Abstract
Current screening and detection of asymptomatic aortic aneurysms is largely based on uniform cut-point diameters. Our objective was to define normal aortic diameters in asymptomatic men and women in a community-based cohort and to determine the association between aortic diameters and traditional risk factors for cardiovascular disease (CVD).Measurements of the diameter of the ascending aorta(AA), descending thoracic aorta (DTA), infrarenal abdominal (IRA) and lower abdominal aorta (LAA) were acquired from 3,431 Framingham Heart Study participants. Mean diameters were stratified by sex, age, and body surface area (BSA). Univariate associations with risk factor levels were examined and multivariable linear regression analysis was used to assess the significance of covariate-adjusted relations with aortic diameters. For men, the average diameter was 34.1 mm for AA, 25.8 mm for DTA, 19.3 mm for IRA and 18.7 mm for LAA.For women, the average diameter was 31.9 mm for AA, 23.1 mm for DTA, 16.7 mm for IRA, and 16.0 mm for LAA. The mean aorticdiameters were strongly correlated (p<0.0001) with age and BSA in age-adjusted analyses, and these relations remained significant in multivariable regression analyses. Positive associations of diastolic BP with AA and DTA in both sexes and pack years of cigarette smoking with DTA in women and with IRA in men and women were observed. In conclusion, average diameters of the thoracic and abdominal aorta by CT are larger in men compared with women, vary significantly with age and BSA, and are associated with modifiable CVD risk factors including diastolic blood pressure and cigarette smoking.
Keywords: Aortic diameter, computed tomography, sex, age, body surface area
In persons without a diagnosed aneurysm, there is a paucity of community-based data in large numbers of men and women regarding aortic diameter thresholds for detection of aortic dilatation above which patients should be monitored or referred for surgical consultation. According to the 2010 Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease, the ascending aortic diameter of 5.5 cm is utilized for most patients without a genetically mediated aortic syndrome (1), despite the knowledge that important patient variables, such as age, sex, and body surface area (BSA), may be associated with the normal aortic dimensions and significance of relative dilatation. While data are sparse regarding normal diameters of various aortic cross sections in the general population, such data are needed for both evaluating the existing standards for aortic aneurysms and for prevention. A recent analysis of the International Registry of Acute Aortic Dissection (IRAD) data base found that 60% of dissection patients had an ascending aortic diameter less than the 5.5 cm cut-off value, demonstrating that a “one size fits all” value may not be sufficient to prevent the majority of thoracic aortic dissections (2).This study establishes the distribution of age- and BSA-specific measurements and cutpoints for normal aortic diameters assessed by computed tomography (CT) in a community-based cohort of adult men and womenand to identify the cardiovascular disease (CVD) risk factor correlates of high aortic diameters.
METHODS
Participants were drawn from an imaging substudy of the community-based Framingham Heart Study (FHS) Offspring and Third-Generation Study cohorts. Beginning in 1948, 5,209 men and women 28 to 62 years of age were enrolled in the original cohort of the FHS. The offspring and spouses of the offspring of the original cohort were enrolled in the Offspring Study starting in 1971 (3,4). Beginning in 2002, 4,095 Third Generation Study participants, who had at least 1 parent in the offspring cohort, were enrolled in the FHS and underwent standard clinic examinations. The standard clinic examination included a physician interview, a physical examination, and laboratory tests, as previously described(5).
For the present analysis, the substudy sample consisted of Offspring and Third Generation Study participants in the Multidetector Computed Tomography (MDCT) substudy. Between June 2002 and April 2005, 3,529 participants underwent MDCT for the assessment of coronary and aortic calcium. 1,418 of the participants were from the offspring generation and 2111 of the participants were from the third generation. Of the 3,529 participants scanned, 3,505 attended Offspring Exam 7 or Generation 3 Exam 1. Of these, 3,496 had a complete risk factor profile and were available for analysis. Of the 3,496 participants, 65 (1.9%) had incomplete CT data available. Thus, the final analysis was performed in 3,431 participants of the overall study cohort.
Participant ascertainment for the MDCT study has been previously described (6). For inclusion, men were ≥ 35 years of age, women were ≥ 40 years of age and not pregnant, and all participants weighed< 350 pounds. The study was approved by the institutional review boards of the Boston University Medical Center and the Massachusetts General Hospital. All participants provided written informed consent.
All participants underwent thoracic electrocardiographically(ECG)-gated, non-contrast-enhanced MDCT scanning in a supine position using an eight-slice MDCT scanner (Light Speed Ultra, General Electric, Milwaukee, WI, USA)(7). In the thorax, contiguous 2.5 mm thick slices (120 kVp, 320 mA if body weight < 220 lbs / 400 mA if body weight > 220 lbs, gantry rotation time 500 ms, table feed 3:1) were acquired from the level of the carina to the level of the diaphragm. Two acquisitions were conducted during end-inspiratory breath holds (typical duration 18 s). Image acquisition was prospectively triggered in early diastole at 50% of the cardiac cycle. In the abdomen, 25 contiguous 5-mm thick slices (120 kVp; 400 mA; gantry rotation time, 500 ms; table feed, 3:1) were acquired covering 125 mm above the level of the first sacral vertebral body (S1).
At each FHS examination, information regarding risk factor levels was obtained from a history and physical examination obtained by a physician, and by laboratory testing, as previously described. Cardiovascular disease outcomes were defined by the presence of coronary heart disease, stroke, peripheral arterial disease or heart failure and were determined by a three physician endpoint committee, as previously described (8). A prior history of valve surgery or of surgery of the thoracic aorta or abdominal aorta was obtained by the physician-examiner during the interview portion of the participant examination by a physician.
