Abstract
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers. Peroxisome proliferator-activated receptor γ (PPARγ) agonists represent a potentially important family of chemopreventive/therapeutic compounds for cancer treatment by affecting cell proliferation, differentiation, and apoptosis. Dual ligands for PPARα and PPARγ, such as netoglitazone (MCC-555), have been developed to improve treatment of metabolic syndromes, including hyperglycemia and hyperlipidemia. Interestingly, these dual ligands also possess anti-proliferative activities against a variety of cancer cell lines with a greater potency than conventional PPARγ specific ligands. In this study, chemopreventive properties of MCC-555 in colorectal tumorigenesis were evaluated using azoxymethane (AOM)-induced colonic aberrant crypt foci (ACF) in A/J mice. We found that MCC-555 suppressed AOM-induced ACF in A/J mice, compared to the control group. Administration of MCC-555 resulted in decreased mitoses and increased apoptotic cells in the colon. Furthermore, expression of tumor suppressor protein MUC2 was increased in MCC-555 treated mice. Our data clearly suggest that MCC-555 has an effect on the early events of colon carcinogenesis, thus providing evidence that MCC-555 could be a potential preventive compound for CRC.
Keywords: MCC-555, Azoxymethane, Aberrant crypt foci, PPAR ligand, Colon cancer
1. Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers on a global level. Despite increasing efforts in many countries to reduce the incidence of CRC, over 600,000 men and women worldwide die of CRC annually (Garcia-Albeniz and Chan, 2011), highlighting the importance of developing effective strategies to prevent and treat this disease. It is one of the most common cancers in developed countries, and its incidence continues to rise. In the United States, colorectal cancer is the third most frequently diagnosed cancer in both men and women, with an estimated 142,570 new cases and 51,370 deaths occurring annually (Ollberding et al., 2012). The etiology of colon cancer is multifactorial, including genetic, behavior, and environmental factors. Despite several advancements in understanding the process of carcinogenesis, available therapies, including surgery, radiation, and chemotherapeutic drugs, limits still exist for advanced-stage colon cancer. In this context, molecular target-based strategies and/or primary prevention, including chemoprevention, could contribute to reduced incidence of CRC. Chemoprevention studies have already led to models that allow the study of cancer biology and the evaluation of pharmacological interventions in animals.
One such rodent model develops aberrant crypt foci (ACF), which are early morphologic changes observed after the administration of a colon-specific carcinogen, such as azoxymethane (AOM) (Suzuki et al., 2004). Similar lesions have also been observed at a high frequency in the colons of patients with sporadic and inherited forms of colon cancer (Stevens et al., 2007). ACF are generally considered preneoplastic lesions and are currently used as a surrogate biomarker to rapidly evaluate the chemopreventive potential of several naturally occurring and synthetic agents in rodent models (Asano et al., 2007, Ravichandran et al., 2010). In addition, ACF accurately replicates many of the clinical, genetic, cellular, and morphologic features of human colorectal cancer (Takahashi and Wakabayashi, 2004). AOM-induced ACF are particularly characterized by an increase in the size of crypts, a thicker epithelial lining, and a pericryptal zone, and they share many morphologic and biochemical characteristics with tumors, including a comparable increase in cell proliferation (Bird, 1987).
It has been previously reported that thiazolidinedione and its derivatives induce apoptosis in human colorectal cancer cells (Dionne et al., 2010, Papi et al., 2010, Yamaguchi et al., 2008, Yamaguchi et al., 2006). Many other studies describe the beneficial effects of peroxisome proliferator-activated receptor γ (PPARγ) agonists for treatment of lung (Tsubouchi et al., 2000), breast (Elstner et al., 1998), pancreas (Motomura et al., 2000) and colorectal cancer (Kopelovich et al., 2002) in vitro and in vivo. Subsequent studies suggest that PPARγ agonists control many genes that are involved in cellular and physiological pathways in PPARγ-dependent and independent manners (Baek et al., 2004, Baek et al., 2003, Rumi et al., 2004, Turturro et al., 2004, Yamaguchi et al., 2008). Thus, apoptosis and/or differentiation induction caused by the PPARγ ligand seems to be a promising approach to cancer prevention research. Among PPARγ agonists, MCC-555 exhibits less cytotoxicity in diabetic patients, and better apoptosis induction in human colorectal cancer cells (Reginato et al., 1998, Yamaguchi et al., 2006).
In the present study, we therefore used the AOM-induced ACF model, a well-known model of carcinogen-induced colorectal cancer, to investigate the possible effect of MCC-555 on colorectal anti-tumorigenesis.
