Abstract
Background
The goal of this study was to investigate differences in socio-emotional processing and functioning in children and adolescents at high risk for bipolar disorder (BD) and healthy control participants.
Methods
Children and adolescents with a parent with bipolar disorder, who had mood dysregulation but not fully syndromal BD (HR, n=24), were compared to participants with no personal or family history of psychopathology (HC, n=27) across several neuropsychological domains. Social reciprocity was measured by the Social Responsiveness Scale, theory of mind was measured by use of the NEPSY, and affect recognition was measured by the NEPSY and the Diagnostic Test of Nonverbal Accuracy 2 (DANVA).
Results
The HR group demonstrated significant impairment in social reciprocity, including impairments in social awareness, social cognition, social communication, social motivation, and autistic mannerisms. There were no significant group differences in performance on theory of mind or affect recognition tasks.
Limitations
Lack of impairment in tasks associated with theory of mind or affect recognition indicate that social functioning difficulties are not likely due to impairments in these areas, or that the measures employed were not sufficiently sensitive to detect group differences.
Conclusions
Youth at high risk for BD demonstrated impairments in numerous social domains, which may be due to innate differences in brain development governing socio-emotional functioning or may be due to disruptions in normal development caused by mood regulation difficulties.
Keywords: pediatric bipolar disorder, bipolar offspring, social reciprocity, theory of mind, affect recognition
Introduction
Bipolar disorder (BD) is a common and debilitating psychiatric disorder, which begins during childhood or adolescence in 50-66% of cases (Leverich et al., 2007; Perlis et al., 2004). Childhood-onset BD compared with adult-onset BD is associated with greater risk for comorbid psychiatric disorders, substance abuse, and suicide (Bellivier et al., 2001; Carter et al., 2003). Youth with mood disorders (Pine et al., 2008; Towbin et al., 2005) including BD (Birmaher and Axelson, 2006; Judd et al., 2005) and offspring of parents with BD (Bella et al., 2011) can have significant impairments in social functioning as well as deficits in social reciprocity, which refers to the ability to understand, and engage in social interactions with others. Deficits in affect recognition have been documented in children with (McClure et al., 2003; McClure et al., 2005) and at risk for BD (Brotman et al., 2008a; Brotman et al., 2008b; Guyer et al., 2007), and are associated with social deficits in youth with BD (Rich et al., 2008). Youth with BD have also demonstrated impaired performance on theory of mind tasks, which may further explain social reciprocity and functioning difficulties (Schenkel et al., 2008).
However, it is unclear if these deficits in socio-emotional functioning occur before the onset of mania. Identification of neuropsychological factors in these youth before the onset of mania would aid in early identification and help target early interventions. Studies of high-risk offspring of parents with BD help address this issue, as family history is currently the clearest risk factor for the development of BD, with an estimated heritability of 59-87% (Smoller and Finn, 2003). These offspring have been found to have social impairment that is more significant than that observed in offspring of parents with non-BD psychopathology and community controls (Bella et al., 2011). Regarding affect recognition, high-risk offspring with no psychopathology were found to require greater intensity of emotion to correctly identify emotional faces compared with healthy control subjects (Brotman et al., 2008b). However, theory of mind (ToM) and social reciprocity specifically, rather than overall social functioning, have not yet been studied in symptomatic high-risk youth who are closer to a potential onset of BD. Therefore, we sought to examine social reciprocity, affect recognition, and ToM in a cohort of symptomatic bipolar offspring compared with healthy controls. The at-risk cohort in this study represents the next gradation of severity and thus may help determine whether these offsprings' mood symptoms lead to further deficits in socio-emotional processing or whether the threshold into mania needs to be crossed before these deficits become apparent. This study is the first to examine such questions among this specific population of children and adolescents.
We hypothesized that children at high risk for BD would demonstrate impairment compared to healthy controls in overall social reciprocity and its components, Social Awareness, Social Cognition, Social Communication, Social Motivation and Autistic Mannerisms. We also expected children at high risk for BD to have poorer performance on ToM tasks and higher rates of errors identifying emotional faces than healthy control participants. Finally, we hypothesized that impairment in social reciprocity would be positively correlated with deficits in ToM and affect recognition.
