Abstract
γ-Aminobutyric acid B (GABAB) receptor activation is a potential therapeutic approach for the treatment of drug addiction, pain, anxiety, and depression. However, full agonists of this receptor induce side-effects, such as sedation, muscle relaxation, tolerance, and cognitive disruption. Positive allosteric modulators (PAMs) of the GABAB receptor may have similar therapeutic effects as agonists with superior side-effect profiles. The present study behaviorally characterized N-([1R,2R,4S]-bicyclo[2.2.1]hept-2-yl)-2-methyl-5-(4-[trifluoromethyl]phenyl)-4-pyrimidinamine (BHF177), a GABAB receptor PAM, in mouse models of anxiety-like behavior, learning and memory. In addition, the effects of BHF177 were compared with the agonist baclofen. Unlike the anxiolytic chlordiazepoxide, baclofen (0.5, 1.5, and 2.5 mg/kg, intraperitoneally) and BHF177 (10, 20, and 40 mg/kg, orally) had no effect on anxiety-like behavior in the elevated plus maze, light/dark box, or the Vogel conflict test. Baclofen increased punished drinking in the Vogel conflict test, however this effect may be attributable to analgesic actions of baclofen. At the highest dose tested (2.5 mg/kg), baclofen-treated mice exhibited sedation-like effects (i.e., reduced locomotor activity) across many of the tests, whereas BHF177-treated mice exhibited no sedation-like effects. BHF177 exhibited pro-convulsion properties only in mice, but not in rats, indicating that this effect may be species-specific. At doses that were not sedative or pro-convulsant, baclofen and BHF177 had no selective effects on fear memory retrieval in contextual and cued fear conditioning or spatial learning and memory in the Barnes maze. These data suggest that BHF177 has little sedative activity, no anxiolytic-like profile, and minimal impairment of learning and memory in mice.
Keywords: GABAB receptor, positive allosteric modulator, anxiety, learning, memory
1. Introduction
γ-Aminobutyric acid (GABA) is the most widely distributed amino acid neurotransmitter in the central nervous system and acts as the primary inhibitory neurotransmitter (Johnston, 1978; Sivilotti and Nistri, 1991). GABA is critically involved in the function of multiple brain sites, and thus dysfunction of the GABAergic system is likely to be involved in the development of dependence on drugs of abuse, such as nicotine and cocaine, and other psychiatric disorders, including anxiety and depression (Cryan and Slattery, 2010; Koob, 2000; Millan, 2003; Reynolds, 2008; Vlachou and Markou, 2010; Xi and Gardner, 2008). GABA signaling is mediated through ionotropic GABAA and GABAC receptors and metabotropic GABAB receptors (Bormann, 1988; Bowery, 1989). GABAA receptors are ligand-gated ion channels responsible for the rapid component of inhibitory postsynaptic potentials. GABAB receptors are G-protein-coupled receptors that inhibit adenylate cyclase activity and mediate the slow and prolonged component of synaptic inhibition (Bormann, 1988; Bowery et al., 2004). The prototypical GABAB receptor agonist baclofen, which is used for the treatment of spasticity and skeletal muscle rigidity, has shown therapeutic promise in a wide range of other indications, including drug dependence, anxiety disorders, and depression (Bettler et al., 2004; Cousins et al., 2002; Cryan and Kaupmann, 2005; Cryan and Slattery, 2010). However, baclofen induces sedation and muscle relaxation, limiting its use as a tool for behavioral research and therapeutic agent, although tolerance develops to some of these effects (Ong and Kerr, 2005).
Accumulating evidence indicates that allosteric positive modulators (PAMs), which act at sites distinct from the orthosteric binding site, may have a better side-effect profile compared with full agonists because PAMs are devoid of substantial intrinsic agonist activity in the absence of the orthosteric agonist. Instead, PAMs potentiate the potency and maximal efficacy of full agonists, such as endogenously released GABA (Christopoulos, 2002; Jensen and Spalding, 2004), thus offering more physiological means to activate the receptors than agonists. This notion is supported by the demonstrated effects of benzodiazepines that are GABAA receptor PAMs. Compared with orthosteric agonists at the GABAA receptor, benzodiazepines have similar pharmacological effectiveness in treating anxiety and sleep disorders, while having improved side-effect profiles (Mohler et al., 2002). Moreover, the GABAB receptor PAM GS39783 showed similar efficacy as the agonist baclofen in rodent models of drug dependence (Lhuillier et al., 2007; Maccioni et al., 2008; Maccioni et al., 2007; Mombereau et al., 2007; Paterson et al., 2008; Slattery et al., 2005) and anxiety-like behavior (Cryan et al., 2004; Mombereau et al., 2004). In contrast to baclofen, GS39783 showed few adverse effects, having no effect on locomotor activity, rotarod performance (Cryan et al., 2004), or food- and sucrose-maintained responding (Maccioni et al., 2008; Maccioni et al., 2007; Paterson et al., 2008). Thus, GABAB receptor PAMs may exhibit similar pharmacological effectiveness as the full agonist baclofen and therefore serve as a useful tool for the evaluation of the role of GABAB receptors in psychiatric disorders.
Although the important role of GABAA receptors in anxiety has been well documented, findings on the involvement of GABAB receptors in anxiety-like behavior have been inconsistent (Cryan and Kaupmann, 2005; Frankowska et al., 2007; Partyka et al., 2007). Thus, we evaluated the effects of GABAB receptor activation by the novel PAM N-([1R,2R,4S]-bicyclo[2.2.1]hept-2-yl)-2-methyl-5-(4-[trifluoromethyl]phenyl)-4-pyrimidinamine (BHF177) on anxiety-like behavior in various mouse models and compare the effects of BHF177 and baclofen. BHF177 is a potent (pEC50 = 5.78 ± 0.03; Emax (%) = 183 ± 4) and selective GABAB receptor PAM with good metabolic stability and ability to cross the blood-brain barrier (Guery et al., 2007). Similar to GS39783, BHF177 showed efficacy in animal models of drug dependence (Maccioni et al., 2009; Paterson et al., 2008; Vlachou et al., 2011a). However, little is known about whether BHF177 has anxiolytic efficacy. The present study evaluated potential anxiolytic properties of BHF177 in the elevated plus maze, the light/dark box and the Vogel conflict test in mice. These tests are standard “first pass” preclinical test for anxiolytic efficacy and commonly used to evaluate the putative anxiolytic properties of compounds (Cryan and Sweeney, 2011; Treit et al., 2010). Both the light/dark box and the elevated plus maze are approach-avoidance-based behavioral paradigms (i.e., they are based on the conflict between innate exploratory drive and avoidance of brightly lit or open, elevated environments in rodents) (Rodgers et al., 1997). The Vogel conflict test is a conflict-based anxiety test, in which the drinking behavior of thirsty rodents is punished by aversive shock delivery (Millan and Brocco, 2003). Although the neurobiology that underlies these models is not identical, all of these tests are sensitive to anxiolytic compounds with a wide spectrum of putative mechanisms, including benzodiazepines, 5-HT1A receptor agonists, and selective serotonin reuptake inhibitors (Cryan and Sweeney, 2011; Treit et al., 2010).
Because the Vogel conflict test is a conflict-based anxiety test which involves aversive learning to a painful stimulus (Millan and Brocco, 2003), the flinch-jump test, was conducted to determine whether analgesic effects of the compound are involved in the effects obtained in this conflict test. Furthermore, activation of the GABAB receptor by baclofen has been reported to disrupt learning and memory (Castellano et al., 1989; McNamara and Skelton, 1996; Nakagawa and Takashima, 1997; Pitsikas et al., 2003; Stackman and Walsh, 1994; Zarrindast et al., 2004). Therefore, the effects of BHF177 and baclofen on fear-related learning and memory, evaluated in the contextual and cued fear conditioning tests, and spatial learning and memory, evaluated in the Barnes maze, were investigated to characterize any potential cognitive deficits induced by these compounds. Finally, we noticed that several mice experienced mild seizures after administration of the highest dose of BHF177 in these behavioral tests. Therefore, the pentylenetetrazole (PTZ)-induced seizure test was conducted to evaluate the potential pro-convulsant properties of this compound in both mice and rats. We conducted the PTZ test in rats to test the hypothesis that the pro-convulsant effects of BHF177 may be species-specific because BHF177-induced seizures in rats have never been observed in our behavioral studies and have not been reported by others who used similar dose ranges (Paterson et al., 2008; Vlachou et al., 2011a; Vlachou et al., 2011b).
2. Materials and Methods
2.1. Subjects
Experimentally naive male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) 12-14 weeks old, and naive male Wistar rats (Charles River, Raleigh, NC, USA) that weighed 300-320 g upon arrival in the laboratory were housed in humidity- and temperature-controlled animal facilities on a reverse 12 h/12 h light/dark cycle (lights off at 7:00 AM) with ad libitum access to food and water except during testing. Behavioral testing was conducted during the dark phase of the light/dark cycle. All of the procedures were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and National Research Council’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of the University of California San Diego and The Scripps Research Institute. An independent group of naive mice was used for each experiment using a between-subjects design for the factor drug dose, with the exception of a few vehicle-treated mice that were used in both the probe and retention tests in the Barnes maze (see below).