2. Materials and methods
2.1. Reagents
AOM, 10% buffered formalin, 0.2% methylene blue, and all other analytic reagents were purchased from Sigma (St. Louis, MO). MCC-555 was obtained from Mitsubishi Pharma Corporation (Tokyo, Japan). TACS®2 TdT blue in situ cell detection kit was purchased from Trevigen (Gaithersburg, MD), and MUC2 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
2.2. Animals and study design
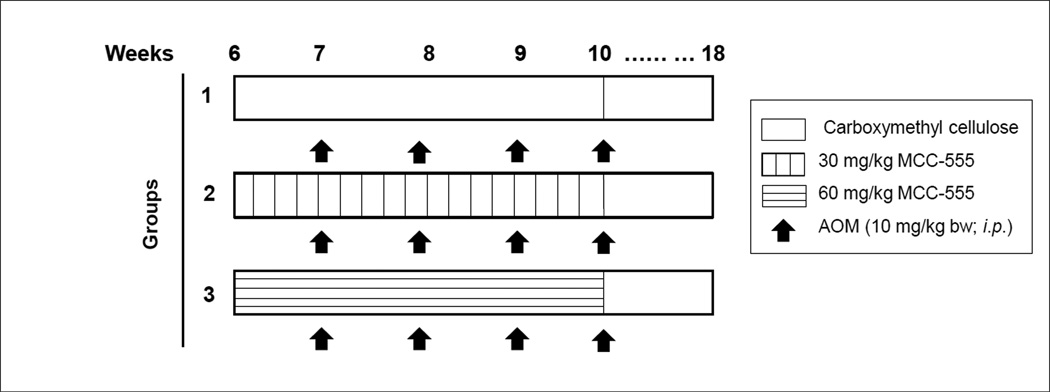
Four-week-old female A/J mice with average body weights (BW) of 15–17 g were purchased from Jackson Laboratory, Bar Harbor, Maine. Mice were maintained at 22 ± 2°C on a 12-h light/dark cycle and with free access to standard rodent chow and water. The experiments were carried out with a protocol approved by the Institutional Animal Care and Use Committee (and were in accordance with NIH guidelines) at the University of Tennessee College of Veterinary Medicine. Food and fluid intake and general health status were monitored weekly. Animals were randomly assigned to three groups (10 mice per group) after a 2-week acclimatization period. MCC-555 was suspended in 1.5% carboxymethylcellulose (CMC) with 0.2% Tween 20. As shown in Fig. 1, the control group was orally gavaged with CMC alone, and the second and third groups received 30 and 60 mg/kg MCC-555 suspended in CMC for 4 weeks, respectively. Starting at 7 weeks of age, mice in all the 3 groups were injected i.p. once a week for a total of 4 weeks with 10 mg/kg AOM. Mice were sacrificed by CO2 asphyxiation 8 weeks after the last AOM treatment.
Fig. 1.
Experimental design for AOM-induced colon tumorigenesis in A/J mice. Four-week-old female A/J mice were purchased and randomly assigned to three groups after 2 week of an acclimatization period. MCC-555 was suspended in 1.5% CMC with 0.2% Tween 20. The control group was gavaged orally with CMC alone; the second and third group received 30 and 60 mg/kg MCC-555 suspended in CMC for 4 weeks. Starting at 7 weeks of age, mice in all three groups were injected i.p. once a week for a total of 4 weeks with 10 mg/kg AOM. Mice were sacrificed by CO2 asphyxiation 8 weeks after their last AOM treatment.
2.3. Sample preparation and determination of ACF
After sacrifice of the animals by CO2 asphyxiation, the entire colons were immediately removed, flushed with ice-cold PBS to remove fecal content, cut open longitudinally from anus to cecum, and fixed flat between sheets of hard cardboard in 10% neutral buffered formalin for a minimum of 24 h. The fixed samples were then stained with 0.2% methylene blue for 5 to 10 min rinsed in PBS to facilitate enumeration of ACF. Colons were placed with the mucosal side up on an observation microscope and counted under 20X magnification. ACF scoring was performed as previously described (Papanikolaou et al., 2000). Aberrant crypts were distinguished from the surrounding normal crypts using a published set of criteria: aberrant crypts are larger, have an increased pericryptal area, have greater staining intensity due to the thickened layer of epithelial cells, and are microscopically elevated above the adjacent normal crypts (Papanikolaou et al., 2000). The total number of ACF, as well as the number of aberrant crypts in each focus, was quantified in the colon under an observation microscope. Total numbers were an average developed based on counts from two different investigators. Dome-shaped areas on the mucosal surface represent lymphoid tissue, based on previous studies conducted in our laboratory (Baek et al., 2006) and by others (Carter et al., 1994). The length and width of these foci were measured and excluded from the measurements used to calculate overall colonic area. The proximal colonic lymphoid tissue was not evaluated because of the natural occurrence of crypt-like structures in untreated animals.
2.4. Measuring mitotic index
After all necessary observations were made with the whole mount tissue, the colons were Swiss rolled and embedded in paraffin. Sections (5 µm) were cut, dewaxed, rehydrated in graded alcohol solutions and distilled water, and then stained with hematoxylin and eosin (H&E) for histological examination. Mitoses were then calculated from the H&E slides by the percentage of cells undergoing mitosis against the total crypt cell count as described previously (Araki et al., 2010).
