Abstract
Intermuscular coupling has been investigated to understand neural inputs to coordinate muscles in a motor performance. However, little is known on the role of nerve innervation on intermuscular coupling. The purpose of this study was to investigate how the anatomy of nerve distribution affected intermuscular coupling in the hand during static grip. Electromyographic (EMG) signals were recorded from intrinsic and extrinsic muscles while subjects performed a static grip. Coherence was computed for muscle pairs innervated by either the same or different nerves. The results did not support the hypothesis that muscles sharing the same nerve exhibit greater coupling than muscles innervated by different nerves. In general, extrinsic muscle pairs displayed higher coherence than intrinsic pairs. The results suggest that intermuscular coupling in a voluntary motor task is likely modulated in a functional manner and that different nerves might transport common neural inputs to functionally coupled muscles.
Keywords: Electromyography, coupling, coherence, hand muscles, innervations
1 INTRODUCTION
Corticomuscular and musculo-muscular coupling analyses are two approaches widely used for the study of neural inputs to coordinated muscles [Grosse et al, 2002]. The former inquires on correlations between electroencephalographic (EEG) signals and surface electromyographic (EMG) signals, whereas the later focuses on correlations across EMG signals from different muscles. Factors affecting corticomuscular coupling include type of task [Kilner et al, 1999; Kilner et al, 2000], magnitude of force [Brown et al, 1998], learning processes [Perez et al, 2006], attention and precision [Kristeva-Feige et al, 2002], pathological conditions [Grosse et al, 2002; Grosse et al, 2003; Leocani and Comi, 1999], and fatigue level [Yang et al, 2009]. A challenging aspect of corticomuscular coupling analysis is the generation of artifact-free recordings (especially in EEG) and the proper matching of their specific frequency bands (low frequency EEG with high frequency EMG). Alternatively, examination of musculo-muscular coupling has proven effective to interrogate the neuromuscular synergies originating from the central drive [Kilner et al, 1999; Farmer et al, 1993]. In general, the results of musculo-muscular coupling analysis might differ depending on the location of the muscle pairs [Poston et al, 2010], hand dominance [Schmied et al, 1994; Semmler and Nordstrom, 1995] and the type of task performed [Kilner et al, 1999].
It has been reported that intrinsic muscles of the hand exhibit weaker coupling than the extrinsic muscles [Poston et al, 2010], suggesting that the strong coupling among the extrinsic muscles is advantageous for synergistic force production and the weaker coupling among intrinsic muscles facilitates independent control of individual fingers for fine motor skills [Poston et al, 2010; Johnston et al, 2009]. Bremner et al. showed a higher degree of motor-unit synchrony between hand muscles that produce similar actions on different digits than muscles producing different actions on the same or different digit [Bremner et al, 1991]. Winges et al. suggested that coupling of specific muscle pairs may be influenced by distinct inputs to motor neuron pools as muscle innervations differ [Winges et al, 2008]. Thus, while multiple factors are known to influence musculo-muscular coupling, the role of nerve innervation remains poorly understood.
The anatomical structure of muscle innervation could conceivably impact the strength of coupling between muscles as nerves are the conveyors of the electrical signals originating from the brain and spinal motoneurons. The brachial plexus, a neural network from the fifth cervical to the first thoracic nerve roots (C5–C8 and T1), descends its pathway and recombines into the median, radial and ulnar nerves to innervate the hand muscles. It is unclear how such neuro-anatomical characteristics affect the musculo-muscular coupling analysis of the hand. The purpose of this study was to investigate the effect of nerve supply on the strength of coupling among hand muscles during a static grip task by means of EMG-EMG coherence analysis. We hypothesized that muscle pairs receiving the same nerve innervation would exhibit a greater level of coupling than a pair of muscles innervated by different nerves.
2 METHODS
Subjects
Ten healthy, right-handed volunteers (age: 27.1 ± 5.49 years; body mass: 77.15 ± 14.67 kg; height: 178.6 ± 7.4 cm; all males) participated in the study. None of them had a history of neurological disorders, neuropathies or trauma to the upper extremities. All participants signed an informed consent approved by the Institutional Review Board prior to their participation in this study.
