following successful cataract surgery (Owsley et al. 2002). Thus reductions in crash risk achieved by cataract surgery are not offset by increases in driving post surgery (Owsley et al. 2002).
So far, these facts have not been linked and analyzed to determine what would happen if the current practice of delaying cataract surgery until a patient complains sufficiently were to be replaced by a proactive policy of making cataract surgery available earlier when symptoms of visual problems initially appear. From the perspective of direct medical costs for cataract surgery, delay reduces the number of surgeries in a particular age cohort because some candidates will die of other causes or become institutionalized before they are eligible for an operation while, in contrast, a proactive program would increase the number of surgeries. For example, for a cohort of persons at age 60, about 6% of them will have died by age 65 and 15% will have died by age 70 (Arias 2007). Thus delaying surgery reduces the number to be performed on a cohort. The financial present value of a surgery would also rise when performed earlier. However, both direct medical costs and deaths due to MVCs may be reduced by shortening the period of exposure to a heightened risk of crash due to cataract. These considerations create a classic cost-effectiveness question of the relationship between changes in cost to changes in crashes avoided. Additionally, consideration should be given to the improvement in quality of life that occurs due to improved vision following cataract surgery.
2.0 Methods
A Monte Carlo model was developed to simulate the motor vehicle collision experience of the US population from age 60 to 89 under alternative protocols for the timing of cataract surgery which we call “Current Practice” (CP) and “Earlier Surgery” (ES). Age-related cataract often starts to cause functional vision problems in adults during their 60s, including being troublesome for driving (Owsley et al. 1999). The model starts 5 years before eligibility for Medicare so as to examine how a protocol permitting earlier surgery might shift expenditures from Medicare and its supplemental insurers and beneficiaries to pre-Medicare age payers. The model tabulates expenditures for ages 60 to 64 and for 65+ without parsing further among specific payers. Variables of interest over this timespan for an individual are the number of MVCs, cost of MVCs, probability of a fatality, probability of having cataract surgery, the age at which surgery occurs, its cost to either private payers or Medicare, and quality of life of the individual. The model adopts a societal viewpoint but also considers the distribution of surgical costs to private and public payers. All dollar values are adjusted to 2010 equivalents, and future events are discounted to present value so that both are measured in the same metric regarding time. Discounting is the reverse of the process of compounding interest. An event in the future is worth less than when it is immediately available. Discounting can be viewed as either a financial or psychological phenomenon (Stokey and Zeckhauser 1978). Discounting both costs and outcomes (at 3%) has been recommended by the U.S. Panel on Cost Effectiveness in Health and Medicine (Weinstein et al. 1996). Nord, however, has recently argued that while discounting outcomes has a role in individual financial decisions, it lacks a strong theoretical foundation for public policy choices (Nord 2011). We find Nord’s argument to be persuasive and we consequently discuss results from both a discounted and un- discounted perspective, although results are similar in both cases.
ICOM Data
Our model uses data from the Impact of Cataracts on Mobility (ICOM) study to derive parameters for our model as described below (Owsley et al. 2002). ICOM was a prospective cohort study designed to address the question, for older drivers who have cataract, of whether cataract surgery impacts the MVC rate. The study compared the crash rates of individuals, age 55 to 84 at enrollment, who elected cataract surgery to those who stated that they did not wish to have the surgery. Participants were recruited from eye clinics affiliated with the University of Alabama at Birmingham. They were interviewed at baseline and at two subsequent annual study visits. Of 277 participants, 140 elected to have surgery at baseline and 137 declined. Eventually 34 of those who declined surgery changed their minds and elected surgery so that in the end there were 174 (140 + 34) who had surgery and 103 who did not have surgery during the follow-up period. An additional 102 patients without evident cataract were followed for comparison. Table 1 shows the demographics and some baseline vision characteristics of the Surgery and No Surgery groups. The average participant was a white non- Hispanic with a high school education. Females were more prevalent in the Surgery Group (53%) and less prevalent (35%) in the No Surgery Group.
Table 1.
Characteristics of ICOM Participants
| Surgery Group (N=174) |
No Surgery (N=103) |
P value | |
|---|---|---|---|
| Mean Age (years) | 71.2 (0.51) | 71.5 (0.52) | 0.670 |
| Mean Education (years) | 12.7 (0.23) | 12.4 (0.32) | 0.420 |
| Race (%) | 0.023 | ||
| White, non-Hispanic | 90.2 | 80.6 | |
| African American | 9.8 | 19.4 | |
| Gender (%) | 0.004 | ||
| Male | 47.1 | 65.0 | |
| Female | 52.9 | 34.9 | |
| Mean ADVS | |||
| Night driving a | 53.7 (2.05) | 70.8 (2.30) | 0.000 |
| Disability glare a | 59.6 (1.89) | 79.3 (1.71) | 0.000 |
| Overall a | 69.6 (1.29) | 82.1 (1.33) | 0.000 |
| Better eye | |||
| Visual Acuity b | 0.26 (0.20) | 0.16 (0.14) | 0.000 |
| Contrast Sensitivity c | 1.34 (0.20) | 1.41 (0.13) | 0.000 |
| Disability Glare b | 1.00 (0.39) | 1.21 (0.22) | 0.000 |
0=No sight, 100=Worst possible
higher scores are worse
lower scores are worse
At each interview, ICOM participants were asked about their visual difficulties, using the Activities of Daily Vision Scale (ADVS) The ADVS is a well validated questionnaire for assessing visual difficulties due to cataract from the patient’s perspective (Mangione CM et al. 1992). Higher ADVS scores denote better vision and fewer complaints of difficulty in doing daily activities including driving. The ADVS ranges in value from 0 (no sight) to 100 (no visual difficulties). It has subscales measuring difficulty in driving by day and by night, difficulties with glare, near vision and far vision. It thus measures the types of complaints that an ophthalmologist would have to consider for the individual to be eligible for health insurance reimbursement for cataract surgery. As shown in Table 1, the ICOM Surgery Group at baseline (before surgery) had significantly worse ADVS scores overall and in regard to night driving and disability glare compared to the No Surgery Group. Compared with the No Surgery Group, visual acuity and contrast sensitivity were worse in the better eye while disability glare was less accentuated in the Surgery Group.
