Abstract
Non-adherence to cardiovascular medications such as statins is a common, important problem. Clinicians currently rely on intuition to identify medication non-adherence. The visit-to-visit variability (VVV) of LDL-C may represent an opportunity to identify statin non-adherence with greater accuracy. We examined the clinical and pharmacy data from 782 members of the Boston Medical Center (BMC) Health Plan, seen at either BMC or its affiliated Community Health Centers, who were taking statins and had at least 3 LDL-C measurements between 2008 and 2011. The LDL-C VVV (defined by the within-patient standard deviation) was categorized into quintiles. Multivariable logistic regression models were generated with statin non-adherence (defined by the standard 80% pharmacy refill based medication possession ratio threshold) as the dependent variable. The proportion of statin non-adherence increased across quintiles of LDL-C VVV (64.3%, 71.2%, 89.2%, 92.3%, 91.7%). Higher quintiles of LDL-C VVV had a strong positive association with statin non-adherence with an adjusted odds ratio of 3.4 (CI: 1.7–7.1) in the highest versus lowest quintile of LDL-C VVV. The age and gender adjusted model had poor discrimination [C-statistic 0.62 (CI: 0.57, 0.67)] while the final adjusted (age, gender, race, mean LDL-C) model demonstrated good discrimination [C-statistic 0.75 (CI: 0.71, 0.79)] between adherent and non-adherent patients. In conclusion, the VVV of LDL-C demonstrated a strong association with statin non-adherence in a clinic setting. Further, a VVV- of LDL-C based model has good discrimination characteristics for statin non-adherence. Research is needed to validate and generalize these findings to other populations and biomarkers.
Keywords: Visit-to-visit variability, statins, medication adherence
Introduction
The visit-to-visit variability (VVV) of cardiovascular risk factors, such as low-density lipoprotein cholesterol (LDL-C), in clinical practice has been thought to be due to random variation or measurement error.1–4 While there are several physiological mechanisms posited to contribute to VVV, medication non-adherence may be a key contributor. 17 Indirect evidence for an effect of non-adherence on VVV comes from a meta-analysis of the effect of different blood pressure medications on VVV which found that diuretics and calcium channel blockers were associated with lower VVV than ACE inhibitors and beta-blockers.5 18 This observation was thought to possibly be due to medication adherence, although few data have tested this hypothesis.6,17 These observations create the possibility that the VVV of a biomarker like LDL-C, which has a strong correlation to a medication effect such as from statins, may demonstrate an observable phenomenon of VVV based on differences in statin adherence. If established, the VVV of LDL-C could be used to detect and trigger interventions to address statin non-adherence in clinical settings where pharmacy claims data are not electronically integrated, as is currently the case in the large majority of the US. To test this hypothesis, we conducted analyses using an integrated pharmacy claims and clinical database from a large urban population of adult medical patients to determine the independent association of VVV in LDL-C and statin adherence.
Methods
The study sample was patients enrolled in the Boston Medical Center Health Plan (BMCHP) during years 2008 – 2011 who received care from Boston Medical Center (BMC) or any of 8 affiliated Community Health Centers during that time. The patient data were drawn from the Massachusetts Health Disparities Repository (MHDR), which uses the Informatics for Integrating Biology & the Bedside (i2b2) system to aggregate de-identified data for BMC and BMCHP. The MDHR currently contains over 650 million EHR-based data elements (medications, diagnoses, labs, visit dates, and clinical observations) as well as claims data (including filled prescriptions) from the BMCHP for the over 1,200,000 individuals who received at least one clinical service at BMC or any of 8 affiliated Community Health Centers during the past 10 years. We utilized i2b2 to access data from the MHDR to examine 74,468 BMCHP members seen at BMC during the sample period. From this group, we limited our analysis to the 2,641 patients taking statin medications between 2008 and 2011. Of those taking statins during this time period, 1,886 had three or more fills; of those with three or more statin prescriptions filled, 782 had three or more LDL-C measurements between their first and last statin fulfillment dates (see Figure 1). LDL-C measurements that fell outside the 0.1th and 99.9th percentiles were top and bottom coded to those values. If multiple LDL-C measures existed for the same date (n=68 dates) then an average of the same day measurements were used as part of the VVV estimation and it was counted only once towards the three measure minimum. The primary exposure variable was the VVV of LDL-C between the first and last statin fulfillment dates over the three year study period. The VVV of LDL-C was defined as within-patient standard deviation over the study period. LDL-C VVV was categorized into quintiles. The within-patient mean LDL-C was calculated by averaging LDL-C measures over the study period.