Experimental Protocol
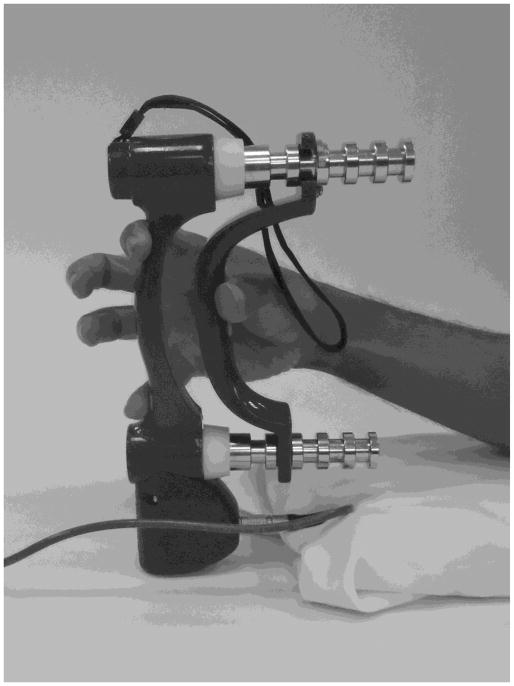
Each participant was seated on a height-adjustable chair in front of the testing table with the right shoulder in a position approximately 50° of flexion and 0° of abduction. The elbow was flexed at 90° and the forearm and wrist were relaxed and supported in a neutral (between pronation and supronation) position. Each participant placed the pulp of their thumb, index and little fingers on the handle marks of a computerized grip dynamometer (Biometrics, UK), while extending the middle and ring finger away from the handle surface (Figure 1). This posture allowed us to specifically target the muscles of the thumb, index finger and little fingers. During the grip performance, the subject was asked to produce sustained (10 seconds), submaximal grip forces at 40% maximum voluntary contraction (MVC). Prior to the MVC force measurement the subjects were allowed to practice until they felt comfortable with the task. The subjects were instructed to maintain the dynamometer handle straight while maintaining a stable grasping. In each trial, the target 40% MVC force and produced forces were displayed on a computer screen for visual feedback. Nine trials were performed with an inter-trial rest time of 30 seconds.
Figure 1.
Gripping on a grip dynamometer with the thumb, index finger and little finger.
EMG Recording
Surface EMG signals were collected for the following three extrinsic muscles: abductor pollicis longus (APL, innervated by radial nerve); extensor digitorum communis (EDC, innervated by radial nerve); flexor digitorum superficialis (FDS, innervated by median nerve); and three intrinsic muscles: abductor pollicis brevis (APB, innervated by median nerve); abductor digiti minimi (ADM, innervated by ulnar nerve); and first dorsal interosseous (FDI, innervated by ulnar nerve). These muscles were strategically selected because they represent key combinations of extrinsic/intrinsic locations, innervations, agonist-antagonist roles, and their accessibility with surface EMG.
The skin sites of the EMG electrode placements were prepared by shaving, abrading and cleaning. Ag/AgCl electrodes (8 mm in diameter) (In Vivo Metric, Healdsburg, CA) were used to record muscle activity. The center-to-center distance was 10 mm according to Kattla et al. [Kattla et al, 2010]. Positioning of the electrodes was confirmed by testing functional movements corresponding to the targeted muscle according to Basmajian and Blumenstein recommendations [Basmajian and Blumenstein, 1980]. For the FDI, APB and ADP muscles, the electrodes were placed directly above the muscles’ belly. For the FDS muscle, electrodes were located midway between the medial epicondyle and the radial styloid process. For the EDC, electrodes were located mid-forearm at radial border of EDC to better target the index finger section [Leinsje et al, 2008]. The positioning of the electrodes was confirmed by observing the EMG signals with specific movement induced by the targeted muscle. The EMG signals were amplified (×1000), bandwidth filtered (1–500 Hz), digitized (1000 samples/s) using a Noraxon MySystem 1400 system (Noraxon Inc., Scottsdale, AZ), and recorded on hard disk of a personal portable computer.
Data Analysis
The objective of this work was to investigate the influence of nerve innervation on EMG-EMG coherence. Therefore, to isolate the effect of nerve supply, only pairs involving muscles of the same group (i.e. extrinsic-extrinsic; intrinsic-intrinsic) were selected for coherence analysis (6 out of 15 possible muscle pairs, Table 1).
Table 1.