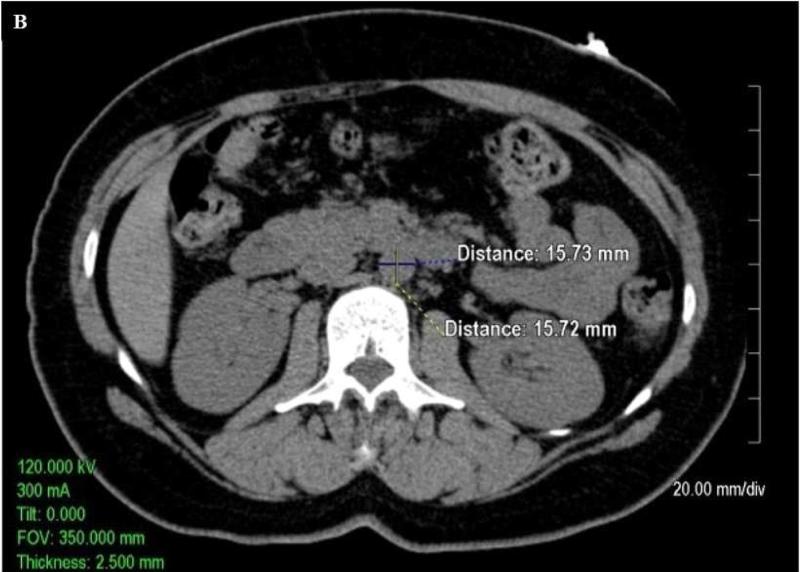
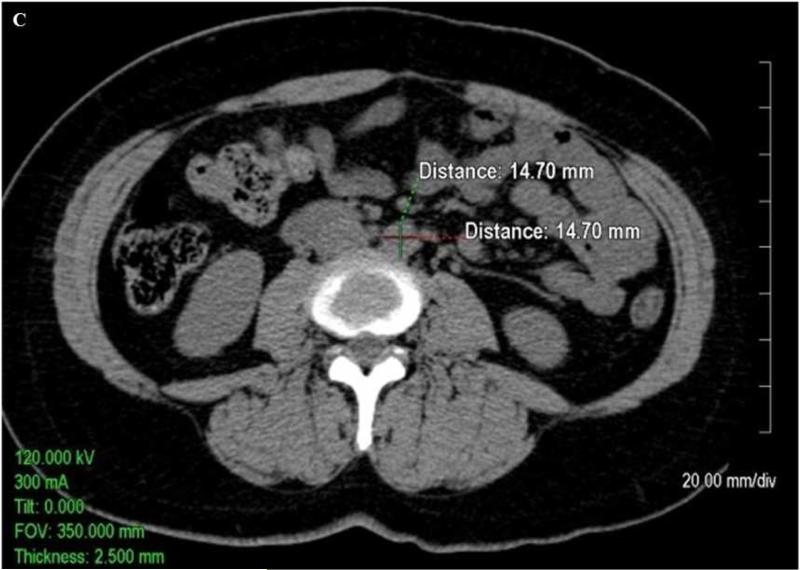
Quantitative measurements of the thoracic and abdominal aortic diameter were acquired utilizing the 2.5 mm axial slices acquired during the scan on an Aquarius 3D Workstation (Tera Recon Inc, San Mateo, CA.).As shown in Figure 1A, measurements of the diameter of the ascending thoracic aorta and descending thoracic aorta were acquired on axial slices at the level of right pulmonary artery. As shown in Figure 1B and Figure 1C, respectively, measurements of the abdominal aorta were acquired on axial slices at one slice level 5 cm above the aorto iliac bifurcation (infrarenal abdominal aorta) and at a slice level above the bifurcation of the abdominal aorta into the common iliac arteries (lower abdominal aorta). The measurements were traced manually from outside wall to outside wall of the aorta in both anterior-posterior and transverse planes. At each of the four locations (i.e. ascending thoracic aorta (AA), descending thoracic aorta (DTA), infrarenal abdominal aorta (IRA), and lower abdominal aorta (LAA)), the mean of anterior-posterior and transverse measurements was calculated (mean AA diameter, mean DTA diameter, mean IRA diameter, and mean LAA diameter, respectively). Mean values were utilized to help control for obliquity of the vasculature on a small number of scans inherent in the use of axial images.
Figure.
Axial non-contrast enhanced CT images (A)of the thoracic ascending aorta (AA, measurements in upper part of figure) and descending aorta (DTA, measurements in lower part of figure) at the level of the right pulmonary artery, (B)the infrarenal abdominal aorta (IRA) 5cm above the aortoiliac bifurcation, and (C)the lower abdominal aorta (LAA) just above the aortoiliac bifurcation. The images demonstrate sample measurements of the aorta from outside wall to outside wall in both the anterior-posterior and transverse planes.
To assess inter-observer and intra-observer measurement reliability, reliability measurements were acquired in a random subset of 100 participants (age range: 37-83 years; 49% women) drawn from the FHS offspring. The random sample included an approximately equal number of men and women and an approximately equal number of participants in the each of the age groups of 35-44, 45-54, 55-64, 65-74, and 75-84 years. To evaluate inter-observer reliability, two of the trained observers performed independent measurements of the 100 participant subset in random order. To evaluate intra-observer reliability, one of the observers independently repeated these measurements 1 week later in random order. The intra-observer intraclass correlation coefficient was greater than 0.97 (range 0.97-0.99), suggesting an excellent correlation between reads, with an average difference of 0.5 mm between reads. There was also excellent inter-observer intraclass correlation between readers (all r greater than 0.96, range 0.96 – 0.99), with an average difference of 0.8 mm between readers across the measurements.