Although the ADVS is not formally used to determine when an individual qualifies for cataract surgery under current guidelines, we use it in our simulation model because it speaks to current practice that requires an individual to complain sufficiently about limitations of activities due to vision problems in order to be eligible for insurance reimbursement for cataract surgery. Previously it has been reported that ADVS change scores were significantly correlated with changes in visual acuity, contrast sensitivity, and disability glare independent of age, sex, race, education, chronic medical conditions and ocular comorbidities for individuals in the ICOM study. Furthermore, in the ICOM surgery group, multiple linear regression models showed that visual acuity in the first-eye surgery had a significant, independent association with the change in the overall ADVS score and with the change in night driving and disability glare subscales (McGwin et al. 2003). Thus the ADVS is indeed associated with objective visual difficulties in the ICOM sample and it is relevant for assessing eligibility for cataract surgery.
Information regarding MVC involvement was collected on enrollees by obtaining accident reports from the Alabama Department of Public Safety. At baseline, a five year prior history of crashes was collected; MVC information was then collected prospectively for an approximately five year period.
Demographics & Vision
The model begins by randomly selecting an individual at age 60 with gender and race corresponding to the U.S. White and Black population. Each year the individual faces a probability of dying based upon U.S. lifetables (Arias 2010) and the possibility of a fatal MVC (Blincoe et al. 2002). Race is restricted to White and Black because they are the only groups in the ICOM sample and because information on the frequency of cataract, progression of vision deterioration with age, and MVC frequency among other racial/ethnic groups is generally not available from other sources.
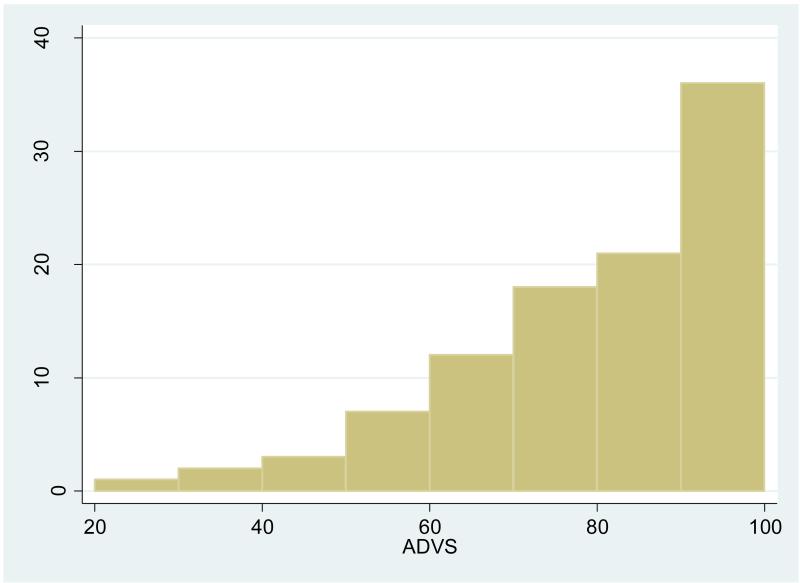
Next, the individual is randomly assigned a value of the ADVS as a measure of vision status. In our base model, as a starting point for testing various alternative specifications, the individual is randomly assigned a value of the ADVS drawn from a right skewed distribution with mean 76.4 (SD =18.6), median 80, an upper bound of 100 and a lower bound of 20 as shown in Figure 1. The distribution is based on the entire population in the ICOM study, including persons who had not yet been found to have cataracts. Thus our simulation model starts off with part of the population immediately eligible for surgery under either the CP or ES criteria while the rest of the population is assumed to gradually deteriorate to a point where they may be eligible for surgery if they are still alive. This distribution is somewhat better than the distribution of the overall ADVS score (mean 67, SD=22) reported by Mangione and colleagues in their development of the ADVS (Mangione CM et al. 1992). Their work however was based on an older population (mean age 75 years, SD= 9) already scheduled for cataract surgery whereas we wish to simulate a cohort beginning at age 60 with cataracts in various stages of development. We are not aware of any study that has tracked ADVS values for individuals over a period as long as the 30 years that we consider. Cross sectional studies show that various measures of vision deteriorate with age, often with periods of stability (Haegerstrom-Portnoy et al. 1999) (Rubin et al. 1997). Because the ADVS indicates how much an individual is bothered by vision issues in daily life, it may be that year to year changes in the ADVS will sometimes show an improvement. This is the case for some individuals in the 3 years monitored by the ICOM study. To allow for gradual deterioration of vision with age and the possibility of being less bothered in some years, our base model has a variable annual rate of depreciation drawn from a triangular distribution which allows the ADVS to improve by as much as 1 % or decline by as much as 3% with no change being the most common occurrence. Following cataract surgery, the individual experiences a change (usually an improvement) in ADVS that is randomly drawn from the distribution of change in the ADVS observed in the ICOM (mean improvement 16.7, SD=17.2, range minimum -28, maximum 75). This new ADVS is assumed to persist for the remaining lifetime. In sensitivity analyses, we have examined situations (see Methods, Sensitivity Analyses “Case 4”) where the population begins with generally high level of ADVS but experiences a faster rate of decline than in our base model.
Figure 1.
Frequencies of initial ADVS values
Distribution of ADVS at Start of Simulation Model, age 60.
Timing of Surgery
An individual’s ADVS can be used to determine when and if the individual has surgery. Under the CP and ES protocols, respectively, surgery occurs in the base model at ADVS values of 70 and 80. These thresholds are based upon the median ADVS scores of persons in the ICOM with cataracts who initially choose to have surgery (70) or declined to have surgery (80). The model assumes that everyone receives surgery immediately upon becoming eligible.
Mileage
The U.S. Department of Transportation reports that men and women ages 55-64 respectively drive an average of 15,859 and 7,780 miles per year and roughly a third less at older ages (U.S. Department of Transportation and Federal Highway Administration 2011). To more precisely take account of the effects on miles driven of age, gender, ADVS score and whether or not the person has yet had cataract surgery, our model computed an individual’s miles driven per year from a regression equation based on ICOM data. In the model, mileage is positively related to ADVS but it falls with age and falls by 1,646 miles per year after the occurrence of cataract surgery despite the overall improvement in ADVS due to surgery. For example, in our base model a 65 year old male with ADVS=80 who has not yet had surgery will drive 12,796 miles per year.