2.5. Terminal deoxyribonucleotidyl transferase-mediated dUTP nick-end labeling staining for apoptotic cells
Formalin-fixed, paraffin-embedded sections were stained for apoptotic cells by the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining method using an in situ cell detection kit (TACS®2 TdT Blue kit, Trevigen, Gaithersburg, MD) according to the manufacturer’s instructions. The sections on the slides were deparafinized and permeabilized with proteinase K solution. Thereafter, the sections were quenched of endogenous peroxidase activity, immersed in 1 X TdT labeling buffer, and incubated in a labeling reaction mix containing TdT dNTP mix and TdT enzyme. After 1 h, the sections were immersed in streptavidin-horseradish peroxidase (strep-HRP) solution for 10 min and then were incubated in TACS®-Blue label solution. The slides were counterstained with Nuclear Fast Red solution, and the stained slides were evaluated and photographed under a magnification of x100 for TUNEL-positive cells (blue color). Quantitative evaluation of apoptotic cells was done under light microscopy by counting the total number of TUNEL-positive cells in the total numbers of cells per 10 crypt columns (vertically oriented colonic crypts) for each mouse. The apoptotic index was calculated as the percentage of the mean of the number of positively stained cells per crypt column [(number of positively stained cells/number of cells per crypt column) × 100] (Dirisina et al., 2011).
2.6. Immunostaining for MUC2
Tissue sections were deparaffinized in xylene and rehydrated in ethyl alcohol, followed by treatment with citrate buffer (pH 6.0) in a microwave for 5 min at full power for antigen retrieval. The sections were then washed in TBS for 5 min and quenched of endogenous peroxidase activity for 10 min at room temperature. The sections were incubated in 1.5% blocking serum in phosphate-buffered saline (PBS) for 1 h at room temperature in a humidity chamber followed by overnight incubation at 4°C with MUC2 rabbit polyclonal antibody (1:100 dilution; Santa Cruz Biotechnology). The sections were then incubated with appropriate biotinylated secondary antibody for 30 min at room temperature followed by 30 min incubation with horseradish peroxidase-conjugated streptavidin. Sections were then incubated in a peroxidase substrate for 30 sec to 10 min or until desired stain intensity developed, followed by washing in deionized water for 5 min, then counterstaining for 5–10 sec with hematoxylin followed by dehydration and cover-slipping. Observation was carried out under a light microscope, and pictures were taken under ×100 magnification.
2.7. Statistical analysis
Data were analyzed using SAS for window (v9.2; SAS institute, Inc.). Results were analyzed by analysis of variance (ANOVA) and expressed as means ± standard error of the mean (SEM). Significant differences (p < 0.05.) were determined using one-way ANOVA.
3. Results
3.1. Quantification of ACF
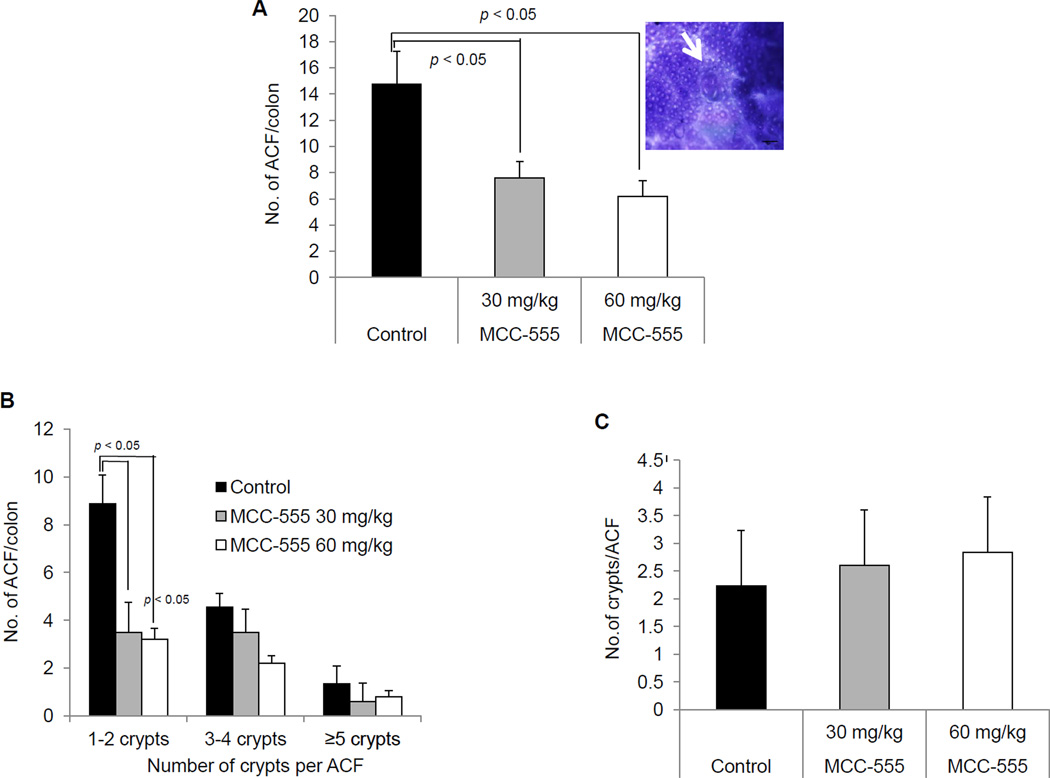
The number and size of ACF were determined in methylene blue-stained distal colons. The results were from ACF counts of two individuals with strong agreement. The administration of MCC-555 at doses of 30 and 60 mg/kg significantly (p < 0.05) reduced ACF formation, compared with the control group (Fig. 2A). Although there is a trend in a dose-dependent manner, there is no statistical difference between 30 mg/kg and 60 mg/kg treatment. The incidence and multiplicity of ACF were also evaluated. ACF were classified as small (1–2 crypts/focus), medium (3–4 crypts/focus) and large (≥ 5 crypts/focus). There were significant differences (p < 0.05) in the incidence of ACF with 1–2 crypts per focus among mice treated with MCC-555 and the control group (Fig. 2B). However, there was no statistical significance with 3–4 and ≥ 5 crypts per ACF. Finally, we investigated the number of crypts per ACF in each group. As shown in Fig. 2C, both control and treated mice exhibit a similar number of crypts per ACF, indicating that MCC-555 did not have an inhibitory effect on crypt numbers.