2.2. Drugs
Chlordiazepoxide and R(+)-baclofen hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in sterile 0.9% saline and injected intraperitoneally (i.p.) 30 min before testing. N-([1R,2R,4S]-bicyclo[2.2.1]hept-2-yl)-2-methyl-5-(4-[trifluoromethyl]phenyl)-4-pyrimidinamine (BHF177) was synthesized as described below. This compound was suspended in 0.5% methylcellulose and administered orally (p.o.) 60 min before testing. The convulsant drug PTZ (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterile 0.9% saline and injected i.p. immediately before testing. The doses of baclofen (0.5, 1.5, and 2.5 mg/kg) and route of administration (i.p.) were chosen based on previously published studies (Cryan et al., 2004; Dalvi and Rodgers, 1996; Frankowska et al., 2007; Umezu, 1999; Zarrindast et al., 2001) that all reported a narrow effective dose range of this compound before sedative effects were manifested. The doses of BHF177 (10, 20, and 40 mg/kg) and route of administration (p.o.) were also chosen based on previous studies (Maccioni et al., 2009; Paterson et al., 2008; Vlachou et al., 2011a; Vlachou et al., 2011b). All of the drugs were administered in volumes of 0.1 ml/10 g body weight in mice and 0.1 ml/100 g body weight in rats. The effects of baclofen and BHF177 were investigated using a between-subjects design for the factor Dose, with n = 12 per group for most of the behavioral tests, with the exception of the flinch-jump test and rat PTZ-induced seizure test, in which eight subjects per group were used.
2.2.1. Synthesis of BHF177
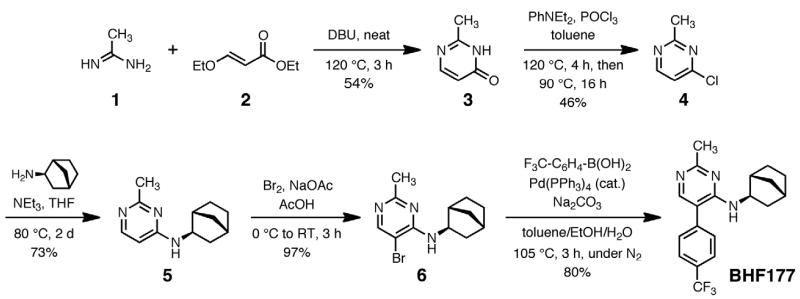
The previous synthesis of BHF177 (Guery et al., 2007) required seven steps from commercially available 2,4-dichloro-5-bromobenzene, during which the C2-methyl, C5-aryl, and C4-amine groups were introduced in succession. We chose instead to assemble the pyrimidine core with the methyl group pre-installed, resulting in the five-step procedure shown in Fig. 1 (see Supplemental Material for detailed synthesis and compound characterization information). The final product showed nuclear magnetic resonance, mass spectrometry, and chromatographic data identical to BHF177 prepared previously by the published route, and consistent with its proposed structure. Under similar administration parameters and doses the same batch of BHF177 synthesized following this procedure had behavioral effects on startle reactivity in rats (unpublished observations).
Figure 1.
Synthetic pathway to BHF177.
2.3. Anxiety tests
2.3.1. Elevated plus maze
The elevated plus maze apparatus has four arms (5 × 30 cm) at right angles to each other and is elevated 30 cm from the floor. Two of the arms have 16 cm high walls (enclosed arms) and two arms have a 0.5 cm lip but no walls (open arms). The mice were placed onto the center of the maze and allowed free access to all four arms for 5 min. Behavior was recorded using a camera mounted above the apparatus. The percentage of time spent on the open arms, number of open arm entries, and number of total arm entries were scored from videotapes at a later time by an observer blind to each mouse treatment. An arm entry was defined as all four paws entering an arm.
2.3.2. Light/dark box
The light/dark box consisted of a rectangular box made of Plexiglas divided by a partition into two environments. One compartment (14.5 × 27 × 26.5 cm) was dark (8-16 lux), and the other compartment (28.5 × 27 × 26.5 cm) was brightly illuminated (600-800 lux) by a 60 W light source located above it. The compartments were connected by an opening (7.5 × 7.5 cm) located at floor level in the center of the partition. The mice were placed in the dark compartment, facing away from the opening, and allowed to freely explore the apparatus for 5 min. A video camera mounted above the apparatus was used to record mouse behavior. The total time spent in the light compartment and number of transitions into the light compartment were scored from videotapes at a later time by an observer blind to treatment groups. The mouse was considered to have entered the light compartment when all four paws were inside the light compartment. Similarly, the mouse was considered to have entered the dark compartment when all four paws were inside the dark compartment.
2.3.3. Vogel conflict test
The Vogel conflict test was conducted in operant chambers (Med Associates) fitted with fluid tubes that contained water, lickometers (to record separate lick events), and a shock delivery apparatus. The mice were deprived of water for 23 h prior to each exposure to the apparatus to motivate fluid seeking and, ultimately, licking behavior. On the training days, no shock was applied, and the mice were tested in 5 min sessions (timing started after the first lick). Immediately after each session, water was given to the mice. Once the mice were licking more than 100 times per session and initiating the sessions within 5 min of placing them into the operant boxes, they were considered trained and ready to be tested after drug manipulations in the conflict (shock) component. All of the mice were trained in 2-3 sessions with the test session occurring on day 4. In the test session, the mice received a mild (0.3 mA) shock to the tongue on every 20th lick. The number of licks throughout this 5-min experimental session was recorded.
2.4. Nociception test: flinch-jump test
Because of the nature of the Vogel conflict test, in which the drinking behavior of thirsty animals is punished by shock delivery, increased drinking may be induced by either the anxiolytic or analgesic effects of the tested compound. Therefore, the flinch-jump test was conducted to determine whether putative analgesic effects are involved in the actions of the compound in the conflict test. The apparatus consisted of an operant chamber (Freeze Monitor, Med Associates; 26 × 26 × 17 cm) made of Plexiglas with a shockable grid floor. After the mouse was placed into the chamber, an initial shock was given at 0.05 mA (2 s duration) and increased in steps of 0.03 mA every 20 s. Behavior was recorded using a camera mounted above the apparatus. The footshock intensities that were required to induce “flinch” and “jump” responses were observed from videotapes at a later time by an observer blind to each mouse treatment. The highest shock intensity that any mouse received was one step above the intensity that elicited a jump response (in this case 0.74 mA for one mouse and considerably lower for many of the mice). The flinch response is regarded as the first behavioral sign that indicates that a nociceptive stimulus was felt. With increasing intensities of footshock stimulation, however, the behavioral pattern changes from flinch to vocalization and jump responses. After the first appearance of a given nociceptive response, the intensity of the footshock was increased and decreased to accurately determine the “pain” threshold.
2.5. Learning and memory tests
2.5.1. Contextual and cued fear conditioning
The fear conditioning system consisted of Freeze Monitor chambers (Med Associates) housed in sound-proofed boxes. The conditioning chambers (26 × 26 × 17 cm) were made of Plexiglas with speakers and lights mounted on two opposite walls. The chambers were installed with a grid floor for footshock presentation. On day 1, the mice were individually placed in the conditioning chamber for 5 min to habituate them to the apparatus. On day 2, the mice were reexposed to the context (i.e., chamber), and a tone conditioned stimulus (30 s, 3000 Hz, 80 dB) was presented in association with a footshock (0.70 mA, 2 s, scrambled current). Specifically, a 5.5 min session was conducted in which the mice received two shock exposures, both during the last 2 s of a 30 s tone presentation. The two shock and tone pairing trials were separated by 2 min. On day 3, testing of contextual memory was conducted by placing the mice in the chamber in which they were trained (contextual fear test). Neither a tone nor shock was delivered because this session examined the recognition of the association between the shock and context. Contextual conditioning was measured as the time spent freezing (in seconds) during the 5-min test. On day 4, the mice were tested for cued conditioning (CS+ fear test). For this test, the chamber was disguised with new walls (i.e., white opaque plastic that created a rounded compartment in contrast to a clear plastic square compartment) and a new floor (i.e., white opaque plastic in contrast to metal grid). The mice were placed in this novel context for 3 min, after which they were exposed to the conditioned stimulus (tone) for 3 min to test the association between the footshock and cue tone. Freezing behavior (i.e., the absence of all voluntary movements except breathing) was measured in all of the sessions by real-time digital video recordings calibrated to distinguish between subtle movements, such as whisker twitches, tail flicks, and freezing behavior. Videos of the animals’ behavior were later scored by an observer blind to treatment group.
2.5.2. Barnes maze
The Barnes maze apparatus (Ridout Plastics, San Diego, CA) is an opaque Plexiglas disk (75 cm diameter) elevated 58 cm above the floor by a tripod. Twenty holes (5 cm diameter) are located 5 cm from the perimeter, and a black Plexiglas escape box (19 × 8 × 7 cm) is placed under one of the holes. Distinct spatial cues are located all around the maze and kept constant throughout the study.
On the first day of testing, the mice were put in the escape box for 1 min, after which the first session began. The mouse was placed in the middle of the maze in a 10 cm high cylindrical black start chamber. After 10 s, the start chamber was removed, a buzzer (80 dB) and light (400 lux) were turned on, and the mouse was set free to explore the maze. The session ended when the mouse entered the escape tunnel or after 3 min elapsed, whichever occurred first. When the mouse entered the escape tunnel, the buzzer was turned off, and the mouse was allowed to remain in the dark for 1 min. When the mouse did not enter the tunnel by itself, it was gently put in the escape box for 1 min. The tunnel was always located underneath the same hole (stable within the spatial environment), which was randomly determined for each mouse. The mice were tested once per day for 9 days for the acquisition portion of the study. The measures recorded in each session included the latency to enter the escape box with all four paws and number of errors made before entering the escape hole. Errors were defined as nosepokes and head deflections over any hole that did not have the tunnel beneath it.
On day 10 (probe test), the escape tunnel was removed, and the mouse was allowed to freely explore the maze for 3 min. The time spent in each quadrant was determined, and the percent time spent in the target quadrant (i.e., the one that originally contained the escape box) was compared with the average percent time in the other three quadrants. This probe test directly measures spatial memory because there is no potential for local cues to be used in the mouse’s behavioral decision.
