Abstract
Upon injury, the skin must quickly regenerate to regain its barrier function. In mammals, wound healing is rapid and scar-free during embryogenesis, whereas in adults it involves multiple steps including blood clotting, inflammation, re-epithelialization, vascularization, and granulation tissue formation and maturation, resulting in a scar. We have established a rapid and robust method to introduce full-thickness wounds onto the flank of adult zebrafish, and show that apart from external fibrin clot formation, all steps of adult mammalian wound repair also exist in zebrafish. Wound re-epithelialization is extremely rapid and initiates with no apparent lag-phase, subsequently followed by the immigration of inflammatory cells and the formation of granulation tissue, consisting of macrophages, fibroblasts, blood vessels and collagen. The granulation tissue later regresses, resulting in minimal scar formation. Studies after chemical treatment or with transgenic fish further suggest that wound re-epithelialization occurs independently of inflammation and Fibroblast growth factor (FGF) signaling, whereas both are essential for fibroblast recruitment and granulation tissue formation. Together these results demonstrate that major steps and principles of cutaneous wound healing are conserved among adult mammals and adult zebrafish, making zebrafish a valuable model for studying vertebrate skin repair.
Introduction
Full-thickness wounds to the skin must be promptly repaired to prevent blood loss and contamination of underlying tissues by foreign particles and pathogens. Cutaneous wound healing in adult mammals is a complex, multi-step process involving overlapping stages of blood clot formation, inflammation, re-epithelialization, granulation tissue formation, neovascularization and re-modeling, usually leaving a scar behind (Martin, 1997; Shaw and Martin, 2009; Singer and Clark, 1999). In comparison, wounds in mammalian embryos heal via rapid re-epithelialization and in the absence of inflammation, granulation tissue formation and scarring (Redd et al., 2004).
Wound healing studies in mammalian systems, although of high medical relevance, are costly, technically challenging and time-consuming. Given that major principles of wound repair are conserved, using “lower” organisms would aid in initial steps of the study. During recent years, the zebrafish (Danio rerio) has emerged as a model organism for various aspects of human development and disease (Lieschke and Currie, 2007, Li et al., 2011). Wounds in the epidermis of zebrafish and mammalian embryos use similar principles to close (Redd et al., 2004). However, cutaneous wound repair in adult zebrafish has not been studied as yet. During embryonic and larval stages, the zebrafish epidermis is bi-layered, consisting of a flattened superficial layer known as the enveloping layer (EVL) or periderm, which forms tight junctions and fulfills particular barrier functions (Kiener et al., 2008), and a basal layer, which is attached to the underlying basement membrane (Sonawane et al., 2005). A multi-layered epidermis is only obtained during metamorphosis, commencing at approximately 20 and 25 days post fertilization. At the same time, fibroblasts invade the dermis, take over collagen production from basal keratinocytes and form localized thickenings (dermal papilla) to initiate scale formation (Le Guellec et al., 2004; Sire and Akimenko, 2004; Sire et al., 1997).
Studies in mammalian systems have led to the identification of a number of cytokines and growth factors, which regulate the initial blood clotting response, re-epithelialization, inflammation or granulation tissue formation (Barrientos et al., 2008; Werner and Grose, 2003). Fibroblast growth factors (FGFs) comprise a family of structurally related secreted proteins that signal through high-affinity transmembrane tyrosine kinase receptors (FGFr1-4) (Ornitz and Itoh, 2001). In mammals, expression of FGF1, FGF2, FGF5, FGF7 and FGF10 was found in normal and wounded skin, and expression of all these FGFs increased after skin injury (Barrientos et al., 2008; Werner and Grose, 2003). Loss of FGF2 signaling by application of blocking antibodies (Broadley et al., 1989) or in Fgf2 mutant mice (Ortega et al., 1998) results in decelerated wound closure and compromised granulation tissue formation, while topical application of FGF2 to wounds of diabetic mice increases granulation tissue formation and wound healing capacity (Greenhalgh et al., 1990).
In this paper we describe the development of an assay for studying in vivo wound healing using zebrafish as a model system. With a laser, full-thickness wounds can be quickly and reproducibly introduced on the flank of adult zebrafish. Wounds are re-epithelialized extremely rapidly and independently of blood clot formation and inflammation. Furthermore, granulation-like tissue is formed and later largely cleared, resulting in minimal scar formation. Chemical treatment and transgenic studies reveal essential roles of wound inflammation and FGF signaling for granulation tissue formation, demonstrating genetic and mechanistic conservation of vital wound healing processes between fish and mammals.
Results
The organization of unwounded skin in the trunk of adult zebrafish
Histological and immunofluorescent analysis with a variety of markers (Supplementary Figure S1) demonstrates that the trunk skin of adult zebrafish is composed of overlapping scales, each of which is wrapped by a thin layer of dermal fibroblasts and a multi-layered epidermis. Epidermis and dermis are separated by a basement membrane, and dermis and underlying muscle by a layer of subcutaneous adipocytes.
Full thickness skin wounds are re-epithelialized within hours
We have established a rapid and reproducible technique for introducing wounds of approximately 2 mm in diameter onto the flank of adult zebrafish, using a clinical dermatology laser (Figure 1a). A vital dye penetration assay, where methylene blue is absorbed by damaged tissue but not undamaged or regenerated epidermis, reveals rapid re-establishment of the barrier by 12 hours post-wounding (hpw) (Figure 1b-e). Sections reveal that introduced wounds have initially lost all epidermal and dermal cells, including the scales, and the subcutaneous adipocytes, while underlying muscle tissue is undamaged (Figure 1f,i). At 7 hpw, a thin neo-epidermis covers most of the wounded surface (Figure 1g,j) and by 24 hpw the wound is completely re-epithelialized, with a neo-epidermis of multiple cell layers (Figure 1h,k).