Fig. 2.
Effect of treatment with MCC-555 on number of colonic ACF in A/J mice. The number and size of ACF were determined in methylene blue-stained, whole mount distal colons. (A) Number of ACF per colon. A representative picture of methylene blue-stained ACF is shown in the right. (B) Number of ACF per colon based on numbers of crypts per ACF. (C) Average number of crypts per ACF for each group. Results were expressed as mean ± SEM from 10 mice per group. Data were analyzed using SAS for Windows (v9.2; SAS Institute, Inc.) statistical analysis software for statistical difference between different groups. Significance was determined by one-way ANOVA with Tukey’s test for multiple comparisons. Results were considered statistically significant at p < 0.05.
3.2. Quantification of mitotic and apoptotic cells
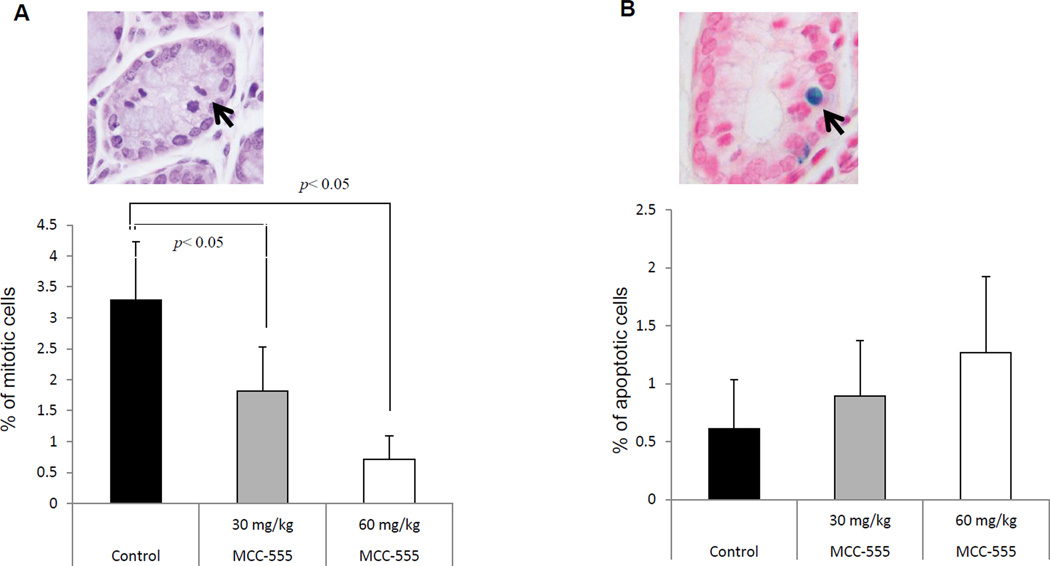
To measure proliferative and apoptotic cells in the colon tissue, we examined mitotic and TUNEL-positive cells. Mitotic cells were identified by H&E staining, exhibiting condensed DNA (Fig. 3A, top panel). We calculated the ratio of the mitotic cells over total cell count in the crypt. There was sporadic mitosis present in the colon tissue in the control mice. However, mitoses were less frequent in the group of both mice administered MCC-555 (Fig. 3A). The apoptotic response in the colonic tissue of AOM-induced mice was investigated by TUNEL staining. Quantitative analyses of the TUNEL positive cells revealed that MCC-555 increased apoptotic cells in a MCC-555 treated samples (Fig. 3B); however, there was no significant difference between the control and treated groups. The apoptotic induction was more profound in the group of mice that were administered MCC-555 at 60 mg/kg.