Two weeks later (on day 25), the mice were tested again with the escape box in the original position (retention test). This test examines long-term memory. Finally, on the day after this test, the escape tunnel was moved to a new location (90° from the original position), and the behavior of the mouse was recorded. This test is called the reversal test and measures perseveration at the old hole. Each session was videotaped and scored by an experimenter blind to the treatment of the mice. The measures recorded in the retention and reversal tests included the latency to enter the escape hole and number of errors made per session.
2.6. Pentylenetetrazole-induced seizures
Because several mice experienced mild seizures after administration of the highest dose of BHF177 in the above behavioral tests, the PTZ-induced seizure test was conducted to evaluate the potential pro-convulsant properties of this compound in rodents. Mice and rats were injected with PTZ at doses of 50 and 70 mg/kg (i.p.), respectively, and observed for convulsions for up to 30 min. Five convulsion types were possible, including: (1) flat posture, characterized by the mouse’s abdomen touching the floor, (2) myoclonic jerk, characterized by a sudden involuntary muscle twitch often accompanied by a head twitch or squeak, (3) face and forelimb clonus (writhe), characterized by rapid writhing movements of the head and neck and clonic forelimb movements, (4) running bouncing clonus, characterized by a violent whole-body clonus, and (5) tonic hindlimb extension, characterized by extreme rigidity, caudal extension of the forelimbs and hindlimbs, head held perpendicular to the body, ears laid back, and eyes closed. The latencies to the first three signs were recorded because they occurred in the majority of mice, and the most severe sign observed was used to determine overall severity. The mice or rats that did not die within 30 min after PTZ administration were immediately euthanized.
2.7. Statistical analysis
All of the data are expressed as mean ± SEM. t-tests were performed for the anxiety tests with chlordiazepoxide. One-way analysis of variance (ANOVA) was performed, with Dose as the between-subjects factor for the rest of behavioral tests, with the exception of the Barnes maze, in which the drugs were chronically administered. Data from the chronic treatment groups that were assessed in the Barnes maze were analyzed by two-way ANOVA, with Dose as the between-subjects factor and Time (day) as the within-subjects factor. Individual group comparisons were performed using the Student-Newman-Keuls test.
3. Results
3.1. Anxiety tests
3.1.1. Effects of chlordiazepoxide, baclofen and BHF177 on anxiety-like behavior in the elevated plus maze
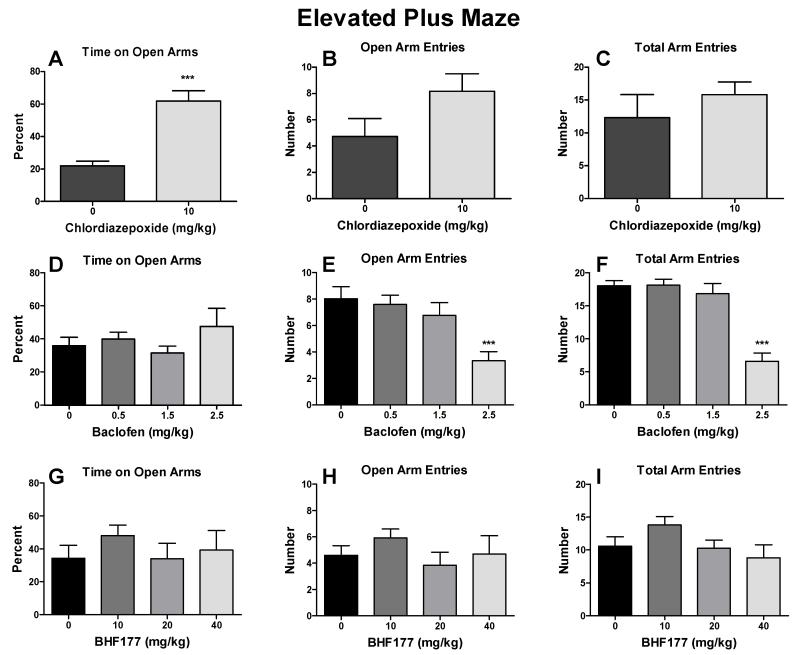
Chlordiazepoxide significantly increased the percentage of time spent on the open arms (Fig. 2A; t = 5.98, p < 0.001), but had no effect on the number of open arm entries (Fig. 2B) and total number of arm entries (Fig. 2C). Baclofen had no effect on the percentage of time spent on the open arms (Fig. 2D) but decreased the number of open arm entries (Fig. 2E; F3,44 = 6.56, p < 0.01) and total number of arm entries (Fig. 2F; F3,44 = 22.98, p < 0.001) in mice. The post hoc tests revealed statistically significant reductions of the number of open arm entries after 2.5 mg/kg (Fig. 2E; q = 5.64, p < 0.001) and total arm entries after 2.5 mg/kg (Fig. 2F; q = 9.85, p < 0.001) but not after 0.5 or 1.5 mg/kg baclofen for either of the two measures. BHF177 had no effect on any measure in the elevated plus maze (Fig. 2G-2I). Two mice had seizures after administration of 40 mg/kg BHF177 and thus were excluded from the data analysis.
Figure 2.
Effects of chlordiazepoxide, baclofen and BHF177 on anxiety-like behavior in the elevated plus maze. Chlordiazepoxide (i.p.) significantly increased the percentage of time spent on the open arms (A) but had no effect on the number of open arm entries (B) and number of total arm entries (C) in mice. Baclofen (i.p.) had no effect on the percentage of time spent on the open arms (D) but decreased the number of open arm entries (E) and number of total arm entries (F) at the highest dose tested in mice. BHF177 (p.o.) had no effect on the percentage of time spent on the open arms (G), number of open arm entries (H), or number of total arm entries (I) in mice. ***p < 0.001, compared with vehicle control group.
3.1.2. Effects of chlordiazepoxide, baclofen and BHF177 on anxiety-like behavior in the light/dark box
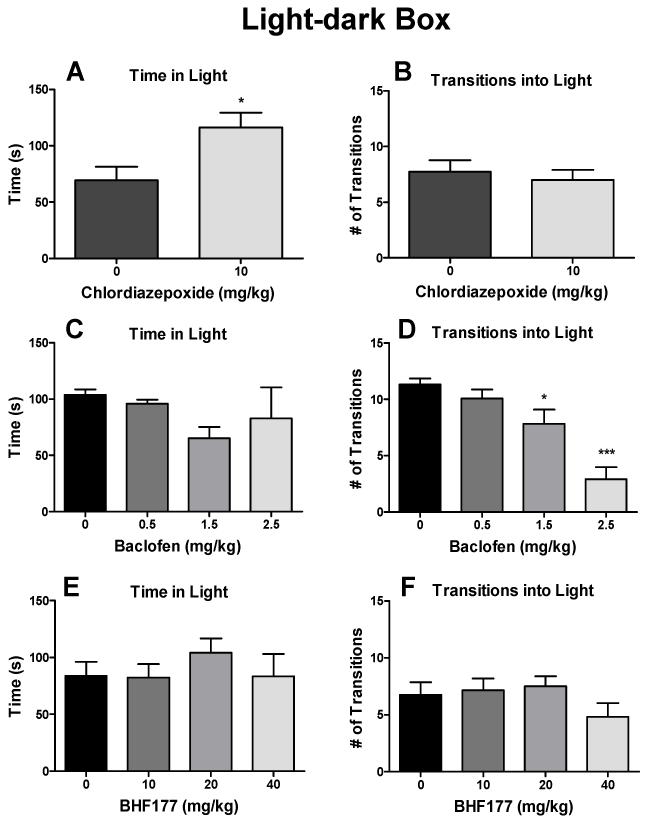
Chlordiazepoxide significantly increased the time spent in the light compartment (Fig. 3A; t = 2.64, p < 0.05) but had no effect on the number of light/dark transitions (Fig. 3B). Baclofen had no effect on the time spent in the light compartment (Fig. 3C) but dose-dependently decreased the number of light/dark transitions (Fig. 3D; F3,44 = 15.21, p < 0.001). The post hoc tests revealed statistically significant reductions of the number of transitions into the light compartment after 1.5 mg/kg (q = 3.68, p < 0.05) and 2.5 mg/kg (q = 8.84, p < 0.001) but not after 0.5 mg/kg baclofen (Fig. 3D). BHF177 had no effect on the time spent in the light compartment (Fig. 3E) or number of light/dark transitions (Fig. 3F). One mouse experienced seizures after administration of 40 mg/kg BHF177 and thus was excluded from the data analysis.
Figure 3.
Effects of chlordiazepoxide, baclofen and BHF177 on anxiety-like behavior in the light/dark box. Chlordiazepoxide (i.p.) significantly increased the time spent in the light compartment (A) but had no effect on the number of light/dark transitions (B) in mice. Baclofen (i.p.) had no effect on the time spent in the light compartment (C) but dose-dependently decreased the number of light/dark transitions (D) in mice. BHF177 (p.o.) had no effect on the time spent in the light compartment (E) or number of light/dark transitions (F) in mice. *p < 0.05, ***p < 0.001, compared with vehicle control group.
