Abstract
Aim
We hypothesized that liposomes modified with lysosomotropic octadecyl-rhodamine B (Rh) and loaded with therapeutic glucocerebroside velaglucerase alfa (VPRIV™) will improve lysosomal delivery of the enzyme into Gaucher’s cells.
Materials & methods
Confocal microscopy and flow cytometry were used to evaluate the ability of Rh-modified liposomes loaded with VPRIV to improve the lysosomal targeting in monocyte-derived macrophages and Gaucher’s fibroblasts.
Results
Confocal microscopy demonstrated that Rh-modified liposomes localized primarily in the lysosomes. As confirmed by flow cytometry using specific substrate 5-(pentafluorobenzoylamino)fluorescein diglucoside, intralysosomal accumulation of VPRIV in the cells treated with Rh-modified liposomes was significantly increased (up to 68%) relative to the cells treated with plain liposomes or free VPRIV.
Conclusion
Rh-modified lysosomotropic liposomes can improve lysosomal accumulation of liposomal enzymes both in nonphagocytic Gaucher’s fibroblasts and phagocytic monocyte-derived macrophages.
Keywords: enzyme replacement therapy, glucocerebroside, liposomes, lysosomal targeting, rhodamine B, velaglucerase alfa, VPRIV®
Lysosomal storage diseases (LSD), a set of more than 40 inherited disorders associated with the deficiency of certain lysosomal enzymes, continue to pose a serious medical problem [1-3]. The main approach for the treatment of LSD is enzyme replacement therapy (ERT) by the administration of exogenous enzymes. However, ERT remains limited and expensive because of poor delivery and low stability of therapeutic enzymes [4].
Gaucher’s disease is the most common lysosomal storage disorder [1]. It is characterized by a deficiency of the lysosomal enzyme glucocerebrosidase (GlcCerase). Residual levels of GlcCerase activity in Gaucher’s disease patients have been estimated to be between 5 and 25% of normal activity [5]. Such a deficiency leads to accumulation of the substrate glucocerebroside (GlcCer) within the lysosomes, primarily in the monocyte/macrophage lineage [5]. The GlcCer-laden cells demonstrate a characteristic morphology with the presence of a huge amount of lipids in tubular deposits [6]. These storage cells are called Gaucher’s cells and are present in various locations, predominantly in the bone marrow, spleen, liver and parenchyma of the lymphatics [7].
The treatment of Gaucher’s disease is based on the administration of active GlcCerase [4]. Velaglucerase alfa (VPRIV™; Shire Human Genetic Therapies, Inc., MA, USA), is one of the therapeutic enzymes available for treatment of Gaucher’s disease [8]. Clinical trials with VPRIV have produced significant improvements in the condition of patients [9]. In general, the ERT for Gaucher’s disease is considered very safe and effective for patients, but ERT does have its share of limitations, including the poor therapeutic response on neurologic and bone symptoms [10], elevated dosages of injected enzymes (up to 60 units/kg) and short half-life (9.8 min), which leads to the high cost of the treatment [11].
The use of liposomes loaded with therapeutic enzymes has been considered as a promising way to improve ERT for some time [12]. Bio-distribution studies of β-fructofuranosidase-loaded liposomes demonstrated good enzyme accumulation in the liver with up to 50% of intra cellular enzyme activity localized in the lysosomal fraction [13]. Similar data have been obtained for intravenously administered liposome-encapsulated αmannosidase [14], neuraminidase [13] and β-glucuronidase [15]. Liposome-encapsulated β-glucosidase of human origin demonstrated the ability to degrade GM1-ganglioside in lysosomes of feline fibroblasts with pathological accumulation of this substrate [16]. The β-galactosidase-containing liposomes caused the breakdown of 70–80% of intra cellular galactocerebroside in mice [17]. However, these liposome-based preparations are not in general clinical use for ERT, and there remains a clear need to sharply increase the efficiency of the delivery of liposomal enzymes to lysosomes within cells.
One improvement in liposome-based enzyme delivery can be achieved by using liposomes specifically targeted to lysosomes. Previously, we demonstrated that modification of the liposomal surface with the lysosomotropic octadecyl-rhodamine B (Rh) significantly increases the delivery of the liposome-loaded model marker, FITC-dextran, to lysosomes in HeLa cells [18].
Here, we hypothesized that the application of lysosomotropic liposomes would significantly increase the delivery of encapsulated enzymes to lysosomes of Gaucher’s model cells and Gaucher’s fibroblasts. With this in mind, we prepared liposomes modified with lysosomotropic Rh, loaded them with VPRIV and investigated their intracellular delivery as well as the efficiency of delivery of enzymes into lysosomes.