Figure 1.
Flow diagram of study inclusion criteria
The primary outcome is medication adherence to statins as determined from the medication possession ratio (MPR). The MPR, also known as the proportion of days covered, is calculated as the sum of days’ supply of the medication (in this case statin) obtained between the first fill and the last fill divided by the total number of days in this time period.7 19 This method was used as the main measure of medication adherence. The MPR was calculated using all statin fills during the study period. Statin MPR was dichotomized as non-adherent and adherent according to the standard cutoff of <80% and >= 80%, respectively.8,8
Covariate data of previously reported weak correlates of statin adherence were obtained from the MHDR i2b2 portal and included: age at first statin fill during the study period; sex; race/ethnicity (White, Black, Hispanic, Other); total number of outpatient visits during the study period; mean LDL-C; number of LDL-C measurements during the study period; number of days between first and last statin fills; and diagnosis of diabetes mellitus (ICD-9 codes 250.0× – 250.9×), ischemic heart disease (410.0× – 414.9×), hypertension (401.0× – 405.0×), chronic liver disease (571.0× – 571.9×), or cerebrovascular disease (430.0× – 438.9×) any time during the study period.9
Descriptive data are reported as percentages or means with standard deviations as appropriate. All variables were examined for normality and outliers. Bivariate associations between covariates and quintiles of LDL-C VVV, and between covariates and statin adherence, were tested through chi-square statistics for categorical variables, and the Wilcoxon rank-sum test for continuous variables.
Multivariable logistic regression models were used to examine the association between VVV of LDL-C and statin non-adherence (MPR <80%). We performed an unadjusted model with VVV of LDL-C alone, and an adjusted model including age at first statin fill, sex, race/ethnicity (define above), and within-patient mean LDL-C. Additional covariates were examined for inclusion in models but were not included as they did not materially affect the results (number of LDL-C measurements during the study period; diagnoses of diabetes mellitus, ischemic heart disease, hypertension, chronic liver disease, cerebrovascular disease any time during the study period). For all logistic regression models, odds ratios (ORs) and 95% confidence intervals (CI) were calculated. The significance level was set at P-value <0.05. The performance of each model for predicting statin MPR of <80% was assessed by plotting the ROC curve and calculating the C-statistic (area under the ROC curve).
Sensitivity analyses were conducted by dichotomizing the VVV of LDL-C quintiles into the 1st and 2nd quintiles vs. the 3rd through 5th quintiles. We also substituted quintiles of VVV of total cholesterol for quintiles of VVV of LDL-C and we dichotomized statin MPR as <50% vs. 50% or higher. We also conducted analyses restricted to the 1st three LDL-C measures to examine the impact of patients with larger numbers of LDL-C measures. We also conducted an analysis dropping the first LDL-C measure as an indirect control for the effect of a “first statin fill” effect in which there should be a substantial variation between LDL-C measures; an effect that might dilute the relationship of adherence and LDL-C VVV. We also repeated all analyses using coefficient of variation (CV) instead of standard deviation as the measure of VVV and using a continuous measure of LDL-C VVV. Data analyses were conducted using SAS/STAT software, Version 9.2 of the SAS System for Windows (2002–2008,SAS Institute Inc., Cary, NC, USA).
Results
After restricting the dataset to patients with at least three statin pharmacy claims and three LDL-C values within the dates of the first and last statin fill, the final analytic dataset contained 782 patients. The average within-patient mean LDL-C for our sample was 109.2 (SD±32.9) mg/dL.