Selected muscle pairs for coherence analysis.
| Pair index | Muscle pairs | Nerve innervation | Group |
|---|---|---|---|
| 1 | APL-EDC | Radial - Radial | Extrinsic |
| 2 | APL-FDS | Radial - Median | Extrinsic |
| 3 | EDC-FDS | Radial - Median | Extrinsic |
| 4 | APB-FDI | Median - Ulnar | Intrinsic |
| 5 | APB-ADM | Median - Ulnar | Intrinsic |
| 6 | FDI-ADM | Ulnar - Ulnar | Intrinsic |
The EMG signals were filtered and rectified off-line using a Matlab program (The MathWorks, Natick, MA) by a zero-lag Butterworth filter (4th order, band-pass from 5 to 500 Hz). EMG-EMG coherence (Cxy) at a given frequency (ω) between EMG signals of two muscles was calculated as follows:
| (1) |
where Sxy is the cross-spectrum of the EMG signals of muscle x and muscle y; Sxx and S yy are the auto-spectra of the EMG signals of muscles x and y, respectively. Coherence values ranges between 0 and 1. A bivariate autoregressive model was applied to each EMG-EMG pair, and the coefficients were estimated by solving the Yule-Walker equation in ARfit MATLAB software [Schneider and Neumaier, 2001]. Akaike’s Final Prediction Error Criterion [Akaike, 1971] was used to calculate the optimal order. A Boxcar window (1024 points length and 1000 overlap) was then used to generate time-dependent coefficients. Coherence was presented in the time-frequency domain. The coherence values were normalized by computing the Fisher’s Z transform (Zxy = tanh−1(Cxy)).
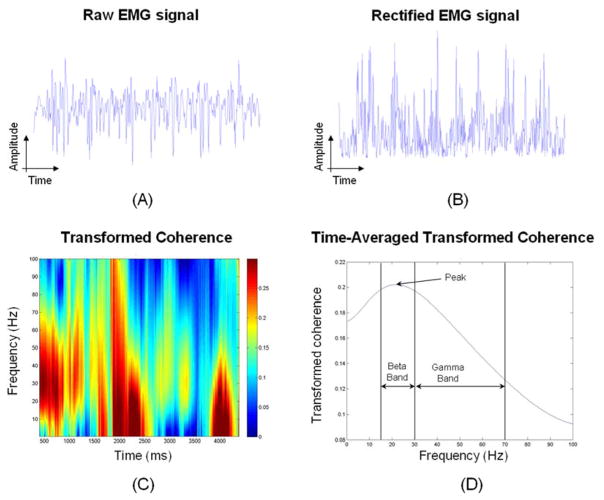
The coherence analysis was performed during a 5-second period following the first 4-second after the trial beginning to avoid non-stationary effects at the beginning and end of the trial. Figure 2 shows a flow diagram of the data analysis. The raw surface EMG was filtered (Figure 2-A) and rectified (Figure 2-B) before coherence analysis. Figure 2-C shows a color map of the transformed coherence values (indicated by the color bar) as a function of time and frequency. The transformed coherence presented in time-frequency domain was averaged in time to indicate the EMG-EMG coherence at each frequency point (Figure 2-D). The peak of the transformed coherence value was determined for each trial. The time-averaged of the transformed coherence values for the beta-range (15–30 Hz) and the gamma-range (30–70 Hz) were also calculated (Figure 2-D) since these are frequency ranges mainly associated with the primary motor cortex [Grosse et al, 2002]. To obtain a measure of the overall coupling between muscle pairs, the time-averaged of the transformed coherence values were further averaged across all the frequencies.
Figure 2.
Flow diagram of the data analysis. (A) EMG signal after filtering. (B) Filtered, rectified EMG signal. (C) Transformed coherence color map as a function of time and frequency. The right axis represents the color-coded scale for the transformed coherence values. (D) Time-averaged transformed coherence as a function of frequency.
The coherence results (peak, beta-range, gamma-range and average) were averaged across trials for each subject and muscle pairs (60 coherence values per parameter). Since the transformed coherence values were not normally distributed, non-parametric tests were used (Friedman and Wilcoxon Signed Ranks) to identify significant differences among muscle pairs (p < 0.05). Although adjusting the p values by standard methods (i.e. the Bonferroni correction) rendered these differences non-significant, clear trends were observed for certain muscle pairs as will be discussed below. The reliability of multiple comparisons adjustments for establishing the overall stringency of the analysis is a matter of active debate [Perneger, 1998; Bender and Lange, 1999; Feise, 2002]. Herein, we utilized non-adjusted p values to avoid Type II error, which would increase the chances of excluding findings of physiologically relevance. All the statistical analyses were run on SPSS (IBM, Armonk, NY).