Methods
The study was approved by Stanford University's Human Subjects Institutional Review Board at Stanford University. Participants were drawn from two ongoing studies of pediatric offspring, aged 9 to 18 years, of parents with BD. One child per family was included. Participants at high risk for BD (HR, n=24) were recruited through referral and from the surrounding community, and healthy control (HC, n=27) participants were recruited through internet advertisements and from the surrounding community. Participants were included in the HR group if they had a biological parent with BD, and if they themselves had past or current major depression, or, if they were diagnosed with Attention-Deficit/Hyperactivity Disorder (ADHD) and also had moderate mood symptoms (Youth Manic Rating Scale Score>12 (YMRS, Young et al., 1978) or Children's Depression Rating Scale score>30 on the day of assessment (CDRS, Poznanski et al., 1984) that fell short of diagnostic criteria for a manic episode. Exclusion criteria included past manic episode, substance dependence, history of developmental or autism spectrum disorders, seizures, or head injury with loss of consciousness. Eligibility for the HC participants required the child and his/her first-degree relatives to be free of present or past psychiatric illness. A telephone screening interview with a parent or guardian established that all participants were fluent in English, and had no history of head injury with loss of consciousness lasting more than five minutes, no seizures, and no developmental or substance dependence disorders.
Diagnosis of BD was verified for the affected parent of an HR participant using the Structured Clinical Interview for DSM-Disorders (SCID, First et al., 1996) Participants' diagnoses were assessed by use of the Washington University Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS, Geller et al., 1996; Geller et al., 2001) for assessment of mood disorder symptoms, and the Kiddie Schedule for Affective Disorders and Schizophrenia – Present and Lifetime version (KSADS-PL, Kaufman et al., 1997) for non-mood disorder symptoms. Board-certified psychiatrists or masters-level clinicians with high inter-rater reliability (kappa>0.9) interviewed parents and participants separately; final diagnoses were reviewed at weekly consensus conferences attended by the Principal Investigators (KDC, MKS). The Wechsler Abbreviated Scale of Intelligence was used to estimate Intelligence Quotient (IQ, Weschler, 1999) Lifetime exposure to psychotropic medications was gathered via family and participant self-report and was reviewed by study clinicians.
Neuropsychiatric measures included the following
Social Responsiveness Scale (SRS, Constantino et al., 2003)
A parent of each participant completed the SRS to describe the child's social functioning. For children in the HR group, the BD-affected parent was typically the reporter due to parental availability at the time of the study visit. The SRS is a multiple-choice, 56-item questionnaire that produces a total score scaled on the basis of age and gender, and subtest scaled scores for each of the following domains: Social Awareness, Social Cognition, Social Communication, Social Motivation, and Autistic Mannerisms. Higher scores indicate more difficulty with social functioning. The scale is validated to capture social impairment among children with symptoms of autism spectrum disorders. It has been used previously to characterize social reciprocity in adolescents with BD (Pine et al., 2008; Rich et al., 2008) but not in an at risk population.
Diagnostic Test of Nonverbal Accuracy 2 (DANVA, Nowicki and Duke, 1994)
Participants were shown twenty-four photos of child faces followed by twenty-four photos of adult faces on a computer, and were asked to identify which emotion (happy, sad, angry or fearful) was displayed after the offset of each photograph. This task has been previously used in children and adolescents with (McClure et al., 2003; McClure et al., 2005) and at risk for (Brotman et al., 2008a) BD.
NEPSYII, Korkman et al., 2007)
The Affect Recognition and ToM substest were administered to participant by trained study staff, and raw and scaled scores were calculated. The Affect Recognition task is designed to assess participants' ability to identify happy, sad, neutral, fearful, angry and disgusted expressions in four tasks. The first task requires participants to evaluate whether two photographs of children's faces display the same affect. The second task involves identifying a pair of faces displaying the same affect from a group of three or four emotional faces. The third task requires participants to select which one of four emotional faces displays the same emotion as a singular stimulus face. Finally, the participant is shown a photograph of a child's face displaying a certain emotion for five seconds then from memory is asked to select two faces from a set displayed that match the affect of the face previously shown.
The NEPSY ToM subtest is designed to assess the following constructs: seeing leads to knowing, first- and second-order false beliefs, recognizing mental states, imitation/pretending, mental-physical distinction, bluff and double-bluff, appearance-reality, and understanding figurative language. To assess ToM in the verbal domain, children are read stories or shown pictures of social scenarios then asked questions regarding the characters' varying perspectives. For assessment in the contextual domain, pictures of social scenes are shown to participants and they are asked questions to measure understanding of the emotional content of the scenes. Lower scores indicate more impaired ToM.