3.1.3. Effects of chlordiazepoxide, baclofen and BHF177 on anxiety-like behavior in the Vogel conflict test
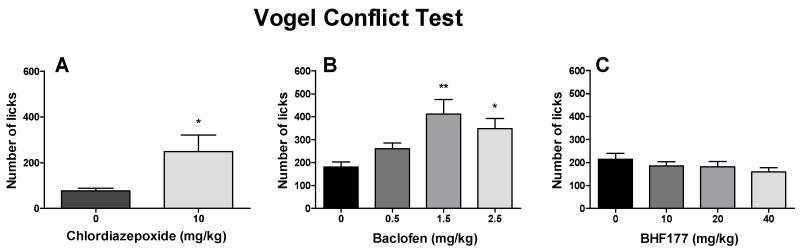
Chlordiazepoxide significantly increased the number of licks, despite the simultaneous shock delivery (Fig. 4A; t = 2.71, p < 0.05). Baclofen also dose-dependently increased the number of licks, despite the simultaneous shock delivery (Fig. 4B; F3,44 = 5.63, p < 0.01). The post hoc tests revealed statistically significant increases in the numbers of licks after 1.5 mg/kg (q = 5.43, p < 0.01) and 2.5 mg/kg (q = 3.92, p < 0.05) but not after 0.5 mg/kg baclofen. BHF177 had no effect on the number of licks (Fig. 4C).
Figure 4.
Effects of chlordiazepoxide, baclofen and BHF177 on anxiety-like behavior in the Vogel conflict test. Chlordiazepoxide (i.p.) significantly increased the number of licks (A). Baclofen (i.p.) also dose-dependently increased the number of licks (B). BHF177 (p.o.) had no effect on the number of licks (C). *p < 0.05, **p < 0.01, compared with vehicle control group.
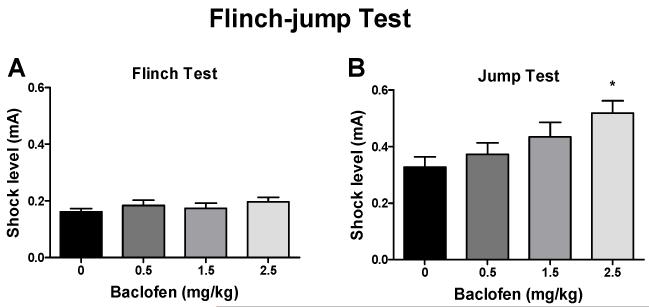
3.2. Effects of baclofen on footshock sensitivity in the flinch-jump test
Baclofen had no effect on flinch thresholds (Fig. 5A) but dose-dependently increased jump thresholds (Fig. 5B; F3,31 = 3.60, p < 0.05). The post hoc tests revealed statistically significant increases in the jump thresholds after 2.5 mg/kg (q = 4.38, p < 0.01) but not after 0.5 or 1.5 mg/kg baclofen.
Figure 5.
Effects of baclofen on footshock sensitivity in the flinch-jump test. Baclofen (i.p.) had no effect on the flinch threshold (A) but dose-dependently increased the jump threshold (B) in the flinch-jump test in mice. *p < 0.05, compared with vehicle control group.
3.3. Learning and memory tests
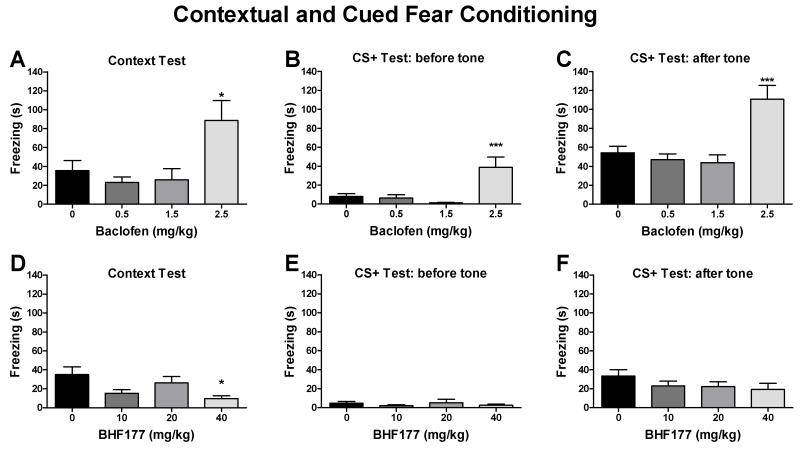
3.3.1. Effects of baclofen and BHF177 on retrieval of fear memory in the contextual and cued fear conditioning test
Baclofen significantly enhanced the freezing response in the contextual fear test in mice (Fig. 6A; F3,44 = 5.17, p < 0.01). The post hoc tests revealed statistically significant increases in freezing behavior after 2.5 mg/kg (q = 3.91, p < 0.05) but not after 0.5 or 1.5 mg/kg baclofen. The CS+ fear test data were divided into two parts, with the first 3 min representing baseline activity in the novel context and the last 3 min assessing memory retrieval of the cued stimulus by exposing mice to the conditioned tone previously associated with footshock delivery. Similar to the contextual fear test, baclofen significantly enhanced the freezing response both before the tone (Fig. 6B; F3,44 = 8.39, p < 0.001) or during the tone (Fig. 6C; F3,44 = 10.80, p < 0.001) in the CS+ fear test. The post hoc tests revealed statistically significant increases in freezing behavior after 2.5 mg/kg (q = 5.25, p < 0.001) but not after 0.5 or 1.5 mg/kg baclofen in the CS+ test before the tone (Fig. 6B) and after 2.5 mg/kg (q = 5.89, p < 0.001) but not after 0.5 or 1.5 mg/kg baclofen in the CS+ test after the tone (Fig. 6C).
Figure 6.
Effects of baclofen and BHF177 on retrieval of fear memory in the contextual and cued fear conditioning test. Baclofen (i.p.) significantly enhanced the freezing response in the contextual fear test (A) and cued fear test (CS+ test) when administered either before the tone (B) or during the tone (C) at the highest dose tested. BHF177 (p.o.) attenuated the freezing response in the contextual fear test at the highest dose tested (D) but had no effect on the freezing response when administered either before the tone (E) or during the tone (F) in the CS+ fear test in mice. *p < 0.05, ***p < 0.001, compared with vehicle control group.
BHF177 significantly attenuated the freezing response in the contextual fear test (Fig. 6D;F3,42 = 3.73, p < 0.05). The post hoc tests revealed statistically significant decreases in freezing behavior after 40 mg/kg (q = 4.29, p < 0.05) but not after 10 or 20 mg/kg BHF177. However, BHF177 had no effect on the freezing response either before the tone (Fig. 6E) or during the tone (Fig. 6F) in the CS+ fear test. Two mice had seizures after administration of 40 mg/kg BHF177 in the contextual fear test and thus were excluded from the data analysis.
3.3.2. Effects of baclofen and BHF177 on spatial learning and spatial memory in the Barnes maze
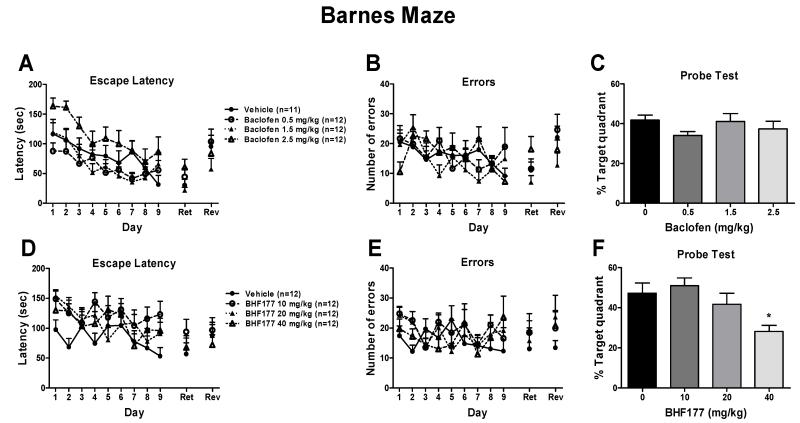
3.3.2.1. Effects of chronic administration of baclofen on spatial learning and subsequent expression of spatial memory in the Barnes maze
Chronic treatment with baclofen during acquisition training from day 1 to day 9 had no effect on the latency to enter the escape box (Fig. 7A) or number of errors made before entering the escape hole (Fig. 7B), suggesting that baclofen had no effect on spatial learning in the Barnes maze. A two-way ANOVA, with Time as the repeated measure and Dose as the between-subjects factor, revealed a significant main effect of Time for both latency (Fig. 7A; F8,344 = 10.83, p < 0.001) and errors (Fig. 7B; F8,344 = 2.95, p < 0.01), indicating that mice showed decreased latencies and errors across sessions. A significant effect of Dose on escape latency was found after baclofen administration (F3,43 = 6.74, p < 0.001), with no Dose × Time interaction. The post hoc individual group comparisons revealed no statistically significant differences between the vehicle group and any of the baclofen-treated groups (Fig. 7A). No statistically significant effect of baclofen on errors was observed (Fig. 7B). Chronic baclofen administration during acquisition had no effect on performance in the subsequent probe, retention, or reversal tests (Fig. 7A-C), suggesting that chronic baclofen administration had no effect on the subsequent expression of spatial memory in the Barnes maze. One mouse became ill during training and was excluded from the data analysis.
Figure 7.
Effects of chronic administration of baclofen and BHF177 on spatial learning and the subsequent expression of spatial memory in the Barnes maze. Chronic treatment with baclofen (i.p., once daily before testing) from day 1 to day 9 had no effect on the latency to enter the escape box (A) or number of errors made before entering the escape hole (B) during acquisition training and did not affect performance in the subsequent probe (C), retention (Ret) (A), or reversal (Rev) (B) tests in mice. Chronic treatment with BHF177 (p.o., once daily before testing) from day 1 to day 9 had no effect on the latency to enter the escape box (D) or number of errors made before entering the escape hole (E) during acquisition training and did not affect performance in the subsequent retention (Ret) or reversal (D and E) tests but inhibited performance in the probe test (F) at the highest dose tested in mice. *p < 0.05, compared with vehicle control group.
