Abstract
Anxiety negatively affects quality of life and psychosocial functioning. Previous research has shown that anxiety symptoms in healthy individuals are associated with variations in the volume of brain regions, such as the amygdala, hippocampus, and the bed nucleus of the stria terminalis. Brain lesion data also suggests the hemisphere damaged may affect levels of anxiety. We studied a sample of 182 male Vietnam War veterans with penetrating brain injuries, using a semi-automated voxel-based lesion-symptom mapping (VLSM) approach. VLSM reveals significant associations between a symptom such as anxiety and the location of brain lesions, and does not require a broad, subjective assignment of patients into categories based on lesion location. We found that lesioned brain regions in cortical and limbic areas of the left hemisphere, including middle, inferior and superior temporal lobe, hippocampus, and fusiform regions, along with smaller areas in the inferior occipital lobe, parahippocampus, amygdala, and insula, were associated with increased anxiety symptoms as measured by the Neurobehavioral Rating Scale (NRS). These results were corroborated by similar findings using Neuropsychiatric Inventory (NPI) anxiety scores, which supports these regions’ role in regulating anxiety.
In summary, using a semi-automated analysis tool, we detected an effect of focal brain damage on the presentation of anxiety. We also separated the effects of brain injury and war experience by including a control group of combat veterans without brain injury. We compared this control group against veterans with brain lesions in areas associated with anxiety, and against veterans with lesions only in other brain areas.
Keywords: anxiety, traumatic brain injury, voxel-based lesion symptom mapping
1. Introduction
Anxiety is an emotional response that arises in situations of conflict and uncertainty (Gray, 1982). The symptoms of anxiety include hyperarousal and worry (Bishop, 2007). In stress-provoking situations, the behavioral expressions of anxiety, such as displays of tension, increased agitation and locomotion, and defensive hostile behavior may be observed and reliably quantified in humans (Lippert-Gruner, Kuchta, Hellmich, & Klug, 2006) and other species (Kalin & Shelton, 2003).
In this study, we wished to investigate areas in the brain that when lesioned affect anxiety levels. We used voxel-based lesion-symptom mapping (VLSM) to determine where lesions are associated with higher NRS anxiety ratings. VLSM computes a t-value comparing behavioral scores in patients with and without damage to a voxel. It performs a whole-brain, voxel-by-voxel, hypothesis-free analysis, rather than attempting to categorize patients based on lesion location. Because TBI patients have been shown to have increased prevalence and severity of anxiety (Fann, Katon, Uomoto, & Esselman, 1995; Rapoport, McCauley, Levin, Song, & Feinstein, 2002), we expected that the NRS would reveal increased anxiety in veterans with TBI. We used a control group of veterans who did not suffer brain injury, but who otherwise shared a similar war experience.
Studies have reported increased anxiety and PTSD (a form of anxiety) in those with war experience and TBI. In one study, 74% percent of Vietnam veterans who experienced combat also reported symptoms of PTSD (Buydens-Branchey, Noumair, & Branchey, 1990). This same study found that longer combat exposure and increased combat intensity were both associated with increased likelihood of PTSD. Also, while 62% of those not wounded in the war reported a lifetime occurrence of PTSD, 92% who suffered physical injuries of any type in the war reported a lifetime occurrence of PTSD. Epstein & Ursano (1994) found that 29% of brain-injured patients were clinically diagnosed with anxiety following their traumatic brain injuries (TBI). Carlson et al. (2010) found increased anxiety disorders and PTSD in war veterans with TBI compared to war veterans without TBI.
While there are factors other than brain damage that can increase anxiety after TBI, including memories of the event that caused the TBI (R. S. Epstein & Ursano, 1994), Jorge et al. (1993) suggest that anxiety in brain-injured patients may be related to the extent and location of brain damage. Anxiety and fear are mediated at least partially by different brain regions. Fear is a biologically adaptive physiological and behavioral response to an actual or anticipated occurrence of an explicit threatening stimulus (Bishop, 2007). Anxiety is, in many ways, similar to fear, although it is less stimulus-specific, has a slower onset, and is longer lasting (Davis, 1998; Walker, Toufexis, & Davis, 2003). The difference can be portrayed as follows: anxiety occurs during approach or movement toward a dangerous situation and increases awareness and preparedness, while fear occurs during escape from a dangerous situation or threat (R. J. Blanchard, Yudko, Rodgers, & Blanchard, 1993; Gray & McNaughton, 2000). Early research found that the destruction of the amygdala and temporal lobes leads to reduced anxiety in both humans and animals. Monkeys who had their bilateral temporal lobe and amygdala removed had no fear of approaching other animals or objects, and were less fearful and hostile toward humans (Kluver & Bucy, 1939; Weiskrantz, 1956). More recent work in humans found that removal of the temporal lobes impaired recognition of vocal fear (Dellacherie, Hasboun, Baulac, Belin, & Samson, 2011). Lesions of smaller regions provide more precise results. Reviews of both animal and human literature report that the bed nucleus of the stria terminalis (part of the extended amygdala; Fox et al., 2010; Walker et al., 2003) is involved in anxiety, and the amygdala is involved in fear (Davis, 1998; Walker et al., 2003). Truitt et al. (2009) found that lesions of interneurons in the anterior and posterior divisions of the basolateral amygdala in rats resulted in increased anxiety-like behaviors.
Other brain regions involved in anxiety include the hippocampus (in animals: Barkus et al., 2010; and in PTSD patients: Bossini et al., 2008), insula (Simmons, Strigo, Matthews, Paulus, & Stein, 2006; Uchidi et al., 2000), medial prefrontal cortex (PFC) (in rats: Blanco et al., 2009) and the orbitofrontal cortex (Kringelbach & Rolls, 2004; Milad & Rauch, 2007).
Anxiety levels may also be affected by the hemisphere of the lesions. From the same cohort as the current study, those TBI veterans with right orbitofrontal lesions reported higher anxiety than those with left orbitofrontal lesions and controls (Grafman, Vance, Weingartner, Salazar, & Amin, 1986). In patients with both closed head injury and depression, those with right hemisphere lesions were more likely to have anxiety in addition to their depression (Jorge et al., 1993). Patients with tumors in the right hemisphere had higher anxiety than those with tumors in the left hemisphere (Mainio et al., 2003).
The picture is not so simple however. Using the same cohort as the current study, Koenigs et al. (2008) found that veterans with lesions in vmPFC and amygdala were less likely to have PTSD, while those with posterior lesions had a rate similar to controls.
2. Material and Methods
2.1 Subjects
Veterans were drawn from Phase III of the W.F. Caveness Vietnam Head Injury Study registry (VHIS), a longitudinal study of brain-injured veterans, mainly with focal penetrating injuries, and uninjured combat control veterans (Raymont, Salazar, Krueger, & Grafman, 2011). In Phase I, 56% of brain-injured veterans were working compared with 82% of control veterans (Schwab, Grafman, Salazar, & Kraft, 1993). Equal percentages of brain-injured and control veterans were living with their wives (74%). Phase III (2003–2006) was conducted at the National Naval Medical Center in Bethesda, MD. The veteran population offers a number of methodological advantages including its large sample size, relative uniformity, and access to pre-injury data for comparison with post-injury performance.