The VVV of LDL-C ranged from 0.6 to 79.6, with mean 21.7 (SD ± 13.1). There was equal distribution by sex, race, number of outpatient visits and days between first and last statin fills across VVV quintiles (Table 1). Mean age at first statin fill declined as the quintile of VVV increased with a more significant decline with increasing VVV quintile when age was dichotomized at 55 years (data not shown). The proportion of the sample with diabetes also significantly declined as the quintile of VVV increased. The number of LDL-C measurements [mean=4.6 (SD ± 1.8)] and the within patient mean LDL-C level both significantly increased with increasing LDL-C VVV.
Table 1.
Descriptive Statistics, Overall and by Within Patient Low Density Lipoprotein-C SD (Quintiles), 2008–2011
| Quintiles | ||||||
|---|---|---|---|---|---|---|
| Variable | ≤ 10.4 (N=157) |
>10.4–≤16.4 (N=156) |
>16.4–≤23.0 (N=157) |
>23.0–≤32.3 (N=155) |
>32.3 (N=157) |
P value |
| Female | 77 (49%) | 87 (56%) | 77 (49%) | 91 (59%) | 94 (60%) | 0.15 |
| Male | 80 (51%) | 69 (44%) | 80 (51%) | 64 (41%) | 63 (40%) | |
| Age at 1st Rx Fill (Years) Mean (SD) |
5 (8) | 53 (7) | 53 (7) | 52 (8) | 52 (8) | <0.01 |
| White | 39 (25%) | 44 (28%) | 36 (23%) | 32 (21%) | 34 (22%) | 0.29 |
| Black | 59 (38%) | 59 (38%) | 66 (42%) | 58 (38%) | 58 (40%) | |
| Hispanic | 18 (12%) | 25 (16%) | 28 (18%) | 37 (24%) | 32 (21%) | |
| Other | 39 (25%) | 27 (17%) | 27 (17%) | 27 (18%) | 29 (19%) | |
| Number of outpatient visits Mean (SD) |
46 (40) | 46 (39) | 45 (36) | 49 (58) | 42 (31) | 0.78 |
| Diagnoses | ||||||
| Diabetes mellitus | 108 (69%) | 117 (75%) | 104 (66%) | 95 (61%) | 88 (56%) | <0.01 |
| Coronary heart disease | 38 (24%) | 36 (23%) | 31 (20%) | 30 (20%) | 33 (21%) | 0.8 |
| Hypertension | 148 (94%) | 145 (93%) | 142 (91%) | 134 (87%) | 129 (82%) | <0.01 |
| Chronic liver disease | 7 (5%) | 6 (4%) | 6 (1%) | 13 (8%) | 11 (7%) | 0.27 |
| Cerebrovascular disease | 17 (11%) | 16 (10%) | 14 (9%) | 12 (8%) | 17 (11%) | 0.86 |
| Framingham risk index Mean (SD) |
0.17 (0.11) | 0.19 (0.13) | 0.20 (0.14) | 0.16 (0.13) | 0.19 (0.14) | <0.01 |
| # LDL measurements Mean (SD) |
4.1 (1.6) | 4.5 (1.6) | 4.7 (1.6) | 4.9 (2.0) | 5.0 (2.2) | <0.01 |
| Within patient mean LDL Mean (SD) |
89 (26) | 101 (32) | 109 (27) | 115 (27) | 133 (34) | <0.01 |
| Number of days between 1st and last fill dates Mean (SD) |
983 (360) | 1027 (351) | 979 (361) | 979 (355) | 1003 (343) | 0.67 |
| Statin MPR | ||||||
| <80% | 101 (64%) | 111 (71%) | 140 (89%) | 143 (92%) | 144 (92%) | <0.01 |
| 80%+ | 56 (36%) | 45 (29%) | 17 (11%) | 12 (8%) | 13 (8%) | |
There was no significant association between statin non-adherence and gender, number of outpatient visits, comorbidities, number of LDL measurements or number of days between first and last statin fills (Table 2). Younger age was associated with statin non-adherence. Race also demonstrated a significant relationship with statin non-adherence with Blacks having a higher proportion of statin non-adherence as compared to all other race groups. Higher within-patient mean LDL was significantly associated with statin non-adherence.