Fig. 3.
Mitosis and apoptosis in the colonic crypts of AOM-induced A/J mice treated with MCC-555. (A) To quantify mitosis, we calculated the percentage of the crypt count with mitosis from total cell count in the crypt. A representative mitotic cell is shown in the top. (B) To quantify the apoptotic cells, we randomly calculated the TdT-positive cell count under a magnification of ×100. A representative apoptotic cells have shown in the top. Results were expressed as mean ± SEM. ANOVA was used with Tukey post hoc analysis. The results were considered statistically significant at p < 0.05.
3.3. MUC2 expression by MCC-555 in colon tissue
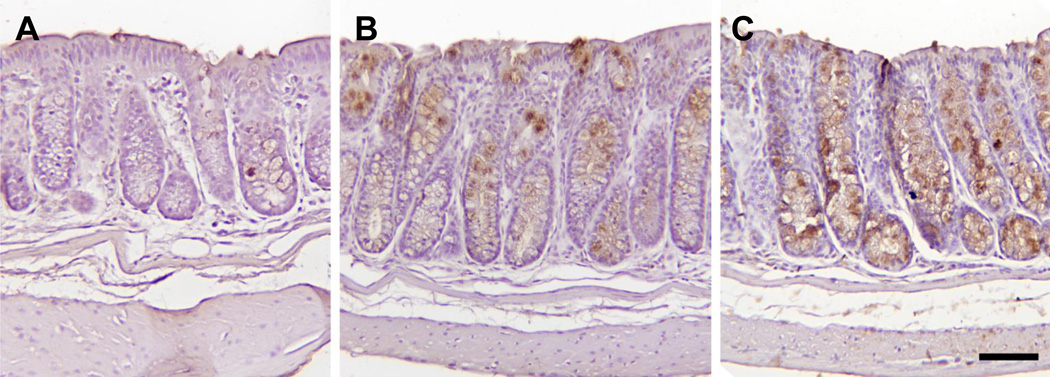
MUC2 is known to have a tumor suppressor function in colorectal cancer (Velcich et al., 2002), and MCC-555 induces MUC2 expression in APCMin mice models (Yamaguchi et al., 2008) . Therefore, we examined if MUC2 is induced by MCC-555 in the AOM-induced animal models. Paraffin-embedded colon tissue samples from control, 30 mg/kg, or 60 mg/kg MCC-555-treated mice were stained with MUC2 antibody. Microscopic examination of colonic tissue sections revealed that MUC2 was expressed in the colon tissue. However, MUC2 staining was considerably increased at a higher MCC-555 treatment, as shown in Fig. 4.
Fig. 4.
Representative images of MUC2 immunohistochemical staining of colon of A/J mice. The bar is equal to 75 micrometers. Immunohistochemical analysis was done as described in the Material and methods section. Microscopic photograph of colon tissue from (A) vehicle-treated mice, (B) 30 mg/kg MCC-555-treated mice, and (C) 60 mg/kg MCC-555-treated mice.
4. Discussion
The PPARγ agonists affect cell proliferation, differentiation, and apoptosis in a PPARγ-dependent and/or -independent manner, and thereby represent a potentially important family of therapeutic compounds for cancer treatment. Many studies show that PPARγ and PPARα/γ agonists such as thiazolidinedione have anti-tumorigenic properties in colorectal cancer by increasing the expression of tumor suppressor genes (Baek et al., 2003, Chintharlapalli et al., 2007, Yamaguchi et al., 2006). MCC-555 is a relatively new member of the thiazolidinediones and exhibits anti-tumorigenic activity in prostate cancer (Kumagai et al., 2004) and colorectal cancer cells (Yamaguchi et al., 2008). In addition, we have shown that MCC-555 suppresses polyps in a genetic model of human colorectal cancer (Yamaguchi et al., 2008). However, it remains unclear how MCC-555 affects anti-tumorigenesis in carcinogen-induced animal models.
In the present study, we investigated the effect of MCC-555 in AOM-induced colorectal tumorigenesis using A/J mice. The AOM model is an excellent and wellstudied pre-clinical model for colorectal cancer chemoprevention efficacy studies, and is also known for its relevance with respect to the clinical, histopathologic, and molecular features of human colorectal cancer (Papanikolaou et al., 2000, Tammali et al., 2009). We chose A/J mice since these strains are very sensitive to AOM, compared to other strains of mice (Papanikolaou et al., 2000, Ravichandran et al., 2010). The results of our study showed that administration of MCC-555 at 30 and 60 mg/kg body weight per day for 4 weeks significantly inhibited AOM-induced ACF formation. The inhibitory effect of MCC-555 on ACF formation was particularly seen in small crypts (Fig. 2B), with the reduction in crypt multiplicity. These findings suggest that MCC-555 suppresses the pre-initiation phase of chemically-induced colon carcinogenesis, and that MCC-555 probably did not contribute to the inhibition of crypt numbers. A similar effect of MCC-555 in Apcmin mice, an animal model for human familial adenomatous polyposis, has also been reported (Yamaguchi et al., 2008). In addition, there are several reports on anti-tumorigenesis of naturally occurring phytochemicals in AOM-induced models, and AOM-induced ACF formation and crypt multiplicity by phytochemicals were particularly observed in a small number of ACF-containing crypts (Velmurugan et al., 2008, Volate et al., 2005), which is consistent with our result.