Descriptive statistics were computed for each group using t-test or chi-squared analyses as appropriate. Data were examined visually to determine normality of distribution with Shapiro-Wilk used for verification. Since there were group differences in IQ, Analysis of Covariance (ANCOVA) with IQ as a covariate, and Multivariate Analysis of Covariance (MANCOVA) were employed to assess the effect of group on outcome measures. Cohen's d was calculated to determine the effect size for between-group differences. Pearson's r was computed to describe relationships between continuous variables.
Results
Twenty-four subjects at high risk for BD (HR) were compared to twenty-seven healthy control subjects (HC). The major outcome variables were non-normally distributed (Shapiro-Wilk, p < 0.05). For HR participants, the mean age was 12.7±2.9 years, and 46% were female. For HC participants, the mean age was 13.3±2.6 years and 60% were female. The groups did not differ in terms of age (p=0.4) or gender (p=0.4). IQ was higher (p=0.04) by an average of 8 points in the HC group compared to the HR group. The groups also differed in terms of race (p=0.01); the HC group included participants who were white, non-Hispanic (n=14), Hispanic (n=5), mixed races (n=4), Asian (n=3), and black, non-Hispanic (n=1) whereas the HR group included participants who were white-non-Hispanic (n=23) and Hispanic (n=1). The HR participants were all diagnosed with Axis I disorders and 68% had previous exposure to psychotropic medications. Demographics and clinical characteristics are presented in Table 1.
Table 1. Demographic and clinical characteristics.
| Variable | Healthy Control (n = 27) | High Risk (n = 24) | Statistic |
|---|---|---|---|
|
| |||
| Age: Mean ± standard deviation | 13.3 ± 2.6 | 12.7 ± 2.9 | t (49) = 0.8, p = 0.4 |
|
| |||
| Gender: n, % | |||
| -Female | 16, 60% | 11, 46% | χ2(n = 51) = 0.9, p = 0.4 |
| -Male | 11, 40% | 13, 54% | |
|
| |||
| IQ: Mean ± standard deviation** | 117.6 ± 12.8 | 109.8 ± 13.2 | t (47) = 2.2, p = 0.04 |
|
| |||
| Race: n, %** | χ2(n = 51) = 12.7, p = 0.01 | ||
| -Asian | 3, 11% | 0, 0% | |
| -Black, Non-Hispanic | 1, 4% | 0, 0% | |
| -Hispanic | 5, 19% | 1, 4% | |
| -White, Non-Hispanic | 14, 52% | 23, 96% | |
| - Mixed | 4, 15% | 0, 0% | |
|
| |||
| Axis I Diagnoses: n, % | 0, 0% | 22, 100%a | |
| -Bipolar Disorder not otherwise specified (NOS) | 5, 23% | ||
| -Major depressive episode | |||
| -Current | 12, 56% | ||
| -Past | 3, 14% | ||
| -Dysthymia | 2, 9% | ||
| -Adjustment disorder | 1, 5% | ||
| -Attention-deficit/hyperactivity disorder | 15, 68% | ||
| -Oppositional defiant disorder | |||
| -Generalized anxiety disorder | 5, 23% | ||
| -Anxiety disorder NOS | 4, 18% | ||
| -Specific phobia | 2, 9% | ||
| 3, 14% | |||
|
| |||
| Psychotropic medication exposure: n, % | 0, 0% | 15, 68%a | |
Data missing for 2 HR participants so is reported for n = 22
p < 0.05
A MANCOVA was performed with each of the five main measures as outcome variables (SRS total t-score, NEPSY ToM total raw score, NEPSY affect recognition total raw score, DANVA child faces total errors, DANVA adult faces total errors), group as a fixed factor, and IQ as a covariate. There was a significant effect of group (Wilk's λ <0.001), and so the five outcome measures were examined individually with one-way ANOVAs.