MVC Frequency, Severity, Fatalities and Costs
In our model, the probability of an individual experiencing a MVC in any year is positively correlated with miles driven, being male, and the square of age and negatively correlated with the ADVS score, age and the receipt of cataract surgery. The computed probability is based on a panel data logistic regression model estimated from ICOM data. Age 72 is the crossover point where the probability of an MVC begins to rise after initially falling from age 60. Whether or not a MVC occurs for a given individual is determined, following standard practice in simulation modeling, by comparing the computed probability to a random number (Stokey and Zeckhauser 1978). If the MVC occurs, another random number is drawn to determine the probability of a fatality and the corresponding total cost of the MVC, adjusted into dollar values for 2010, based upon our calculations of the frequencies of these events reported by (Blincoe et al. 2002) as shown in Table 2 .
Table 2.
Types of MVC, their probability of occurrence, total cost and subcomponent cost due to Medical and Emergency Service in 2010 dollars
| Type of MVC | Probability | Medical & Emergency | Total Cost |
|---|---|---|---|
| Fatal | 0.0023 | 28,733 | 1,224,633 |
| MAIS 5 | 0.0002 | 417,701 | 1,373,704 |
| MAIS 4 | 0.0008 | 165,588 | 436,279 |
| MAIS 3 | 0.0028 | 58,728 | 233,216 |
| MAIS 2 | 0.0096 | 19,847 | 83,739 |
| MAIS 1 | 0.1031 | 3,104 | 13,236 |
| MAIS 0 | 0.0564 | 29 | 2,459 |
| PDO | 0.8248 | 40 | 3,173 |
| Total | 1.0000 |
Source: Authors’ computations based on Blincoe et al. 2002, page 9
MAIS = Maximum Abbreviated Injury Scale
PDO= Property Damage Only
NOTE: TOTAL Costs consist of: Medical, Emergency Services, Market Productivity, Insurance Administration, Workplace, Legal, Travel Delay and Property Damage
Surgery Expenditures
Our main interest in modeling direct surgery costs is to determine how a policy of earlier surgery would increase the incidence of surgery and shift expenditures into the years prior to Medicare eligibility. Our base model assumes that all subjects who become eligible for surgery receive surgery at a total cost of $2,000 (in 2010 dollars) (Cutler and McClellan 2001). We do not explicitly model whether surgery is one eye or both eyes but assume that whatever is performed occurs within a given year and achieves a lasting effect on the ADVS. We ignore routine annual eye exam expenses and their indirect costs (e.g. travel time) because we expect them to occur with similar frequency before and after surgery (Naeim et al. 2006). We assume that an individual receives surgery promptly whenever a threshold is reached. That is, we do not model delays or refusals of surgery.
Medical and Emergency Services Costs
Whatever the societal benefit of a policy of ES might be compared to CP, it is likely to generate increase cataract surgery expenditures for the parties – private insurers before age 65 and Medicare thereafter - who pay for direct medical expenses. Would the policy also generate savings for these parties in terms of the direct medical expenses that arise from MVCs? To explore this issue, our model explicitly tracks the Medical and Emergency Services (M&ES) expenditures attributed to MVCs based on the itemization found in (Blincoe et al. 2002) as shown in Table 2.
Total cost
Total cost in our model is defined as the sum of direct cataract surgery expenditures plus the 9 costs attributable to MVCs itemized in Blincoe et al.: Medical, Emergency Services, Market Productivity, Household Productivity, Insurance Administration, Workplace, Legal, Travel Delay and Property Damage (Blincoe et al. 2002). As noted earlier, our model explicitly tracks total MVC costs and the sum of the components for M&ES.
Quality of Life
Our model estimates the QALYs that an individual experiences based upon his or her ADVS score. Our base model converts ADVS scores to QALYs using the formula QALYs = 0.270121 + 0.005578* ADVS which we derive by regression from Table 1 in Naeim et al.’s study that used the Health Utilities Index 3 (HUI3) to measure QALYs as a function of the ADVS (Naeim et al. 2006). Their study examined 250 patients with a low probability (under 30%) of benefiting from cataract surgery who were randomly assigned to surgery or watchful waiting. Although a large portion of their study population is less likely to benefit from cataract surgery than most persons with cataracts, our formula seems to be reasonable for simulation modeling. Note that our regression fitted formula for QALYs does not yield perfect quality of life (QALYs=1.0) when the ADVS has a perfect vision score of 100 because of the presence of other comorbidities (heart disease, arthritis, etc.) in the population susceptible to cataracts. Thus QALYs is measured on a scale, appropriate to public policy resource allocation, where perfect health, not merely perfect vision, equals 1.0 and death equals zero (Kymes and Lee 2007). In examining data from the ICOM study, we have found that the average ADVS score before and after cataract surgery was respectively 76 and 87 which would move the patient from a QALYs of 69 to 76 for a gain of about 8%. Insight into the reasonableness of this change can be gained from a recent study by Kaplan et al. who compared the changes in QALYs that occur after cataract surgery for 376 adults using five generic instruments for measuring utility (Kaplan et al. 2010). They found that, depending on the instrument used, the total improvement in QALYs in the six months following cataract surgery ranged from virtually zero to about 9% with a median of about 3.7%. (These percentage changes are our own calculations based upon Kaplan.) Of particular interest to our simulation work was their finding that the improvement in QALYs using the HUI3 was about 9% which is reasonably close to what our formula predicts.