Table 2.
Descriptive Statistics, Overall and by Statin MPR, 2008–2011
| Statin MPR | ||||
|---|---|---|---|---|
| Variable | <80% (N=639) |
80%+ (N=143) |
Overall (N=782) |
p-value |
| Female | 353 (55%) | 73 (51%) | 426 (55%) | 0.36 |
| Male | 286 (45%) | 70 (49%) | 356 (46) | |
| Age at 1st Rx Fill Mean (SD) |
52 (8) | 55 (7) | 53 (8) | <0.01 |
| White | 135 (21%) | 50 (36%) | 185 (24%) | <0.01 |
| Black | 258 (41%) | 42 (30%) | 300 (39%) | |
| Hispanic | 122 (19%) | 18 (13%) | 140 (18%) | |
| Other | 118 (19%) | 31 (22%) | 149 (19%) | |
| Number of outpatient visits Mean (SD) |
45 (41) | 47 (44) | 46 (42) | 0.96 |
| Diagnoses | ||||
| Diabetes mellitus | 422 (66%) | 90 (63%) | 512 (66%) | 0.48 |
| Ischemic heart disease | 137 (21%) | 31 (22%) | 168 (22%) | 0.95 |
| Hypertension | 568 (89%) | 130 (91%) | 698 (89%) | 0.48 |
| Chronic liver disease | 33 (5%) | 10 (7%) | 43 (6%) | 0.39 |
| Cerebrovascular disease | 62 (10%) | 14 (10%) | 76 (10%) | 0.97 |
| Framingham risk index Mean (SD) |
0.18 (0.13) | 0.19 (0.13) | 0.18 (0.13) | 0.24 |
| Number of LDL measurements Mean (SD) |
4.6 (1.8) | 4.5 (1.8) | 4.6 (1.8 | 0.36 |
| Within patient mean LDL Mean (SD) |
112 (33) | 96 (29) | 109 (33) | <0.01 |
| Number of days between 1st and last fill dates Mean (SD) |
1000 (338) | 968 (418) | 994 (354) | 0.97 |
The prevalence of statin non-adherence increased across higher quintiles of LDL-C VVV (64.3%, 71.2%, 89.2%, 92.3%, 91.7%). In unadjusted logistic regression models, a strong positive and significant association was noted between increasing quintiles of VVV and statin non-adherence (Table 3). When adjusted for gender, age at first statin fill, race, and within-patient mean LDL-C, the association was attenuated but remained statistically significant. Inclusion of the number of LDL-C measurements, number of outpatient visits, number of days between first and last statin fills, or comorbidities to the model did not appreciably change the association of VVV of LDL-C and adherence (data not shown).