Although colon carcinogenesis is a multistage process, enhanced cell proliferation and reduced apoptosis are early events in the progression of tumorigenesis (Patel et al., 2009). An increase in cell turnover accompanied by epithelial cell damage can increase mitotic aberrations and induce changes at both genetic and epigenetic levels, that favor cancer promotion (Kohno et al., 2006). Cell proliferation and apoptosis biomarkers are generally used to test the efficacy of any chemopreventive agent (Naumov et al., 2006). H&E staining of the colons of mice revealed a significant decrease in mitotic cell count in both groups (30 and 60 mg/kg) treated with MCC-555, compared to the control group. This finding suggests that a hyper-proliferative response to a carcinogen within the normal mucosa may be an important early feature of tumor initiation. On the other hand, apoptosis and associated cellular events have a profound effect on progression from a benign to a malignant phenotype, and can be targeted for the therapy of various malignancies including colon cancer (Schmelz et al., 2007). Hence, the apoptosis-inducing effect of MCC-555 was evaluated using a TUNEL-positive index during AOM-induced ACF formation. The results of the present study indicate that MCC-555 induces apoptosis in the colon tissue. We found a marked increase in the number of apoptotic cells within the colons of the mice, treated with MCC-555 compared to the control group. Although it has been reported that MCC-555 has an apoptosis-inducing effect in human colorectal cancer cells (Yamaguchi et al., 2006), there was no significant difference in the apoptotic index between control and treated mice. It could be explained that MCC-555 treatment in A/J mice may inhibit mitosis, but not affect the induction of apoptosis, in our experiments. Another explanation is that the dose and time for MCC-555 treatment may not reach the threshold to significantly increase apoptosis in the colon tissue of the mice. Therefore, further analysis may be needed to conclude that MCC-555 enhances apoptosis in AOM-induced animal model.
Finally, we investigated the possible effect of MCC-555 on MUC2 expression. It has been suggested that an alteration in mucin gene expression is likely associated with the early steps of colon cancer development and later tumor progression (Velcich et al., 2002). Inactivation of MUC2 causes tumor formation accompanied by reduced apoptosis and increased proliferation and migration of intestinal adenocarcinoma cells (Velcich et al., 2002). The transcriptional activity of MUC2 is negatively and positively regulated by oncogenic SOX9 and tumor suppressor p53, respectively (Blache et al., 2004, Ookawa et al., 2002), indicating that MUC2 can be associated with tumor suppression. Our results showed increased MUC2 expression in the mice treated with MCC-555 at doses of 30 and 60 mg/kg. These results are consistent with previous reports that MCC-555 induces MUC2 in human cancer cells and in Apcmin mice (Yamaguchi et al., 2008). Thus, MUC2 may be, in part, responsible for the anti-tumorigenic activity of MCC-555 in the AOM-induced ACF animal model.
Our combined findings suggest that MCC-555 could be a potent chemopreventive agent against human colorectal cancer, and therefore warrants further studies for broadening the scope of its application to colorectal cancer patients. These promising results suggest the importance of conducting further investigations with MCC-555 in pre-clinical colon cancer models, especially long-term in vivo efficacy studies, to support the clinical usefulness of MCC-555 against colon cancer development.
Acknowledgements
We thank Misty Bailey for her critical reading, Anik Vasington for her technical assistance and Dr. M. McEntee for his valuable advice of this manuscript.
Financial Support: This work was supported by grants from the National Institutes of Health (R01CA108975), and the University of Tennessee Center of Excellence in Livestock Diseases and Human Health to SJ Baek. TI is a recipient of the National Overseas Scholarship Award [No. 11016/07/2010-Education] from the Ministry of Tribal Affairs, New Delhi, India.