Sex-specific, age-adjusted Pearson correlation coefficients were used to assess univariable correlations between aortic diameters and traditional CVD risk factors (age, BSA, systolic blood pressure, diastolic blood pressure, pack years of smoking, fasting plasma glucose, total cholesterol, triglycerides, and HDL cholesterol). Sex-specific multivariable stepwise linear regression, with age forced into the model, was used to identify traditional risk factors independently associated with aortic diameters; a significance level of 0.05 was used in the stepwise model.
Risk factor data acquired at FHS examinations were then used to create a healthy reference sample after excluding participants with the following conditions from the overall study sample: atherosclerotic CVD, hypertension, and previous surgery of the heart valves, thoracic aorta, or abdominal aorta. Mean aortic diameters and percentiles were then calculated for the healthy reference sample, stratified by age (<45, 45-54, 55-64, and > 65 years), sex and by BSA (< 1.9, 1.9-2.09, and ≥ 2.1 m2for men and < 1.7, 1.7-1.89, and ≥ 1.9 m2 for women, respectively). Similar calculations were made for the entire sample.
The 90thpercentile aortic diameter was defined at each measurement location. Multivariable logistic regression analysis in the entire sample was used to further identify independent risk factors for dilatation, defined as aortic diameter >90thpercentile of the healthy referent cohort. Covariates included in the multivariable model included age, BSA, hypertension, dyslipidemia, smoking, and diabetes. Finally, we employed sex- and age-specific simple linear regression of each diameter versus BSA to create nomograms to demonstrate expected aortic diameter versus BSA by sex and age group and to create a formula for calculating the predicted normal range of aortic diameters at each location, accounting for age, sex, and BSA.
RESULTS
As displayed in Table 1, the mean age and BSA was 52 ± 10 years and 1.8 ± 0.2 m2, respectively, for women,and 50 ± 11 years and 2.1 ± 0.2 m2, respectively, for men. Approximately 27% of women and 32% of men had hypertension. The healthy referent cohort consisted of a total of 2,343 participants after excluding 1,088 participants with CVD, hypertension, and/or previous surgery of heart valves, thoracic aorta, or abdominal aorta.
Table 1.
Clinical characteristics of the study population
| Variable | Overall (n=3431) | Women (n=1664) | Men (n=1767) |
|---|---|---|---|
| Age (years) | 50.9 ± 10.4 | 52.2 ± 9.9 | 49.8 ± 10.7 |
| Body surface area (m2) | 1.9 ± 0.3 | 1.8 ± 0.2 | 2.1 ± 0.2 |
| BMI > 30kg/m2 | 947 (27.6%) | 430 (25.8%) | 517 (29.3%) |
| Systolic blood pressure (mmHg) | 122 ± 16 | 120.3 ± 18 | 123.6 ± 15 |
| Diastolic blood pressure (mmHg) | 76 ± 9 | 74 ± 9 | 78 ± 9 |
| Hypertension | 1006 (29.4%) | 443 (26.6%) | 563 (31.9%) |
| Smoking status | |||
| Current | 444 (12.9%) | 204 (12.3%) | 240 (13.6%) |
| Former | 1345 (39.2%) | 704 (42.3%) | 641 (36.3%) |
| Never | 1642 (47.9%) | 756 (45.4%) | 886 (50.1%) |
| Smoking amount (pack-years) | |||
| Current* | 37.7 ± 21.2 | 35.5 ± 18.3 | 40.1 ± 23.9 |
| Former* | 21.5 ± 19.7 | 16.8 ± 16.5 | 26.4 ± 21.5 |
| Diabetes mellitus | 220 (6.4%) | 90 (5.4%) | 130 (7.4%) |
| Fasting plasma glucose (mg/dL) | 99.2 ± 21.4 | 95.7 ± 17.9 | 102.4 ± 23.8 |
| Total cholesterol (mg/dL) | 196.4 ± 35.3 | 198.1 ± 36.3 | 194.8 ± 34.3 |
| Triglycerides (mg/dL) | 128.5 ± 91.5 | 113.5 ± 68.3 | 142.6 ± 107.1 |
| HDL cholesterol (mg/dL) | 53.3 ± 16.6 | 61.3 ± 16.8 | 45.9 ± 12.4 |
| Coronary artery disease | 205 (6%) | 71 (4.3%) | 134 (7.6%) |
| Valvular heart disease | 35 (1%) | 14 (0.8%) | 21 (1.1%) |
denotes data only available for Offspring generation. BMI: body mass index; HDL: high-density lipoprotein.
For all men, the average diameter was 34.1 mm (standard deviation (SD) 3.9 mm, range 23.1 - 49.6 mm) for AA, 25.8 mm (SD 3.0 mm, range 17.9 - 55.2 mm) for DTA, 19.3 mm (SD 2.9 mm, range 12.8 - 69.9 mm) for IRA, 18.7 mm (SD 2.7 mm, range 12.9 - 48.5 mm) for LAA. For all women, the average diameter was 31.9 mm (SD 3.5 mm, range 21.6 - 48.0 mm) for AA, 23.1 mm (SD 2.6 mm, range 16.8 - 47.2 mm) for DTA, 16.7 mm (SD 1.8 mm, range 12.0 - 33.0 mm) for IRA, 16.0 mm (SD 1.7 mm, range 11.1 - 33.5 mm) for LAA.