One hundred and eighty-two brain-injured male combat veterans and 51 uninjured combat veterans with CT scans for whom the NRS was completed were included in this study. The TBI and control groups did not differ significantly in age or level of education (see Table 1). All subjects gave informed written consent in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All study procedures were approved by Institutional Review Boards at the National Naval Medical Center and the National Institute of Neurological Disorders and Stroke.
Table 1.
Comparison between veterans with TBI and control veterans on means and standard deviations for demographic information and neurobehavioral scores, and medians and mean ranks for anxiety and depression scores.
| Variable | Veterans with TBI | Control veterans | Statistics (2- tailed) |
|---|---|---|---|
| Age (years) | 58.32 ± 3.09 | 59.08 ± 3.52 | t(231)= 1.50; p = .14 |
| Education (years) | 14.74 ± 2.59 | 15.25 ± 2.50 | t(228)= 1.24; p = .22 |
| Post-injury AFQT percentile score | 52.71 ± 25.01 | 67.96 ± 22.04 | t(227)= 3.94; p < .001** |
| WMS-III working memory primary index percentile score | 49.19 ± 28.37 | 62.70 ± 27.76 | t(225)= 2.99; p = .003* |
| NRS anxiety (medians) | 1.00 | 1.00 | U = 4087, z = 1.55, p = .12 |
| NPI anxiety (medians) | 0 | 0 | U = 3830, z = 0.72, p = .47 |
| State anxiety scaled scores (medians) | 48.00 | 49.50 | U = 4407, z = 0.64, p = .52 |
| Trait anxiety scaled scores (medians) | 51.00 | 54.50 | U = 4135, z = 1.34, p = .18 |
| SCID: PTSD Lifetime Prevalence (medians) | 2.00 | 2.00 | U = 3777, z = 2.23, p = .03* |
| SCID: Major Depressive Disorder Lifetime Prevalence (medians) | 1.00 | 1.00 | U = 4194, z = 1.35, p = .18 |
| BDI total score (medians) | 6.00 | 9.00 | U = 4079, z = 1.47, p = .14 |
AFQT = Armed Forces Qualification Test. BDI = Beck Depression Inventory. NPI = Neuropsychiatric Inventory. NRS = Neurobehavioral Rating Scale. PTSD = Post-traumatic stress disorder. SCID= Structured Clinical Interview for DSM Disorders. U = Mann-Whitney. WMS-III = Wechsler Memory Scale.
Significant at p = .01,
Significant at p = .001.
2.2 CT-imaging and lesion identification
MRIs were precluded as many of the veterans had retained metal shrapnel in their heads; therefore axial CT scans were acquired. These were performed without contrast in helical mode on a GE LightSpeed Plus CT scanner, and images were reconstructed with an in-plane voxel size of 0.4 × 0.4 mm, an overlapping slice thickness of 2.5 mm, and a 1 mm slice interval. Lesion location was determined from CT images by manual tracing on each slice using the Analysis of Brain Lesions (ABLe) software implemented in MEDx v3.44 (Medical Numerics) (Makale et al., 2002; Solomon, Raymont, Braun, Butman, & Grafman, 2007). Lesion volume was calculated by summing the traced areas and multiplying by slice thickness. The tracing was performed by a physician with clinical experience reading CT scans (VR), and reviewed by an experienced observer (JG) who was blind to the results of the clinical evaluation and neuropsychological testing. The TBI veteran’s CT scans were normalized to a CT template in Montreal Neurological Institute (MNI) space, using the AIR algorithm with a 12-parameter affine fit and linear transformation (Woods, Grafton, Watson, Sicotte, & Mazziotta, 1998). To optimize efficacy of the registration procedure, the brain images were first automatically skull-stripped. Voxels inside the traced lesion were not included in the spatial normalization procedure. The spatial transformation was applied to the traced lesion, bringing it into MNI space. See Supplementary Figure 1 for examples of brain lesions in our participants, and Supplementary Figure 2 for assessments of registration accuracy. For each subject, the resulting normalized lesion mask image was used in the VLSM analysis using ABLe software. Specifically, the VLSM analysis computed a t-value comparing NRS anxiety scores in those with and without damage to a voxel. We performed VLSM on all voxels in the brain where at least four subjects had a lesion. We used two different brain atlases to determine the anatomic location of the voxels that significantly correlated with the anxiety scores. These atlases were the AAL atlas database for grey matter (Tzourio-Mazoyer et al., 2002) and the White Matter atlas database for white matter (Mori, Wakana, Nagae-Poetscher, & van Zijl, 2005).
2.3 Neuropsychological Tests
A range of neuropsychological functions, including memory and executive functioning were assessed in a battery of tests administered over 5–7 days. This study used a subset of these, as follows. Post-injury general intelligence was assessed with the Armed Forces Qualification Test (AFQT-7A, United States Department of Defense, 1960). Scores on this test correlate highly with the Wechsler Adult Intelligence Scale (WAIS) intelligence quotient scores (Grafman et al., 1988; Wechsler, 1997a). Working memory was assessed with the Wechsler Memory Scale- III working memory primary index scores (WMS-III, Wechsler, 1997b). Depression was assessed with both the Beck Depression Inventory scores (BDI; Beck, Steer, & Brown, 1996) and the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID) major depression lifetime prevalence scores (First, Spitzer, Gibbon, & Williams, 2002). PTSD was assessed with the SCID PTSD lifetime prevalence scores (First et al., 2002).
After approximately 25 hours of formal testing and general interactions, an experienced research assistant rated each participant using the NRS (Levin et al., 1987), based on observations of the participant’s spontaneous behavior. The NRS is a validated (Corrigan, Dickerson, Fisher, & Meyer, 1990) 27-item instrument used to measure the severity of behavioral sequelae following TBI. Each item required a rating on a scale from 1 (not present) to 7 (extremely severe). We used the NRS anxiety subscale, which measures worry, fear, or over-concern for the present or future. For the UCLA Neuropsychiatric Inventory (NPI, Cummings et al., 1994), the examiner read the questions aloud to a companion who knew the participant well. Companions were generally spouses, partners, or adult children, most of whom gave their answers via telephone. For each abnormal behavior present, a rating for that behavior was elicited on a severity scale from 1 (mild) to 3 (severe), and on a frequency scale from 1 (occasionally, less than once a week) to 4 (very frequently, once or more per day or continuously). We used the frequency x severity scores for NPI anxiety in our analyses. In addition, participants completed the state and trait anxiety inventories (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). We used standard scores for this; higher scores mean higher anxiety.