Abbreviations
- ACF
aberrant crypt foci
- ANOVA
analysis of variance
- AOM
azoxymethane
- CMC
carboxyl methyl cellulose
- CRC
colorectal cancer
- H&E
hematoxylin and eosin
- PPAR
peroxisome proliferator-activated receptor
- TUNEL
TdT-mediated deoxyuridine triphosphate nick-end labeling
- TdT
terminal deoxynucleotidyl transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki Y, Mukaisyo K, Sugihara H, Fujiyama Y, Hattori T. Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol Rep. 2010;24:869–874. doi: 10.3892/or.2010.869. [DOI] [PubMed] [Google Scholar]
- Asano N, Kuno T, Hirose Y, Yamada Y, Yoshida K, Tomita H, et al. Preventive effects of a flavonoid myricitrin on the formation of azoxymethane-induced premalignant lesions in colons of rats. Asian Pac J Cancer Prev. 2007;1:73–76. [PubMed] [Google Scholar]
- Baek SJ, Kim J, Nixon JB, DiAugustine RP, Eling TE. Expression of NAG-1, a Transforming Growth Factor-β Superfamily Member, by Troglitazone Requires the Early Growth Response Gene EGR-1. J Biol Chem. 2004;279:6883–6892. doi: 10.1074/jbc.M305295200. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Okazaki R, Lee SH, Martinez J, Kim JS, Yamaguchi K, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology. 2006;131:1553–1560. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor gamma (PPAR gamma) ligand, selectively induces the early growth response-1 gene independently of PPAR gamma. A novel mechanism for its anti-tumorigenic activity. J Biol Chem. 2003;8:5845–5853. doi: 10.1074/jbc.M208394200. [DOI] [PubMed] [Google Scholar]
- Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: Preliminary findings. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund J-N, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Biol Chem. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JW, Lancaster HK, Hardman WE, Cameron IL. Distribution of Intestine-associated Lymphoid Tissue, Aberrant Crypt Foci, and Tumors in the Large Bowel of 1,2-Dimethylhydrazine-treated Mice. Cancer Res. 1994;54:4304–4307. [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Jutooru I, McAlees A, Safe S. Structure-dependent activity of glycyrrhetinic acid derivatives as peroxisome proliferator–activated receptor γ agonists in colon cancer cells. Mol Cancer Ther. 2007;6:1588–1598. doi: 10.1158/1535-7163.MCT-07-0022. [DOI] [PubMed] [Google Scholar]
- Dionne S, Levy E, Levesque D, Seidman EG. PPARγ Ligand 15-Deoxy-delta 12,14-Prostaglandin J2 Sensitizes Human Colon Carcinoma Cells to TWEAK-induced Apoptosis. Anticancer Res. 2010;30:157–166. [PubMed] [Google Scholar]
- Dirisina R, Katzman RB, Goretsky T, Managlia E, Mittal N, Williams DB, et al. p53 and PUMA Independently Regulate Apoptosis of Intestinal Epithelial Cells in Patients and Mice With Colitis. Gastroenterology. 2011;141:1036–1045. doi: 10.1053/j.gastro.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner E, Müller C, Koshizuka K, Williamson EA, Park D, Asou H, et al. Ligands for peroxisome proliferator-activated receptorγ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad of Sci. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Albeniz X, Chan AT. Aspirin for the prevention of colorectal cancer. Best Practice & Res Clin Gastroenterol. 2011;25:461–472. doi: 10.1016/j.bpg.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno H, Suzuki R, Curini M, Epifano F, Maltese F, Gonzales SP, et al. Dietary administration with prenyloxycoumarins, auraptene and collinin, inhibits colitis-related colon carcinogenesis in mice. Int J Cancer. 2006;118:2936–2942. doi: 10.1002/ijc.21719. [DOI] [PubMed] [Google Scholar]
- Kopelovich L, Fay JR, Glazer RI, Crowell JA. Peroxisome Proliferator-activated Receptor Modulators As Potential Chemopreventive Agents. Mol Cancer Ther. 2002;5:357–363. [PubMed] [Google Scholar]
- Kumagai T, Ikezoe T, Gui D, O’Kelly J, Tong X-J, Cohen FJ, et al. RWJ-241947 (MCC-555), A Unique Peroxisome Proliferator-Activated Receptor-γ Ligand with Antitumor Activity against Human Prostate Cancer in Vitro and in Beige/Nude/ X-Linked Immunodeficient Mice and Enhancement of Apoptosis in Myeloma Cells Induced by Arsenic Trioxide. Clin Cancer Res. 2004;10:1508–1520. doi: 10.1158/1078-0432.ccr-0476-03. [DOI] [PubMed] [Google Scholar]
- Motomura W, Okumura T, Takahashi N, Obara T, Kohgo Y. Activation of peroxisome proliferator-activated receptor gamma by troglitazone inhibits cell growth through the increase of p27KiP1 in human. Pancreatic carcinoma cells. Cancer Res. 2000:5558–5564. [PubMed] [Google Scholar]
- Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- Ollberding N, Wilkens L, Henderson B, Kolonel L, Marchand LL. Meat consumption, heterocyclic amines and colorectal cancer risk: The Multiethnic Cohort Study. Int J Cancer. 2012 doi: 10.1002/ijc.27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookawa K, Kudo T, Aizawa S, Saito H, Tsuchida S. Transcriptional Activation of the MUC2 Gene by p53. J Biol Chem. 2002;277:48270–48275. doi: 10.1074/jbc.M207986200. [DOI] [PubMed] [Google Scholar]