Table 3.
Logistic regression models predicting the odds of statin MPR <80%, quintiles of within-patient standard deviation (SD) of LDL Subjects with at least 3 statin fills and at least 3 LDL measurements within dates of first/last statin fill
| Unadjusted* OR (95% CI) |
p-value | Adjusted** OR (95% CI) |
p-value | |
|---|---|---|---|---|
| VVV (LDL SD quintiles) | ||||
| 1st quintile (ref) | 1.00 (−) | <0.0001 | 1.00 (−) | <0.0001 |
| 2nd quintile | 1.37 (0.85, 2.20) | 1.12 (0.68, 1.85) | ||
| 3rd quintile | 4.57 (2.51, 8.32) | 3.44 (1.83, 6.46) | ||
| 4th quintile | 6.61 (3.37, 12.95) | 4.47 (2.19, 9.10) | ||
| 5th quintile | 6.14 (3.19, 11.82) | 3.43 (1.65, 7.14) | ||
| Age at 1st statin fill (1 year increase) | 0.96 (0.94, 0.99) | 0.01 | ||
| Sex | 1.00 (−) | 0.76 | ||
| Female (ref) | 0.94 (0.63, 1.40) | |||
| Male | ||||
| Race | 0.95 (0.51, 1.77) | 0.01 | ||
| Hispanic | 0.68 (0.39, 1.17) | |||
| Other | 0.46 (0.28, 0.75) | |||
| White | 1.00 (−) | |||
| Black (ref) | ||||
| Within-patient mean | 1.01 (1.00, 1.02) | 0.01 | ||
| LDL |
Hosmer and Lemeshow Goodness-of-Fit Test p-value 1.000
Hosmer and Lemeshow Goodness-of-Fit Test p-value 0.3129
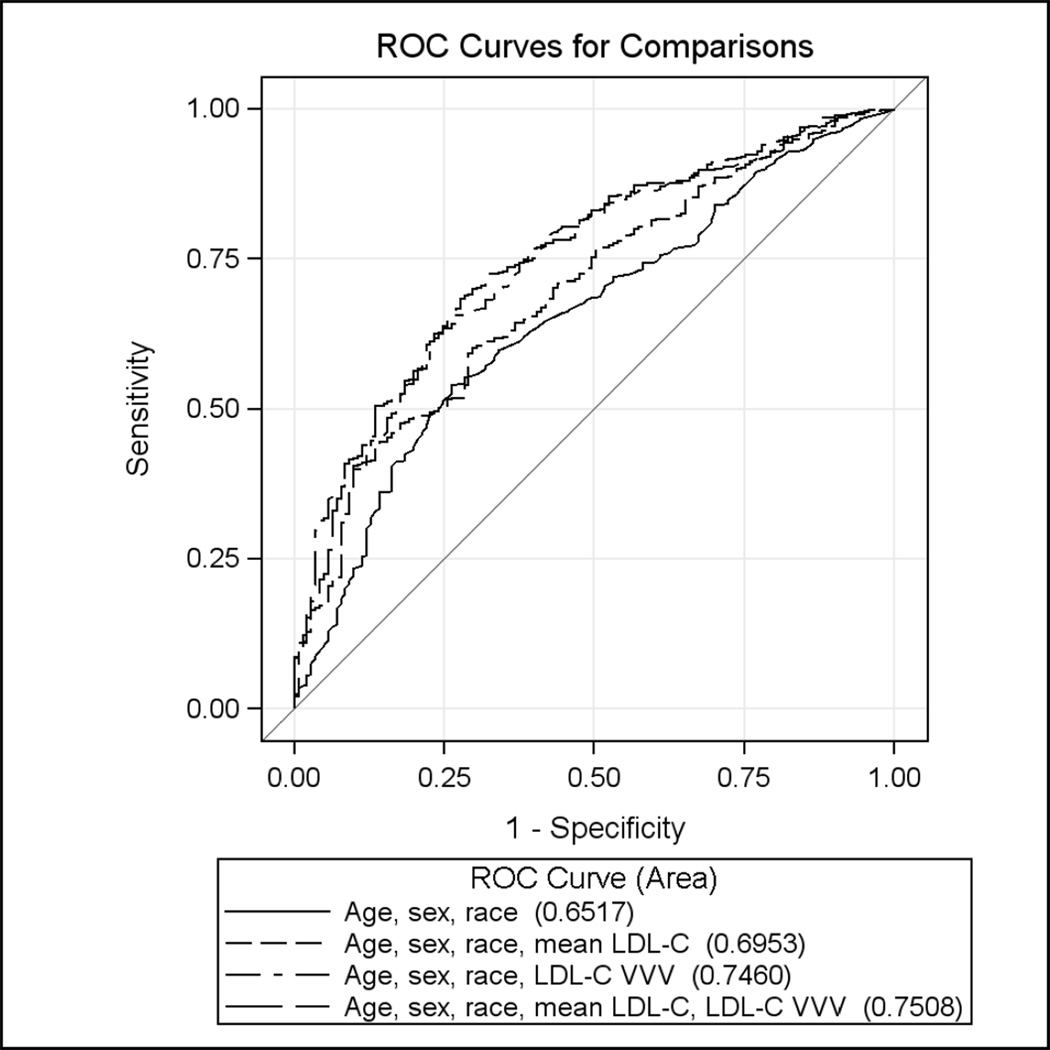
Figure 2 depicts the ROC curves for discrimination between adherence and non-adherent patients between four models: 1) age and sex (C-statistic 0.62, CI: 0.57, 0.67); 2) age, sex, race and mean LDL-C (C-statistic 0.70, CI: 0.65, 0.74); 3) age, sex, race, and VVV of LDL-C (C-statistic 0.75, CI: 0.70, 0.79); and 4) age, sex, race, mean LDL-C and VVV of LDL-C (C-statistic 0.75, CI: 0.71, 0.79).