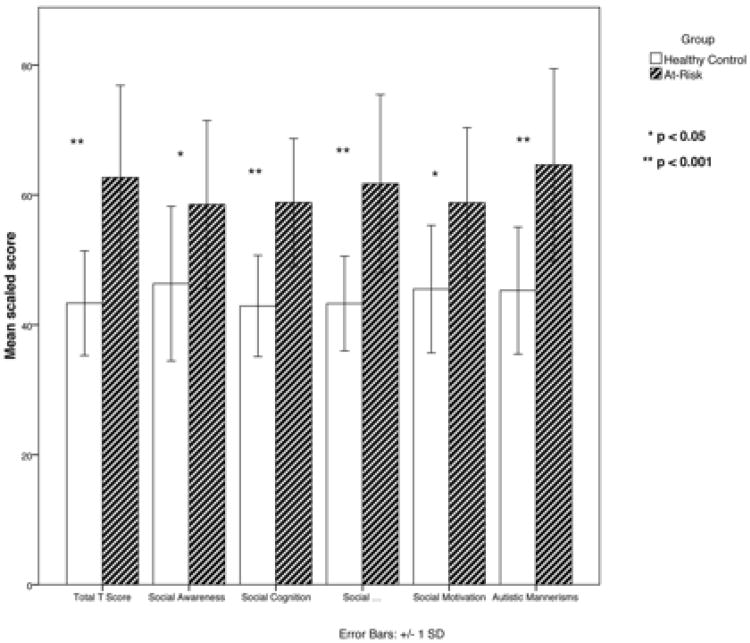
Thirty-five participants (17 HC and 18 HR) had the SRS completed about them. Participants about whom the SRS was completed did not differ from the sample as a whole or from participants without the SRS on file in terms of age, race, gender, IQ or diagnostic subject category (all p>0.05). ANOVA revealed a significant main effect of group on social reciprocity total t-score (F(1,35)=25.6, p<0.001; Cohen's d=1.7), with HR participants displaying more impaired social reciprocity. To investigate which subscale might be driving this finding, a MANCOVA was performed, with group as the fixed factor, IQ as a covariate, and SRS subscales t-scores as the outcome variables. There was a significant effect of group (Wilk's λ=0.001), and post-hoc examination of individual subscale scores revealed a significant group by subscale interaction for all five subscales including Social Awareness, (F(1,35)=8.9, p=0.005; Cohen's d=1.0), Social Cognition (F(1,35)=29.6, p<0.001; Cohen's d=1.8), Social Communication (F(1,35)=25.7, p<0.001; Cohen's d=1.7), Social Motivation (F(1,35)=14.1, p=0.001; Cohen's d=1.2), and Autistic Mannerisms (F(1,35)=21.7, p<0.001; Cohen's d=1.5). Figure 1 shows the mean total score and subscale scores by group.
Figure 1. Social Responsiveness Scale total and subscale scores by group.
Forty-eight participants, 25 HC and 23 HR, completed the NEPSY ToM task. Of these, 43 participants (23 HC and 20 HR) were within the age range for which scaled scores could be completed. Total ToM raw scores were 25.2±2.2 for HR and 25±1.8 for HC. ANOVA revealed no differences between groups on ToM total raw score (F(1,48)=0.1, p=0.7; Cohen's d=0.1). An exploratory MANCOVA was performed, covarying for IQ with group as a fixed factor, but did not allow (Wilk's λ=0.3) for post-hoc ANOVAs to examine the effect of group on ToM verbal or contextual subscale scores. After limiting the sample to the 43 participants for whom a scaled score could be calculated, ANOVA revealed no main effect of group on total scaled score (p=0.7), and MANCOVA with IQ as a covariate and group as a fixed factor did not permit post-hoc ANOVAs (Wilk's λ=02).
Forty-eight participants, 25 HC and 23 HR, completed the Affect Recognition subtest of the NEPSY. ANOVA found no significant effect of group on Affect Recognition total raw score (p=0.2). MANCOVA was performed, covarying for IQ with group as a fixed factor, and did not permit (Wilk's λ=0.6) individual ANOVAs to examine the effect of group on affect recognition subscale scores (i.e. number of errors identifying happy, sad, neutral, fearful, angry, or disgusted faces). When the sample was limited to the 43 (23 HC and 20 HR) participants who were in the age range for which scaled scores could be reliably calculated (under 17 years old), there was no significant effect of group on total scaled score (F(1,48)=2.1, p=0.2; Cohen's d=0.4), and MANCOVA again did not permit post-hoc ANOVAs of subtest scores (Wilk's λ=0.5).