3 RESULTS
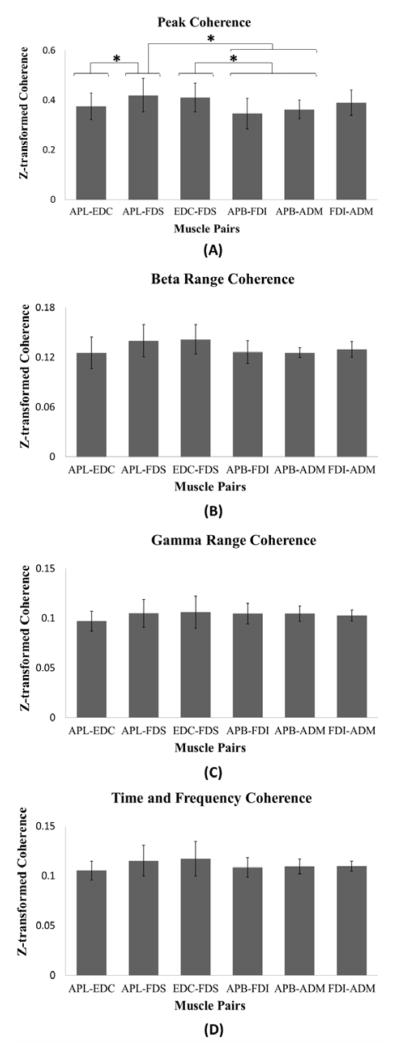
The Friedman test showed that the muscle pairs had significantly different transformed coherence values for the peak of the time-averaged coherence (p = 0.007). Figure 3 shows the means and standard deviations for the Z-transformed values for the peak of the time-averaged coherence (A), the beta-range coherence (B), the gamma-range coherence (C) and the time-frequency averaged coherence (D).
Figure 3.
Means and standard deviations for the transformed coherence. Pairs that are statistically different (p < 0.05) are marked by an asterisk. (A) Peak of time-average coherence. (B) Time and frequency averaged coherence for beta-range. (C) Time and frequency averaged coherence for gamma-range. (D) Time and frequency averaged coherence.
The APL-FDS and EDC-FDS showed the highest transformed coherence values, indicating a higher musculo-muscular coupling in comparison with the others muscle pairs. High coherence values for the EDC-FDS pair confirm its known functional relationship. Neither the muscles in the APL-FDS pair nor the muscles in the EDC-FDS pair share the same nerve innervations and these muscles belong to the extrinsic group.
Regarding EMG-EMG coherence in specific frequency bands, the results showed no significant differences for the beta (p = 0.061) and gamma (p = 0.138) ranges regardless of muscles groups (intrinsic/extrinsic) or nerve innervations (Figure 3-B, C). No significant differences were found for the time-frequency averaged coherence values (p = 0.419) (Figure 3).
Table 2 summarizes the multiple comparison results from the Wilcoxon Signed Ranks post hoc tests performed on the transformed coherence values for the peak of the time-averaged coherence. The transformed coherence values of the muscle pairs in the A column were found to be statistically higher than that of the muscle pairs in the B column (p < 0.05).
Table 2.
Comparisons of the Z-transformed coherence values for the peak of the time-averaged coherence. The Z-transformed coherence values in Pair A column are statistically larger (p < 0.05) than in Pair B column. The muscles highlighted in bold share the same nerve innervations.
| Pair A | Pair B | P value | |
|---|---|---|---|
| ZAPL-FDS | > | ZAPL-EDC | 0.047 |
| ZAPL-FDS | > | ZAPB-ADM | 0.028 |
| ZAPL-FDS | > | ZAPB-FDI | 0.007 |
| ZEDC-FDS | > | ZAPB-FDI | 0.037 |
| ZEDC-FDS | > | ZAPB-ADM | 0.028 |
Extrinsic muscle pairs consistently showed statistically greater coherence than intrinsic muscle pairs (last four comparisons in Table 2). However, there was no significant difference between any extrinsic pair and the FDI-ADM (intrinsic pair). In addition, among the extrinsic muscle pairs, muscles innervated by the same nerve did not show stronger coherence than those with different innervations (first comparison in Table 2).