Figure 2.
Sequential ROC curves for identification of statin adherence (defined as statin MPR <80% vs. 80%+) using age, sex, race, mean LDL-C, and VVV of LDL-C with final model c-statistic 0.75 (CI: 0.71, 0.79).
The logistic regression results were similar when VVV of total cholesterol was used in place of LDL-C (data not shown). Dichotomizing statin MPR at <50% and 50% or higher also did not change the results. Collapsing of the VVV quintiles into two groups that appeared to cluster (quintiles 1–2 versus quintiles 3–5) led to no change in results, with the upper quintiles showing odds about four times higher for statin non-adherence in unadjusted and adjusted models in comparison to lower quintiles (data not shown). Mean LDL-C divided into quintiles was not a significant correlate of non-adherence (p=.16) with odds ratios of 1.3 (0.7–2.2), 1.4 (0.7–2.6), 1.2 (0.6–2.2), and 2.7 (1.2–5.9) across increasing quintiles. Restricting analyses to only the first 3 LDL measures to remove any potential confounding from outliers with many LDL-C measures provided similar results (Supplement Table 1). Analyses with the 1st LDL-C measure dropped for each patient also found similar, if not stronger, findings (Supplement Table 2). There were no significant interactions between mean LDL-C and VVV and analyses using coefficient of variation instead of standard deviation as the measure of VVV were similar (Supplement Table 3 and Supplement Figure 1). Analyses using VVV of LDL-C as a continuous measure are presented in Supplement Table 4).
Discussion
These data show a positive association between increasing VVV of LDL-C and statin non-adherence, measured using pharmacy refill data. The relationship was maintained in adjusted models that incorporated key covariates that have traditionally been associated with non-adherence, including mean within patient LDL-C and number of LDL-C measurements. The magnitude of the relationship was substantial, with the adjusted odds of being non-adherent to statins near four in the higher quintiles of VVV. The ROC curve including VVV demonstrated good adherence discrimination characteristics – creating the potential that a VVV based prediction model may be useful in identifying statin non-adherence.
Approaches used to identify medication non-adherence include self-report, pill counts, pharmacy records, and electronic medication event monitoring systems.8,8 Pill counts and electronic monitoring systems commonly fail and are too costly and burdensome for use in routine clinical settings.8 Self-reported questionnaires, while simple, have poor reliability and are difficult to administer in busy clinical settings.8 Databases in which clinical and pharmacy data are linked allow for generation of objective medication adherence estimates and are frequently used by researchers.10,9 However, this metric is unavailable in most US healthcare systems due to the lack of integration between clinical and pharmacy claims data systems, particularly those serving disadvantaged and minority populations in whom non-adherence is both common and associated with morbidity.11 There is a great need for real-time, point-of-care tools for helping clinicians improve their ability to identify and intervene on non-adherent patients.
The observed relationship between VVV of LDL-C and statin non-adherence has the potential to be just such a tool as it has a relationship to non-adherence substantially stronger than previously identified correlates. In a prior meta-analysis of 22 cohort studies, the significant markers of statin non-adherence (age, gender, income, history of cardiovascular disease, diabetes, hypertension) all demonstrated relatively modest relationships with peak odds ratios of about 30%.9,4 As a result, prior clinical and socio-demographic variables are not likely to be useful markers of medication non-adherence.12,12 The VVV of LDL-C is a novel method to transform clinical data into a useful marker of statin adherence. To our knowledge, this has not been previously exhibited.
The substantial discrimination ability of VVV in combination with the other significant variables represents a potentially important finding. We began our model building with the variables previously identified as associated with statin adherence in the prior literature. The covariates included in our final model have previously been identified as weak markers of statin adherence but have never been successfully used to discriminate between adherence and non-adherence.9,13,14 We then added VVV to the model, which was the most significant variable included. For the first time we created a prediction model with a strong association with statin adherence.