Figure 1. Wounds of adult zebrafish undergo rapid re-epithelialization.
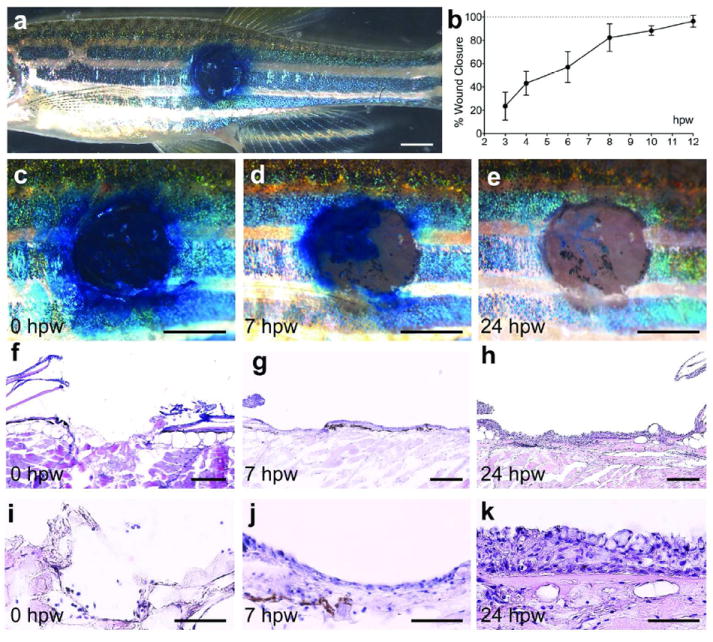
(a) Overview of the left flank of an adult zebrafish with an approximately 2 mm circular wound stained with methylene blue. (b) Graphical illustration of time course of wound closure (see Material and Methods); mean values of closure and standard deviations were determined for at least 6 individuals per time point using Excel software. (c-e) Magnified superficial views of the wound. At 0 hpw (a, c), methylene blue penetrates the entire wound, while the epidermal barrier has been partly recovered by 7 hpw (d), and fully recovered by 24 hpw (e). (f-k) H&E staining of longitudinal sections through the wound reveals the removal of epidermis, dermis and scales via the applied wounding protocol (f and I; n=4). At 7 hpw, a thin neo-epidermis is observed on the surface of the wound (g and j; n=6). At 24 hpw, the recovered epidermis appears re-stratified (h and k; n=10). Scale bars: a,c-e = 1 mm; f-h = 200 μm; i-k = 50 μm.
Wounds exhibit a strong inflammatory response, granulation tissue formation and neo-vascularization
To analyze the inflammatory response of adult zebrafish, we made use of transgenic lines expressing GFP under the control of the mpx promoter to label neutrophils (Tg(mpx:GFP)) (Renshaw et al., 2006), or the lyz (formerly called lysC) promoter, labeling leukocyte lineages that include neutrophils and macrophages (Tg(lyz:DsRED2/GFP)) (Feng et al., 2010; Hall et al., 2007). At 24 hpw, wound inflammation is massive and readily visible at lowest magnification (Supplementary Figure S2). Live imaging was performed to study the kinetics of inflammation. Indirect co-labeling of the neo-epidermis via the methylene blue penetration assay at 4 hpw reveals that neutrophils remain behind the leading edge of the re-epithelializing epidermis (Figure 2a-d). Consistently, in Tg(mpx:GFP)/Tg(lyz:DsRED2) double transgenic fish, inflammatory cells are only present in marginal regions, but absent from the center of the wound at 4 hpw (Figure 2e). More inflammatory cells are present at 8 hpw, when the wound is largely re-epithelialized (Figure 2f,i). During the following days, numbers of inflammatory cells slowly drop, leaving mainly macrophages in the wound at 4 days post-wounding (dpw) (Figure 2g-i).
Figure 2. Adult zebrafish exhibit a strong inflammatory response and granulation tissue formation.
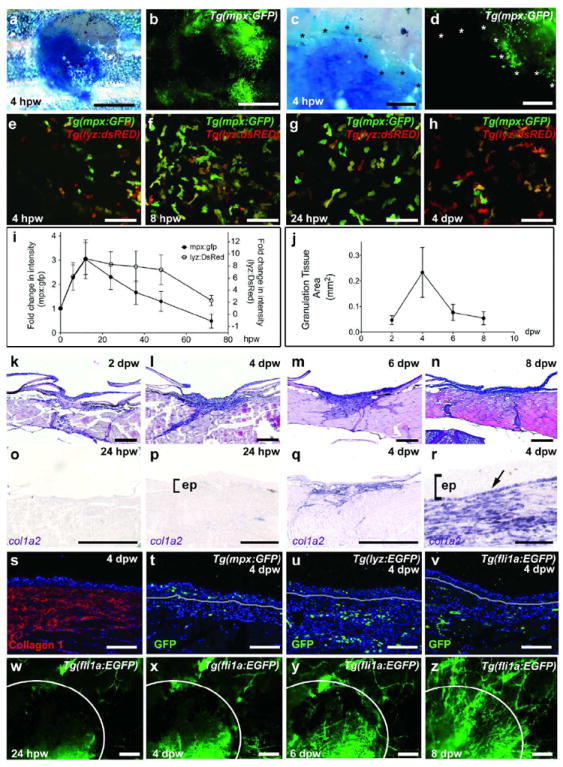
(a-d) Brightfield (a,c) and fluorescent (b,d) images of a Tg(mpx:GFP) fish at 4 hpw; GFP-positive neutrophils are present in re-epithelialized (methylene-blue excluding) regions of the wound (edges marked by asterisks in c and d; n=6/6). (e-h) Live images of the wound centre of Tg(mpx:GFP)i114/Tg(lyz:DsRED2)117 double transgenic fish reveal inflammatory cells, with a progressive relative increase of macrophages (h). (i,j) Graphical illustrations of time course of wound inflammation (i) and granulation tissue formation (j); mean values and standard deviations of relative fluorescent intensities (i) or granulation tissue areas (j) were determined for at least 6 individuals per time point using Excel software. (k-n) H&E staining reveals formation of granulation tissue beneath the wound from 2-6 dpw, which then regresses (8 dpw, n). (o-r) col1a2 expression beneath the wound is sparse at 24 hpw (o,p; n=4/4), but very prominent at 4 dpw (q,r; n=6/6). In addition to dermal fibroblasts, col1a2 is expressed by basal keratinocytes of the neo-epidermis (indicated by arrow in r). (s-v) Immunofluorescence analysis at 4 dpw reveals Collagen 1 deposition (s; n=4/4), leukocytes (t,u; n=4/4) and blood vessels (v; n=4/4) within the granulation tissue. mpx-positive neutrophils are also present in the neo-epidermis (t; n=4/4). (w-z) Superficial views of a Tg(fli1a:EGFP) fish shows progressive wound vascularization from 24 hpw to 8 dpw (n=4/4). Scale bars: a,b,o,q = 1mm; c,d, w-z = 250 μm; e-h = 50 μm; k-n = 500 μm; p,r,s-v = 100 μm. Abbreviation: ep, epidermis.