3.3.2.2. Effects of acute administration of baclofen on spatial memory in the Barnes maze
Acute administration of baclofen before the probe test had no effect on the percent time spent in the target quadrant (Table 1). All of the mice spent approximately 40% of their time in the target zone. Acute administration of baclofen significantly increased the latency to enter the escape box (retention test, F3,44 = 14.67, p < 0.001; reversal test, F3,44 = 4.94, p < 0.001) but had no effect on the number of errors made before entering the escape hole in both the retention and reversal tests. The post hoc tests revealed statistically significant increases in latency after 2.5 mg/kg (retention test, q = 7.98, p < 0.001; reversal test, q = 3.99, p < 0.05) but not after 0.5 or 1.5 mg/kg baclofen.
Table 1.
Effects of acute administration of baclofen on spatial memory in the Barnes maze.
| Test | Baclofen (mg/kg) |
||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.5 | 2.5 | ||
| Probe test | 36.72 ± 2.78 | 36.72 ± 5.20 | 42.59 ± 3.47 | 38.22 ± 7.08 | |
| Retention test | Latency | 34.25 ± 5.18 | 34.92 ± 6.55 | 48.08 ± 14.42 | 134.92 ± 21.49*** |
| Number of errors | 11.42 ± 2.87 | 8.67 ± 1.76 | 6.17 ± 1.31 | 11.00 ± 5.16 | |
| Reversal test | Latency | 66.00 ± 16.92 | 88.67 ± 22.09 | 45.17 ± 13.91 | 135.75 ± 20.78* |
| Number of errors | 19.92 ± 6.65 | 17.67 ± 4.69 | 8.00 ± 2.43 | 10.33 ± 3.11 | |
p < 0.05,
p < 0.001, compared with vehicle control group.
3.3.2.3. Effects of chronic administration of BHF177 on spatial learning and subsequent expression of spatial memory in the Barnes maze
Chronic treatment with BHF177 during acquisition training had no effect on the latency to enter the escape box (Fig. 7D) or number of errors made before entering the escape hole (Fig. 7E), suggesting that BHF177 had no effect on spatial learning in the Barnes maze. The two-way ANOVA, with Time as the within-subjects factor revealed a significant main effect of Time on latency (Fig. 7D; F8,352 = 3.38, p < 0.001). Although chronic administration of BHF177 during acquisition decreased the percent time spent in the target quadrant at the highest dose tested in the subsequent probe test (Fig. 7F), the compound did not have an effect on performance in the subsequent retention or reversal tests (Fig. 7D-E).
3.3.2.4. Effects of acute administration of BHF177 on spatial memory in the Barnes maze
Acute administration of BHF177 had no effect on the percent time spent in the target quadrant in the probe test (Table 2). Consistent with the data in the baclofen tests, all of the mice spent approximately 40% of their time in the target zone. Acute BHF177 had no effect on latency or the number of errors in the retention and reversal tests.
Table 2.
Effects of acute administration of BHF177 on spatial memory in the Barnes maze.
| Test | BHF177 (mg/kg) |
||||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | ||
| Probe test | 34.17 ± 4.59 | 37.38 ± 6.28 | 39.57 ± 4.00 | 40.85 ± 7.13 | |
| Retention test | Latency | 94.92 ± 21.15 | 92.83 ± 19.79 | 101.83 ± 21.94 | 124.83 ± 22.25 |
| Number of errors | 19.00 ± 4.77 | 15.67 ± 4.94 | 17.42 ± 4.26 | 14.67 ± 6.04 | |
| Reversal test | Latency | 69.50 ± 22.42 | 108.25 ± 20.74 | 115.42 ± 17.87 | 134.83 ± 18.34 |
| Number of errors | 11.92 ± 3.29 | 15.92 ± 3.21 | 16.92 ± 3.25 | 17.75 ± 3.55 | |
3.4. Effects of BHF177 on susceptibility to PTZ-induced seizures
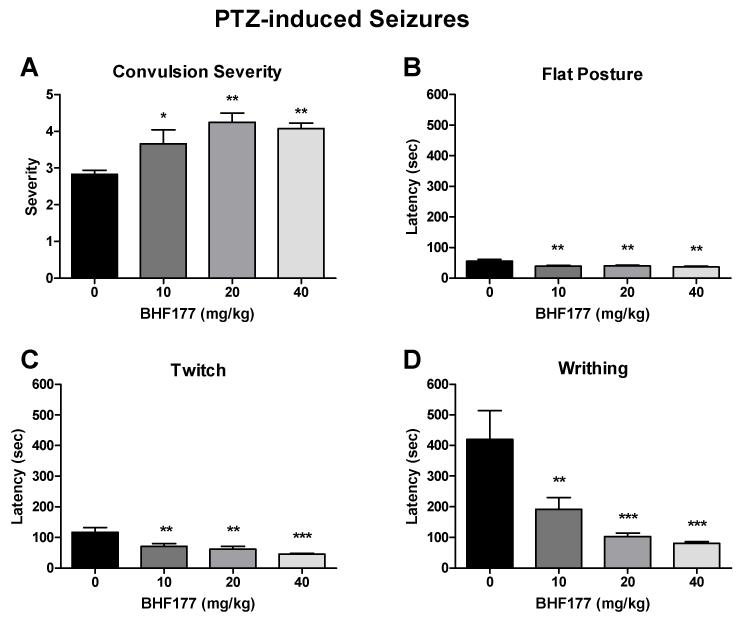
3.4.1. Effects of BHF177 on susceptibility to PTZ-induced seizures in mice
BHF177 significantly increased the severity of convulsions induced by PTZ in mice (Fig. 8A; F3,44 = 6.71, p < 0.001). The post hoc tests revealed statistically significant increases in convulsion severity after BHF177 doses of 10 mg/kg (q = 3.41, p < 0.05), 20 mg/kg (q = 5.80, p < 0.01), and 40 mg/kg (q = 5.12, p < 0.01). Moreover, BHF177 dose-dependently decreased the latency to flat posture (Fig. 8B; F3,44 = 5.94, p < 0.01), myoclonic jerks (twitches; Fig. 8C; F3,44 = 9.16, p < 0.001), and face and forelimb clonus (writhes; Fig. 8D; F3,44 = 9.21, p < 0.001). The post hoc tests revealed statistically significant decreases in the latency to flat posture after BHF177 doses of 10 mg/kg (q = 4.61, p < 0.01), 20 mg/kg (q = 4.42, p < 0.01), and 40 mg/kg (q = 5.38, p < 0.01; Fig. 8B), latency to twitch after BHF177 doses of 10 mg/kg (q = 4.59, p < 0.01), 20 mg/kg (q = 5.42, p < 0.01), and 40 mg/kg (q = 7.07, p < 0.001; Fig. 8C), and latency to writhe after BHF177 doses of 10 mg/kg (q = 4.47, p < 0.01), 20 mg/kg (q = 6.21, p < 0.001), and 40 mg/kg (q = 6.64, p < 0.001; Fig. 8D).
Figure 8.
Effects of BHF177 on susceptibility to PTZ-induced seizures. BHF177 (p.o.) dose-dependently increased the severity of convulsions (A) and decreased the latency to flat posture (B), myoclonic jerk (twitch) (C), and face and forelimb clonus (writhing) (D) induced by PTZ in mice. *p < 0.05, **p < 0.01, ***p < 0.001, compared with vehicle control group.
3.4.2. Effects of BHF177 on susceptibility to PTZ-induced seizures in rats
BHF177 had no effect on the severity of convulsions or latency to twitch and writhe induced by PTZ in rats (Table 3).
Table 3.
Effects of BHF177 on susceptibility to PTZ-induced seizures in rats.
| Tests | BHF177 (mg/kg) |
F, P values | |||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | ||
| Severity | 2.50 ± 0.20 | 2.63 ± 0.20 | 2.75 ± 0.17 | 2.75 ± 0.17 | Fb,31 = 0.47, p =NS |
| Latency to twitch | 65.75 ± 7.27 | 48.00 ± 3.09 | 73.00 ± 33.11 | 37.88 ± 3.88 | F3,31 = 1.16, p =NS |
| Latency to writhing | 88.63 ± 15.02 | 56.71 ± 3.32 | 110.29 ± 63.07 | 60.63 ± 16.24 | F3,31 = 0.74, p =NS |
4. Discussion
The present study behaviorally characterized the potent GABAB receptor PAM BHF177 and compared its effects with the agonist baclofen in mouse models of anxiety, learning, and memory. Both baclofen and BHF177 had no specific effects on anxiety-like behavior in the elevated plus maze, the light/dark box, or the Vogel conflict test in mice at the dose range tested, while the anxiety-like behavior in these tests was significantly diminished by the anxiolytic chlordiazepoxide. Baclofen increased punished drinking in the Vogel conflict test, however this effect may be attributable to its analgesic action because baclofen also decreased shock sensitivity in the flinch-jump test. Baclofen and BHF177 did not exert selective effects on fear memory retrieval in the contextual and cued fear conditioning procedure or spatial learning and memory in the Barnes maze in mice. These data suggest that these compounds do not affect anxiety-like behavior or learning and memory processes. Finally, unlike BHF177, baclofen produced consistent reductions of activity measures across many of the tasks, consistent with the sedative side effects reported for full GABAB receptor agonists but not PAMs.