4 DISCUSSION
The main purpose of this study was to investigate the effect of nerve innervation on EMG-EMG coherence as a measurement of musculo-muscular coupling. The results did not support the hypothesis that hand muscles with shared nerve innervation exhibit a greater coupling than muscles innervated by different nerves during our specific isometric grip task. A potential explanation for this phenomenon might be found in the recombination of the efferent signal pathways after passing through the nerve roots. Different nerves might likely transport common neural inputs from nerve fibers of the same spinal cord level. Hence, muscles innervated by different nerves can still exhibit strong EMG-EMG coherence to fulfill functional needs that lack anatomical continuity. Our studies suggest that more than one mechanism exists for controlling the output of musculo-muscular coupling. The distribution of neural inputs to hand muscles might be influenced by the functional role of the muscles. Johnston et al. proposed that common neural inputs distribute depending on functional relationships among muscles [Johnston et al, 2009]. The finding that the APL-FDS pair (associated with different nerves) had stronger coupling than the APL-EDC, APB-ADM and APB-FDI pairs may be explained by their functional roles specific to the grip task. For the experimental task, the thumb opposes the index and little fingers to produce grip forces, requiring synergistic and coordinated thumb abduction by the APL and finger flexion by the FDS. Moreover, the finding that the EDC-FDS pair exhibited higher coherence than the APB-FDI and the APB-ADM pairs further demonstrates a function-dependent coupling of muscles. The function of the FDS is to flex the metacarpophalangeal and the proximal interphalangeal joints of the fingers. EDC is an extrinsic extensor of the fingers. Our experimental task requires that the middle and ring fingers do not touch the handle. As the flexor muscles of the index and little finger (i.e. FDS) are activated to produce force on the handle, they also flex the middle and ring fingers [Li et al, 2002]. Thus, the extensor muscles (i.e. EDC) must be activated to maintain these two fingers away from the handle. Because this enslaving effect is an intrinsic property of the hand’s function, the role of nerve innervation on EMG-EMG coherence cannot be perfectly isolated in the case of EDC-FDS. In contrast, the biomechanical coordination could be less stringent for the APL-EDC, APB-ADM and APB-FDI pairs under our experimental conditions.
Musculo-muscular coupling analysis within the hand has been shown to be location dependent, i.e. extrinsic muscles are more closely coupled than intrinsic muscles [Poston et al, 2010; Johnston et al, 2009]. Our results showed a higher EMG-EMG coherence between extrinsic muscles than between intrinsic muscles. Studies on motor unit coherence showed that coupling between extrinsic-extrinsic muscles and intrinsic-intrinsic muscles are related to mechanical features, such as the dependency of muscle length on wrist posture [Johnston et al, 2009]. It was also proposed that the location-dependent coupling is beneficial to hand function as stronger coupling of extrinsic muscles facilitate for synergistic force production while less coupled intrinsic muscles contribute to finger dexterity [Johnston et al, 2009]. In contrast, Maier and Hepp-Reymond reported high synchronization between intrinsic muscles during precision grip [Maier and Hepp-Reymond, 1995] (even though with high intra- and inter-subject variability). Further research is needed to clarify the reasons for higher musculo-muscular coupling in particular muscle groups (i.e. intrinsic/extrinsic muscles).
In summary, our study shows that hand muscles sharing the same nerve innervation do not necessarily display stronger coupling than muscles innervated by different nerves. We confirmed that extrinsic muscles exhibited higher coupling than intrinsic muscles for the task studied. It is conceivable that neural drive influences EMG-EMG coherence in a functional manner as pairs of muscles sharing functional roles exhibited stronger coupling.