Materials & methods
Materials
Egg phosphatidylcholine and cholesterol were purchased from Avanti Polar Lipids (AL, USA) and used without further purification. VPRIV was a generous gift from Shire Human Genetic Therapies Inc. Fluorescein isothiocyanate–dextran (FD; 40 kDa) and Rh were purchased from Sigma Aldrich (MO, USA). Bio-Gel A-1.5 m was purchased from Bio-Rad (CA, USA). DyLight™ 350-conjugated goat anti-mouse IgG was obtained from Pierce Biotechnology (IL, USA). Lysotracker® Red and 5-(pentafluorobenzoylamino)fluorescein diglucoside (PFB-FDGlu; P-11947) were purchased from Invitrogen/Molecular Probes, Inc. (OR, USA). Mouse monoclonal (H4B4) anti-lysosome-associated membrane protein antibody (anti-Lamp2) was purchased from Abcam (MA, USA). Fluoromount-G mounting medium was from SouthernBiotech (AL, USA). Human histiocytic lymphoma cells (U-937) and human fibroblasts (PCS 201–010) were purchased from American Type Culture Collection (VA, USA). Phorbol-12-myristate 13-acetate (P 8139) and conduritol B epoxide (CBE; C5424) were obtained from Sigma Aldrich. Gaucher’s Type 1 fibroblasts (GM00372) were purchased from Corriell Cell Repository (NJ, USA). Cell culture media and supplements were from CellGro (MO, USA). All other chemicals and buffer components were analytical grade preparations. Reverse osmosis purified and deionized water was used in all experiments.
Preparation of liposomes
For all preparations, the lipid films were first obtained from a mixture of egg phosphatidylcholine and cholesterol (69:30 mol%) in chloroform. Rh dissolved in ethanol was added to the lipid mixture in chloroform at 1 mol%. Chloroform was removed on a rotary evaporator followed by freeze-drying on a Freeze Dry System Freezone 4.5 (Labconco, MO, USA). The thin film formed was hydrated by vigorous vortexing with phosphate-buffered saline (PBS; pH 7.4) or PBS supplemented with either fluorescein isothiocyanate (FITC)-dextran-40k (45 mg/ml) or with VPRIV (10 units/ml). To produce liposomes with a narrow size distribution, the hydrated lipid films were extruded 20 times through double-stacked 200-nm pore size Nuclepore™ polycarbonate membranes (Whatman, NJ, USA) using a manual extrusion device (Avanti Polar Lipids). The liposomes and nonincorporated loads were separated on a BioGel 1.5 M column.
To estimate the enzyme content, liposomes loaded with VPRIV were lysed with 0.1% CHAPS (v/v) and the activity of the released VPRIV was measured using the fluorogenic substrate PFB-FDGlu; the reaction was allowed to proceed for 1 h at 37°C. The resulting fluorescence was measured on a microplate reader Synergy™ HT (Biotek, VT, USA; ex/em: 488/520 nm) and normalized for lipid content. The enzyme content in liposomes was calculated using a calibration curve of known VPRIV concentrations.
Physicochemical characterization of liposomes
Liposome size and size distribution were determined with a Coulter N4 MD Submicron Particle Size Analyzer (Beckman-Coulter, CA, USA). The ζ-potential of the various liposomal preparations were measured at 25°C in water (0.167–0.330 μg lipids/ml) using a Zeta-Plus ζ-potential analyzer (Brookhaven Instruments, NY, USA).
Cell culture
U937 monocytes cells were grown at 37°C in 5% CO2 and 95% humidity in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine and 1× minimum essential medium (MEM) nonessential amino acids. Gaucher’s fibroblasts were grown at 37°C in 5% CO2 and 95% humidity in MEM supplemented with 15% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine and 1× MEM nonessential amino acids. The fibroblasts were detached by trypsinization with 0.25% trypsin in PBS containing 0.025% EDTA. The cells were used for all experiments up to passage 9.
Development of monocyte-derived macrophages
U937 monocytes were differentiated into macrophages by culturing in the presence of 10-nM phorbol myristate acetate (PMA) in RPMI medium for 48 h at 37°C. To cause inhibition of the cellular β-glucocerebrosidase, the monocyte-derived macrophages (MDMs) were then incubated with 200-μM conduritol B epoxide (CBE) in RPMI medium for 72 h [19].