3.0 Calculations
The Monte Carlo simulation model was run simultaneously for both the CP and ES protocols by drawing a hypothetical 60 year old person at random with race and gender based on the U.S. population. Our modeling was done in Excel 2010 using XLSim Commercial Electronic Version 3.2.8c (McDonald et al. 2010). Because automobile fatalities are rare events in a population with the low mileage characteristics of our age group, the model was run for 100,000 persons even though runs with 10,000 were sufficient to achieve stable results. For each individual, regression coefficients in the equations for mileage and the probability of an MVC were drawn from a multivariate normal distribution governed by the covariance matrix of the regression. This procedure adjusts for model uncertainty or individual idiosyncrasies. Our reported results show t and p statistics for significance assuming unequal variances for each group. Because our model uses a large number of observations, small differences in outcomes will very often be significant at conventional levels of confidence. To see if statistically significant differences have substantive significance, a Dissimilarity Index was calculated to compare the overlap of the distributions of the variables of interest. If two distributions have a perfect overlap the Dissimilarity Index is zero. If the distributions are far apart with virtually no overlap, their Dissimilarity Index approaches 1.0 (Goldstein 1994) (Inman and Bradley 1989).
Sensitivity Analyses
To explore the sensitivity of our base model, further simulations were run for four alternative scenarios:
Case 1: A reduction by half in the probability of dying in any year for non-motor vehicle causes such that more people would be eligible for cataract surgery before their natural death;
Case 2: A wider gap between the CP and ES criteria for when to do cataract surgery, specifically a change in the threshold ADVS scores for CP from 70 to 65 and for ES from 80 to 85;
Case 3: Higher costs of cataract surgery, specifically a change in the cost for surgery from the base case of $2,000 to $6,000 to allow for any potential unmeasured or underestimated costs.
Case 4: Faster rate of deterioration of the ADVS for a population with generally better initial vision. Williams et al. report an annual rate of cataract surgery of 5.3% based upon longitudinal analysis of the same individuals over time in the Asset and Health Dynamics Among the Oldest Old (AHEAD) national panel survey of US households for waves 1998, 2000 and 2002. (Williams et al. 2006) The AHEAD surveys did not collect the ADVS but they did have self-reported quality of vision (legally blind, poor, fair, good, very good or excellent) and information on whether the individual had cataract surgery on one or both eyes since the previous wave of the survey. We attempted to mimic the reported longitudinal pattern of cataract surgery frequency for individuals by setting the initial ADVS distribution at a median of 90 with lower and upper bounds of 80 and 100 and an annual deterioration of ADVS of 5.3%.
4.0 Results
Table 3 shows simulation base model results in terms of undiscounted mean values per person over the period from age 60 to 89 for the CP and ES thresholds for cataract surgery eligibility. Mean values per person can be multiplied by convenient factors to yield estimates such as fatalities per 10,000 persons. For example, under CP and ES, respectively, the mean number of MVCs per 10,000 persons over the 30 year period of age 60 to 89 is 14,601 (1.461 per person times 10,000 persons) and 11,487 (1.1487*10,000). The base model finds that switching to ES from CP reduces by 20% to 21% the average number of MVCs, fatalities, and MVC cost per person. The switch would increase expenditure for ages 65+ by about 8% and expenditures for ages 60 to 64 by about 47%. The net effect on total cost - MVC costs plus cataract surgery expenditures -- is a reduction of about 16.3%. QALYs would increase by about 5%. With ES the mean age at which surgery occurs falls from 64.7 to 63.6 years while the probability that someone will obtain surgery during this time span rises from 0.55 to 0.77. Due to the large sample sizes in the simulation model, all of these results are statistically significant at least the 3% level. The Dissimilarity Index shows that a randomly selected person would have a different outcome under ES from 6% to 11% of the time in terms of the occurrence of MVCs, fatalities, MVC cost, total cost or QALYs. Furthermore about 16% of the subjects would have higher cataract surgery expenditures in their pre-Medicare years while 2% would have higher surgery expenditures after age 65. Changes in the probability of surgery and the age at which it occurs would affect 8% and 19% of the individuals respectively. Discounting the results to present value at 3% has only a modest effect on these results as shown in Table 4.
Table 3.
Mean Motor Vehicle Collision Events and Costs per Person for 30 Year Period Age 60 to 89 for Current Practice and Earlier Surgery from Base Case Simulation Model
| Current Practice |
Early Practice | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | t | p | Dis-similarity Index |
% change |
|
| Motor Vehicle Collisions | 1.4601 (1.5074) |
1.1487 (1.3025) |
49.42 | 0.0000 | 0.11 | −21.3% |
| Fatalities | 0.0033 (0.0569) |
0.0026 (0.0507) |
2.78 | 0.0254 | 0.06 | −20.6% |
| MVC Cost | 12,969 (77,161) |
10,252 (68,741) |
8.32 | 0.0000 | 0.06 | −21.0% |
| Surgery Cost age 60-64 | 862 (990) |
1,266 (964) |
−92.39 | 0.0000 | 0.16 | 46.8% |
| Surgery Cost age 65+ | 243 (653) |
264 (677) |
−7.15 | 0.0000 | 0.02 | 8.8% |
| Total Cost | 14,074 (77,118) |
11,782 (68,700) |
7.02 | 0.0000 | 0.06 | −16.3% |
| QALY | 15.7 (6.2) |
16.5 (6.5) |
−27.73 | 0.0000 | 0.05 | 5.0% |
| Mean Age at Surgery | 64.7 (8.3) |
63.6 (7.3) |
33.58 | 0.0000 | 0.08 | −1.8% |
| Probability of Surgery | 0.55 (0.50) |
0.77 (0.42) |
−100.00 | 0.0000 | 0.19 | 38.5% |
Table 4.
Mean Motor Vehicle Collision Events and Costs per Person for 30 year period age 60-89 for Current Practice compared to Earlier Surgery from Base Case Simulation Model, Present Values at 3%.