5 LIMITATIONS
The results in this study report on a task involving static force production using hand grip. Task-dependency has been shown to affect motor unit coherence [Johnston et al, 2005] and coherence between motor cortex and hand muscles [Kilner et al, 2000]. Hence, the results found for the isometric task performed in this study may differ from those during a dynamic task (i.e. slip-prevention). The recording of surface EMG signals presents limitations in that they rely on the conditions at the skin-electrode interface and are prone to cross-talk among muscles. Only a subset of muscle pairs was analyzed in the present study given their accessibility with surface EMG, nerve innervation as well as their combination of intrinsic/extrinsic pairs. In order to minimize the cross-talk of untargeted muscle compartments, electrode locations were specifically adjusted and the pinch force was kept rather low (40% MVC). In spite of these setbacks, our data indicates that muscles innervated by the same nerve do not necessarily display higher EMG-EMG coherence than muscle pairs supplied by different nerves. This is especially relevant from a clinical point of view because patients who suffer from neuropathies such as carpal tunnel syndrome are assessed using surface EMG only. Further experimentation should be conducted to determine if there are differences in coupling between dominant and non-dominant hands and to discriminate between left and right hand dominant groups.
Acknowledgments
The project described was supported by Grant Number R01AR056964 from NIAMS/NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH.
Biographies

Dr. Cristian Pasluosta received his degree in Electrical Engineering from the National University of Cordoba, Argentina, in 2005, and his MSc in Engineering and Ph.D in Biomedical Engineering from Louisiana Tech University, LA, USA, in 2010. He is currently a Postdoctoral Research Fellow at the Lerner Research Institute, Cleveland Clinic in Cleveland, USA. His areas of interest are bio-signal processing and machine learning techniques for biomedical applications, motion analysis, mechatronic design, control systems as well as analog and digital electronic circuit design.

Dr. Mathieu Domalain received his MSc in Kinesiology from the University of Savoie in 2006 and his PhD in Movement Sciences from Aix-Marseilles University (France) in 2010. He worked as a Postdoctoral Research Fellow at the Lerner Research Institute, Cleveland Clinic (Cleveland OH, USA) in 2010–2011. He currently holds an Assistant Professor position in the Department of Kinesiology and Sport Sciences at the University of Poitiers (France). His areas of interest are biomechanics, motor control, ergonomics and sport engineering with an emphasis on hand function.
Dr. Yin Fang – a photograph and biography are not available at this time.

Guang H. Yue received his Ph.D. from the University of Iowa. From 1991 to 1994, he was a Postdoctoral Fellow at the University of Arizona and Cleveland Clinic. From 1994 to 2011 he was a faculty member in the Department of Biomedical Engineering at the Cleveland Clinic and Department of Molecular Medicine at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University. He is now the Director of Human Performance and Engineering Laboratory at Kessler Foundation/Kessler Institute for Rehabilitation, and Professor at University of Medicine and Dentistry of New Jersey – New Jersey Medical School. Dr. Yue’s research program is focused primarily on human motor control in health and disease and on finding neural mechanisms underlying movement disorders and recovery of motor function as a result of medical intervention.

Zong-Ming Li received his Ph.D. from the Pennsylvania State University in 1998. He is currently an Associate Staff in the Departments of Biomedical Engineering, Orthopaedic Surgery, and Physical Medicine and Rehabilitation at the Cleveland Clinic. He is also an Associate Professor at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University. Dr. Li has a broad research interest in musculoskeletal biomechanics and rehabilitation with a particular focus on the hand and upper extremity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akaike H. Autoregressive Model Fitting for Control. Ann I Stat Math. 1971;23:163–180. [Google Scholar]
- 2.Basmajian JV, Blumenstein R. Electrode placement in EMG biofeedback. Williams & Wilkins; Baltimore: 1980. [Google Scholar]
- 3.Bender R, Lange S. Multiple test procedures other than Bonferroni’s deserve wider use. British Medical Journal. 1999;318:600–601. doi: 10.1136/bmj.318.7183.600a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremner FD, Baker JR, Stephens JA. Effect of task on the degree of synchronization of intrinsic hand muscle motor units in man. J Neurophysiol. 1991;66:2072–2083. doi: 10.1152/jn.1991.66.6.2072. [DOI] [PubMed] [Google Scholar]