There is a compelling clinical rationale for why the VVV of LDL-C appears to have a strong relationship to statin adherence. Statins, particularly newer generic statins such as simvastatin, have a potent effect on mean LDL-C. As such, non-adherence to these drugs will likely have a relatively dramatic effect on mean LDL-C. Underlying drivers of non-adherence such as concerns about side-effects, doubts about the need for drug therapy, problems with costs and other psychosocial variables are difficult to detect in a busy clinical setting.13,15 Since VVV likely incorporates the impact of these variables in addition to other more modest epidemiologic variables, this may explain the more substantial relationship. Moreover, VVV of LDL-C could easily be computed at the point-of-care in a modern electronic health record making it a potentially powerful and scalable non-adherence screening strategy. With the ability to more reliably screen for statin non-adherence, clinicians may be able to avoid unnecessary dose titration in patients who are non-adherence and target these patients with specific adherence interventions.16
Our findings were robust to several sensitivity analyses. Using total cholesterol instead of LDL-C and changing the adherence thresholds from 80% to 50% did not alter the results. Altering the categorization of the VVV grouping from quintiles into 2 categories also did not alter the findings; this further strengthens the validity of the observed association.
The study findings need to be viewed within the context of several limitations. First, the dataset applies to a large, urban medical cohort with a disproportionate number of minorities and a high percentage of Medicaid enrollees. Future studies will need to replicate these findings in other datasets to ensure their validity and generalizability to other populations. However, Massachusetts health reform which was enacted before 2008 helps minimize the impact of cost on non-adherence in the sample. The study sample was also limited to those enrolled in a specific Medicaid health insurance plan which represents another limitation to the generalizability of the findings but is difficult to predict in what direction this could bias the data. The use of pharmacy claims to assess medication adherence is a standard practice, but does suffer from issues of patient pill dumping or storing medications and does not take into account nor differentiate between patient versus physician directed discontinuation. Therefore there is likely some misclassification of the exposure which would bias our results to the null. Furthermore, as this is a clinical database, the LDL-C sample cannot be verified as fasting which may reduce the accuracy of the LDL-C estimates. The sample also had a high prevalence of diabetes and hypertension which is likely due to the lower threshold for use of statins in these populations and the need for frequent visits among both groups which gives greater opportunity for LDL-C measures. We used quintiles of VVV as there are no published clinically relevant cut-points for VVV of LDL-C. These limitations are counterbalanced by several study strengths including the use of a practice based clinical sample that incorporates a population that is disproportionately affected by cardiovascular disease and non-adherence.17
Next steps for this research include analyses to identify optimal thresholds of VVV to detect statin non-adherence. Fortunately, as the intervention for non-adherence is very low risk (often enhanced counseling), the sensitivity can, in theory, be maximized in favor of specificity. These relationships must also been validated in other datasets with different clinical populations and potential interactions with other medications examined. The impact of whether patients are currently at goal for statin therapy or not also needs to be examined in future studies. The relationship between VVV of other cardiovascular biomarkers such as hemoglobin A1C and SBP also needs to be examined to determine if the observed relationship with VVV of LDL-C, if validated, is a unique phenomenon of statins or is an example of a more robust association between the variability in cardiovascular biomarkers and medication adherence. More work is also needed to identify the number of LDL-C measurements needed to get a reproducible estimate of VVV and to maximize its non-adherence discrimination ability.
Supplementary Material
Supplement Figure 1. Sequential ROC curves for identification of statin adherence (defined as statin MPR <80% vs. 80%+) using age, sex, race, mean LDL-C, and VVV of LDL-C (quintiles of CV) with final model c-statistic 0.75 (CI: 0.71, 0.79).