| Current Practice |
Early Surgery | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | t | p | Dis-similarity Index |
% change | |
| Motor Vehicle Collisions |
1.0677 (1.0801) |
0.8466 (0.9491) |
48.61 |
0 |
0.102 |
− 20.70% |
| Fatalities | 0.0024 (0.0428) |
0.0019 (0.0381) |
2.71 | 0.0254 | 0.056 | −20.7% |
| MVC Cost | $9,488 (58,103) |
$7,534 (51,734) |
7.94 | 0.0000 | 0.057 | −20.6% |
| Surgery Expenditures | ||||||
| age 60-64 | $849 (976) |
$1,251 (953) |
−93.19 | 0.0000 | 0.165 | 47.3% |
| Surgery Expenditures | ||||||
| age 65+ | $142 (386) |
$158 (410) |
−9.07 | 0.0000 | 0.033 | 11.4% |
| Total Cost | $10,479 (58,060) |
$8,943 (51,691) |
6.25 | 0.0011 | 0.057 | −14.7% |
| QALY | 11.4 (3.8) |
11.9 (4.0) |
−31.51 | 0.0000 | 0.059 | 4.8% |
Medical and Emergency Services
Table 5 shows for the base model the mean M&ES cost per person of MVCs. Under CP, the undiscounted mean M&ES cost per person summed over age 60 to 89 is $1,421 (SD=10,941); under ES, it is $1,124 (SD=8,990). Thus ES reduces mean M&ES costs by $297 or 20.9%. The difference is statistically significant (p=0.0003); in terms of practical significance, the distribution of costs is dissimilar for about 3 % of the cases (Dissimilarity Index = 0.0269). From the perspective of parties who typically pay for health care, a policy of ES increases mean direct expenditures per person for cataract surgery (see Table 3) by $425 (1266 + 264 - 862 – 243) with an offsetting reduction in M&ES of $297 (291 + 833 - 362 – 1059) per person for a net increase in mean health care costs per person of $128 (=425 - 297). When results are decomposed by age, under ES the pre-Medicare years have a net increase of $333 in direct health care spending consisting of an increase in cataract surgery expenses of $404 (1266-862) that is only partly offset by a reduction of $71 (291-362) in M&ES. For the Medicare years, however, ES has a net reduction of $205 because increased cataract surgery expenditures of $21 (264-243) are more than offset by reductions of $226 (833-1059) in M&ES.
Table 5.
Mean Medical and Emergency Costs per person by age for Motor Vehicle Collisions, Current Practice compared to Earlier Surgery from Base Case Simulation Model.
| Current Practice |
Early Surgery | |||||
|---|---|---|---|---|---|---|
| Mean (sd) | Mean (sd) | t | p | Dis-similarity Index |
% change | |
| Undiscounted Total, Age 60-89 |
$1,421 (10,091) |
$1,124 (8,990) |
3.65 | 0.0003 | 0.0269 | −20.9% |
| Age 60-64 | $362 (5,358) |
$291 (4,745) |
1.19 | 0.2323 | 0.0281 | −19.5% |
| Age 65-89 | $1,059 (8,554) |
$833 (7,633) |
3.49 | 0.0005 | 0.0266 | −21.4% |
| Discounted at 3 % Total, Age 60-89 |
$1,041 (7,703) |
$828 (6,868) |
3.09 |
0.002 |
0.0245 |
−20.5% |
| Age 60-64 | $343 (5,052) |
$276 (4,487) |
1.16 | 0.2459 | 0.0254 | −19.4% |
| Age 65-89 | $786 (6,545) |
$621 (5,847) |
3.16 | 0.0016 | 0.0237 | −21.0% |
Similar results hold when dollars are discounted as shown in the respective tables 3 and 4. In terms of dollars discounted at 3%, the net effect of ES in the years before age 65 is an increase in mean surgery expenditures per person of $402 (= 1,251 for ES minus 849 for CP) offset by a reduction in M&ES of $67 (276 -343) for a net health care cost increase of $335. For age 65 and thereafter, the respective net effect of ES is a mean per person reduction of $149 because increased mean surgery expenditures per person of $16 (158 - 142) are more than offset by lower M&ES costs of $165 (=621 - 786). Thus, with discounting at 3%, the net effect of a policy of ES across all age groups is that higher mean cataract surgery expenditures of $418 are partly offset by reduced M&ES expenditures of $237 with the Medicare years benefiting from this policy.
Sensitivity
The results for the sensitivity analyses are shown in Table 6. For all of the cases, a policy of ES continues to be favorable (a dominant solution for a societal viewpoint). For Case 1, where general mortality rates are reduced by half, a switch to Earlier Surgery modestly improves the reduction in MVCs fatalities, MVC cost, total cost and QALYs found in the base case. Likewise surgery costs pre and post age 65, and the probability of surgery both increase somewhat more for ES. For Case 2, a wider criteria gap, the percentage difference between CP and ES is about twice as large. For example, the percentage difference in MVCs changes to a reduction of 38% in favor of ES compared to the 21% change found for the base case. For Case 3, where costs for cataract surgery ($6,000) are 3 times the base case, results change modestly relative to the base case in terms of the percentage difference attributable to ES. For Case 4, where the population begins with a better ADVS distribution but deteriorates more rapidly, MVCs, fatalities and MVC costs decline for ES relative to CP by 15% to 16%; surgery expenditures are higher for ES by 148% in the years before age 65 and lower by 50% in the years after age 65; and the lifetime probability of cataract surgery is respectively 94% and 96% for CP and ES.
Table 6.