Forty participants, 21 HC and 19 HR, completed the DANVA. There were two subjects who completed the DANVA child faces who did not complete the adult faces. Participants in the HR group made 4±2.3 errors in identifying the emotion displayed on children's faces, and participants in the HC group made 4.2±2.3 errors. ANOVA revealed no difference between groups on the total number of identifying emotions on children's faces (F(1,40)=0.1, p=0.8; Cohen's d=0.1). Using group as a fixed factor and IQ as a covariate there was no effect of group on the total number of errors or types of errors by emotion (i.e. errors identifying happy, sad, angry, or fearful faces, Wilk's λ=0.7). When identifying the emotion displayed on adults' faces, the HR group made 5.2±2.5 errors and the HR group made 5.6±2.7 errors; there was no significant effect of group (F(1,38)=0.3, p=0.6; Cohen's d=0.2). MANCOVA did not permit individual post-hoc ANOVAs to examine the effect of group on total errors or type of errors by emotion (Wilk's λ=0.1).
Among HR participants, diagnostic subject category at the time of enrollment (ADHD without history of depression vs. current or past major depressive episode) and previous medication exposure was examined using MANOVA. The sample was limited to HR participants, and there was no main effect of diagnostic subject category or previous medication exposure on any of the outcome measure (all p>0.05).
Exploratory correlations for the HR group were calculated between SRS and ToM or affect recognition scores to determine whether there was an association between poor social skills and impaired ToM or affect recognition. Higher SRS scores and higher DANVA affect recognition scores indicate poorer performance, whereas lower NEPSY ToM and affect recognition scores indicate poor performance. Among HR participants, there was a significant negative association between SRS total score and NEPSY ToM (r=-0.5, p=0.03), indicating that poorer social reciprocity was associated with worse performance in ToM. There was a significant positive association between NEPSY ToM and affect recognition scores (r=0.6, p=0.001), indicating that worse ToM was associated with worse affect recognition. There was also a significant positive association between total errors in identification of child and adult faces on the DANVA (r=0.5, p=0.02), indicating that participants who had difficulty identifying child faces also had difficulty identifying adult faces.
Discussion
As hypothesized, symptomatic bipolar offspring at high risk for BD had deficits in social reciprocity by parent report. However, these offspring did not exhibit deficits when tested for ToM and affect recognition ability. Exploratory correlations suggested that for youth at high risk for BD, poorer social reciprocity was associated with impaired performance on ToM tasks. Differences in IQ, diagnostic subject category, and medication exposure did not account for the group differences across five domains of social functioning nor did these seem to have contributed to the lack of group differences in ToM and affect recognition.
Children and adolescents with bipolar spectrum disorders have previously been shown to have global impairments in social functioning (Birmaher and Axelson, 2006; Goldstein et al., 2009; Judd et al., 2005; Pine et al., 2008). A large study of offspring of parents with BD also reported social impairment in these youth, 26% of whom had an Axis I disorder, including 16% with ADHD, 3% with a mood disorder, and 11% with an anxiety disorder (Birmaher et al., 2010). Our subjects were similar to those in this latter study, but were likely at even higher risk for developing mania in that they all had either ADHD with moderate mood symptoms or a history of a major depressive episode (Chang et al., 2006; Miklowitz et al., 2011). Thus, our results suggest that the social impairment associated with BD may appear before the onset of mania, consistent with the commonly long prodromal period that youth experience before their first full manic episode (Correll et al., 2007). However, it is not clear whether this social impairment is due to the disruption in normal socio-cognitive development from having psychiatric disturbances such as ADHD and depression, which may be socially isolating, or due to underlying abnormalities in neural development that may have predated symptom onset.
A more detailed examination of underlying socio-emotional processing may help to solve the question of whether deficits in ToM and affect recognition could be driving social impairment in youth at high risk for BD in concert with the socially-isolating effects of having a psychiatric disorder. While ToM has not previously been assessed in children at risk for BD, one study found ToM to be impaired in a cohort of twenty-six children with BD, demonstrated as difficulty in understanding emotionally-valenced false beliefs and understanding the internal thoughts of characters in a story (Schenkel et al., 2008). Our examination of high-risk youth indicated that they were able to put themselves in the mental state of fictional characters as well as healthy children. Thus, it does not appear that for these youth, ToM deficits begin before the first manic episode. However, those high-risk youth that did have relatively poorer ToM scores also had more impairment in social reciprocity, indicating that perhaps for some of these individuals, ToM impairments could lead to poor social functioning, a logical outcome.