2.4 Statistical Analysis
Analysis of the behavioral data was performed with IBM© SPSS© 19.0 (www.spss.com), and alpha was set to .05 for all analyses. Two-tailed independent samples t-tests were performed to compare veterans with TBI and controls in age, years of education, AFQT scores, and WMS-III measures. Kolmogorov-Smirnov testing on NRS anxiety, NPI anxiety, STAI, and BDI scores revealed that both groups had significantly non-normal distributions, because most individuals showed no overt signs of anxiety or depression. We therefore used non-parametric tests for these. Non-parametric tests were also used for lifetime prevalence variables since they were not scalar.
Using the VLSM software in ABLe, we created a lesion density map to show how many veterans with TBI had lesions at each voxel by overlaying their individual normalized lesion maps. We then performed one-tailed t-tests at each voxel to find any significant positive associations of lesioned voxels and NRS anxiety, NPI anxiety, and STAI for grey matter and again for white matter. An FDR correction of .05 for multiple comparisons was used, and a minimum cluster size of 10 voxels.
Because not every injured brain is lesioned at every voxel, statistical power is often lacking in VLSM analyses. We tolerated low power in order to be able to test lesion locations over much of the brain. For our study, we chose the minimum number of cases with overlapping lesions to be 4 at any voxel (see Gläscher et al., 2009, who used a similar criterion). If fewer than 4 injured veterans had a lesion in a given voxel, that voxel was excluded from our analyses.
After the VLSM analysis, we divided the brain-injured veterans into two groups: those who had lesions in significant grey matter areas resulting from the VLSM analysis for NRS anxiety, and those who had lesions only in other grey matter areas. We used two-tailed Mann-Whitney tests to compare median NRS, NPI, STAI scores, BDI scores, PTSD lifetime prevalence, and depression lifetime prevalence for each of the two TBI groups resulting from our VLSM analysis with those of the control veterans and with each other. To investigate the potential impact of brain volume loss, we performed Pearson correlations between percentage of brain volume loss and NRS anxiety in all injured veterans, and between volume loss in each hemisphere and NRS anxiety.
3 Results
3.1 Behavioral Results
Controls had significantly higher AFQT and WMS-III scores than injured veterans, although the scores of the injured veterans were within the normal range (see Table 1). Injured and control veterans did not differ significantly on the NRS, NPI, or STAI anxiety measures. Injured and control veterans did not differ significantly in their likelihood of suffering from depression, but brain injured veterans were less likely to have had PTSD in their lifetimes than control veterans.
3.2 VLSM Results
Figure 1 shows the lesion density overlap map for all 182 veterans with TBI. The maximum overlap of 32 subjects occurred in prefrontal areas.
Figure 1.
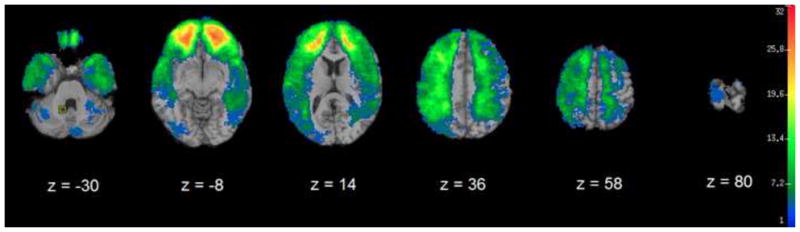
Color indicates the number of participants’ lesions overlapping at each voxel. A minimum overlap of four lesions is required for VLSM analysis.
Table 2 and Figure 2 show the results of the VLSM analysis for NRS anxiety. Lesioned regions in the left temporal lobe, fusiform, inferior occipital lobe, and insula, along with limbic structures including the hippocampus, parahippocampus, and amygdala were associated with increased NRS anxiety. In the right hemisphere, a small lesioned region in the superior and middle temporal lobes was associated with increased NRS anxiety.
Table 2.
Clusters of lesioned voxels associated with NRS anxiety (t-test, FDR = .05 correction for multiple comparison). AAL structures, hemisphere, x, y, z coordinates of the voxel with the large Z-value (note that if there is more than one voxel with this Z-value in the cluster, we used the most inferior), cluster volume, and Z-value. Corresponds with Figure 1.
| Location | Hemisphere | x | y | z | Volume (voxels) | Z |
|---|---|---|---|---|---|---|
| Middle, inferior, and superior temporal lobe, hippocampus, fusiform, superior temporal pole, inferior occipital, parahippocampus, amygdala, insula | Left | −38 | −44 | −12 | 3017 | 5.34 |
| Superior and middle temporal lobe | Right | 70 | −10 | −6 | 40 | 3.65 |
x, y, z, Talairach coordinates.
Figure 2.
Color indicates brain regions where the association between lesion location and NRS anxiety score is statistically significant, based on t-tests and after correction for multiple comparisons (FDR). The left is on the viewer’s right.
VLSM results for NPI anxiety overlapped considerably with those for NRS anxiety. Lesioned regions in the left temporal lobe, fusiform, middle occipital lobe, lingual gyrus, precuneus, and cerebellum, along with limbic structures including the hippocampus and parahippocampus were associated with increased NPI anxiety. There were no lesioned regions in the right hemisphere associated with increased NPI anxiety. There were no lesioned regions associated with STAI scores.
Comparisons of NRS anxiety between the resulting three groups (veterans with lesions in areas significantly associated with NRS anxiety, veterans with lesions only in other areas, and control veterans) revealed a trend toward a significant difference only between veterans with lesions in areas significantly associated with NRS anxiety (mean 2.14 ± 1.58) and control veterans (mean 1.49 ± 1.07) (Mann-Whitney U = 760, n1 = 37, n2 = 51, p = .059, 2-tailed) with veterans with significant lesions having higher anxiety than control veterans. This was expected based on our VLSM results. Veterans with lesions only in other areas had a mean of 1.68 ± 1.13 which was in between the other two groups. There were no significant differences for the NPI anxiety measure (p > .37) or the STAI measures for the three groups (state: p > .47, trait: p > .13).
Comparisons of PTSD lifetime prevalence revealed that veterans with lesions in significant areas had a significantly lower likelihood of PTSD (mean 1.62 ± 0.83) than control veterans (mean 2.17 ± 0.88) (Mann-Whitney U = 643, n1 = 37, n2 = 52, p = .004). PTSD comparisons between veterans with lesions in significant areas and those with lesions only in other areas (mean 1.97 ± 1.07), and between those with lesions only in other areas and control veterans revealed trends toward significance (Mann-Whitney U = 2150, n1 = 37, n2 = 142, p = .07; Mann-Whitney U = 3134, n1 = 142, n2 = 52, p = .08, respectively).
Comparisons of major depression lifetime prevalence between the three groups revealed no significant differences (p > .17). Similarly, comparisons of BDI scores revealed no significant differences (p > .11).