Multiple procedures employed in the present study indicated that the highest dose of baclofen tested (2.5 mg/kg) exerted nonspecific effects on locomotor performance in mice. First, 2.5 mg/kg baclofen decreased the number of open arm entries and total arm entries but had no effect on the percentage of time spent on the open arms in the elevated plus maze. Decreases in the time spent on the open arms and number of open arm entries reflect increased anxiety-like behavior, whereas changes in total arm entries reflect nonspecific effects on locomotor activity (Hogg, 1996; Treit et al., 2010). Therefore, the results indicated that 2.5 mg/kg baclofen impaired locomotor activity in mice. Second, in the light/dark box, baclofen only decreased the number of transitions into the light compartment but had no effect on the time spent in the light compartment. Third, in the Barnes maze, acute administration of the highest baclofen dose increased the latency to find the escape hole in the retention and reversal tests but had no effect on the numbers of errors in either test. Therefore, the effects induced by the highest dose of baclofen in these procedures are most likely attributable to sedation and not a selective anxiolytic effect or disruption of learning or memory. In contrast to baclofen, BHF177 did not exert an inhibitory effect on any of the locomotion-related parameters in these procedures. Consistent with the lack of locomotor suppression by BHF177 in mice in the present study, BHF177 had no effect on food- or sucrose-maintained operant behavior in rats (Maccioni et al., 2009; Paterson et al., 2008). Thus, the results indicate that BHF177 is not as sedative as baclofen.
GABAergic pathways exert an inhibitory influence upon anxiogenic actions. Supporting this notion, the anxiolytic chlordiazepoxide diminished the anxiety-like behavior in the light/dark box, the elevated plus maze, and the Vogel conflict test in mice in the present study, indicating that the procedures used can detect anxiolytic activity. However, neither the GABAB receptor agonist baclofen nor the PAM BHF177 exhibited anxiolytic-like profiles in these tests. There are inconsistencies in reports of anxiolytic efficacy of GABA-B ligands in rodent tests of anxiety. The present findings are consistent with previous reports in which baclofen did not exhibit anxiolytic-like properties in the elevated plus maze in mice (Dalvi and Rodgers, 1996) and rats (Zarrindast et al., 2001), elevated zero maze in rats (Frankowska et al., 2007), or Vogel conflict test in mice (Umezu, 1999) or rats (Agmo et al., 1991). Furthermore, the GABAB receptor PAM GS39783 had no effect on anxiety-like behavior in the modified Geller-Seifter task, in which food-taking behavior in food-deprived rats is punished by footshock, a similar conflict test as Vogel’s (Paterson and Hanania, 2010). Additionally, another PAM, CGP7930, did not have anxiolytic-like effects in the elevated plus maze in rats (Jacobson and Cryan, 2008). In contrast to these negative reports, both baclofen and PAMs, such as GS39783 and CGP7930, have been shown to produce anxiolytic-like effects in rodents (Andrews and File, 1993; Cryan et al., 2004; Jacobson and Cryan, 2008; Mombereau et al., 2004; Shephard et al., 1992). Finally, the agonist CGP44532 exerted anxiogenic-like effects in the four-plate test in mice (Partyka et al., 2007). To add to this complexity, controversial findings have been reported about the anxiolytic activity of GABAB receptor antagonists, showing either anxiolytic effects (Frankowska et al., 2007; Partyka et al., 2007) or no effects (Partyka et al., 2007; Paterson and Hanania, 2010).
Although the differences in species, strains, and protocols may contribute to the contradictory results on the role of GABAB receptor ligands in anxiety-like behavior (Cryan and Sweeney, 2011), another plausible explanation is that these ligands may have different binding affinities for the various GABAB receptor subtypes (Vlachou et al., 2011b). Fully functional GABAB receptors are heterodimers that consist of GABAB1 and GABAB2 subunits. The GABAB1 subunit also contains two isoforms, GABAB1a and GABAB1b (Jones et al., 1998; Kaupmann et al., 1998). GABAB1a/2 receptors are located mainly presynaptically and regulate the release of neurotransmitters, such as GABA and glutamate, whereas GABAB1b/2 receptors are located primarily postsynaptically and mediate long-lasting inhibitory postsynaptic potential (Bettler and Tiao, 2006; Vigot et al., 2006). Although the GABAB receptor ligands available to date have significant selectivity for GABAB receptors, the binding affinity of these compounds for the two different isoforms is unclear. Therefore, these ligands may differentially bind to presynaptic GABAB1a/2 and postsynaptic GABAB1b/2 isoforms, thus producing either predominantly inhibition (via activation of the autoreceptor to reduce endogenous GABA release) or facilitation (via activation of the postsynaptic receptor) of GABAergic signaling. Further, in situ hybridization studies indicate that the GABAB1a and GABAB1b isoforms are distributed differentially in some brain areas (Benke et al., 1999; Liang et al., 2000), including corticolimbic structures which are involved in the modulation of anxious states. Therefore, activation of different GABAB1 isoforms across specific brain circuits may produce different actions in regulation of anxiety-like behavior in animals. This hypothesis may also explain the similar effects of GABAB receptor agonists and antagonists on various behaviors, such as anxiety-like behavior (Andrews and File, 1993; Frankowska et al., 2007; Ketelaars et al., 1988; Partyka et al., 2007; Shephard et al., 1992), cognitive tasks (Castellano et al., 1993; Escher and Mittleman, 2004), and the depressive-like state associated with nicotine withdrawal (Vlachou et al., 2011b). Thus, the development and preclinical testing of isoform-selective GABAB receptor ligands will significantly contribute to uncovering the role of each isoform in brain function.
Baclofen increased punished drinking in the Vogel conflict test. Because of the nature of this test, increased drinking may be induced by either the anxiolytic or analgesic effects of the test compound. Further studies that used the flinch-jump test to assess nociception (Evans, 1961; Lehner et al., 2010; Pamplona et al., 2010) indicated that baclofen increased jump thresholds, reflecting pain tolerance, but had no effect in the flinch test that reflects pain sensitivity, indicating that baclofen has analgesic effects in mice. Our finding is consistent with previous reports of the analgesic effects of baclofen (Balerio and Rubio, 2002; Cutting and Jordan, 1975; Sawynok, 1987; Vaught et al., 1985). Given the lack of anxiolytic effects of baclofen in all other tests, the positive effects of baclofen in the Vogel test is most likely attributable to its analgesic and not anxiolytic effects in mice. This conclusion is consistent with the report by Umezu, in which a lack of anxiolytic effects of baclofen was found in the Vogel conflict test in mice (Umezu, 1999).
We noticed that several mice experienced mild seizures (primarily forelimb clonus) after administration of the highest dose of BHF177 tested (40 mg/kg), indicating that BHF177 may have pro-convulsant properties in mice. Further formal experimentation confirmed that BHF177 increased seizure susceptibility produced by PTZ in mice. The mechanism for BHF177-induced seizures in mice is unknown. At high doses, BHF177 shows binding affinity for 5-HT2C (IC50 = 8.40 μM) and 5-HT6 receptors (IC50 = 9.35 μM) (Guery et al., 2007). Therefore, the pro-convulsant effect of BHF177 might be due to its off-target effects. However, enhanced GABAB receptor activity has been shown to exacerbate seizures in models of absence seizures (Marescaux et al., 1992; Vergnes et al., 1997), a type of seizure that is non-convulsive but characterized by an arrest of behavior and disconnection from environmental inputs and associated with bilateral and synchronous cortical spike-and-wave discharges. Conversely, baclofen and GS39783 have also been reported to reduce the incidence of seizures in rat hippocampal slices and mice, respectively (Ault and Nadler, 1983; Pacey et al., 2011). The PAMs GS39783 and BHF177 are structurally different and may have different preferential affinity for presynaptic vs. postsynaptic GABAB receptor isoforms, thereby exhibiting different pro-convulsant profiles. Moreover, the pro-convulsant properties of BHF177 may contribute to its lack of anxiolytic action in mice. However, BHF177 did not affect PTZ-induced seizure in rats, and seizures were not casually observed in our laboratory in several rat studies with BHF177 (Paterson et al., 2008; Vlachou et al., 2011a; Vlachou et al., 2011b), suggesting species differences in the pro-convulsant properties of BHF177 that may be attributable to differential metabolism of the compound or differential receptor distribution in mice and rats.
Similar to the inconsistent findings with regard to baclofen efficacy in anxiety tests, the effects of baclofen on learning and memory have also been variable, ranging from impairment (Castellano et al., 1989; McNamara and Skelton, 1996; Nakagawa and Takashima, 1997; Pitsikas et al., 2003; Stackman and Walsh, 1994; Zarrindast et al., 2004) to facilitation (Georgiev et al., 1988; Saha et al., 1993) in rodents. In contrast, a recent clinical study indicated that baclofen produced bi-directional effects on reinforcement learning (i.e., low doses facilitated and high dose did not affect learning in humans) (Terrier et al., 2011). In the present study, two different procedures, the contextual and cued fear conditioning procedure and Barnes maze, were used to assess the effects of baclofen and BHF177 on learning and memory in mice.
The contextual and cued fear conditioning procedure is a commonly used behavioral technique to assess fear-related learning and memory (Maren, 2001). Using this procedure, we have demonstrated that activation of the GABAA receptor by benzodiazepines, such as flunitrazepam, impaired contextual fear and cued fear memory in mice (Treweek et al., 2008). This finding is consistent with the report that benzodiazepines elicited anterograde amnesia, reflected in weakened associations between the context/cue and footshock in rodents (DeLorey et al., 2001; Guscott et al., 2000), and impaired emotional memory in human studies (Brignell and Curran, 2006; Buchanan et al., 2003). In the present studies, the highest dose of baclofen increased freezing in both the contextual and cued fear tests, while the highest dose of BHF177 decreased freezing in the contextual fear tests. Given that the same dose of baclofen also reduced activity in numerous tests, as discussed above, the increased freezing response is likely attributable to the fact that baclofen reduced activity instead of increased recall or anxiety-like behavior. In contrast, the decreased freezing induced by 40 mg/kg BHF177 may be due to its pro-convulsant properties in mice. Future studies in rats, which are not sensitive to the pro-convulsant effects of BHF177, are needed to determine if BHF177 has consistent effects on fear conditioning in rodents.