Sensitivity Analyses of Mean Motor Vehicle Collision Events and Undiscounted Costs per Person for 30 year period age 60-89 for Current Practice (CP) compared to Earlier Surgery (ES)
| Case 1 - Mortality Rate Reduced by Half |
Case 2 - Wider Thresholds | Case 3 - Surgery Expenditure $6,000 |
Case 4 - Better Initial ADVS distribution with 5.3% annual depreciation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP | ES | % difference |
CP | ES | % difference |
CP | ES | % difference |
CP | ES | % difference |
|
| Mean (sd) |
Mean (sd) |
Mean (sd) |
Mean (sd) |
Mean (sd) |
Mean (sd) |
Mean (sd) |
Mean (sd) |
|||||
| Motor Vehicle Collisions |
1.7204 (1.65) |
1.3433 (1.42) |
−21.9% |
1.6398 (160) |
1.0210 (1.19) |
−37.7% |
1.4615 (151) |
1.1506 (130) |
−21.3% |
1.1436 (1.19) |
0.9659 (1.09) |
−15.5% |
| Fatalities | 0.0037 (0.06) |
0.0028 (0.05) |
−23.2% | 0.0039 (0.06) |
0.0026 (0.05) |
−34.2% | 0.0032 (0.06) |
0.0025 (0.05) |
−22.4% | 0.0025 (0.05) |
0.0021 (0.05) |
−14.7% |
| MVC Cost | $15,378 (83,745) |
$11,989 (73,618) |
−22.0% | $15,024 (84,975) |
$9,538 (68,584) |
−36.5% | $13,184 (78,236) |
$10,367 (68,998) |
−21.4% | $9,991 (67,167) |
$8,440 (61,936) |
−15.5% |
| Surgery Expenditure | ||||||||||||
| age 60-64 | $867 (991) |
$1,269 (963) |
46.4% | $522 (878) |
$1,293 (956) |
147.7% | $2,587 (2,971) |
$3,790 (2,894) |
46.5% | $504 (868) |
$1,247 (969) |
147.5% |
| Surgery Expenditure | ||||||||||||
| age 65+ | $314 (727) |
$331 (743) |
5.5% | $355 (764) |
$425 (818) |
19.5% | $721 (1,951) |
$791 (2,029) |
9.6% | $1,373 (928) |
$681 (948) |
−50.4% |
| Total Cost | $16,559 (83,690) |
$13,590 (73,578) |
−17.9% | $15,901 (84,935) |
$11,255 (68,557) |
−29.2% | $16,492 (78,152) |
$14,948 (68,912) |
−9.4% | $11,868 (67,147) |
$10,368 (61,921) |
−12.6% |
| QALY | 18.3 (5.5) |
19.2 (5.8) |
5.1% | 15.3 (6.1) |
16.8 (6.6) |
9.7% | 15.7 (6.2) |
16.4 (6.5) |
5.0% | 15.6 (6.2) |
16.5 (6.5) |
5.7% |
| Mean Age at Surgery |
65.73 (9.0) |
64.37 (8.2) |
−2.1% |
65.91 (8.4) |
63.53 (7.0) |
−3.6% |
64.71 (8.3) |
63.57 (7.3) |
−1.8% |
65.13 (17) |
63.15 (16) |
−3.0% |
| Probability of Surgery |
0.59 (0.49) |
0.80 (0.40) |
35.5% |
0.44 (0.50) |
0.86 (0.35) |
95.8% |
0.55 (0.50) |
0.76 (0.42) |
38.5% |
0.94 (0.24) |
0.96 (0.19) |
2.7% |
5.0 Discussion
We have used a Monte Carlo simulation model to examine a hypothetical situation where the rules currently in place in the U.S. for authorizing payment for cataract surgery were relaxed in favor of allowing surgery earlier for less bothersome complaints of visual difficulties in doing daily activities. Our base model finds that ES is a dominant strategy over CP from a societal viewpoint: it reduces by about 21% the number of MVCs, fatalities and MVC related costs; it reduces total costs by 16% and improves QALYS by approximately 5%. Our analysis using dissimilarity rates shows that for most people, changing from CP to ES would have no effect on whether they have a motor vehicle collision or sustain serious injury. However, for 2% to 6% of individuals changing the rules for when to allow cataract surgery is important and it produces the resultant reductions in collisions and their subsequent expense.
From the narrower perspective of parties who typically pay for health care, ES increases their payments for cataract surgery especially for the years prior to Medicare eligibility at age 65 where reductions in the costs of M&ES are roughly half of the increase in cataract surgery expenditures. For the period after age 65, higher cataract surgery expenditures are more than offset by a tenfold reduction in M&ES; before age 65, however, the increased expenditure for cataract surgery are almost six times the reduction in M&ES. Thus it seems that Medicare would find it to be advantageous to encourage a policy of earlier cataract surgery whereas pre-Medicare health insurers might be reluctant. However, the potential savings to auto insurers suggest an accommodation. For example, health insurers might allow ES subject to a copayment that could be offset by a “cataract correction discount” offered by auto insurers. The exact specifications of such arrangements are worth further study.
Our study has the following limitations. Because there is no large data set that tracks vision characteristics and MVCs over the 30 year span that our model considers, we have built its equations and estimated its parameter values by drawing information from various cross sectional studies or comparatively short longitudinal studies as previously described (Blincoe et al. 2002) (Kaplan et al. 2010) (Naeim et al. 2006) (U.S. Dept of Transportation and National Highway Traffic Safety Administration 1993) (Owsley et al. 2002) (U.S. Department of Transportation and Federal Highway Administration 2011). This opens the danger that vision characteristics and driving behavior may change more or less rapidly, or be more or less strongly correlated, than we specify. Additionally, we assume that everyone who qualifies for cataract surgery according to our specified criteria will immediately have it. The reality, of course, is that individuals may delay cataract surgery due to comorbidities, personal expense, or natural human inertia. However, delays in seeking surgery would only affect our results about the comparative effects of early surgery if people behaved very differently when offered surgery under earlier compared to current practice.
Our model is also sensitive to the measure of vision that is used. In this paper, we have based the model upon the ADVS, a well validated measure, which reports how an older individual with cataract feels that vision difficulties affect his or her daily activities. Previous research with the ICOM study data has shown that changes in the ADVS are strongly correlated with clinical measures of vision such as disability glare and contrast sensitivity which are important predictors of MVCs. Although the ADVS is not formally used to determine when an individual qualifies for cataract surgery under current guidelines, it speaks to current practice that requires an individual to complain sufficiently about limitations of activities due to vision problems in order to be eligible for insurance reimbursement for cataract surgery. Thus the ADVS is indeed associated with objective visual difficulties in the ICOM sample but it is more relevant for assessing eligibility for cataract surgery. In earlier modeling exercises we used contrast sensitivity in the better eye as our measure of vision because this has been shown to be a statistically significant predictor of MVCs and because it is measured with a precise clinical protocol (Mennemeyer et al. 2010) (Owsley et al. 2002). There we found that a policy of earlier surgery was cost effective but not dominant relative to current practice. In that model, ES would again reduce mean MVCs per person (by 2%), increase payments for cataract surgery (by 22%) and increase QALYs (by 1%) but also have higher costs for the MVCs (by 0.5%). We consider our current model to be an improvement over the earlier work because changes in the ADVS speak more directly to actual practice regarding the eligibility for cataract surgery (namely, the symptoms and complaints communicated by patients). It is not surprising that simulation models can differ but this emphasizes the importance for future work to understand more precisely the complex relationship between a measure of vision, the rate at which vision deteriorates, miles driven, the probability of an MVC and the resultant extent of damage.