During mammalian wound healing, inflammation and re-epithelialization coincides with granulation tissue formation, characterized by the invasion of fibroblasts, macrophages and blood vessels into the wound space underneath the neo-epidermis (Singer and Clark, 1999). Histologically, a granulation-like tissue in zebrafish wounds is first visible at 2 days post wounding (dpw) (Figure 2j and k), reaches maximal size at 4 dpw (Figure 2j,l), and has started to regress again at 6 dpw (Figure 2j,m,n). col1a2 in situ hybridization reveals that the granulation tissue largely consists of fibroblasts, which are hardly present at 24 hpw (Figure 2o,p), but in large numbers at 4 dpw (Figure 2q,r). Immunofluorescent analysis at 4 dpw further reveals the presence of collagen type I fibers (Figure 2s) and macrophages in the granulation tissue (Figure 2h,u), while neutrophils are largely confined to the neo-epidermis (Figure 2t). Furthermore, analysis of Tg(fli1a:EGFP) fish shows weak wound neo-vascularization at 4 dpw (Figure 2v-x), which becomes more prominent at 6 and 8 dpw (Figure 2v,y,z).
Adult wounded zebrafish exhibit minimal scar formation
Analysis at later stages after wounding demonstrates the progressive regression of the granulation tissue so that at 10 dpw very few cells can be seen beneath the neo-epidermis. New scales are forming, and the collagen distribution resembles that of an unwounded region (Figure 3a-c). Analysis of Tg(mpx:GFP) (Figure 3d,e) and Tg(fli1a:EGFP) (Figure 3f,g) transgenic fish at 10 dpw reveals a reduction in the number of leukocytes and blood vessels back to levels as in unwounded skin. At 28 dpw, the healed wound (Figure 3h) is largely indistinguishable from unwounded skin (Figure 3i), with a normal epidermis and dermal compartment, and with completely recovered subcutaneous adipocytes and scales (Figure 3m-o). Even the striped pigmentation is largely recovered (Figure 3j-l). The only remaining indication of the wound is a small region of collagen deposition in the muscle layer, as indicated by AFOG staining (arrowed in Figure 3h) although this–was not apparent in all cases.
Figure 3. Adult zebrafish exhibit minimal scar formation.
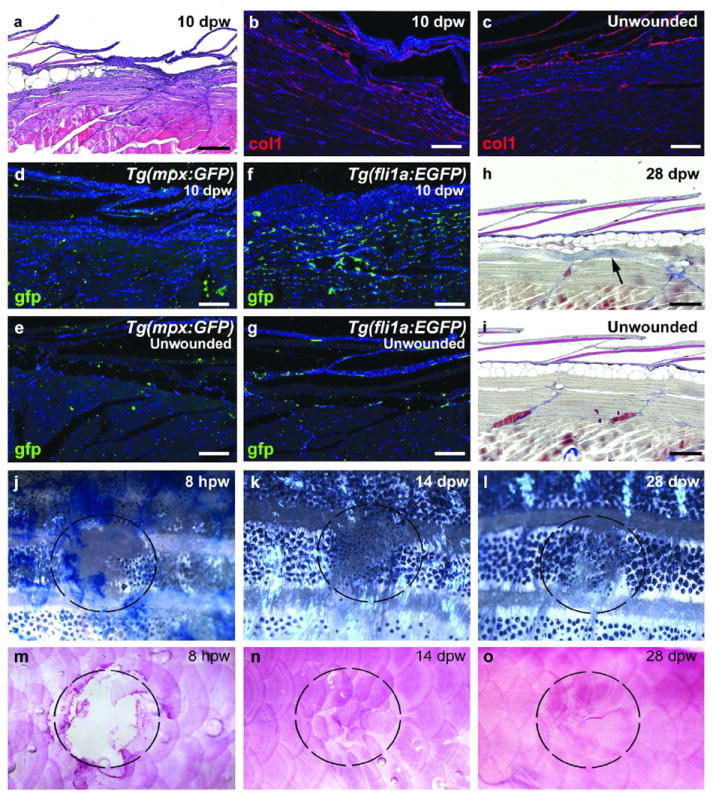
(a) H&E staining at 10 dpw reveals minimal remaining granulation tissue and newly forming scales. (b,c) At 10 dpw, minimal Collagen1 deposition is observed beneath the regenerating scales (b), similar to an unwounded region (c) (n=6/6). (d-g) Tg(mpx:GFP) (d,e; n=4/4) and Tg(fli1a:EGFP) (f,g; n=4/4) transgenic fish at 10 dpw display normal number of leukocytes (d) and reduced numbers of blood vessels (f) at the regenerating wound site when compared to an unwounded region (e and g). (h,i) AFOG staining at 28 dpw indicates the complete recovery of epidermis, dermis, scales and adipocytes with rarely occurring collagen deposits within the muscle layer beneath (h; n=4/4), compared to unwounded fish (i). (j-o) Superficial views of wounded fish demonstrate the almost complete recovery of stripe pattern (j-l) and scales (m-o; alizarin red) by 28 dpw (n=6/6). Dashed circles mark the position of the wound. Scale bars: a,h,i = 500 μm; b-g = 200 μm.