The sex-specific, age-adjusted univariable Pearson correlations between continuous measures of CVD risk factors and aortic were calculated. The mean diameters of the AA and DTA were correlated (p < 0.0001) with age (0.44-0.66), BSA (0.31-0.41), SBP (0.15-0.20), and DBP (0.18-0.26) in both sexes. The mean diameters of IRA and LAA were correlated (p < 0.0001) with age (0.32-0.48) and BSA (0.22-0.35) in both sexes. Weaker correlations (0.03-0.14) were noted for abdominal aortic diameters with blood pressure measurements in both sexes, with both SBP (0.09) and DBP (0.14) associated with IRA mean diameter in women and DBP (0.10) associated with LAA mean diameter in women. There were positive correlations of pack-years cigarette smoking with IRA (0.34, 0.45) and LAA(0.25, 0.28) in men and women and of fasting plasma glucose with IRA (0.08), LAA(0.06) and DTA(0.09) in women and with AA (0.08) and DTA (0.10) in men. Inverse correlations were noted of HDL cholesterol with IRA (-0.08), LAA (-0.07) and DTA(-0.10)for men and with AA (-0.05) and DTA(-0.09) for women. Paradoxical inverse correlations of very small magnitude were noted for total cholesterol with IRA (-0.07, - 0.05) and LAA (-0.08, -0.07) in men and women and with DTA (-0.08) in men.
Multivariable stepwise linear regression analysis was performed between continuous CVD risk factors and aortic diameters. For every one year increase in age, the mean diameterof the AA was higher by 0.16 mm in women and 0.2 mm in men, the mean DTA diameterwas higherby 0.16 mm in women and 0.19 mm in men, the mean IRA diameterwas higher by 0.09 mm in women and 0.13 mm in men, and mean LAA diameterwas higher by 0.07 mm in women and 0.12 mm in men. For every 0.1 m2increase in BSA, the mean AA diameter was higher by 4.14mm in women and 5.8 mm in men, themean DTA diameter was higher by 3.61 mm in women and 4.15 mm in men, the mean IRA diameter was higherby 2.38 mm in women and 2.92 mm in men, and the mean LAA diameter was higherby 3.11 mm in women and 2.90 mm in men. Of note, for every one mmHg increase in DBP, the mean AA diameter was higher 0.06 mm in women and 0.08mm in men, and the mean DTA diameter was higher 0.03 mm in both women andmen. The mean IRA diameterwas higher by 0.3mm in women and 0.27 mm in men with a cigarette smoking history.
The distributions of aortic diameters by sex, age, and BSA, are displayed for all men and all women in Tables 2a-2d for AA, DTA, IRA and LAA, respectively. The distributions of aortic diameters by sex, age, and BSA, along with the prevalence of aortic diameters >90th of the healthy referent percentile by sex, age and BSA, are displayed for men and women in the health referent cohortin Supplemental Tables 1a-1d for AA, DTA, IRA and LAA, respectively. Within each sex, diameter increased with increasing age and BSA. Diameters for participants in the healthy referent group are similar to those of the entire cohort across the spectrum of age and BSA categories and measurement locations. Diameters indexed to BSA by age and sex are presented in Supplemental Tables 2a – 2b.
Table 2a.
Mean and percentiles of the mean diameter of the ascending (mean AA) thoracic aorta (mm) by age and BSA in all women (left) and all men (right).
| All Women | All Men | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | BSA | Mean | 25 | 50 | 75 | 90 | BSA | Mean | 25 | 50 | 75 | 90 |
| < 45 | < 1.7 | 28.8 | 27.0 | 28.5 | 30.9 | 32.1 | < 1.9 | 30.3 | 28.2 | 30.1 | 32.5 | 34.2 |
| 1.7-1.89 | 30.2 | 28.0 | 30.3 | 31.8 | 33.8 | 1.9-2.09 | 31.1 | 29.4 | 31.1 | 32.5 | 34.5 | |
| ≥ 1.9 | 31.1 | 28.9 | 31.1 | 33.2 | 35.4 | ≥ 2.1 | 33.2 | 30.8 | 32.8 | 34.9 | 37.0 | |
| 45-54 | < 1.7 | 29.8 | 27.9 | 29.7 | 32.0 | 33.8 | < 1.9 | 32.6 | 30.1 | 33.0 | 34.5 | 36.4 |
| 1.7-1.89 | 31.5 | 29.7 | 31.5 | 33.3 | 35.0 | 1.9-2.09 | 33.5 | 31.1. | 33.5 | 35.7 | 37.4 | |
| ≥ 1.9 | 32.8 | 30.8 | 32.5 | 35.1 | 36.8 | ≥ 2.1 | 34.9 | 32.6 | 34.5 | 36.8 | 39.3 | |
| 55-64 | < 1.7 | 32.3 | 30.1 | 32.3 | 34.0 | 37.2 | < 1.9 | 34.5 | 32.4 | 34.7 | 36.5 | 38.1 |
| 1.7-1.89 | 33.5 | 31.5 | 33.5 | 35.3 | 37.2 | 1.9-2.09 | 34.7 | 32.5 | 34.3 | 36.6 | 39.0 | |
| ≥ 1.9 | 33.7 | 31.6 | 33.6 | 35.7 | 37.6 | ≥ 2.1 | 36.8 | 34.5 | 36.4 | 38.6 | 40.7 | |
| ≥ 65 | < 1.7 | 33.9 | 32.1 | 33.7 | 35.7 | 38.0 | < 1.9 | 36.2 | 33.3 | 35.3 | 38.4 | 42.2 |
| 1.7-1.89 | 33.9 | 32.3 | 33.6 | 35.9 | 37.9 | 1.9-2.09 | 36.7 | 34.5 | 36.3 | 39.0 | 40.7 | |
| ≥ 1.9 | 35.1 | 33.0 | 34.6 | 37.1 | 39.6 | ≥ 2.1 | 38.6 | 36.1 | 38.3 | 41.3 | 43.4 | |
BSA: body surface area
Table 2d.