Difficulty with identification of emotional faces has also been found in youth with and at-risk for BD (Brotman et al., 2008a; Brotman et al., 2008b; Guyer et al., 2007; McClure et al., 2003; McClure et al., 2005; Rich et al., 2008; Schenkel et al., 2007). Brotman et al., (Brotman et al., 2008b) showed that asymptomatic children with familial risk for BD had difficulties with affect recognition in that they required greater intensity of emotion to identify faces correctly. Therefore, based on this study and the others conducted with at risk samples (Brotman et al., 2008a; Brotman et al., 2008b; Guyer et al., 2007; Rich et al., 2008), we hypothesized that our cohort of symptomatic children with familial risk would share in deficits in affect recognition. However, we did not find any affect recognition differences as measured by the NEPSY and DANVA. There are several possible reasons for this discrepancy. First, the total number of errors made by our subjects completing the NEPSY was low, raising the possibility that the questions were not difficult (i.e. sensitive) enough to detect a group difference. Second, examination of DANVA error rates published previously for HC and youth with BD (McClure et al., 2003) and for HC and asymptomatic children with familial risk for BD (Brotman et al., 2008a) highlights a unique characteristic of these HC subjects. Error rates for this HR group (child faces: 4.0±2.3, adult faces: 5.6±2.7) were similar to error rates published in youth with BD (child faces: 4.3±2.1, adult faces: 5.7±2.4, (McClure et al., 2003) and to asymptomatic bipolar offspring (child faces: mean 3.67, adult faces: mean 5.42, (Brotman et al., 2008a). However, error rates for our HC group (child faces: 4.2±2.3, adult faces: 5.2±2.5) were higher than the error rates for HC from these previous studies: child faces: 2.9±1.9, adult faces: 3.9±2.5 (McClure et al., 2003); child faces: mean 2.20, adult faces: mean 3.82 (Brotman et al., 2008a). Therefore, the relatively elevated rate of errors committed by HC appears to have resulted in similar error rates between our two groups. However, it is not clear why HC subjects in our sample would have these elevated error rates.
Another reason for our findings may be due to the lack of sufficient variability in our facial emotion instruments used. For example, the Emotional Expression Multimorph has previously been used in children with and at-risk for BD and has thirty-nine gradations of intensity for emotional faces. At-risk children required three more gradations of intensity than their healthy counterparts to identify emotional faces in one study (Brotman et al., 2008b) whereas in another study both the at-risk and the BD group required on average four more gradations of intensity to correctly identify emotional faces (Rich et al., 2008). The difference of three or four out of thirty-nine gradations of intensity before identification of an emotional face may reflect subtle neural differences that may or may not be clinically significant. Nonetheless, a logical next step in measuring affect recognition in our high-risk sample is to use a more continuous and detailed measurement. However, it is also possible that our findings were discrepant due to different samples in that our participants were symptomatic and not free of psychiatric disturbance. The fact that the SRS was completed by the affected parent about his/her child and that the child alone completed the NEPSY and DANVA could have also affected our results if a parent's mood influenced perceptions of a child. Finally, offspring of parents with BD with mood disorders themselves may simply not have affect recognition difficulties.
It is possible that social reciprocity is impaired sooner in the course of BD development than ToM and affect recognition. It remains unclear whether social deficits early in the course of illness might predispose to later development of BD or whether intrinsic brain differences underlie the observed social deficits and mood symptoms. Future studies are needed to help understand why youth at high risk for BD demonstrate such profound social impairment. It has been previously suggested that social deficits, though not social reciprocity specifically, demonstrated by bipolar offspring can be explained by parental symptomatology (Bella et al., 2011). Deficits in social reciprocity may reflect the family environment or alternatively, in the context that the SRS was a parent-reported measure and the NEPSY and DANVA were administered directly to study participants, it is possible that an observer effect contributed to either over- or under-reporting of symptoms by participants' parents. Future studies are needed to help understand why youth at high risk for BD have deficits in social reciprocity. It is possible that social deficits are a core reason for much of their functional impairment, just as social impairment appears to drive the functional impairment for many children with autism spectrum disorders (Dawson et al., 1998; Klin et al., 2003; Mundy et al., 1990).