Wound depth affects the extent of tissue regeneration
In order to determine if the extent of regeneration in the adult fish was due to the shallowness of the wounds we introduced, we also analyzed deeper wounds, reaching into the muscle tissue (Supplementary Figure S3). According to the vital dye assay and histological analysis at 8 and 24 hpw, deeper wounds re-epithelialize at normal rates, yielding a fully re-stratified wound epidermis (Supplementary Figure S3a-i). Furthermore, dermis and scales are fully regenerated at 28 dpw (Supplementary Figure S3m-o). By contrast, the regeneration of the lost muscle tissue beneath the deeper wounds is incomplete, with damaged regions often filled with additional subcutaneous adipocytes (Supplementary Figure S3m-o). Furthermore, pigmentation regenerates in more irregular patterns (Supplementary Figure S3j-l). This indicates that also in deeper wounds, the skin and its appendages readily regenerate with minimal scarring.
Blood clotting and inflammation play no role in re-epithelialization of adult zebrafish wounds
To gain insights into functional interdependences of the different steps of wound healing and into possible evolutionary conservation of molecular control mechanisms, we carried out a series of chemical treatments and transgenic analyses. In adult mammals, the formation of an external fibrin clot is a critical initial wound healing response, serving as a temporary seal for the damaged tissue, a source of signals that stimulate later processes of wound healing, and a substrate for invading cells. We treated adult fish with 150 μM Sodium Warfarin, an inhibitor of the blood clotting process, which has previously been used in zebrafish (Hanumanthaiah et al., 2001; Jagadeeswaran and Sheehan, 1999) and which causes localized internal bleeding of treated fish (Figure 4 m-p). However, Warfarin treatment has no effect on the re-epithelialization of introduced wounds, which close with the same rate and yield a multi-layered neo-epidermis as in controls (Figure 4a-l). Additionally, we failed to detect an external fibrin clot during our histological examinations (Figure 2), indicating that re-epithelialization of cutaneous zebrafish wounds occurs independently of blood clotting. Similarly, abrogation of inflammation by treatment of Tg(mpx:GFP) and Tg(mpx:GFP)/Tg(lyz:DsRED2) fish with hydrocortisone has no effect on the rate of epidermal barrier recovery (Figure 5), suggesting that re-epithelialization is uncoupled from wound inflammation.
Figure 4. Blood clot formation plays no role in the re-epithelialization process.
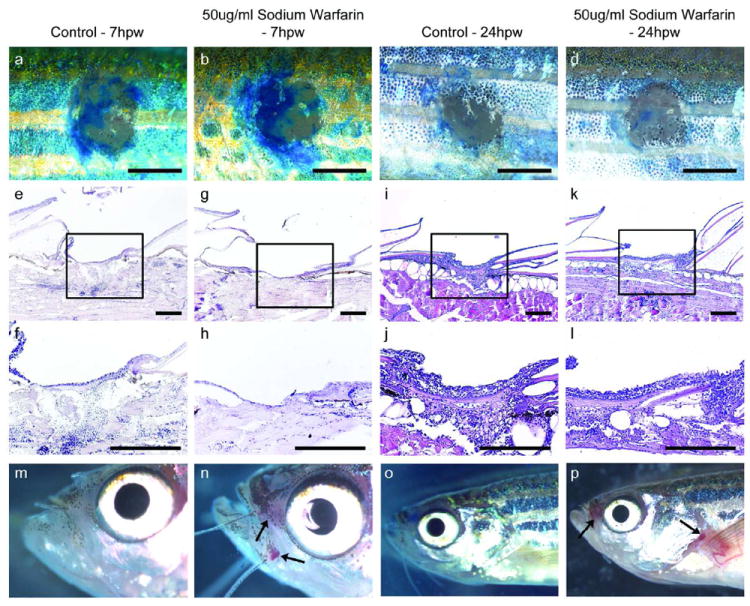
Methylene blue penetration assay (a-d) and H&E stained longitudinal sections (e-l) of wounds from control fish (a, c, e, f, i, j) and fish treated with 150 μM Sodium Warfarin (b, d, g, h, k, l), demonstrating no differences in the degree of barrier recovery or wound re-epithelialization at 7 hpw (a, b, e-h) or 24 hpw (c, d, i-l) (n=18/18), even though warfarin-treated fish show localized internal bleeding, particularly around the mouth, gills and pec fins at 7 and 24 hpw (m-p). Scale bars: a-d = 1 mm; e-l = 100 μm.
Figure 5. Reducing the inflammatory response does not affect re-epithelialization.