Mean and percentiles of the mean diameter of the lower abdominal (mean LAA) aorta (mm) by age and BSA in all women (left) and all men (right).
| All Women | All Men | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | BSA | Mean | 25 | 50 | 75 | 90 | BSA | Mean | 25 | 50 | 75 | 90 |
| < 45 | < 1.7 | 14.7 | 13.9 | 14.7 | 15.4 | 16.4 | < 1.9 | 16.7 | 15.7 | 16.6 | 17.4 | 18.1 |
| 1.7-1.89 | 15.6 | 14.7 | 15.7 | 16.2 | 17.1 | 1.9-2.09 | 17.2 | 16.4 | 17.3 | 18.1 | 18.8 | |
| ≥ 1.9 | 16.0 | 15.0 | 15.7 | 16.8 | 17.8 | ≥ 2.1 | 18.2 | 17.1 | 18.1 | 19.0 | 19.8 | |
| 45-54 | < 1.7 | 15.2 | 14.2 | 15.0 | 15.9 | 16.8 | < 1.9 | 17.7 | 16.4 | 17.4 | 18.5 | 20.2 |
| 1.7-1.89 | 15.8 | 14.9 | 15.7 | 16.7 | 17.5 | 1.9-2.09 | 18.2 | 17.1 | 18.1 | 19.0 | 20.2 | |
| ≥ 1.9 | 16.4 | 15.4 | 16.2 | 17.4 | 18.1 | ≥ 2.1 | 18.8 | 17.8 | 18.7 | 19.8 | 20.7 | |
| 55-64 | < 1.7 | 15.6 | 14.6 | 15.6 | 16.4 | 17.3 | < 1.9 | 18.4 | 17.1 | 18.2 | 19.5 | 20.8 |
| 1.7-1.89 | 16.1 | 15.2 | 16.1 | 16.9 | 17.4 | 1.9-2.09 | 18.4 | 17.4 | 18.3 | 19.3 | 20.2 | |
| ≥ 1.9 | 16.9 | 15.7 | 16.9 | 17.6 | 18.9 | ≥ 2.1 | 19.8 | 18.3 | 19.3 | 20.9 | 22.5 | |
| ≥ 65 | < 1.7 | 16.5 | 15.2 | 16.5 | 17.6 | 18.4 | < 1.9 | 20.3 | 18.0 | 19.0 | 20.5 | 26.5 |
| 1.7-1.89 | 16.9 | 15.7 | 16.6 | 17.5 | 18.7 | 1.9-2.09 | 21.0 | 18.5 | 19.7 | 21.2 | 24.9 | |
| ≥ 1.9 | 17.2 | 16.1 | 17.0 | 17.9 | 19.4 | ≥ 2.1 | 21.5 | 19.3 | 20.9 | 22.3 | 25.3 | |
BSA: body surface area
Supplemental Figure 1 A-H provides nomograms of expected aortic diameter (± 95% CI) versus BSA in men and women by age groupsfor the AA, DTA, IRA and LAA, respectively. The formula for predicting aortic diameter is 14.8 + 0.16 (age) + -1.04 (sex) + 5.34 (BSA) in the ascending thoracic aorta, 8.86 + 0.16 (age) + -1.79 (sex) + 4.25 (BSA) in the descending thoracic aorta, 8.6 + 0.1(age) + -2.03 (sex) + 2.64 (BSA) in the IRA, and 8.71 + 0.08(age) + -2.11 (sex) + 2.78 (BSA) in the LAA. The nomograms demonstrate the higher values of aortic diameter with increasing age, BSA, and male sex.
In multivariable-adjusted logistic regression analysis for >90th percentile thoracic (Supplemental Table 3a) and abdominal (Supplemental Table 3b) aortic diameters, hypertension was independently associated with the upper decile for the ascending and descending thoracic aorta in both men (OR 2.01 and 1.86, respectively) and women (OR 1.50 and 1.73, respectively). Current or former smoking was independently associated with the upper decile for IRA diameter in women (OR 1.63).There was no association with hyperlipidemia, defined by high cholesterol or lipid lowering treatment.
DISCUSSION
In this study of MDCT imaging in men and women in a community-based cohort, we provide estimates of the strong correlations of aortic diameters with sex, age, and BSA and we provide sex-specific reference distributions and cutpoints of thoracic and abdominal aortic diameters stratified by age and BSA in asymptomatic men and women in the community. We further present normal values of thoracic and abdominal aortic diameter, stratified by sex, age, and BSA, in all participants in the study and in a healthy reference sample of those free of CVD, hypertension, and previous history of valvular, thoracic aortic, or abdominal aortic surgery. This is the first study that we are aware of to present such community-based data in both the thoracic and abdominal aorta.