- 5.Brown P, Salenius S, Rothwell JC, Hari R. Cortical correlate of the Piper rhythm in humans. J Neurophysiol. 1998;80:2911–2917. doi: 10.1152/jn.1998.80.6.2911. [DOI] [PubMed] [Google Scholar]
- 6.Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. The Journal of physiology. 1993;470:127–155. doi: 10.1113/jphysiol.1993.sp019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosse P, Cassidy MJ, Brown P. EEG-EMG, MEG-EMG and EMG-EMG frequency analysis: physiological principles and clinical applications. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2002;113:1523–1531. doi: 10.1016/s1388-2457(02)00223-7. [DOI] [PubMed] [Google Scholar]
- 9.Grosse P, et al. Abnormal corticomuscular and intermuscular coupling in high-frequency rhythmic myoclonus. Brain. 2003;126:326–342. doi: 10.1093/brain/awg043. [DOI] [PubMed] [Google Scholar]
- 10.Johnston JA, Winges SA, Santello M. Periodic modulation of motor-unit activity in extrinsic hand muscles during multidigit grasping. Journal of Neurophysiology. 2005;94:206–218. doi: 10.1152/jn.01134.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston JA, Winges SA, Santello M. Neural control of hand muscles during prehension. Advances in experimental medicine and biology. 2009;629:577–596. doi: 10.1007/978-0-387-77064-2_31. [DOI] [PubMed] [Google Scholar]
- 12.Kattla S, Lowery M. Fatigue related changes in electromyographic coherence between synergistic hand muscles. Exp Brain Res. 2010;202(1):89–99. doi: 10.1007/s00221-009-2110-0. [DOI] [PubMed] [Google Scholar]
- 13.Kilner JM, et al. Task-dependent modulation of 15–30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol. 1999;516 (Pt 2):559–570. doi: 10.1111/j.1469-7793.1999.0559v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci. 2000;20:8838–8845. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristeva-Feige R, Fritsch C, Timmer J, Lucking CH. Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during a maintained motor contraction task. Clin Neurophysiol. 2002;113:124–131. doi: 10.1016/s1388-2457(01)00722-2. [DOI] [PubMed] [Google Scholar]
- 16.Leijnse J, Campbell-Kyureghyan N, Spektor D, Quesada P. Assessment of individual finger muscle activity in the extensor digitorum communis by surface EMG. J Neurophysiol. 2008;100(6):3225–35. doi: 10.1152/jn.90570.2008. [DOI] [PubMed] [Google Scholar]
- 17.Leocani L, Comi G. EEG coherence in pathological conditions. J Clin Neurophysiol. 1999;16:548–555. doi: 10.1097/00004691-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Li ZM, Zatsiorsky VM, Latash ML, Bose NK. Anatomically and experimentally based neural networks modeling force coordination in static multi-finger tasks. Neurocomputing. 2002;47:259–275. [Google Scholar]
- 19.Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. II. Muscular synergies in the spatial and temporal domain. Exp Brain Res. 1995;103:123–136. doi: 10.1007/BF00241970. [DOI] [PubMed] [Google Scholar]
- 20.Perez MA, Lundbye-Jensen J, Nielsen JB. Changes in corticospinal drive to spinal motoneurones following visuo-motor skill learning in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perneger TV. What’s wrong with Bonferroni adjustments. British Medical Journal. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poston B, Danna-Dos Santos A, Jesunathadas M, Hamm TM, Santello M. Force-independent distribution of correlated neural inputs to hand muscles during three-digit grasping. J Neurophysiol. 2010;104:1141–1154. doi: 10.1152/jn.00185.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmied A, Vedel JP, Pagni S. Human spinal lateralization assessed from motoneurone synchronization: dependence on handedness and motor unit type. The Journal of physiology. 1994;480 (Pt 2):369–387. doi: 10.1113/jphysiol.1994.sp020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider T, Neumaier A. Algorithm 808: ARfit - A matlab package for the estimation of parameters and eigenmodes of multivariate autoregressive models. Acm T Math Software. 2001;27:58–65. [Google Scholar]
- 25.Semmler JG, Nordstrom MA. Influence of Handedness on Motor Unit Discharge Properties and Force Tremor. Experimental Brain Research. 1995;104:115–125. doi: 10.1007/BF00229861. [DOI] [PubMed] [Google Scholar]
- 26.Winges SA, Kornatz KW, Santello M. Common input to motor units of intrinsic and extrinsic hand muscles during two-digit object hold. J Neurophysiol. 2008;99:1119–1126. doi: 10.1152/jn.01059.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Q, et al. Weakening of functional corticomuscular coupling during muscle fatigue. Brain Res. 2009;1250:101–112. doi: 10.1016/j.brainres.2008.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]