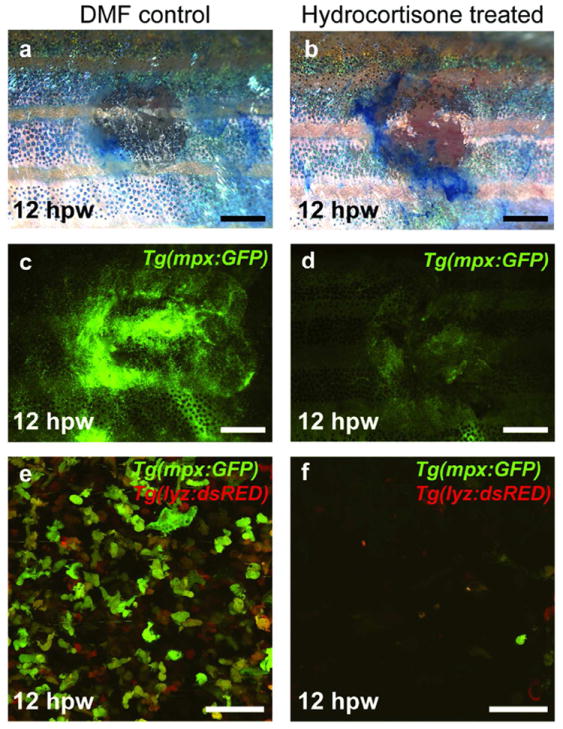
(a-f) Tg(mpx:GFP) (a-d) and Tg(mpx:GFP)/Tg(lyz:DsRED2) fish (e and f) treated with 275 μM Hydrocortisone show no delay in re-epithelialization (a,b; n=19/19), although the number of inflammatory cells at the wound is clearly reduced at 12 hpw when compared to control fish (c-f). Scale bars: a-d = 1 mm; e-f = 50 μm.
Impairment of inflammation and transgenic inhibition of FGF signaling results in compromised granulation tissue formation
In mammals, formation of granulation tissue has been shown to depend both on wound inflammation (Eming et al., 2009; Leibovich and Ross, 1975) and FGF2 function (Broadley et al., 1989; Ortega et al., 1998). Indeed, hydrocortisone-induced impairment of inflammation in wounds of the zebrafish results in reduced granulation tissue (Figure 6a-d). To investigate the role of FGF signaling during granulation tissue formation, we made use of Tg(hsp70l:dnfgfr1-EGFP) transgenic zebrafish for temporally controlled ubiquitous expression of a C-terminally truncated mutant form of Fgf receptor 1 in which the cytoplasmic tyrosine kinase domain is replaced by GFP. Upon dimerization with endogenous receptors, this truncated receptor is predicted to block all FGF receptor subtypes in a dominant-negative manner, as shown for Fgfr1, Fgfr2 and Fgfr4-dependent processes in larval and adult zebrafish (Lee et al., 2005; Lepilina et al., 2006). Heatshock-treatment of Tg(hsp70l:dnfgfr1-EGFP) fish results in a normal neo-epidermis, but almost complete absence of granulation tissue at 4 dpw (Figure 6f,I,j), while granulation tissue of normal size is present in heat-shocked non-transgenic control fish (Figure 6e,g,h). col1a2 in situ hybridization analysis further indicates the absence of wound fibroblasts in heat-shocked Tg(hsp70l:dnfgfr1-EGFP) fish (Figure 6k,l). However, blockage of FGF signal transduction does not affect wound macrophage numbers at 1 dpw (Figure 6m-p), or wound vascularization (Figure 6q-t).
Figure 6. Inflammation and FGF signaling are required for granulation tissue formation.
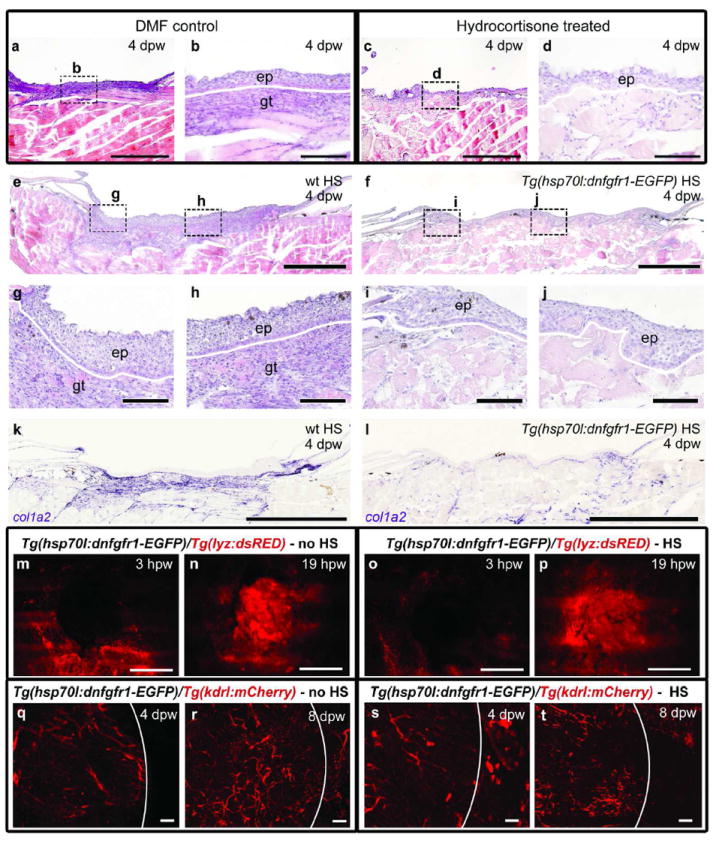
(a-d) Hydrocortisone-treated fish at 4 dpw, lacking granulation tissue beneath the wound (c,d; n=9/9) in comparison to DMF-treated controls (a,b). (e-j) H&E staining demonstrates granulation tissue beneath the wound epidermis in heat-shocked wild-type fish (e, g, h; n=6/6), but not in the heat-shocked Tg(hsp70l:dnfgfr1-EGFP) fish (f, i, j; n=6/6) at 4 dpw. (k, l) in situ hybridization analysis reveals normal col1a2 expression in a heat-shocked wild-type fish (k; n=4/4), but strongly reduced expression in a heat-shocked Tg(hsp70l:dnfgfr1-EGFP) fish at 4 dpw (l; n=4/4). (m-t) Analysis of Tg(hsp70l:dnfgfr1-EGFP),Tg(lyz:dsRED) (m-p) or Tg(hsp70l:dnfgfr1-EGFP), Tg(kdrl:HSRAS-mCherry) (q-t) double transgenic fish reveals normal inflammatory responses (m-p) and normal vascularization (q-t) in non-heat-shocked (m,n; n=8/8; q,r; n=8/8) and heat-shocked Tg(hsp70l:dnfgfr1-EGFP) fish (o,p; n=8/8; s,t; n=8/8). Scale bars: a,c,e,f = 500 μm; b,d, g-j, q-t = 100 μm; k-p = 1 mm.