The few small studies that have characterized normal thoracic diameters in males and females have been largely confined to pediatric and young adult populations, such as a Finnish study in 2003 of 168 children (mean age 11.1 years)(9), a study in 1993 of 182 US college students (mean age 21 years)(10), and a 1989 study of 52 children (mean age 9 ± 5 years) and 135 adults (mean age 43 ± 15 years) scanned to exclude structural heart disease(11). Additionally, many studies have measured only thoracic aortic root dimensions, using ultrasound technology(12). While ultrasound is reliable in detecting aneurysms in the aortic root and portions of the abdomen, CT and magnetic resonance imaging (MRI) are frequently utilized in clinical medicine and allow complete imaging of the entire aorta and therefore may incidentally detect patients with dilated aortas. Moreover, current standard of care for surveillance imaging of aortic dilatation often includes CT or MRI. As a consequence, physicians must extrapolate normal aortic dimensions in patients with a range of ages, sexes, heights, and weights, and must often do so using a different modality (i.e., CT or MRI) than from which the original data was obtained. Comparison of measurements among modalities is confounded by the distinct conventional methods by which measurements are made with each modality.
The FHS MDCT substudy was initially designed with the primary intent of studying the distribution and determinants of coronary calcium and thoracic and abdominal aortic calcium(13,14).As such, the available thoracic and abdominal aortic images represented a rich opportunity to examine aortic diameters on MDCT in an unselected community-based cohort. Previous work by Lin et al examined aortic diameter by CT in the thorax alone in 103 subjects who underwent “clinically indicated” CT angiography(15). A major limitation of this study was the small number of patients. Wolak et al (16) examined aortic diameter by CT in the thorax alone in a cohort that was either self-referred or physician-referred or recruited for the Early Identification of Subclinical Atherosclerosis using Noninvasiv E Imaging Research (EISNER) research protocol. Although potential subjects with known CAD were excluded, the examined cohort was subject to referral bias. In contrast, the community-based nature of the FHS allowedus to study a truly unselected cohort with reliably collected longitudinal risk factor data that is unbiased by aorta measurements. The span of subject age across the Offspring and Third Generation study participants provided a robust sample from which generalization to most men and women in the community in whom aortic diameter measurement is feasible. Moreover, we present diameter data for both the entire cohort and the healthy referent subset.
We measured and reported ascending and descending thoracic aortic diameters at the level of the pulmonary artery, using a commonly used landmark for measurement of diameters on routine axial images in the thorax. We demonstrated that measurement at this location is highly reproducible, even in the absence of intravenous contrast. While vascular tortuosity favors 3-dimensional reconstruction to precisely measure areas of interest, particularly in the aortic root, the aorta tends to be the least oblique on axial images at the level of the pulmonary artery. Thus, axial diameters commonly remain the first line measurements for screening. We chose to measure both anteroposterior and transverse diameters in both the thorax and the abdomen and report the average diameter to help control for any obliquity of the vessel. However, double oblique measurements should be performed in dilated aortas being monitored for potential surgical repair to control for potential tortuosity and angulation, particularly in the annulus, in the root (sinuses of Valsalva and sinotubular junction), and in the arch, which have an angular orientation in almost all patients.In the clinical setting of known aortic dilation or disease where surgical repair may be under consideration, double oblique measurements may have better concordance with planimetry than axial images, particularly in the aortic root(17).As the CT scans in our study were obtained from the level of the carina to the level of the diaphragm, measurements of aortic diameters within the arch were precluded. However, the known prevalence of isolated arch aneurysms is low.
While aortic diameter was correlated with sex, age, and BSA across all measurements, ascending and descending thoracic diameter also correlated with blood pressure. The strength of correlation with blood pressure did not persist across the abdominal measurements. Instead, the strength of univariate correlation in the abdominal aorta was most notable with number of pack-years in smokers, particularly in current smokers, and with cholesterol values to a lesser extent. These observations fit with theories that thoracic aortic aneurysms, in the absence of select heritable conditions, tend to stem from a hypertensive acceleration of medial degeneration(18), whereas abdominal aneurysms stem from an atherosclerotic mechanism with tobacco use as an associated exacerbant (19). However, sex, age, and BSA outweigh the effects of these traditional CVD risk factors in predicting aortic diameter. While we observed paradoxical inverse correlations of increasing cholesterol with aortic diameters, the magnitude of correlation was small and it is possible that confounding by treatment accounts for these association. Indeed, this hypothesis is supported by the absence of any association of hyperlipidemia, defined by high cholesterol or lipid-lowering therapy, with high aortic diameters.
This analysis is limited by the inclusion of only Caucasian participants. Our findings may not generalize to non-Caucasian ethnic and racial groups. Analogous studies in ethnically diverse cohorts, such as Multi-Ethnic Study of Atherosclerosis (MESA), will be needed to provide complementary information. While the MDCT scans are ECG-gated, allowing for much more precise analysis of the ascending aorta than with non-gated scans, intravenous contrast was not utilized. As such, vascular structures were less defined than they would be on contrast-enhanced exams, which may have introduced a small margin of error. Serial imaging of the aorta with CT may result in significant cumulative radiation exposure. According to the 2010 Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease, radiation dose should be minimized and alternative imaging modalities that do not involve exposure to ionizing radiation, such as magnetic resonance imaging, should be considered as alternatives to CT when clinically appropriate(1).
Supplementary Material
Supplemental Figure 1 A-H. Nomograms displaying predicted diameter (orange line) by BSA for each age strata in the (A) ascending aorta in men, (B) ascending aorta in women, (C) descending aorta in men, (D) descending aorta in women, (E) infrarenal abdominal aorta in men, (F) infrarenal abdominal aorta in women, (G) lower abdominal aorta in men, and (H) lower abdominal aorta in women. Lower and upper limits indicate the two-sided 95% confidence intervals of the predicted values.