Baclofen did not have effects on spatial learning and memory in the Barnes maze in mice. In contrast, previous studies showed that baclofen impaired spatial learning and memory in the Morris water maze in rats (McNamara and Skelton, 1996; Nakagawa and Takashima, 1997). The reason for this discrepancy may be that the Morris water maze involves swimming in opaque water to locate a hidden platform, which is more stressful and physically demanding for the animals than the Barnes maze, thereby producing more motivated escape responses (Harrison et al., 2009). Thus, the mechanisms that underlie these two procedures may not be identical. Furthermore, different species (rats) and a higher dose of baclofen (> 3 mg/kg) were used in these studies, which may also contribute to these different findings. Similar to the present study, Cryan et al. (Cryan et al., 2004) reported that neither baclofen nor GS39783 affected performance in the passive avoidance test when administered before the training trial in both mice and rats, indicating that these GABAB receptor ligands had no effect on learning. Furthermore, GS39783 did not affect performance in the retention test in either mice or rats, indicating that this compound did not disrupt memory recall. Conversely, baclofen administration during the retention test disrupted recall in mice but not rats (Cryan et al., 2004). The present studies did find that the 40mg/kg chronic administration of BHF177 reduced time spent at the target quadrant during the probe trial, however there were not effects on acquisition, retention or reversal (Figure 7F). Similar to the effects of this high dose on fear conditioning, it is possible that the pro-convulsant effects in mice of this dose are causing some behavioral disruption. Again, future tests in rats are needed to determine if this disruption is a consistent effects of high doses of BHF177 or if it is specific to mice. Overall, evidence of the influence of GABAB receptors on learning and memory is limited and inconsistent. One possible explanation for this inconsistency is that behavioral paradigms that assess learning and memory differentially involve stress- or anxiety-related behavior; therefore, the underlying neurobiology and pharmacology are not identical. Second, large species and strain differences exist in these paradigms (Sharma et al., 2010). For example, rats and mice are inherently different in terms of the physical and motivational factors that influence performance in learning and memory tasks. Thus, different species or strains and multiple paradigms should be applied to generate reliable data (Sharma et al., 2010). Third, the most common pharmacological tool that previous studies used to evaluate the role of GABAB receptors in learning and memory is baclofen, which has a narrow efficacy window before confounding side-effects, such as sedation and analgesia, are manifested in these behavioral paradigms (Cryan and Kaupmann, 2005; Dalvi and Rodgers, 1996). However, GABAB receptor PAMs exhibit similar pharmacological effectiveness as full agonists but have superior side-effect profiles with regard to sedation, muscle relaxant effects, and tolerance. Therefore, future studies that use PAMs are recommended to characterize the role of the GABAB receptor in psychiatric disorders.
Supplementary Material
Baclofen and BHF177 do not affect anxiety-like behavior in mice.
Baclofen and BHF177 do not affect learning and memory processes in mice.
Unlike baclofen, BHF177 has no sedative activity in mice.
Acknowledgements
This work was supported by National Institutes of Health grant U19DA026838 to AM.
FINANCIAL DISCLOSURES
Athina Markou has received contract research support from Bristol-Myers Squibb Co., F. Hoffman-La Roche, Pfizer, and Astra-Zeneca and honoraria/consulting fees from Abbott GmbH and Company, AstraZeneca, and Pfizer during the past 3 years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmo A, Pruneda R, Guzman M, Gutierrez M. GABAergic drugs and conflict behavior in the rat: lack of similarities with the actions of benzodiazepines. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:314–322. doi: 10.1007/BF00183006. [DOI] [PubMed] [Google Scholar]
- Andrews N, File SE. Handling history of rats modifies behavioural effects of drugs in the elevated plus-maze test of anxiety. Eur J Pharmacol. 1993;235:109–112. doi: 10.1016/0014-2999(93)90827-5. [DOI] [PubMed] [Google Scholar]
- Ault B, Nadler JV. Anticonvulsant-like actions of baclofen in the rat hippocampal slice. Br J Pharmacol. 1983;78:701–708. doi: 10.1111/j.1476-5381.1983.tb09423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balerio GN, Rubio MC. Baclofen analgesia: involvement of the GABAergic system. Pharmacol Res. 2002;46:281–286. doi: 10.1016/s1043-6618(02)00147-0. [DOI] [PubMed] [Google Scholar]
- Benke D, Honer M, Michel C, Bettler B, Mohler H. gamma-aminobutyric acid type B receptor splice variant proteins GBR1a and GBR1b are both associated with GBR2 in situ and display differential regional and subcellular distribution. J Biol Chem. 1999;274:27323–27330. doi: 10.1074/jbc.274.38.27323. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bettler B, Tiao JY. Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol Ther. 2006;110:533–543. doi: 10.1016/j.pharmthera.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988;11:112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci. 1989;10:401–407. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Bowery N, Enna SJ, Olsen RW. Six decades of GABA. Biochem Pharmacol. 2004;68:1477–1478. doi: 10.1016/j.bcp.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Brignell CM, Curran HV. Drugs, sweat, and fears: a comparison of the effects of diazepam and methylphenidate on fear conditioning. Psychopharmacology (Berl) 2006;186:504–516. doi: 10.1007/s00213-006-0363-x. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Karafin MS, Adolphs R. Selective effects of triazolam on memory for emotional, relative to neutral, stimuli: differential effects on gist versus detail. Behav Neurosci. 2003;117:517–525. doi: 10.1037/0735-7044.117.3.517. [DOI] [PubMed] [Google Scholar]
- Castellano C, Brioni JD, Nagahara AH, McGaugh JL. Post-training systemic and intra-amygdala administration of the GABA-B agonist baclofen impairs retention. Behav Neural Biol. 1989;52:170–179. doi: 10.1016/s0163-1047(89)90285-9. [DOI] [PubMed] [Google Scholar]
- Castellano C, Cestari V, Cabib S, Puglisi-Allegra S. Strain-dependent effects of post-training GABA receptor agonists and antagonists on memory storage in mice. Psychopharmacology (Berl) 1993;111:134–138. doi: 10.1007/BF02245514. [DOI] [PubMed] [Google Scholar]
- Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Roberts DC, de Wit H. GABA(B) receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend. 2002;65:209–220. doi: 10.1016/s0376-8716(01)00163-6. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K. Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, Froestl W, Bettler B, Kaupmann K, Spooren WP. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N’-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther. 2004;310:952–963. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. GABAB receptors and depression. Current status. Adv Pharmacol. 2010;58:427–451. doi: 10.1016/S1054-3589(10)58016-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Sweeney FF. The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol. 2011;164:1129–1161. doi: 10.1111/j.1476-5381.2011.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting DA, Jordan CC. Alternative approaches to analgesia: baclofen as a model compound. Br J Pharmacol. 1975;54:171–179. doi: 10.1111/j.1476-5381.1975.tb06926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvi A, Rodgers RJ. GABAergic influences on plus-maze behaviour in mice. Psychopharmacology (Berl) 1996;128:380–397. doi: 10.1007/s002130050148. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Lin RC, McBrady B, He X, Cook JM, Lameh J, Loew GH. Influence of benzodiazepine binding site ligands on fear-conditioned contextual memory. Eur J Pharmacol. 2001;426:45–54. doi: 10.1016/s0014-2999(01)01199-2. [DOI] [PubMed] [Google Scholar]
- Escher T, Mittleman G. Effects of ethanol and GABAB drugs on working memory in C57BL/6J and DBA/2J mice. Psychopharmacology (Berl) 2004;176:166–174. doi: 10.1007/s00213-004-1875-x. [DOI] [PubMed] [Google Scholar]
- Evans WO. A technique for the investigation of some effects of psychotropic and analgesic drugs on relfexive behavior in the rat. Rep US Army Med Res Lab. 1961;476:1–9. [PubMed] [Google Scholar]
- Frankowska M, Filip M, Przegalinski E. Effects of GABAB receptor ligands in animal tests of depression and anxiety. Pharmacol Rep. 2007;59:645–655. [PubMed] [Google Scholar]
- Georgiev VP, Yonkov DI, Kambourova TS. Interactions between angiotensin II and baclofen in shuttle-box and passive avoidance performance. Neuropeptides. 1988;12:155–158. doi: 10.1016/0143-4179(88)90047-9. [DOI] [PubMed] [Google Scholar]
- Guery S, Floersheim P, Kaupmann K, Froestl W. Syntheses and optimization of new GS39783 analogues as positive allosteric modulators of GABA B receptors. Bioorg Med Chem Lett. 2007;17:6206–6211. doi: 10.1016/j.bmcl.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guscott MR, Cook GP, Bristow LJ. Contextual fear conditioning and baseline startle responses in the rat fear-potentiated startle test: a comparison of benzodiazepine/gamma-aminobutyric acid-A receptor agonists. Behav Pharmacol. 2000;11:495–504. doi: 10.1097/00008877-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Evaluation of the anxiolytic-like profile of the GABAB receptor positive modulator CGP7930 in rodents. Neuropharmacology. 2008;54:854–862. doi: 10.1016/j.neuropharm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Spalding TA. Allosteric modulation of G-protein coupled receptors. Eur J Pharm Sci. 2004;21:407–420. doi: 10.1016/j.ejps.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Johnston GA. Neuropharmacology of amino acid inhibitory transmitters. Annu Rev Pharmacol Toxicol. 1978;18:269–289. doi: 10.1146/annurev.pa.18.040178.001413. [DOI] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Ketelaars CE, Bollen EL, Rigter H, Bruinvels J. GABA-B receptor activation and conflict behaviour. Life Sci. 1988;42:933–942. doi: 10.1016/0024-3205(88)90393-1. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Lehner M, Wislowska-Stanek A, Maciejak P, Szyndler J, Sobolewska A, Krzascik P, Plaznik A. The relationship between pain sensitivity and conditioned fear response in rats. Acta Neurobiol Exp (Wars) 2010;70:56–66. doi: 10.55782/ane-2010-1774. [DOI] [PubMed] [Google Scholar]