- Papanikolaou A, Wang Q-S, Papanikolaou D, Whiteley HE, Rosenberg DW. Sequential and morphological analyses of aberrant crypt foci formation in mice of differing susceptibility to azoxymethane-induced colon carcinogenesis. Carcinogenesis. 2000;21:1567–1572. [PubMed] [Google Scholar]
- Papi A, Rocchi P, Ferreri AM, Orlandi M. RXRγ and PPARγ ligands in combination to inhibit proliferation and invasiveness in colon cancer cells. Cancer Lett. 2010;297:65–74. doi: 10.1016/j.canlet.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Patel BB, Yu Y, Du J, Rishi AK, Sarkar FH, Tarca AL, et al. Schlafen 3, a novel gene, regulates colonic mucosal growth during aging. Am J Physiol Gastrointest Liver Physiol. 2009;296:G955–G962. doi: 10.1152/ajpgi.90726.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran K, Velmurugan B, Gu M, Singh RP, Agarwal R. Inhibitory Effect of Silibinin against Azoxymethane-Induced Colon Tumorigenesis in A/J Mice. Clin Cancer Res. 2010;16:4595–4606. doi: 10.1158/1078-0432.CCR-10-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginato MJ, Bailey ST, Krakow SL, Minami C, Ishii S, Tanaka H, et al. A Potent Antidiabetic Thiazolidinedione with Unique Peroxisome Proliferator-activated Receptor γ-activating Properties. J Biol Chem. 1998;273:32679–32684. doi: 10.1074/jbc.273.49.32679. [DOI] [PubMed] [Google Scholar]
- Rumi MAK, Ishihara S, Kadowaki Y, Ortega-Cava CF, Kazumori H, Kawashima K, et al. Peroxisome proliferator-activated receptor γ-dependent and -independent growth inhibition of gastrointestinal tumour cells. Genes Cells. 2004;9:1113–1123. doi: 10.1111/j.1365-2443.2004.00793.x. [DOI] [PubMed] [Google Scholar]
- Schmelz EM, Xu H, Sengupta R, Du J, Banerjee S, Sarkar FH, et al. Regression of Early and Intermediate Stages of Colon Cancer by Targeting Multiple Members of the EGFR Family with EGFR-Related Protein. Cancer Res. 2007;67:5389–5396. doi: 10.1158/0008-5472.CAN-07-0536. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Swede H, Heinen CD, Jablonski M, Grupka M, Ross B, et al. Aberrant crypt foci in patients with a positive family history of sporadic colorectal cancer. Cancer Lett. 2007;248:262–268. doi: 10.1016/j.canlet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Kohno H, Sugie S, Tanaka T. Sequential observations on the occurrence of preneoplastic and neoplastic lesions in mouse colon treated with azoxymethane and dextran sodium sulfate. Cancer Sci. 2004;95:721–727. doi: 10.1111/j.1349-7006.2004.tb03252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004;95:475–480. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammali R, Reddy ABM, Ramana KV, Petrash JM, Srivastava SK. Aldose reductase deficiency in mice prevents azoxymethane-induced colonic preneoplastic aberrant crypt foci formation. Carcinogenesis. 2009;30:799–807. doi: 10.1093/carcin/bgn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi Y, Sano H, Kawahito Y, Mukai S, Yamada R, Kohno M, et al. Inhibition of Human Lung Cancer Cell Growth by the Peroxisome Proliferator-Activated Receptor-γ Agonists through Induction of Apoptosis. Biochem Biophys Res Commun. 2000;270:400–405. doi: 10.1006/bbrc.2000.2436. [DOI] [PubMed] [Google Scholar]
- Turturro F, Friday E, Fowler R, Surie D, Welbourne T. Troglitazone Acts on Cellular pH and DNA Synthesis through a Peroxisome Proliferator-Activated Receptor γ-Independent Mechanism in Breast Cancer-Derived Cell Lines. Clin Cancer Res. 2004;10:7022–7030. doi: 10.1158/1078-0432.CCR-04-0879. [DOI] [PubMed] [Google Scholar]
- Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;5560:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- Velmurugan B, Singh RP, Tyagi A, Agarwal R. Inhibition of Azoxymethane-Induced Colonic Aberrant Crypt Foci Formation by Silibinin in Male Fisher 344 Rats. Cancer Prev Res (Phila) 2008;1:376–384. doi: 10.1158/1940-6207.CAPR-08-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin) Carcinogenesis. 2005;26:1450–1456. doi: 10.1093/carcin/bgi089. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Cekanova M, McEntee MF, Yoon J, Fischer SM, Renes IB, et al. Peroxisome proliferator-activated receptor ligand MCC-555 suppresses intestinal polyps in ApcMin/+ mice via extracellular signal-regulated kinase and peroxisome proliferator-activated receptor-dependent pathways. Mol Cancer Ther. 2008;7:2779–2787. doi: 10.1158/1535-7163.MCT-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Lee SH, Eling TE, Baek SJ. A novel peroxisome proliferator-activated receptor gamma ligand, MCC-555, induces apoptosis via posttranscriptional regulation of NAG-1 in colorectal cancer cells. Mol Cancer Ther. 2006:1352–1361. doi: 10.1158/1535-7163.MCT-05-0528. [DOI] [PubMed] [Google Scholar]