Table 2b.
Mean and percentiles of the mean diameter of the descending (mean DTA) thoracic aorta (mm) by age and BSA in all women (left) and all men (right).
| All Women | All Men | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | BSA | Mean | 25 | 50 | 75 | 90 | BSA | Mean | 25 | 50 | 75 | 90 |
| < 45 | < 1.7 | 20.5 | 19.5 | 20.5 | 21.4 | 22.2 | < 1.9 | 22.5 | 21.5 | 22.2 | 23.4 | 25.0 |
| 1.7-1.89 | 21.5 | 20.5 | 21.4 | 22.6 | 23.6 | 1.9-2.09 | 23.5 | 22.4 | 23.2 | 24.4 | 25.6 | |
| ≥ 1.9 | 22.2 | 21.2 | 22.1 | 23.2 | 24.6 | ≥ 2.1 | 24.7 | 23.2 | 24.6 | 25.9 | 27.0 | |
| 45-54 | < 1.7 | 21.5 | 20.2 | 21.5 | 22.6 | 23.8 | < 1.9 | 24.3 | 23.2 | 24.0 | 25.5 | 26.8 |
| 1.7-1.89 | 22.7 | 21.5 | 22.5 | 23.8 | 24.8 | 1.9-2.09 | 24.9 | 23.6 | 24.8 | 26.2 | 27.5 | |
| ≥ 1.9 | 23.6 | 22.2 | 23.6 | 24.8 | 26.0 | ≥ 2.1 | 26.0 | 24.6 | 26.0 | 27.2 | 28.5 | |
| 55-64 | < 1.7 | 23.1 | 21.7 | 23.1 | 24.4 | 25.3 | < 1.9 | 25.8 | 24.1 | 25.6 | 27.3 | 28.5 |
| 1.7-1.89 | 24.0 | 22.6 | 24.0 | 25.1 | 26.3 | 1.9-2.09 | 26.4 | 25.0 | 26.3 | 27.7 | 28.8 | |
| ≥ 1.9 | 24.8 | 23.9 | 24.8 | 26.0 | 27.3 | ≥ 2.1 | 27.7 | 26.1 | 27.9 | 29.2 | 30.3 | |
| ≥ 65 | < 1.7 | 25.0 | 23.2 | 24.8 | 26.1 | 27.7 | < 1.9 | 28.4 | 26.8 | 28.2 | 29.6 | 30.9 |
| 1.7-1.89 | 25.4 | 24.2 | 25.3 | 27.0 | 28.1 | 1.9-2.09 | 28.8 | 27.2 | 28.2 | 30.1 | 31.6 | |
| ≥ 1.9 | 27.1 | 25.5 | 26.5 | 28.4 | 30.3 | ≥ 2.1 | 30.3 | 28.4 | 29.9 | 32.0 | 33.0 | |
BSA: body surface area
Table 2c.
Mean and percentiles of the mean diameter of the infrarenal abdominal (mean IRAA) aorta (mm) by age and BSA in all women (left) and all men (right).
| All Women | All Men | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | BSA | Mean | 25 | 50 | 75 | 90 | BSA | Mean | 25 | 50 | 75 | 90 |
| < 45 | < 1.7 | 15.2 | 14.3 | 15.2 | 16.1 | 16.6 | < 1.9 | 16.9 | 16.1 | 16.8 | 17.8 | 18.5 |
| 1.7-1.89 | 16.0 | 15.0 | 15.9 | 16.8 | 17.7 | 1.9-2.09 | 17.6 | 16.8 | 17.5 | 18.5 | 19.5 | |
| ≥ 1.9 | 16.3 | 15.4 | 16.2 | 17.2 | 17.8 | ≥ 2.1 | 18.5 | 17.6 | 18.5 | 19.5 | 20.2 | |
| 45-54 | < 1.7 | 15.8 | 15.0 | 15.7 | 16.5 | 17.5 | < 1.9 | 18.4 | 17.4 | 18.5 | 19.1 | 20.7 |
| 1.7-1.89 | 16.6 | 15.6 | 16.5 | 17.6 | 18.4 | 1.9-2.09 | 18.8 | 17.8 | 18.7 | 19.8 | 20.9 | |
| ≥ 1.9 | 17.0 | 16.1 | 17.0 | 18.0 | 19.0 | ≥ 2.1 | 19.4 | 18.5 | 19.3 | 20.3 | 21.2 | |
| 55-64 | < 1.7 | 16.4 | 15.2 | 16.4 | 17.5 | 18.6 | < 1.9 | 18.9 | 17.5 | 18.8 | 20.1 | 21.5 |
| 1.7-1.89 | 17.2 | 16.3 | 17.3 | 18.1 | 19.0 | 1.9-2.09 | 19.4 | 18.0 | 19.0 | 20.6 | 21.5 | |
| ≥ 1.9 | 17.6 | 16.6 | 17.6 | 18.5 | 19.3 | ≥ 2.1 | 20.7 | 19.0 | 20.2 | 21.7 | 22.9 | |
| ≥ 65 | < 1.7 | 17.8 | 16.2 | 17.5 | 18.7 | 20.7 | < 1.9 | 21.0 | 19.1 | 20.2 | 21.7 | 24.6 |
| 1.7-1.89 | 17.8 | 16.8 | 17.7 | 18.6 | 20.0 | 1.9-2.09 | 21.8 | 19.5 | 20.7 | 22.6 | 25.5 | |
| ≥ 1.9 | 18.7 | 17.1 | 18.3 | 19.5 | 21.8 | ≥ 2.1 | 22.5 | 19.8 | 21.4 | 23.3 | 26.0 | |
BSA: body surface area
Acknowledgments
Sources of Funding: From the Framingham Heart Study of the National Heart, Lung, and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This work was supported by the National Heart, Lung, and Blood Institute's Framingham Heart Study (contract No. N01-HC-25195). Drs. Rogers and Truong received salary support from National Institutes of Health grant 1T32 HL076136. Dr. Truong also received salary support from NIHgrants K23 HL098370 and L30 HL093806.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. American Association for Thoracic Surgery. American College of Radiology. American Stroke Association. Society of Cardiovascular Anesthesiologists. Society for Cardiovascular Angiography and Interventions. Society of Interventional Radiology. Society of Thoracic Surgeons. Society for Vascular Medicine 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology,American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons,and Society for Vascular Medicine. J Am Coll Cardiol. 2010;655:e27–e129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Pape LA, Tsai TT, Isselbacher EM, Oh JK, O'gara PT, Evangelista A, Fattori R, Meinhardt G, Trimarchi S, Bossone E, Suzuki T, Cooper JV, Froehlich JB, Nienaber CA, Eagle KA, International Registry of Acute Aortic Dissection (IRAD) Investigators Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2007;116:1120–1127. doi: 10.1161/CIRCULATIONAHA.107.702720. [DOI] [PubMed] [Google Scholar]