To investigate whether our VLSM results could be partially due to differences in brain volume loss, we performed t-tests comparing percentages of volume loss between the two resulting groups. While there was not a significant difference in total brain volume between groups (1.35 ± 0.11 vs. 1.36 ± 0.12) [t(180) = .76, p = .45]), we found that veterans with damage in significant lesion areas had a significantly greater percentage of volume loss than those with damage only in other areas (5.77% ± 5.25% vs. 2.41% ± 2.61%) [t(40.62) = 3.77, p = .001]. To investigate the potential impact of brain volume loss, we performed Pearson correlations between percentage of brain volume loss and NRS anxiety in all injured veterans. There were trend-level positive relationships between total brain volume loss and NRS anxiety (r = .13, p = .08) and left hemisphere volume loss and NRS anxiety (r = .12, p = .10); but not between right hemisphere volume loss and NRS anxiety (r = .04, p = .60).
Injury to several white matter tracts was significantly associated with NRS anxiety. These were the sagittal stratum, posterior thalamic radiation, superior longitudinal fasciculus, fornix/stria terminalis, and uncinate fasciculus, all on the left (see Table 3 for z-values). The superior longitudinal fasciculus, fornix/stria terminalis, and uncinate fasciculus all connect grey matter regions implicated in modulating anxiety based on our results: the superior longitudinal fasciculus is located in the dorsolateral part of the corona radiata and contains connections between the frontal, parietal, occipital, and temporal lobes. The fornix and stria terminalis are both connected to the limbic system: the fornix to the hippocampus, and the stria terminalis to the amygdala (Weller & Smith, 1982). The uncinate fasciculus connects the frontal lobe (orbital cortex) and the anterior temporal lobe (Kier, Staib, Davis, & Bronen, 2004).
Table 3.
White matter tracts where damage was associated with NRS anxiety.
| % | Structure | Hemisphere | Z-value | Cohen’s d |
|---|---|---|---|---|
| 5.56 | Sagittal stratum | Left | 5.01 | 2.64 |
| 0.82 | Posterior thalamic radiation | Left | 3.99 | 1.58 |
| 0.07 | Superior longitudinal fasciculus | Left | 3.34 | 1.32 |
| 0.33 | Fornix/stria terminalis | Left | 3.27 | 1.69 |
| 0.16 | Uncinate fasciculus | Left | 3.27 | 1.69 |
4 Discussion
We studied a sample of 182 male Vietnam War veterans with penetrating brain injuries, using voxel-based lesion-symptom mapping which examined whether there are any significant associations between anxiety and location of brain lesions. Injured and control veterans did not differ significantly from each other on behavioral measures of anxiety including the NRS, NPI or STAI anxiety measures. This may be due to several reasons. The first is that TBI, per se, may not result in increased anxiety in general. The second is that the controls were also war veterans and both groups may have had elevated anxiety due to their war experience.
Our analysis found specific brain areas that when lesioned resulted in higher NRS anxiety. Damage to similar regions also was associated with another measure of anxiety, NPI anxiety scores. The correspondence of results from these two measures of anxiety supports these regions’ role in the regulation of anxiety. Our significant lesion results are specific to anxiety as measured by NRS and NPI, since we found no lesioned areas associated with STAI scores. Therefore our results are limited to observer-completed rating scales rather than self-reports, as the NPI is completed by another person who knows the veteran well, and the NRS by a researcher, while the STAI is completed by the patient. These differing results may be due to diminished self-insight in those with brain damage (Levin et al., 1987) which is evident on self-reports, or to differences in internal versus external symptoms of anxiety.
Our VLSM results included areas of lesion in bilateral temporal lobe. Consistent with this, a study of pediatric TBI patients found that temporal lobe damage was positively correlated with PTSD (Vasa et al., 2004). Also, people with panic disorder were found to have significantly smaller temporal lobes than normal controls (Vythilingam et al., 2000). On the other hand, in another study of pediatric patients, those with generalized anxiety had larger superior temporal gyri than did normal controls (De Bellis et al., 2002). Our results included mesial temporal lobe, including the hippocampus, parahippocampus, amygdala, and insula, along with the fusiform gyrus, and inferior occipital lobe. While it is difficult to pinpoint exact regions of the brain with certainty using CT images of penetrating brain injuries, the mesial temporal lobes have been shown to be involved in anxiety and fear processing. The hippocampus plays a role in contextual fear processing both in animals (Anagnostaras, Gale, & Fanselow, 2001; Kim & Fanselow, 1992; Phillips & LeDoux, 1992; Selden, Everitt, Jarrard, & Robbins, 1991) and humans (Shin & Liberzon, 2010). The hippocampus projects to the PFC and to the bed nucleus of the stria terminalis/hypothalamus and the amygdala; hence, it is well-placed for a role in emotion, including anxiety (Bannerman et al., 2004; Barkus et al., 2010; Davis, 1998; Goldman-Rakic, Selemon, & Schwartz, 1984; Gray & McNaughton, 2000; Petrovic, Canteras, & Swanson, 2001; Swanson & Cowan, 1977).
Consistent with our results, a VBM study in monozygotic twins found smaller left parahippocampal regions in those at high risk for anxiety (de Geus et al., 2006). Our results are also consistent with Syal et al.’s (2012) in that those with social anxiety disorder have reduced fusiform grey matter thickness. Parahippocampal, fusiform, and inferior occipital regions are involved in recognition of objects, faces, and spaces (R. Epstein, Harris, Stanley, & Kanwisher, 1999; R. Epstein & Kanwisher, 1998; Haxby, Hoffman, & Gobbini, 2000; Schiltz et al., 2006). Damage to these areas could lead to increased anxiety due to feeling less grounded in social and other scenarios. The veterans’ brain damage might have affected the way in which they reacted to novel stimuli in the testing environment (Sander, Grafman, & Zalla, 2003)
Our results are consistent with a large body of literature pointing to the amygdala’s role in the acquisition of fear conditioning and expression of fear responses in monkeys (Kluver & Bucy, 1939; Weiskrantz, 1956). Anxiety is associated with amygdala hyperactivity in humans with PTSD and in anxious and depressed children (Rauch et al., 2000; Thomas et al., 2001). The amygdala is a complex structure composed of several nuclei (Adolphs, 2010; Davidson et al., 2002; Davis, 1994; File, Gonzalez, & Gallant, 1998; Kalin, Shelton, & Davidson, 2004). For a review, see LeDoux (2000). Our methods did not allow for a fine-grained analysis of the locations within the amygdala. The amygdala has connections to the superior temporal gyri (De Bellis et al., 2002), an area that was also associated with anxiety in our study. The central nucleus of the amygdala (CeA) connects to subcortical regions such as the hypothalamus, basal forebrain, and brainstem and is therefore well positioned to mediate anxiety as these areas are involved in the stress response (Amaral, Price, Pitkänen, & Carmichael, 1992; Davis, 1992; Kalin et al., 2004). The basolateral regions of the amygdala connect to the cortex, including orbitofrontal and ventromedial regions, as shown in primates (Amaral et al., 1992). These regions regulate negative emotions in humans and animals (Quirk & Beer, 2006). As work performed mainly in rats shows, the bed nucleus of the stria terminalis, part of the extended amygdala, has been implicated in long lasting anxiety but not acute fear (Davis, 1998; Walker et al., 2003).