Another limitation is that we do not include here the benefits that earlier cataract surgery would produce apart from its effect on have on MVCs such as a reduction in falls and depression. One randomized study of 306 elderly women in Great Britain found that the rate of falling was reduced by 34% in a group that received cataract surgery compared to a control group that did not. In particular, 4 participants in the operated group had fractures (3 %) compared to 12 (8 %) in the control group (p=0.004) (Harwood R. H et al. 2005). Because the British National Health Service has different criteria for when to perform cataract surgery, we cannot assume that these findings about the frequency of fractures are directly transferable to the United States. Nonetheless, fractures are expensive. The National Osteoporosis Foundation found in the U.S. that the total per patient costs of a hip fracture in 1995 dollars ranged from $27,318 to $35,104 (Ray et al. 1997). Adjustment for inflation, without considering changes in technology, would make these costs $41,186 to $52,924 in 2012 dollars (U.S. Department Of Labor and Bureau of Labor Statistics 2012). Additionally, the British study found that “Activity, anxiety, depression, confidence, visual disability, and handicap all improved in the operated group compared to the control group” (Harwood R. H et al. 2005). We are not aware of any study that has specifically determined the health care costs that are due to cataract related depression. However, one study of 11,679 patients over age 60 at 2 large primary care clinics in Seattle found that total ambulatory and impatient costs were 47% to 51% higher in depressed compared with non-depressed elderly patients after adjustment for chronic medical illness (Katon et al. 2003). Considering that these patients would have been living under the current practice regimen for cataract surgery, it is reasonable to suppose that at least a small fraction of these depression related costs might have been avoided with earlier cataract surgery.
6.0 Conclusion
Our simulation base model and several variations show that MVC, fatalities, and total societal costs can all be reduced by encouraging individuals with cataracts to have cataract surgery when bothersome symptoms are at an earlier rather than later stage. A policy of earlier surgery would increase cataract surgery expenditures because more people would qualify for it during their natural lifespan. However, surgical costs are offset by the overall reduction in costs related to MVCs and they are substantially offset after age 65 by the reduction in the medical and emergency services component of MVC costs.
Highlights.
Insurance rules and procrastination delay cataract surgery for older drivers.
We model how earlier cataract surgery would reduce motor vehicle crashes.
Earlier surgery reduces motor vehicle crashes by about 21 percent.
Higher costs for more surgeries are partly offset by reduced accident costs.
Acknowledgement
This project was supported by grant number R03 HS17962 from the Agency for Healthcare Research and Quality. The ICOM study was supported by National Institutes of Health grant P50-AG11684. Supplementary support was provided by Research to Prevent Blindness Inc., and the EyeSight Foundation of Alabama. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or any other agencies supporting this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For submission to: Accident Analysis and Prevention, Special Issue: Mobility for Older Persons
References
- Arias E. United States life tables, 2004. National Vital Statistics Reports. 2007;56(9) [PubMed] [Google Scholar]
- Arias E. United States life tables, 2006. National Vital Statistics Reports. 2010;58(21) [PubMed] [Google Scholar]
- Ball K, Owsley C, Stalvey B, Roenker DL, Sloane ME, Graves M. Driving avoidance and functional impairment in older drivers. Accident Analysis and Prevention. 1998;30(3):313–22. doi: 10.1016/s0001-4575(97)00102-4. [DOI] [PubMed] [Google Scholar]
- Blincoe L, Seay A, Zaloshnja E, Miller T, Romano E, Luchter S, Spicer R. U.S. Department of Transportation, National Highway Traffic Safety Administration. Washinton, D.C.: 2002. The economic impact of motor vehicle crashes, 2000. Report No. DOT HS 809 446. [Google Scholar]
- Centers for Disease Control and Prevention . Vision Health Initiative (VHI), common eye disorders. Atlanta, Goergia: 2011. [Google Scholar]
- Corcoran KJ, Johnson MP. Cataract reimbursement revisited. Ophthalmology Management. 2005 Jun; [Google Scholar]
- Cutler DM, Mcclellan M. Is technological change in medicine worth it? Health Affairs. 2001;20(5):11–29. doi: 10.1377/hlthaff.20.5.11. [DOI] [PubMed] [Google Scholar]
- Eby DW, Molnar LJ. Driving fitness and cognitive impairment: Issues for physicians. JAMA. 2010;303(16):1642–1643. doi: 10.1001/jama.2010.495. [DOI] [PubMed] [Google Scholar]
- Escarce JJ. Would eliminating differences in physician practice style reduce geographic variations in cataract surgery rates? Medical Care. 1993;31(12):1106–1118. doi: 10.1097/00005650-199312000-00004. [DOI] [PubMed] [Google Scholar]
- Evans L. Risk of fatality from physical trauma versus sex and age. Journal of Trauma. 1988;28:368–378. doi: 10.1097/00005373-198803000-00013. [DOI] [PubMed] [Google Scholar]
- Friedman SM, Munoz B, Rubin GS, West SK, Bandeen-Roche K, Fried LP. Characteristics of discrepancies between self-reported visual function and measured reading speed. Salisbury eye evaluation project team. Investigative Ophthalmology & Visual Science. 1999;40(5):858–64. [PubMed] [Google Scholar]
- Gerald Mcgwin J, Gewant HD, Modjarrad K, Hall TA, Owsley C. Effect of cataract surgery on falls and mobility in independently living older adults. Journal of the American Geriatrics Society. 2006;54(7):1089–1094. doi: 10.1111/j.1532-5415.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- Goldstein R. The overlapping coefficient and an “improved” rank-sum statistic. Stata Statistical Bulletin. 1994;22(SG27):132–136. [Google Scholar]