Discussion
The different steps of wound repair in adult mammals and fish
Mammalian wound healing involves several phases and processes that overlap in time: blood clotting, inflammation, re-epithelialization, granulation tissue formation, neo-vascularization, and tissue remodeling/scar resolution (Shaw and Martin, 2009; Singer and Clark, 1999). Our data indicate that, apart from blood clot formation, all processes also take place during wound repair in adult zebrafish. However, the time course of individual process initiation appears slightly different. In mammals, blood clotting occurs first, providing chemotactic factors that attract inflammatory leukocytes and extracellular matrix proteins that serve as a migration substrate for immigrating cells. Neutrophils cleanse the wounded area and are eventually phagocytosed by macrophages which enter the wound slightly later (Kim et al., 2008). Macrophages further secrete various growth factors to attract keratinocytes, fibroblasts and blood vessels into the wound, promoting re-epithelialization, granulation tissue formation and vascularization (Shaw and Martin, 2009; Singer and Clark, 1999).
Although there is some debate over the lineage specificity of the lyz promoter in zebrafish, it has been suggested that mpx-expressing cells are neutrophils and lyz-expressing cells are macrophages (Feng et al., 2010). Therefore, our results suggest that as in mammals, macrophages remain in healing wounds longer than neutrophils, and that inflammation precedes granulation tissue formation and vascularization (Figure 2). However, re-epithelialization starts even before inflammation and in the absence of blood clotting (warfarin treatment; Figure 4), inflammation (hydrocortisone treatment; Figure 5) or granulation tissue (Figure 6). Thus, re-epithelialization seems to be largely independent of ECM proteins supplied by the blood clot or the granulation tissue. This might be due to a predominant impact of tissue-autonomous extension movements within the re-epithelializing epidermis (R.R. and M.H., unpublished data), a process that is likely to also occur in wounds of mammalian embryos (Caddy et al., 2010). In addition, re-epithelialization is independent of wound debridement by neutrophils and chemotactic signals from macrophages. Interestingly, also in mouse, wounds close with almost normal rates in the absence of neutrophils and macrophages (Dovi et al., 2003; Martin et al., 2003), suggesting that, similar to our findings in zebrafish, re-epithelialization does not require an inflammatory response.
Conserved roles of inflammation and FGF signaling for granulation tissue formation in mammals and fish
By contrast, inflammation seems necessary for fibroblast recruitment, granulation tissue formation and wound vascularization both in mammals and fish (Figure 6). Thus, hydrocortisone treatment in zebrafish compromises granulation tissue formation similar to the effects caused by similar treatments in mammals (Leibovich and Ross, 1975) and pointing to the existence of fibroblast-stimulating signals from inflammatory cells both in fish and mammals. However, as the effects of hydrocortisone treatment may not be restricted to inflammation more sophisticated transgenic ablation experiments, as performed in the mouse (Lucas et al., 2010), will be necessary to provide ultimate proof for the requirement of inflammatory cells and to identify the relevant stimulatory signals. Our data obtained upon global transgenic inhibition of FGF signal reception point to a thus far not further specified FGF as essential for granulation tissue formation, again in agreement with results obtained via loss of FGF2 function in mouse (Broadley et al., 1989; Ortega et al., 1998). By contrast, FGF signaling is dispensable for wound re-epithelialization, inflammation and vascularization, pointing to a direct effect of FGF signaling on fibroblasts. Indeed, cultured mammalian wound fibroblasts can directly respond to FGF2 (Rahimi et al., 2005; Sasaki, 1992), while fibroblasts of the zebrafish granulation tissue show strong expression of the FGF receptor fgfr1a (CK and MH, unpublished results). FGFs are well known stimulators of angiogenesis (Lieu et al., 2011). That FGF signaling is dispensable for the neo-vascularization of zebrafish wounds might be due to other related signals, such as Vascular Endothelial Growth factors (VEGFs), which are crucially involved in the vascularization of mouse wounds (Barrientos et al., 2008).
Together, this points to an evolutionary conservation of crucial molecular mechanisms underlying granulation tissue formation in different vertebrate classes.
Adult zebrafish exhibit scar-free skin regeneration
A striking difference between mammalian and fish wound repair is that wound tissue remodeling in zebrafish is completed with minimal scarring (Figure 3 and Supplementary Figure S3). The granulation tissue resolves completely, inflammation terminates, and blood vessels regress within 10 dpw. Even in deeper wounds with damage of underlying muscle, the skin regenerates almost perfectly, while the damaged muscle does not (Supplementary Figure S3).
We can only speculate about the mechanisms of the perfect skin remodeling and possible differences between fish and mammals. Macrophageless PU.1 null neonatal mice repair wounds without an apparent fibrotic response (Martin et al., 2003). Furthermore, the capability of scar-free wound healing during mouse embryogenesis is lost at the same stage (E15) when first inflammatory responses can be observed. Based on these observations, a causative relationship between inflammation and scarring has been proposed (Martin and Parkhurst, 2004). Our finding of scar-free wound healing in adult zebrafish, despite a massive inflammatory response, speaks against this notion. The speed of re-epithelialization, the lack of cornification in outer epidermal layers (Supplementary Figure S1), which only evolved in higher, land-based vertebrates, and the exposure to a liquid environment could be crucial factors in this regeneration capability. In mammals, similar conditions apply to mucosal injuries and to fetal cutaneous wounds, which, strikingly, also close rapidly and with minimal scar formation (Szpaderska et al., 2003; Rolfe and Grobbelaar, 2012).