The insula is part of the extended limbic system, and it is connected to some of the other regions we found associated with anxiety, including the temporal lobe, hippocampus, and amygdala (Augustine, 1996). The insula’s functioning is altered in anxiety disorders (Paulus & Stein, 2006). It responds to fearful versus neutral faces in participants with specific phobias (Wright, Martis, McMullin, Shin, & Rauch, 2003). An increase in blood flow in the insula was found during symptom provocation that was shared across three anxiety disorders: obsessive-compulsive disorder, simple phobia, and PTSD (Rauch, Savage, Alpert, Fischman, & Jenike, 1997). Another study found increased blood flow in the insula during high anxiety (shock) compared to low anxiety (no shock) conditions in humans (Chua, Krams, Toni, Passingham, & Dolan, 1999).
Our white matter results also have support in the literature. The uncinate fasciculus links the PFC, which is involved in top-down regulation of emotion (Davidson, 2002), with the hippocampus and amygdala (Montag, Reuter, Weber, Markett, & Schoene-Bake, 2012). Fractional anisotropy (FA) in the right uncinate fasciculus was found to be lower in patients with generalized social anxiety disorder (Phan et al., 2009). Higher FA in the left superior longitudinal fasciculus and uncinate fasciculus has been shown to be correlated with higher trait anxiety in males (Montag et al., 2012).
Our results support a role for the temporal lobe, hippocampus, fusiform and parahippocampal regions, amygdala, and insula in anxiety; however, the direction of change in our results differs from many, but not all, other studies. In our findings, damage to these areas is associated with increased anxiety. Some studies have shown results similar to ours, in that altered function and anatomy in limbic structures resulted in increased anxiety. For example, in a voxel-based morphometry study of healthy subjects, our group found that smaller left amygdala and parahippocampal gyrus volume was associated with increased anxious symptoms as measured by the STAI (Spampinato, Wood, De Simone, & Grafman, 2009). Further, when inhibitory basolateral amygdala interneurons were lesioned, rats had increased anxiety-like behavior in social situations (Sanders & Shekhar, 1995a, 1995b; Truitt et al., 2009; Truitt et al., 2007).
On the other hand, some studies show decreased fear and anxiety after lesions to amygdala and hippocampus (see Bannerman et al., 2004; Davis, 1992). For instance, lesions of the amygdala are known to block several measures of innate fear in rats (cf., D. C. Blanchard & Blanchard, 1972). Monkeys with amygdala lesions show less caution in approaching potential predators, such as snakes, to which they normally have an innate fear response (Machado, Kazama, & Bachevalier, 2009), and show less initial avoidance of human strangers and unfamiliar objects (Mason, Capitanio, Machado, Mendoza, & Amaral, 2006). In patients tested after temporal lobe resection for the relief of epilepsy, Dellacherie et al. (2011) found that amygdala lesions impaired recognition of fear expressed by voice, and led to decreased anxiety. Others have shown that hippocampal lesions in animals led to reduced anxiety on a variety of behavioral tests (Barkus et al., 2010; Deacon, Bannerman, & Rawlins, 2002; Gray & McNaughton, 1983). Bannerman et al. (2004) summarized the differential effects on anxiety for amygdala versus ventral hippocampus lesions. Depending on the extent of destruction of the amygdala, the resulting effects may range from a decrement in one’s ability to regulate anxiety (leading to increased anxiety) to an inability to generate anxiety when there is more substantial amygdala damage. The increased anxiety found in our VLSM analysis may be at least partially explained by the fact that amygdala damage was minimal.
Brain damage may result in increased or decreased anxiety depending on whether inhibitory or excitatory neurons are damaged (see Sanders & Shekhar, 1995a, 1995b; Truitt et al., 2009; Truitt et al., 2007, for their work in rats). The effects of damage may also vary depending on whether fear or anxiety is being measured. There is evidence in both animals and humans that the bed nucleus of the stria terminalis, the hippocampus, and the insula regulate anxiety, as opposed to fear, which is modulated more by the amygdala (Barkus et al., 2010; Deacon et al., 2002; Duvarci, Bauer, & Pare, 2009; Gray & McNaughton, 1983; Paulus & Stein, 2006; Wright et al., 2003). There are also different types of anxiety. For instance, Koenigs et al. (2008), using the same cohort, found that lesions in the amygdala significantly affected one form of anxiety (PTSD) but not others (e.g., panic disorder, agoraphobia, social phobia, etc.). Consistent with Koenigs et al. (2008), the significant lesions in our VLSM analysis, which included the amygdala and other limbic structures, were associated with lower rates of PTSD over one’s lifetime.
Our results showed a much larger area of damage associated with anxiety symptoms in the left hemisphere compared to the right. Other studies have also shown hemispheric effects for anxiety, but there is not a consistent pattern. Consistent with our results, in a study of 160 patients with damage limited to one hemisphere, Gainotti (1972) found anxious mood more frequently in left versus right hemisphere-lesioned patients. However, several studies have found right hemisphere involvement with higher anxiety. Mainio et al.’s (2003) results revealed that patients with right hemisphere tumors had substantially higher anxiety than those with tumors on the left. Dellacherie et al. (2011) found that patients who had undergone a left temporal lobe resection for intractable epilepsy (compared to those patients who had undergone a right temporal lobe resection and to normal controls) judged the valence of fear as less unpleasant. A PET study on panic disorder showed increased blood flow in the right parahippocampal gyrus compared to the left (Reiman, Raichle, Butler, Herscovitch, & Robins, 1984). There are also studies showing mixed results for hemisphere involvement. A PET study in monkeys showed that anxious temperament correlated with higher deoxyglucose uptake in the left hippocampus and the right central nucleus of the amygdala (Oler et al., 2010).