Acknowledgements
This work was supported by RC2HL101628-01 (NR Kressin and WG Adams, Multiple PIs). Dr. Kressin is also supported by a Research Career Scientist award (RCS 02-066-1) from the Health Services Research and Development Service, Department of Veterans Affairs. It was also supported by K23DK081665, a Patient-Oriented Mentored Scientist Award through the National Institute of Diabetes, Digestive, and Kidney Diseases (DM Mann and Boston University’s Clinical and Translational Institute (UL1-TR000157). DMM had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 2.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 3.Gidding SS, Stone NJ, Bookstein LC, Laskarzewski PM, Stein EA. Month-to-month variability of lipids, lipoproteins, and apolipoproteins and the impact of acute infection in adolescents. J Pediatr. 1998;133:242–246. doi: 10.1016/s0022-3476(98)70227-6. [DOI] [PubMed] [Google Scholar]
- 4.Glasziou PP, Irwig L, Heritier S, Simes RJ, Tonkin A. Monitoring Cholesterol Levels: Measurement Error or True Change? Annals of Internal Medicine. 2008;148:656–661. doi: 10.7326/0003-4819-148-9-200805060-00005. [DOI] [PubMed] [Google Scholar]
- 5.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 6.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 7.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: Methods, validity, and applications. Journal of Clinical Epidemiology. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 8.Osterberg L, Blaschke T. Adherence to Medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 9.Mann DM, Woodward M, Muntner P, Falzon L, Kronish I. Predictors of Nonadherence to Statins: A Systematic Review and Meta-Analysis. Ann Pharmacother. 2010;44:1410–1421. doi: 10.1345/aph.1P150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caetano PA, Lam JMC, Morgan SG. Toward a standard definition and measurement of persistence with drug therapy: Examples from research on statin and antihypertensive utilization. Clinical Therapeutics. 2006;28:1411–1424. doi: 10.1016/j.clinthera.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Jha AK, Ferris TG, Donelan K, DesRoches C, Shields A, Rosenbaum S, Blumenthal D. How common are electronic health records in the United States? A summary of the evidence. Health Aff (Millwood) 2006;25:w496–w507. doi: 10.1377/hlthaff.25.w496. [DOI] [PubMed] [Google Scholar]
- 12.Steiner JF, Ho PM, Beaty BL, Dickinson LM, Hanratty R, Zeng C, Tavel HM, Havranek EP, Davidson AJ, Magid DJ, Estacio RO. Sociodemographic and clinical characteristics are not clinically useful predictors of refill adherence in patients with hypertension. Circ Cardiovasc Qual Outcomes. 2009;2:451–457. doi: 10.1161/CIRCOUTCOMES.108.841635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann DM, Allegrante JP, Natarajan S, Halm EA, Charlson M. Predictors of adherence to statins for primary prevention. Cardiovasc Drugs Ther. 2007;21:311–316. doi: 10.1007/s10557-007-6040-4. [DOI] [PubMed] [Google Scholar]
- 14.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal Statin Adherence and Discontinuation in Primary and Secondary Prevention Populations. Should We Target Patients with the Most to Gain? Journal of General Internal Medicine. 2004;19:638–645. doi: 10.1111/j.1525-1497.2004.30516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann D. Resistant disease or resistant patient: problems with adherence to cardiovascular medications in the elderly? Geriatrics. 2009;4:10–15. [PubMed] [Google Scholar]
- 16.Lee JK, Grace KA, Taylor AJ. Effect of a Pharmacy Care Program on Medication Adherence and Persistence, Blood Pressure, and Low-Density Lipoprotein Cholesterol: A Randomized Controlled Trial. JAMA. 2006;296:2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 17.Kanjilal S, Gregg EW, Cheng YJ, Zhang P, Nelson DE, Mensah G, Beckles GLA. Socioeconomic Status and Trends in Disparities in 4 Major Risk Factors for Cardiovascular Disease Among US Adults, 1971–2002. Arch Intern Med. 2006;166:2348–2355. doi: 10.1001/archinte.166.21.2348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1. Sequential ROC curves for identification of statin adherence (defined as statin MPR <80% vs. 80%+) using age, sex, race, mean LDL-C, and VVV of LDL-C (quintiles of CV) with final model c-statistic 0.75 (CI: 0.71, 0.79).