- Lhuillier L, Mombereau C, Cryan JF, Kaupmann K. GABA(B) receptor-positive modulation decreases selective molecular and behavioral effects of cocaine. Neuropsychopharmacology. 2007;32:388–398. doi: 10.1038/sj.npp.1301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Hatanaka Y, Saito H, Yamamori T, Hashikawa T. Differential expression of gamma-aminobutyric acid type B receptor-1a and -1b mRNA variants in GABA and non-GABAergic neurons of the rat brain. J Comp Neurol. 2000;416:475–495. [PubMed] [Google Scholar]
- Maccioni P, Carai MA, Kaupmann K, Guery S, Froestl W, Leite-Morris KA, Gessa GL, Colombo G. Reduction of alcohol’s reinforcing and motivational properties by the positive allosteric modulator of the GABA(B) receptor, BHF177, in alcohol-preferring rats. Alcohol Clin Exp Res. 2009;33:1749–1756. doi: 10.1111/j.1530-0277.2009.01012.x. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Fantini N, Froestl W, Carai MA, Gessa GL, Colombo G. Specific reduction of alcohol’s motivational properties by the positive allosteric modulator of the GABAB receptor, GS39783--comparison with the effect of the GABAB receptor direct agonist, baclofen. Alcohol Clin Exp Res. 2008;32:1558–1564. doi: 10.1111/j.1530-0277.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Pes D, Orru A, Froestl W, Gessa GL, Carai MA, Colombo G. Reducing effect of the positive allosteric modulator of the GABA(B) receptor, GS39,783, on alcohol self-administration in alcohol-preferring rats. Psychopharmacology (Berl) 2007;193:171–178. doi: 10.1007/s00213-007-0776-1. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Marescaux C, Vergnes M, Bernasconi R. GABAB receptor antagonists: potential new anti-absence drugs. J Neural Transm Suppl. 1992;35:179–188. doi: 10.1007/978-3-7091-9206-1_12. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. Baclofen, a selective GABAB receptor agonist, dose-dependently impairs spatial learning in rats. Pharmacol Biochem Behav. 1996;53:303–308. doi: 10.1016/0091-3057(95)02025-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M. The Vogel conflict test: procedural aspects, gamma-aminobutyric acid, glutamate and monoamines. Eur J Pharmacol. 2003;463:67–96. doi: 10.1016/s0014-2999(03)01275-5. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Lhuillier L, Kaupmann K, Cryan JF. GABAB receptor-positive modulation-induced blockade of the rewarding properties of nicotine is associated with a reduction in nucleus accumbens DeltaFosB accumulation. J Pharmacol Exp Ther. 2007;321:172–177. doi: 10.1124/jpet.106.116228. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Takashima T. The GABA(B) receptor antagonist CGP36742 attenuates the baclofen- and scopolamine-induced deficit in Morris water maze task in rats. Brain Res. 1997;766:101–106. doi: 10.1016/s0006-8993(97)00529-5. [DOI] [PubMed] [Google Scholar]
- Ong J, Kerr DI. Clinical potential of GABAB receptor modulators. CNS Drug Rev. 2005;11:317–334. doi: 10.1111/j.1527-3458.2005.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey LK, Tharmalingam S, Hampson DR. Subchronic administration and combination metabotropic glutamate and GABAB receptor drug therapy in fragile X syndrome. J Pharmacol Exp Ther. 2011;338:897–905. doi: 10.1124/jpet.111.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Pandolfo P, Duarte FS, Takahashi RN, Prediger RD. Altered emotionality leads to increased pain tolerance in amyloid beta (Abeta1-40) peptide-treated mice. Behav Brain Res. 2010;212:96–102. doi: 10.1016/j.bbr.2010.03.052. [DOI] [PubMed] [Google Scholar]
- Partyka A, Klodzinska A, Szewczyk B, Wieronska JM, Chojnacka-Wojcik E, Librowski T, Filipek B, Nowak G, Pilc A. Effects of GABAB receptor ligands in rodent tests of anxiety-like behavior. Pharmacol Rep. 2007;59:757–762. [PubMed] [Google Scholar]
- Paterson NE, Hanania T. The modified Geller-Seifter test in rats was insensitive to GABAB receptor positive modulation or blockade, or 5-HT1A receptor activation. Behav Brain Res. 2010;208:258–264. doi: 10.1016/j.bbr.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A. Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J Pharmacol Exp Ther. 2008;326:306–314. doi: 10.1124/jpet.108.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsikas N, Rigamonti AE, Cella SG, Muller EE. The GABAB receptor and recognition memory: possible modulation of its behavioral effects by the nitrergic system. Neuroscience. 2003;118:1121–1127. doi: 10.1016/s0306-4522(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Reynolds DS. The value of genetic and pharmacological approaches to understanding the complexities of GABA(A) receptor subtype functions: the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 2008;90:37–42. doi: 10.1016/j.pbb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cao BJ, Dalvi A, Holmes A. Animal models of anxiety: an ethological perspective. Braz J Med Biol Res. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- Saha N, Chugh Y, Sankaranaryanan A, Sharma PL. Effects of post-training administration of (-)-baclofen and chlordiazepoxide on memory retention in ICRC Swiss mice: interactions with GABAA and GABAB receptor antagonists. Pharmacol Toxicol. 1993;72:159–162. doi: 10.1111/j.1600-0773.1993.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Sawynok J. GABAergic mechanisms of analgesia: an update. Pharmacol Biochem Behav. 1987;26:463–474. doi: 10.1016/0091-3057(87)90148-1. [DOI] [PubMed] [Google Scholar]
- Sharma S, Rakoczy S, Brown-Borg H. Assessment of spatial memory in mice. Life Sci. 2010;87:521–536. doi: 10.1016/j.lfs.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard RA, Wedlock P, Wilson NE. Direct evidence for mediation of an anticonflict effect of baclofen by GABAb receptors. Pharmacol Biochem Behav. 1992;41:651–653. doi: 10.1016/0091-3057(92)90387-u. [DOI] [PubMed] [Google Scholar]
- Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Markou A, Froestl W, Cryan JF. The GABAB receptor-positive modulator GS39783 and the GABAB receptor agonist baclofen attenuate the reward-facilitating effects of cocaine: intracranial self-stimulation studies in the rat. Neuropsychopharmacology. 2005;30:2065–2072. doi: 10.1038/sj.npp.1300734. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Walsh TJ. Baclofen produces dose-related working memory impairments after intraseptal injection. Behav Neural Biol. 1994;61:181–185. doi: 10.1016/s0163-1047(05)80073-1. [DOI] [PubMed] [Google Scholar]
- Terrier J, Ort A, Yvon C, Saj A, Vuilleumier P, Luscher C. Bi-directional effect of increasing doses of baclofen on reinforcement learning. Front Behav Neurosci. 2011;5:40. doi: 10.3389/fnbeh.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Engin E, McEown K. Animal models of anxiety and anxiolytic drug action. Curr Top Behav Neurosci. 2010;2:121–160. doi: 10.1007/7854_2009_17. [DOI] [PubMed] [Google Scholar]
- Treweek JB, Sun C, Mayorov AV, Qi L, Levy CL, Roberts AJ, Dickerson TJ, Janda KD. Prevention of drug-induced memory impairment by immunopharmacotherapy. J Med Chem. 2008;51:6866–6875. doi: 10.1021/jm800506v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu T. Effects of psychoactive drugs in the Vogel conflict test in mice. Jpn J Pharmacol. 1999;80:111–118. doi: 10.1254/jjp.80.111. [DOI] [PubMed] [Google Scholar]
- Vaught JL, Pelley K, Costa LG, Setler P, Enna SJ. A comparison of the antinociceptive responses to the GABA-receptor agonists THIP and baclofen. Neuropharmacology. 1985;24:211–216. doi: 10.1016/0028-3908(85)90076-0. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Boehrer A, Simler S, Bernasconi R, Marescaux C. Opposite effects of GABAB receptor antagonists on absences and convulsive seizures. Eur J Pharmacol. 1997;332:245–255. doi: 10.1016/s0014-2999(97)01085-6. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Muller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Guery S, Froestl W, Banerjee D, Benedict J, Finn MG, Markou A. Repeated administration of the GABAB receptor positive modulator BHF177 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine seeking in rats. Psychopharmacology (Berl) 2011a;215:117–128. doi: 10.1007/s00213-010-2119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Markou A. GABAB receptors in reward processes. Adv Pharmacol. 2010;58:315–371. doi: 10.1016/S1054-3589(10)58013-X. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Paterson NE, Guery S, Kaupmann K, Froestl W, Banerjee D, Finn MG, Markou A. Both GABA(B) receptor activation and blockade exacerbated anhedonic aspects of nicotine withdrawal in rats. Eur J Pharmacol. 2011b;655:52–58. doi: 10.1016/j.ejphar.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev. 2008;1:303–327. doi: 10.2174/1874473710801030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast M, Rostami P, Sadeghi-Hariri M. GABA(A) but not GABA(B) receptor stimulation induces antianxiety profile in rats. Pharmacol Biochem Behav. 2001;69:9–15. doi: 10.1016/s0091-3057(01)00518-4. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Haidari H, Jafari MR, Djahanguiri B. Influence of beta-adrenoceptor agonists and antagonists on baclofen-induced memory impairment in mice. Behav Pharmacol. 2004;15:293–297. doi: 10.1097/01.fbp.0000137211.95623.07. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.