Numerous human studies implicate the amygdala in emotional processing, but again the hemisphere affected is not consistent. For example, an fMRI study found greater activation of left amygdala and insula after verbal warnings of impending threat (Phelps et al., 2001). Gläscher and Adolphs (2003) note that amygdala activation in response to emotional stimuli has been observed either bilaterally (Hariri, Bookheimer, & Mazziotta, 2000; Liberzon et al., 2000; Taylor, Liberzon, & Koeppe, 2000) or in the left hemisphere (Blair, Morris, Frith, Perrett, & Dolan, 1999; Lane, Chua, & Dolan, 1999; Lane et al., 1997; Morris et al., 1996), whereas several lesion studies implicate the right amygdala in recognizing fear and other negative emotions from faces (Adolphs, Tranel, & Damasio, 2001; Anderson, Spencer, Fulbright, & Phelps, 2000). Meta-analyses of human emotional imaging studies found significantly more activation in left compared to right amygdala (Baas, Aleman, & Kahn, 2004; Sergerie, Chochol, & Armony, 2008; Wager, Phan, Liberzon, & Taylor, 2003); however, Gläscher and Adolphs (2003) suggest there might be an initial, perhaps automatic, emotional reaction mediated by the right amygdala, followed by a more differentiated emotional reaction mediated by the left amygdala. These findings leave a complex picture of laterality for negative emotions. Heller & Nitschke (1998) agree that it is difficult to find a clear hemispheric focal point for anxiety and that several factors may be involved in laterality, including the type of anxiety (anxious arousal, e.g., panic, versus anxious apprehension, e.g., worry or fear about the future), differences in arousal levels, specific brain region affected, emotional valence, and whether there is an increase or a decrease in activation (Davidson, 1992a, 1992b; Gainotti, 1972; Heller, 1990; Lee, Loring, Meador, Flanigin, & Brooks, 1988; Sackeim et al., 1982). Also, as pointed out by Davidson (2002), true hemispheric differences can be found only by statistical tests of the interaction of hemisphere by condition, which have rarely been performed. Some of the lateralization results in the literature may be due to the imaging parameters used. Mathiak et al. (2006) demonstrated a pure method artifact during fMRI that affected the laterality of the resulting amygdala activation, due to susceptibility artifacts from changing the phase-encoding polarity.
It is important to point out limitations of our findings. One relates to our study population of male Vietnam veterans of uniform age, education, and ethnicity. This homogeneity adds to the strength of the findings, but also makes the generalizability of the results uncertain. In addition, anxiety is a multifactorial diagnosis. The anxiety scales we used vary in their focus, subjectivity and other factors. Our lesion groups differed in that those with lesions in significant lesion areas had significantly more volume loss on average than those with lesions in other areas. This makes it difficult to untangle the effects of lesion location and brain volume loss. However, across all lesioned individuals, the correlation between brain volume loss and NRS anxiety was weak (r = .13). Another limitation is that the use of CT scans rather than MRI scans resulted in a less detailed picture of the neuroanatomy involved. Also, the AIR registration process used in ABLe is a 12-parameter linear fit, which may lead to imperfect registration (Solomon et al., 2007). In addition, penetrating injuries can create positional shifts in affected grey matter and white matter tracts, and it is likely that there has been some reorganization of the brain during the recovery process. Further research is needed to differentiate the causes of increased anxiety after injury to these areas in order to determine how much of the increased anxiety is due to the memories of the event that caused the injury, the resulting decrease in functionality, and the social and psychological consequences. Further research is needed to determine more specifically which brain regions lead to increased and decreased anxiety.
In summary, we detected an effect of brain damage on the presentation of anxiety, defined as worry, fear, or over-concern for present or future. Increased anxiety was associated with damage in the middle, inferior and superior temporal lobe, hippocampus, and fusiform regions, along with smaller areas in the inferior occipital lobe, parahippocampus, amygdala, and insula in the left hemisphere, and in a small region of the superior and middle temporal lobe in the right hemisphere. These findings highlight the importance of clearly specifying the type of anxiety being measured. The association of injury to these areas and heightened anxiety suggest that individuals with damage to these areas may be at risk for pathological anxiety.
Supplementary Material
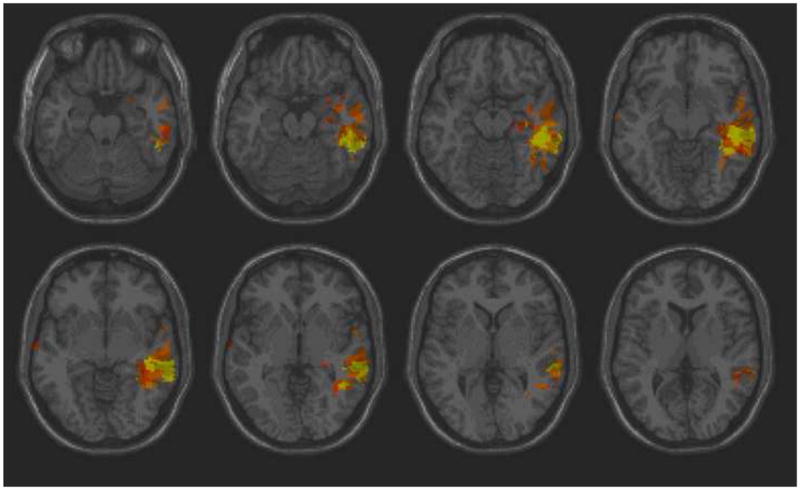
Supplementary Figure 1: Axial slices from CT scans for four brain-injured veterans, randomly chosen. For the top left figure, the lesion volume was 90.88cc, for the top right, 52.90cc, bottom left, 37.80cc, and bottom right, 27.00cc.
Supplementary Figure 2: CT scans from the same slices and brain-injured veterans as in Supplementary Figure 1, deskulled and overlaid onto a CT template. The color overlap shows the registration accuracy qualitatively. For the top left figure, the quantitative registration accuracy was 92%, for the top right, 94%, bottom left, 92%, and bottom right, 94%.
Highlights.
We studied anxiety symptoms in Vietnam War veterans with brain injuries.
Anxiety was measured using the Neurobehavioral Rating Scale.
Damage in temporal lobes and left limbic area associated with increased anxiety.
Acknowledgments
5 Funding
The work was supported by the United States National Institutes of Health, National Institute of Neurological Disorders and Stroke, intramural research program, and a project grant from the United States Army Medical Research and Material Command administrated by the Henry M. Jackson Foundation (Vietnam Head Injury Study Phase III: A 30-year post injury follow-up study, Grant DAMD17-01-1-0675). The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, nor the United States Government.
We especially thank the Vietnam War veterans who participated in this study. We thank the National Naval Medical Center for their support and provision of their facilities and S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, K. Reding, and G. Tasick for their invaluable help with the testing of participants and organization of this study.