The adult zebrafish as a valuable in vivo model of cutaneous wound repair
In our opinion, the described combination of shared and class-specific features of wound healing in fish and mammals makes the adult zebrafish a valuable model for comparative studies which should also increase our understanding of mammalian wound repair, and the cellular, molecular and genetic basis of human wound healing pathologies. Compared to mammals, in which the different processes of wound healing largely overlap in time, they occur in a more subsequent fashion in zebrafish, which should allow us to better dissect direct from indirect effects obtained after chemical or genetic interference. This is exemplified above for the roles of inflammation and FGF signal reception during granulation tissue formation. The established zebrafish wounding protocol is robust and fast and can be used for large-scale forward genetic screening. In addition, the adult zebrafish seems highly suitable for treatments with chemical inhibitors, which are simply added to the water, allowing to screen libraries for drugs interfering with wound healing or alleviating particular wound healing pathologies.
Materials & Methods
Zebrafish and wounding
Adult zebrafish were wounded at an age of 6-12 months. The transgenic lines used, Tg(krt4:egfp)gz7, Tg(mpx:GFP)i114, Tg(lyz:EGFP)nz117, Tg(lyz:dsRED2)nz50, Tg(fli1a:EGFP)y1, Tg(kdrl:HSRAS:mCherry)s896 and Tg(hsp70l:dnfgfr1-EGFP)pd1, have been described previously (Gong et al., 2002; Renshaw et al., 2006; Hall et al., 2007; Lawson and Weinstein, 2002; Chi et al., 2008; Lee et al., 2005).
For wounding, adult fish were anaesthetized in 0.13% Tricaine (w/v) and laid on Whatman paper soaked in system water. A full thickness wound was introduced onto the left flank directly anterior of anal and dorsal fins (Figure 1a). An Erbium:YAG MCL29 Dermablate dermatology laser (Asclepion) was set to a frequency of 5 Hz and two pulses with a strength of 500 mJ for smaller (20-30 mm) or 600 mJ for larger (30-40 mm) specimens were applied, resulting in a pulse strength of 7.1 or 8.5 J/cm2, respectively. For heatshock-induced transgene activation, Tg(hsp70l:dnfgfr1-EGFP) fish were transferred from 28°C to pre-warmed water at 40°C for one hour, returned to water at 28°C and wounded four hours later. Heatshock treatments were repeated every 24 hours.
All zebrafish experiments were approved by the national animal care committee (LANUV Nordrhein-Westfalen; 8.87-50.10.31.08.130; 84-02.04.2012.A253) and the University of Cologne.
Tissue labeling procedures
For methylene blue penetration, fish were anaesthetized in 0.13 % Tricaine and placed on Whatman paper soaked with water. A drop of an aqueous 0.1% (w/v) methylene blue solution was applied to the wound for 1 minute followed by extensive washing. For quantification open (blue) and total wound area was measured using ImageJ software.
For histological and immunofluorescence analysis adult zebrafish were fixed in 4% Paraformaldehyde (PFA) / PBS overnight at 4°C then washed with 1x PBS. For whole-mount immunofluorescence analysis, fish were washed for several hours in dH20 and blocked in PBS+10% fetal calf serum (FCS). Antibody incubations were carried out in PBS+10%FCS and washes in PBS+0.5% Triton-X. Mineralized bone was stained with alizarin red as described (Walker and Kimmel, 2007).
For sectioning samples were fixed in 4% PFA, decalcified in 0.5 M EDTA (pH 7.4) at room temperature for 5 days, dehydrated in a graded series of alcohols, cleared in Roti-Histol (Carl Roth) and embedded in paraffin. 8 μm sections were stained with Hematoxylin and Eosin (H&E), Acidic fucsin orange G (AFOG) or periodic acid-Schiff reaction (PAS), using standard protocols. For quantification of granulation tissue sizes, consecutive sections of wounds were stained with H&E. Sections comprising the center of the wound were selected and granulation tissue areas measured using ImageJ.
Immunofluorescence analysis on paraffin embedded tissue was performed using standard protocols. Primary antibodies used were: anti-p63 (1:100, Santa Cruz, sc-8431), anti-gfp (1:100, Invitrogen, A10262), anti-col1 (1:200, Abcam, ab23730).
In situ hybridization on paraffin embedded sections was performed as described previously (Hyde et al., 2007). col1a2 complete cDNA was excised from EST clone IMAGp998C1714602Q and cloned into pCMV-SPORT6.1. Probe was synthesized with SmaI and T7 RNA polymerase.
Drug Treatments
Adult zebrafish were treated with Sodium Warfarin (150 μM; A4571 - Sigma), or Hydrocortisone (275 μM; H4001 - Sigma) in fish system water. In each case, fish were treated with the drug for at least 12 hours prior to wounding. Drug containing water was exchanged every 24 hours when necessary.
Imaging
Transgenic fish were anaesthetized in 0.13% Tricaine (w/v) and photographed using a Zeiss Apotome, Zeiss Confocal (LSM710 META) or Leica M165 FC microscope. For quantification of inflammation, entire wounds of double transgenic Tg(mpx:GFP)i114/Tg(lyz:dsRED2)nz50 fish were repetitively imaged at indicated time points using identical settings. Mean fluorescent intensity values of the wounded area were measured using ImageJ and expressed as fold change in intensity compared to directly after wounding.
Supplementary Material
Acknowledgments
Excellent technical assistance from Evelin Fahle is gratefully acknowledged. Work was supported by the German Research Foundation (DFG; SFB 829), the European Union (Seventh Framework Programme, Integrated Project ZF-HEALTH, EC Grant Agreement HEALTH-F4-2010-242048), the US National Institute of General Medical Sciences (GM63904) and an EMBO long-term postdoctoral fellowship to RR.