- Haegerstrom-Portnoy G, Schneck ME, Brabyn JA. Seeing into old age: Vision function beyond acuity. Optometry and Vision Science. 1999;76(3):141–158. doi: 10.1097/00006324-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Hall TA, Gerald Mcgwin J, Owsley C. Effect of cataract surgery on cognitive function in older adults. Journal of the American Geriatrics Society. 2005;53(12):2140–2144. doi: 10.1111/j.1532-5415.2005.00499.x. [DOI] [PubMed] [Google Scholar]
- Harwood RH, Foss A, Osborn F, Gregson RM, Zaman A,T,M. Falls and health status in elderly women following first eye cataract surgery: A randomised controlled trial. British Journal of Ophthalmology. 2005;89:53–9. doi: 10.1136/bjo.2004.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman HF, Bradley EL. The overlapping coefficient as a measure of agreement between probability distributions and point estimation of the overlap of two normal densities. Communications in Statistics-Theory and Methods. 1989;18:3851–3874. [Google Scholar]
- Kaplan RM, Tally S, Hays RD, Feeny D, Ganiats TG, Palta M, Fryback DG. Five preference-based indexes in cataract and heart failure patients were not equally responsive to change. Journal of Clinical Epidemiology. 2010 doi: 10.1016/j.jclinepi.2010.04.010. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ, Lin E, Russo J, Unützer J. Increased medical costs of a population-based sample of depressed elderly patients. Archives of General Psychiatry. 2003;60(9):897–903. doi: 10.1001/archpsyc.60.9.897. [DOI] [PubMed] [Google Scholar]
- Klein BEK, Klein R, Linton KLP. Prevalence of agerelated lens opacities in a population: The beaver dam eye study. Ophthalmology. 1992;99:546–552. doi: 10.1016/s0161-6420(92)31934-7. [DOI] [PubMed] [Google Scholar]
- Kymes SM, Lee BS. Preference-based quality of life measures in people with visual impairmen. Optometry andVision Science. 2007;84(8):809–816. doi: 10.1097/OPX.0b013e3181337638. [DOI] [PubMed] [Google Scholar]
- Mangione Cm, Phillips Rs, Seddon Jm, Lawrence Mg, Cook Ef, Dailey R, Goldman L. Development of the ‘activities of daily vision scale’. A measure of visual functional status. Medical Care. 1992;30(12):1111–1126. doi: 10.1097/00005650-199212000-00004. [DOI] [PubMed] [Google Scholar]
- McDonald K, Savage S, Karbasi A. Xlsim simulation software for excel. Vector Economics Inc; Palo Alto, CA: 2010. [Google Scholar]
- Mcgwin G, Scilley K, Brown J, Owsley C. Impact of cataract surgery on self- reported visual difficulties: Comparison with a no-surgery reference group. Journal of Cataract Refract Surg. 2003;29:941–948. doi: 10.1016/s0886-3350(02)01846-1. [DOI] [PubMed] [Google Scholar]
- Mennemeyer ST, Owsley C, Mcgwin G. Cost effectiveness of earlier cataract surgery to prevent motor vehicle collisions; The 32nd Annual Meeting of the Society for Medical Decision Making; Toronto, Ontario, Canada. 2010; Oct 24-27, http://smdm.confex.com/smdm/2010on/webprogram/Session1404.html http://smdm.confex.com/smdm/2010on/webprogram/Paper5648.html. [Google Scholar]
- Naeim A, Keeler EB, Gutierrez PR, Wilson MR, Reuben D, Mangione CM. Is cataract surgery cost-effective among older patients with a low predicted probability for improvement in reported visual functioning? Medical Care Research and Review. 2006;44:982–9. doi: 10.1097/01.mlr.0000228216.18270.3e. [DOI] [PubMed] [Google Scholar]
- Nord E. Discounting future health benefits: The poverty of consistency arguments. Health Economics. 2011;20(1):16–26. doi: 10.1002/hec.1687. [DOI] [PubMed] [Google Scholar]
- Owsley C, Mcgwin G, Jr., Sloane M, Wells J, Stalvey BT, Gauthreaux S. Impact of cataract surgery on motor vehicle crash involvement by older adults. JAMA. 2002;288(7):841–9. doi: 10.1001/jama.288.7.841. [DOI] [PubMed] [Google Scholar]
- Owsley C, Stalvey B, Wells J, Sloane ME. Older drivers and cataract: Driving habits and crash risk. Journal of Gerontology: Medical Sciences. 1999;54A(4):M203–11. doi: 10.1093/gerona/54.4.m203. [DOI] [PubMed] [Google Scholar]
- Owsley C, Stalvey BT, Wells J, Sloane ME, Mcgwin G., Jr. Visual risk factors for crash involvement in older drivers with cataract. Arch Ophthalmol. 2001;119(6):881–7. doi: 10.1001/archopht.119.6.881. [DOI] [PubMed] [Google Scholar]
- Ray NF, Chan JK, Thamer M, Melton LJ. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: Report from the National Osteoporosis Foundation. Journal of Bone and Mineral Research. 1997;12(1):24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- Rubin GS, West SK, Muñoz B, Bandeen-Roche K, Zeger S, Schein O, Fried LP, Team SP. A comprehensive assessment of visual impairment in a population of older americans, the see study. Investigative Ophthalmology and Visual Science. 1997;38(3):557–568. [PubMed] [Google Scholar]
- Stokey E, Zeckhauser R. A primer for policy analysis W.W. Norton & Company. New York and London: 1978. [Google Scholar]
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Medical Care. 2000;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Labor, Bureau of Labor Statistics Consumer Price Index, all urban consumer (CPI-u), U.S. City average, all items. 2012 ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt.
- U.S. Department of Transportation, Federal Highway Administration Average annual miles per driver by age group. 2011 http://www.Fhwa.Dot.Gov/ohim/onh00/bar8.Htm.
- U.S. Dept of Transportation, National Highway Traffic Safety Administration . Addressing the safety issues related to younger and older drivers—a report to Congress. Washington, DC: 1993. [Google Scholar]
- Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- West SK, Munoz B, Schein OD, Duncan DD, Rubin GS. Racial differences in lens opacities: The Salisbury Eye Evaluation (SEE) Project. American Journal of Epidemiology. 1998;148:1033–1039. doi: 10.1093/oxfordjournals.aje.a009579. [DOI] [PubMed] [Google Scholar]
- Williams A, Sloan FA, Lee PP. Longitudinal rates of cataract surgery. Archives of Ophthalmology. 2006;124(9):1308–1314. doi: 10.1001/archopht.124.9.1308. [DOI] [PubMed] [Google Scholar]