Footnotes
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191(1):42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology. 2001;15(3):396–404. doi: 10.1037//0894-4105.15.3.396. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The Amygdala. New York: Wiley; 1992. pp. 1–67. [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Spencer DD, Fulbright RK, Phelps EA. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. 2000;14(4):526–536. doi: 10.1037//0894-4105.14.4.526. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Reviews. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: A systematic review of functional neuroimaging studies. Brain Research Reviews. 2004;45:96– 103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, Feldon J. Regional dissociations within the hippocampus-- memory and anxiety. Neuroscience and Biobehavioral Reviews. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JNP, Bannerman DM. Hippocampal NMDA receptors and anxiety: At the interface between cognition and emotion. European Journal of Pharmacology. 2010;626:49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: An integrative account. Trends in Cognitive Sciences. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. Journal of Comparative and Physiological Psychology. 1972;81(2):281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko EB, Rodgers RJ, Blanchard DC. Defense system psychopharmacology: An ethological approach to the pharmacology of fear and anxiety. Behavioural Brain Research. 1993;58:155– 165. doi: 10.1016/0166-4328(93)90100-5. [DOI] [PubMed] [Google Scholar]
- Blanco E, Castilla-Ortega E, Miranda R, Begega A, Aguirre JA, Arias JL, Santin LJ. Effects of medial prefrontal cortex lesions on anxiety-like behaviour in restrained and non-restrained rats. Behavioural Brain Research. 2009;201:338–342. doi: 10.1016/j.bbr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Bossini L, Tavanti M, Calossi S, Lombardelli A, Polizzotto NR, Galli R, Castrogiovanni P. Magnetic resonance imaging volumes of the hippocampus in drug-naïve patients with post-traumatic stress disorder without comorbidity conditions. Journal of Psychiatric Research. 2008;42(9):752–762. doi: 10.1016/j.jpsychires.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Noumair D, Branchey M. Duration and intensity of combat exposure and posttraumatic stress disorder in Vietnam Veterans. The Journal of Nervous and Mental Disease. 1990;178(9):582– 587. doi: 10.1097/00005053-199009000-00005. [DOI] [PubMed] [Google Scholar]
- Carlson KF, Nelson D, Orazem RJ, Nugent S, Cifu DX, Sayer NA. Psychiatric diagnoses among Iraq and Afghanistan war veterans screened for deployment-related traumatic brain injury. Journal of Traumatic Stress. 2010;23(1):17– 24. doi: 10.1002/jts.20483. [DOI] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Corrigan J, Dickerson J, Fisher E, Meyer P. The Neurobehavioural Rating Scale: Replication in an acute, inpatient rehabilitation setting. Brain Injury. 1990;4(3):215–222. doi: 10.3109/02699059009026170. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega MS, Gray JA, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992a;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Emotion and affective style: Hemispheric substrates. Psychological Science. 1992b;3(1):39–43. [Google Scholar]
- Davidson RJ. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, Peterson BS. Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in emotional learning. International Review of Neurobiology. 1994;36:225–266. doi: 10.1016/s0074-7742(08)60305-0. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44(12):1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Dahl RE, Axelson DA, Ryan ND. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biological Psychiatry. 2002;51:553–562. doi: 10.1016/s0006-3223(01)01375-0. [DOI] [PubMed] [Google Scholar]
- de Geus EJC, van’t Ent D, Wolfensberger SPA, Heutink P, Hoodendijk WJG, Boomsma DI, Veltman DJ. Intrapair differences in hippocampal volume in monozygotic twins discordant for the risk for anxiety and depression. Biological Psychiatry. 2006;61:1062–1071. doi: 10.1016/j.biopsych.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Deacon RMJ, Bannerman DM, Rawlins JNP. Anxiolytic effects of cytotoxic hippocampal lesions in rats. Behavioral Neuroscience. 2002;116(3):494–497. doi: 10.1037//0735-7044.116.3.494. [DOI] [PubMed] [Google Scholar]
- Dellacherie D, Hasboun D, Baulac M, Belin P, Samson S. Impaired recognition of fear in voices and reduced anxiety after unilateral temporal lobe resection. Neuropsychologia. 2011;49:618– 629. doi: 10.1016/j.neuropsychologia.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Pare D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. The Journal of Neuroscience. 2009;29(33):10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: Recognition, navigation, or encoding? Neuron. 1999;23(1):115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598– 601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Epstein RS, Ursano RJ. Anxiety Disorders. In: Silver JM, Hales RE, Yudofsky SC, editors. Neuropsychiatry of Traumatic Brain Injury. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Fann JR, Katon WJ, Uomoto JM, Esselman PC. Psychiatric disorders and functional disability in outpatients with traumatic brain injuries. American Journal of Psychiatry. 1995;152(10):1493–1499. doi: 10.1176/ajp.152.10.1493. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Gallant R. Role of the basolateral nucleus of the amygdala in the formation of a phobia. Neuropsychopharmacology. 1998;19(5):397–405. doi: 10.1016/S0893-133X(98)00035-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, editors. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. The Journal of Neuroscience. 2010;30(20):7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. Emotional behavior and hemispheric side of lesion. Cortex. 1972;8:41–55. doi: 10.1016/s0010-9452(72)80026-1. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. The Journal of Neuroscience. 2003;23(32):10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, Adolphs R. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–691. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and the parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Grafman J, Jonas BS, Martin A, Salazar AM, Weingartner H, Ludlow C, Vance SC. Intellectual function following penetrating head injury in Vietnam veterans. Brain. 1988;111:169–184. doi: 10.1093/brain/111.1.169. [DOI] [PubMed] [Google Scholar]
- Grafman J, Vance SC, Weingartner H, Salazar AM, Amin D. The effects of lateralized frontal lesions on mood regulation. Brain. 1986;109:1127–1148. doi: 10.1093/brain/109.6.1127. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Oxford: Oxford University Press; 1982. [Google Scholar]
- Gray JA, McNaughton N. Comparison between the behavioral effects of septal and hippocampal lesions: A review. Neuroscience & Biobehavioral Reviews. 1983;7:119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety. 2. Oxford: Oxford Medical Publications; 2000. [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: Effects of a neocortical network on the limbic system. NeuroReport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Heller W. The neuropsychology of emotion: Developmental patterns and implications for psychopathology. In: Stein N, Leventhal BL, Trabasso T, editors. Psychological and biological approaches to emotion. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1990. pp. 167–211. [Google Scholar]
- Heller W, Nitschke JB. The puzzle of regional brain activity in depression and anxiety: The importance of subtypes and comorbidity. Cognition and Emotion. 1998;12(3):421–447. http://dx.doi.org/10.1080/026999398379664. [Google Scholar]
- Jorge RE, Robinson RG, Starkstein SE, Arndt SV. Depression and anxiety following traumatic brain injury. Journal of Neuropsychiatry. 1993;5:369–374. doi: 10.1176/jnp.5.4.369. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Annals of the New York Academy of Sciences. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. The Journal of Neuroscience. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: Anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. American Journal of Neuroradiology. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–997. [Google Scholar]
- Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, Grafman J. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nature Neuroscience. 2008;11(2):232– 237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341– 372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lane RD, Chua PML, Dolan R. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee GP, Loring DW, Meador KJ, Flanigin HF, Brooks BS. Severe behavioral complications following intracarotid sodium amobarbital injection: Implications for hemispheric asymmetry of emotion. Neurology. 1988;38:1233–1236. doi: 10.1212/wnl.38.8.1233. [DOI] [PubMed] [Google Scholar]
- Levin HS, High WM, Goethe KE, Sisson R, Overall J, Rhoades H, Gary HE. Neurobehavioural Rating Scale: Assessment of the behavioural sequelae of head injury by the clinician. Journal of Neurology, Neurosurgery, and Psychiatry. 1987;50(2):183–193. doi: 10.1136/jnnp.50.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Fig LM, Decker LR, Koeppe RA, Minoshima S. Limbic activation and psychophysiologic responses to aversive visual stimuli: Interaction with cognitive test. Neuropsychopharmacology. 2000;23(5):508–516. doi: 10.1016/S0893-133X(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Lippert-Gruner M, Kuchta J, Hellmich M, Klug N. Neurobehavioural deficits after severe traumatic brain injury (TBI) Brain Injury. 2006;20(6):569–574. doi: 10.1080/02699050600664467. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9(2):147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainio A, Hakko H, Niemela A, Tuurinkoski T, Koivukangas J, Rasanen P. The effect of brain tumour laterality on anxiety levels among neurosurgical patients. Journal of Neurology and Neurosurgical Psychiatry. 2003;74:1278–1282. doi: 10.1136/jnnp.74.9.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using interactive automated software. Behavior Research Methods, Instruments, and Computers. 2002;34(1):6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys (macaca mulatta): Generality and individual consistency of effects. Emotion. 2006;6(1):73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Mathiak KA, Zvyagintsev M, Ackermann H, Mathiak K. Lateralization of amygdala activation in fMRI may depend on phase-encoding polarity. Magnetic Resonance Materials in Physics, Biology and Medicine. 2006;25(3):177–182. doi: 10.1007/s10334-011-0285-4. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Annals of the New York Academy of Sciences. 2007;1121:546–561. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- Montag C, Reuter M, Weber B, Markett S, Schoene-Bake JC. Individual differences in trait anxiety are associated with white matter tract integrity in the left temporal lobe in healthy males but not females. Neuroscience. 2012;217:77– 83. doi: 10.1016/j.neuroscience.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI Atlas of Human White Matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davison RJ, Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466(7308):864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Petrovic GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Reviews. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, Arfanakis K. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biological Psychiatry. 2009;66:691– 694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rapoport M, McCauley S, Levin H, Song J, Feinstein A. The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2002;15(2):123–132. [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Fischman AJ, Jenike MA. The functional neuroanatomy of anxiety: A study of three disorders using positron emission tomography and symptom provocation. Biological Psychiatry. 1997;42:446– 452. doi: 10.1016/S0006-3223(97)00145-5. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Raymont V, Salazar AM, Krueger F, Grafman J. “Studying injured minds” - The Vietnam head injury study and 40 years of brain injury research. Frontiers in Neurology. 2011;2(15):1–13. doi: 10.3389/fneur.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Raichle ME, Butler FK, Herscovitch P, Robins E. A focal brain abnormality in panic disorder, a severe form of anxiety. Nature. 1984;310(23):683–685. doi: 10.1038/310683a0. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbuhler JP, Geschwind N. Hemispheric asymmetry in the expression of positive and negative emotions: Neurological evidence. Archives of Neurology. 1982;39:210–218. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: An evolved system for relevance detection. Reviews in the Neurosciences. 2003;14(4):303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injections of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biological Psychiatry. 1995a;37:473–476. doi: 10.1016/0006-3223(94)00183-4. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacology Biochemistry and Behavior. 1995b;52(4):701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Schiltz C, Sorger B, Caldara R, Ahmed F, Mayer E, Goebel R, Rossion B. Impaired face discrimination in acquired prosopagnosia is associated with abnormal response to individual faces in the right middle fusiform gyrus. Cerebral Cortex. 2006;16:574–586. doi: 10.1093/cercor/bhj005. [DOI] [PubMed] [Google Scholar]
- Schwab K, Grafman J, Salazar A, Kraft J. Residual impairments and work status 15 years after penetrating head injury: Report from the Vietnam Head Injury Study. Neurology. 1993;43:95–103. doi: 10.1212/wnl.43.1_part_1.95. [DOI] [PubMed] [Google Scholar]
- Selden NRW, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42(2):335– 350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions (ABLe) Computer Methods and Programs in Biomedicine. 2007;86:245– 254. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato MV, Wood JN, De Simone V, Grafman J. Neural correlates of anxiety in healthy volunteers: A voxel-based morphometry study. Journal of Neuropsychiatry and Clinical Neuroscience. 2009;21:199–205. doi: 10.1176/jnp.2009.21.2.199. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Sample Set, Manual, Test, Scoring Key. Palo Alto, CA: Mind Garden, Redwood City, California; 1983. State-Trait Anxiety Inventory (Form Y) for Adults. [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. Journal of Comparative Neurology. 1977;172:49– 84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Syal S, Hattingh CJ, Fouché J-P, Spottiswoode B, Carey PD, Lochner C, Stein DJ. Grey matter abnormalities in social anxiety disorder: A pilot study. Metabolic Brain Disease. 2012 doi: 10.1007/s11011-012-9299-5. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38:1415–1425. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160:284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt WA, Sajdyk TJ, Dietrich AD, Oberlin B, McDougle CJ, Shekhar A. From anxiety to autism: Spectrum of abnormal social behaviors modeled by progressive disruption of inhibitory neuronal function in the basolateral amygdala in Wistar rats. Psychopharmacology. 2007;191:107–118. doi: 10.1007/s00213-006-0674-y. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uchidi RR, Del-Ben CM, Busatto GF, Duran FLS, Guimarães FS, Crippa JAS, Graeff FG. Regional gray matter abnormalities in panic disorder: A voxel-based morphometry study. Psychiatry Research: Neuroimaging. 2000;163:21–29. doi: 10.1016/j.pscychresns.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Vasa RA, Grados M, Slomine B, Herskovits EH, Thompson RE, Salorio C, Gerring JP. Neuroimaging correlates of anxiety after pediatric traumatic brain injury. Biological Psychiatry. 2004;55:208–216. doi: 10.1016/s0006-3223(03)00708-x. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Anderson ER, Goddard A, Woods SW, Staib LH, Charney DS, Bremner JD. Temporal lobe volume in panic disorder -- A quantitative magnetic resonance imaging study. Psychiatry Research: Neuroimaging. 2000;99:75– 82. doi: 10.1016/s0925-4927(00)00055-x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Third Edition. San Antonio, TX: Psychological Corporation; 1997b. [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative and Physiological Psychological. 1956;49(4):381– 391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Research. 1982;232:255– 270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson J, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biological Psychiatry. 2003;54:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Axial slices from CT scans for four brain-injured veterans, randomly chosen. For the top left figure, the lesion volume was 90.88cc, for the top right, 52.90cc, bottom left, 37.80cc, and bottom right, 27.00cc.
Supplementary Figure 2: CT scans from the same slices and brain-injured veterans as in Supplementary Figure 1, deskulled and overlaid onto a CT template. The color overlap shows the registration accuracy qualitatively. For the top left figure, the quantitative registration accuracy was 92%, for the top right, 94%, bottom left, 92%, and bottom right, 94%.