- 3.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Heart Study. Ann NY Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 5.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 6.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 7.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O'Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 8.Murabito JM, Nam BH, D'Agostino RB, Sr, Lloyd-Jones DM, O'Donnell CJ, Wilson PW. Accuracy of offspring reports of parental cardiovascular disease history: the Framingham Offspring Study. Ann Intern Med. 2004;140:434–440. doi: 10.7326/0003-4819-140-6-200403160-00010. [DOI] [PubMed] [Google Scholar]
- 9.Poutanen T, Tikanoja T, Sairanen H, Jokinen E. Normal aortic dimensions and flow in 168 childrenand young adults. Clin Physiol Funct Imaging. 2003;23:224–229. doi: 10.1046/j.1475-097x.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 10.Reed CM, Richey PA, Pulliam DA, Somes GW, Alpert BS. Aortic Dimensions in Tall Men and Women. Am J Cardiol. 1993;71:608–610. doi: 10.1016/0002-9149(93)90523-f. [DOI] [PubMed] [Google Scholar]
- 11.Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64:507–512. doi: 10.1016/0002-9149(89)90430-x. [DOI] [PubMed] [Google Scholar]
- 12.Vasan RS, Larson MG, Benjamin EJ, Levy D. Echocardiographic reference values for aortic root size: the Framingham Heart Study. J Am Soc Echocardiogr. 1995;8(6):793–800. doi: 10.1016/s0894-7317(05)80003-3. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann U, Massaro JM, Fox CS, Manders E, O'Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol. 2008;102:1136–1141. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preis SR, Hwang SJ, Fox CS, Massaro JM, Levy D, Hoffmann U, O'Donnell CJ. Eligibility of individuals with subclinical coronary artery calcium and intermediate coronary heart disease risk for reclassification (from the Framingham Heart Study). Am J Cardiol. 2009;103:1710–1715. doi: 10.1016/j.amjcard.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Jacobs A, Weinsaft JW, Shaw LJ, Berman DS, Gilmore A, Callister TQ, Min JK. Assessment of the thoracic aorta by multidetector computed tomography: age- and sex-specific reference values in adults without evident cardiovascular disease. J Cardiovasc Comput Tomogr. 2008;2:298–308. doi: 10.1016/j.jcct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Wolak A, Gransar H, Thomson LE, Friedman JD, Hachamovitch R, Gutstein A, Shaw LJ, Polk D, Wong ND, Saouaf R, Hayes SW, Rozanski A, Slomka PJ, Germano G, Berman DS. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging. 2008;1:200–209. doi: 10.1016/j.jcmg.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Mendoza DD, Kochar M, Devereux RB, Basson CT, Min JK, Holmes K, Dietz HC, Milewicz DM, LeMaire SA, Pyeritz RE, Bavaria JE, Maslen CL, Song H, Kroner BL, Eagle KA, Weinsaft JW, GenTAC (National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions) Study Investigators Impact of image analysis methodology on diagnostic and surgical classification of patients with thoracic aortic aneurysms. Ann Thorac Surg. 2011;92:904–912. doi: 10.1016/j.athoracsur.2011.03.130. [DOI] [PubMed] [Google Scholar]
- 18.Carlson RG, Lillehei CW, Edwards JE. Cystic medial necrosis of the ascending aorta in relation to age and hypertension. Am J Cardiol. 1970;25:411–415. doi: 10.1016/0002-9149(70)90006-8. [DOI] [PubMed] [Google Scholar]
- 19.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126:441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 A-H. Nomograms displaying predicted diameter (orange line) by BSA for each age strata in the (A) ascending aorta in men, (B) ascending aorta in women, (C) descending aorta in men, (D) descending aorta in women, (E) infrarenal abdominal aorta in men, (F) infrarenal abdominal aorta in women, (G) lower abdominal aorta in men, and (H) lower abdominal aorta in women. Lower and upper limits indicate the two-sided 95% confidence intervals of the predicted values.