Other potential limitations of the present study include a relatively small sample size, sample heterogeneity based on symptomatology of not only mood disorders but also problems anxiety, and medication exposure. We found that diagnosis and medication exposure did not appear to affect outcome measures. Because of the possibility of Type II error given our small sample size, we calculated effect sizes, which were very small for ToM and affect recognition tasks, indicating that reasonably larger sample sizes would still not likely detect significant differences between groups for these measures. As all subjects made relatively few errors in the NEPSY ToM task and in naming emotional faces using the NEPSY and DANVA, it raises the possibility that more difficult questions may have detected more subtle deficits in HR subjects. With regard to affect recognition specifically, finer gradations of emotional intensity than was afforded by the NEPSY or DANVA may trigger unconscious or subconscious differences in the way at risk youth process emotional faces.
The present study documents global impairment in five domains of social reciprocity but not ToM or affect recognition in a population of youth at high risk for BD. Future exploration following a larger sample over a longer period is needed to determine whether deficits in socio-emotional processing resolve, persist, or evolve. Thus, while it is clear that these high-risk youth have significant social impairment, the underlying mechanism for this impairment is not yet clear enough to develop more targeted interventions. A family-based psychotherapeutic intervention has recently been proven to be efficacious in reducing mood symptoms in youth at high risk for BD (Miklowitz et al., 2011) and may therefore also aid in improving social functioning. Regardless of the underlying etiology of social impairment, incorporating social skills training into a therapeutic treatment model may serve to help children at high risk for BD by improving their social functioning, decreasing their stress, improving their relationships, and thereby possibly helping to protect them from future mood episodes and progression to BD.
Acknowledgments
We thank Dr. Ellen Liebenluft and Dr. Melissa Brotman for their early conversations about the aims of this project, and Ms. Erica Weitz and Ms. Elizabeth Adams for their assistance with data collection.
Funding to support this study was provided by the National Institute of Mental Health (R01 MH077047; K23 MH085919) and the Stanford Medical Scholars Fellowship Program.
Footnotes
Dr. Chang is a consultant for GlaxoSmithKline, Eli Lilly and Company, Bristol-Myers Squibb, and Merck; he receives research support from GlaxoSmithKline, Merck, and the National Institute of Mental Health. All other authors declare no conflicts of interest.
Dr. Chang, Dr. Whitney, and Megan Howe designed the study and wrote the protocol in consultation with Dr. Phillips. All authors participated actively in data collection. Dr. Whitney conducted the literature search, statistical analyses, and wrote the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bella T, Goldstein T, Axelson D, Obreja M, Monk K, Hickey MB, Goldstein B, Brent D, Diler RS, Kupfer D, Sakolsky D, Birmaher B. Psychosocial functioning in offspring of parents with bipolar disorder. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellivier F, Golmard JL, Henry C, Leboyer M, Schurhoff F. Admixture analysis of age at onset in bipolar I affective disorder. Arch Gen Psychiatry. 2001;58:510–512. doi: 10.1001/archpsyc.58.5.510. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18:1023–1035. doi: 10.1017/S0954579406060500. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Monk K, Kalas C, Obreja M, Hickey MB, Iyengar S, Brent D, Shamseddeen W, Diler R, Kupfer D. Psychiatric Disorders in Preschool Offspring of Parents With Bipolar Disorder: The Pittsburgh Bipolar Offspring Study (BIOS) Am J Psychiatry. 2010;167:321–330. doi: 10.1176/appi.ajp.2009.09070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Guyer AE, Lawson ES, Horsey SE, Rich BA, Dickstein DP, Pine DS, Leibenluft E. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry. 2008a;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Skup M, Rich BA, Blair KS, Pine DS, Blair JR, Leibenluft E. Risk for bipolar disorder is associated with face-processing deficits across emotions. J Am Acad Child Adolesc Psychiatry. 2008b;47:1455–1461. doi: 10.1097/CHI.0b013e318188832e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter TD, Mundo E, Parikh SV, Kennedy JL. Early age at onset as a risk factor for poor outcome of bipolar disorder. Journal of Psychiatric Research. 2003;37:297–303. doi: 10.1016/s0022-3956(03)00052-9. [DOI] [PubMed] [Google Scholar]