Abbreviations
- hpw
hours post wounding
- dpw
days post wounding
- FGF
Fibroblast growth factor
- H&E
Hematoxylin and Eosin
- AFOG
Acidic fucsin orange G
- PAS
periodic acid-Schiff
- FCS
fetal calf serum
- PFA
Paraformaldehyde
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Broadley KN, Aquino AM, Woodward SC, Buckley-Sturrock A, Sato Y, Rifkin DB, et al. Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Invest. 1989;61:571–575. [PubMed] [Google Scholar]
- Caddy J, WIlanowski T, Darido C, Dworkin S, Ting SB, Zhao Q, Rank G, Auden A, Srivastava S, Papenfuss TA, Murdoch JN, Humbert PO, Parekh V, Boulos N, Weber T, Zuo J, Cunningham JM, Jane SM. Epidermal wound repair is regulated by the planar cell polarity signaling pathway. Dev Cell. 2010;19:138–47. doi: 10.1016/j.devcel.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Shaw RM, De Val S, Kang G, Jan LY, Black BL, Stainier DY. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 2008;22:734–9. doi: 10.1101/gad.1629408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–455. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- Eming SA, Hammerschmidt M, Krieg T, Roers A. Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol. 2009;20:517–527. doi: 10.1016/j.semcdb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol. 2010;8:e1000562. doi: 10.1371/journal.pbio.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Ju B, Wang X, He J, Wan H, Sudha PM, et al. Green fluorescent protein expression in germ-line transmitted transgenic zebrafish under a stratified epithelial promoter from keratin8. Dev Dyn. 2002;223:204–215. doi: 10.1002/dvdy.10051. [DOI] [PubMed] [Google Scholar]
- Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7:42. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanumanthaiah R, Thankavel B, Day K, Gregory M, Jagadeeswaran P. Developmental expression of vitamin K-dependent gamma-carboxylase activity in zebrafish embryos: effect of warfarin. Blood Cells Mol Dis. 2001;27:992–999. doi: 10.1006/bcmd.2001.0472. [DOI] [PubMed] [Google Scholar]
- Hyde G, Dover S, Aszodi A, Wallis GA, Boot-Handford RP. Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev Biol. 2007;304:825–833. doi: 10.1016/j.ydbio.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeeswaran P, Sheehan JP. Analysis of blood coagulation in the zebrafish. Blood Cells Mol Dis. 1999;25:239–249. doi: 10.1006/bcmd.1999.0249. [DOI] [PubMed] [Google Scholar]
- Kiener TK, Selptsova-Friedrich I, Hunziker W. Tjp3/zo-3 is critical for epidermal barrier function in zebrafish embryos. Dev Biol. 2008;316:36–49. doi: 10.1016/j.ydbio.2007.12.047. [DOI] [PubMed] [Google Scholar]
- Kim MH, Liu W, Borjesson DL, Curry FR, Miller LS, Cheung AL, et al. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J Invest Dermatol. 2008;128:1812–1820. doi: 10.1038/sj.jid.5701223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Le Guellec D, Morvan-Dubois G, Sire JY. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio) Int J Dev Biol. 2004;48:217–231. doi: 10.1387/ijdb.15272388. [DOI] [PubMed] [Google Scholar]
- Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development. 2005;132:5173–5183. doi: 10.1242/dev.02101. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Li Q, Frank M, Thisse CI, Thisse BV, Uitto J. Zebrafish: a model system to study heritable skin diseases. J Invest Dermatol. 2011;131:565–571. doi: 10.1038/jid.2010.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu C, Heymach J, Overman M, Tran H, Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res. 2011;17:6130–9. doi: 10.1158/1078-0432.CCR-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–77. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, et al. Wound healing in the PU.1 null mouse--tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci U S A. 1998;95:5672–5677. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi F, Hsu K, Endoh Y, Geczy CL. FGF-2, IL-1beta and TGF-beta regulate fibroblast expression of S100A8. Febs J. 2005;272:2811–2827. doi: 10.1111/j.1742-4658.2005.04703.x. [DOI] [PubMed] [Google Scholar]
- Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci. 2004;359:777–784. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108:3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- Rolfe KJ, Grobbelaar AO. A review of fetal scarless healing. ISRN Dermatol. 2012:698034. doi: 10.5402/2012/698034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T. The effects of basic fibroblast growth factor and doxorubicin on cultured human skin fibroblasts: relevance to wound healing. J Dermatol. 1992;19:664–666. doi: 10.1111/j.1346-8138.1992.tb03755.x. [DOI] [PubMed] [Google Scholar]
- Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Sire JY, Akimenko MA. Scale development in fish: a review, with description of sonic hedgehog (shh) expression in the zebrafish (Danio rerio) Int J Dev Biol. 2004;48:233–247. doi: 10.1387/ijdb.15272389. [DOI] [PubMed] [Google Scholar]
- Sire JY, Allizard F, Babiar O, Bourguignon J, Quilhac A. Scale development in zebrafish (Danio rerio) J Anat. 1997;190(Pt 4):545–561. doi: 10.1046/j.1469-7580.1997.19040545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane M, Carpio Y, Geisler R, Schwarz H, Maischein HM, Nuesslein-Volhard C. Zebrafish penner/lethal giant larvae 2 functions in hemidesmosome formation, maintenance of cellular morphology and growth regulation in the developing basal epidermis. Development. 2005;132:3255–3265. doi: 10.1242/dev.01904. [DOI] [PubMed] [Google Scholar]
- Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res. 2003;82:621–6. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- Walker MB, Kimmel CB. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech Histochem. 2007;82:23–28. doi: 10.1080/10520290701333558. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