- Chang K, Howe M, Gallelli K, Miklowitz D. Prevention of pediatric bipolar disorder: integration of neurobiological and psychosocial processes. Ann N Y Acad Sci. 2006;1094:235–247. doi: 10.1196/annals.1376.026. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Correll CU, Penzner JB, Frederickson AM, Richter JJ, Auther AM, Smith CW, Kane JM, Cornblatt BA. Differentiation in the preonset phases of schizophrenia and mood disorders: evidence in support of a bipolar mania prodrome. Schizophr Bull. 2007;33:703–714. doi: 10.1093/schbul/sbm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. User guide for the structured clinical interview for DSM IV axis I disorders. American Psychiatric Association; Washington, DC: 1996. [Google Scholar]
- Geller B, Williams M, Frazier J. WASH-U-KSADS (Washington University in St Louis Kiddie Schedule for Affective Disorders and Schizophrenia. Washington University; St. Louis, Missouri: 1996. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- Goldstein TR, Birmaher B, Axelson D, Goldstein BI, Gill MK, Esposito-Smythers C, Ryan ND, Strober MA, Hunt J, Keller M. Psychosocial functioning among bipolar youth. J Affect Disord. 2009;114:174–183. doi: 10.1016/j.jad.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure EB, Adler AD, Brotman MA, Rich BA, Kimes AS, Pine DS, Ernst M, Leibenluft E. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Leon AC, Solomon DA, Coryell W, Maser JD, Keller MB. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 2005;62:1322–1330. doi: 10.1001/archpsyc.62.12.1322. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F. The enactive mind, or from actions to cognition: lessons from autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:345–360. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp SL. NEPSY II Clinical and interpretative manual. Psychological Corporation; San Antonio, TC: 2007. [Google Scholar]
- Leverich GS, Post RM, Keck PE, Jr, Altshuler LL, Frye MA, Kupka RW, Nolen WA, Suppes T, McElroy SL, Grunze H, Denicoff K, Moravec MK, Luckenbaugh D. The poor prognosis of childhood-onset bipolar disorder. J Pediatr. 2007;150:485–490. doi: 10.1016/j.jpeds.2006.10.070. [DOI] [PubMed] [Google Scholar]
- McClure EB, Pope K, Hoberman AJ, Pine D, Leibenluft E. Facial Expression Recognition in Adolescents with Mood and Anxiety Disorders. American Journal of Psychiatry. 2003;160:1172–1174. doi: 10.1176/appi.ajp.160.6.1172. [DOI] [PubMed] [Google Scholar]
- McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Twobin KE, Charney DS, Pine D, Leibenluft E. Deficits in Social Cognition and Response Flexibility in Pediatric Bipolar Disorder. American Journal of Psychiatry. 2005;162 doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- Miklowitz D, Chang K, Taylor D, George E, Singh M, Schneck C, Dickinson L, Howe M, Garber J. Early psychosocial intervention for youth at risk for bipolar I or II disorder: a one-year treatment development trial. Bipolar Disord. 2011;13:67–75. doi: 10.1111/j.1399-5618.2011.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. J Autism Dev Disord. 1990;20:115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Duke MP. Individual differences in the nonverbal communication of affect: The diagnostic analysis of nonverbal accuracy scale. Journal of Nonverbal Behavior. 1994;18:9036. [Google Scholar]
- Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, Bowden CL, Sachs GS, Nierenberg AA. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Pine DS, Guyer AE, Goldwin M, Towbin KA, Leibenluft E. Autism spectrum disorder scale scores in pediatric mood and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2008;47:652–661. doi: 10.1097/CHI.0b013e31816bffa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children's depression rating scale. J Am Acad Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol. 2008;20:529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel LS, Marlow-O'Connor M, Moss M, Sweeney JA, Pavuluri MN. Theory of mind and social inference in children and adolescents with bipolar disorder. Psychol Med. 2008;38:791–800. doi: 10.1017/S0033291707002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel LS, Pavuluri MN, Herbener ES, Harral EM, Sweeney JA. Facial Emotion Processing in Acutely Ill and Euthymic Patients With Pediatric Bipolar Disorder. J Am Acad Child Psy. 2007;46:1070–1079. doi: 10.1097/chi.0b013e3180600fd6. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Finn CT. Family, win, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- Towbin KE, Pradella A, Gorrindo T, Pine D, Leibenluft E. Autism Spectrum Traits in Children with Mood and Anxiety Disorder. Journal of Child and Adolescent Psychopharmacology. 2005;15:452–464. doi: 10.1089/cap.2005.15.452. [DOI] [PubMed] [Google Scholar]
- Weschler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; New York: 1999. [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]