Recovery of β-glucocerebrosidase activity in MDMs after treatment with CBE
Recovery of β-glucocerebrosidase activity in MDMs after incubation with CBE was measured by flow cytometry. The MDMs were washed free of CBE and fresh RPMI medium was added. The MDMs were detached by incubation with lidocaine (4 mg/ml) and EDTA (55 μM) at 37°C for 5 min. The cells were pelleted by centrifugation at 1000 g for 5 min and resuspended in RPMI medium. The procedure was repeated twice. To measure the activity of the intracellular GlcCerase, the cells, resuspended in 100 μl of serum-free RPMI medium, were mixed with an equal volume of RPMI medium supplemented with PFB-FDGlu (at a 75 μM final concentration) and incubated at 37°C for 1 h. Lysosomal GlcCerase converts the PFB-FDGlu into the green fluorescent PFB-F dye (ex/em: ~492/516 nm) for which accumulation in the cells was analyzed by flow cytometry. Recovery of enzyme activity was tested by re-incubation of the cells in CBE-free medium at 24, 48 and 72 h after the removal of CBE, and presented as a percentage of the enzyme activity in the control (CBE-untreated) cells.
Interaction of liposomes with cells in vitro
Cells grown to 60–80% confluence were incubated with FD-loaded plain and Rh-modified liposomes (at 50 μg/ml lipid concentration) in complete RPMI medium (15% FBS, 1% antibiotics and 1× MEM nonessential amino acids) for 4 h, washed three-times with RPMI medium to remove unbound liposomes, and used for microscopy or FACS experiments. When required, cells treated with liposomes for 4 h and washed with RPMI medium were incubated additionally for 20 h at 37°C in 5% CO2 in liposome-free complete RPMI medium.
FACS analysis
The binding of FITC-dextran-loaded Rh-modified (Rh-Lip-FD) or plain liposomes (Lip-FD) to MDMs was assessed by flow cytometry. Control (untreated) MDMs or MDMs treated with liposomes were washed twice with RPMI medium, detached by lidocaine/EDTA, resuspended in 1 ml of ice-cold PBS, and their fluorescence determined by FACS. Data acquisition was performed on a Becton Dickinson FACScan™ (Becton Dickinson, CA, USA). Data analysis was performed using CellQuest software (Becton Dickinson). The green fluorescence was determined at the emission wave-length of 520 nm (channel FL-1). To eliminate possible overlap of Rh fluorescence with channel FL-1, a compensation between FL-1 and -2 channels (1–2%) was applied using the cells treated with Rh-modified enzyme-free liposomes as an additional control. A total of 5000 events were acquired for each sample.
Confocal laser scanning microscopy
To evaluate the lysosomal localization of liposomes, MDMs grown on glass coverslips were incubated with Rh-Lip-FD or Lip-FD as described above and then fixed with 4% paraformaldehyde in PBS (pH 7.4) for 15 min at room temperature (RT) followed by a PBS wash, quenching with NaBH4 in PBS for 5 min and another PBS wash. The cells were then permeabilized by incubation with 0.2% saponin and 1% bovine serum albumin in PBS for 10 min at RT, washed three-times with a blocking solution (1% bovine serum albumin in PBS, pH 7.4), and kept for 30 min in the same buffer. The cells were stained with mouse anti-human Lamp2 monoclonal antibody (mAb) diluted with blocking solution (1:50) for 60 min at RT, and washed five times with the blocking solution. Visualization was achieved by cell incubation with DyLight 350-conjugated goat anti-mouse IgG (1:100 dilution) for 60 min at RT followed by five washes with the blocking solution.
Owing to the poor fluorescent stability of FD under conditions accompanying staining with Lamp2 mAb, Lysotracker Red was applied to visualize lysosomes in the case of cells treated with Lip-FD. Briefly, MDMs treated initially with plain (non-modified) liposomes loaded with FD were incubated with 100 nM Lysotracker Red (ex/em: 577/590 nm) for 60 min in complete RPMI medium. The nuclei were visualized by cell staining with Hoechst 33342 (10 μg/ml) for 15 min in PBS. The cells were washed five times with PBS and fixed with 4% paraformaldehyde in PBS (pH 7.4) for 15 min at RT, followed by a PBS wash.
The coverslips with the cells were then mounted on glass slides with Fluoromount-G medium and sealed using a nail polish. The slides were observed with a Zeiss (Oberkochen, Germany) LSM 700 inverted confocal microscope equipped with a 63×, 1.4-numerical aperture plane-apochromat oil-immersion objective. To characterize the co-localization of liposomes and lysosomal markers, Pearson’s correlation coefficient (PCC) and Mander’s overlap coefficient (MOP) were calculated using ImageJ for a least 20 randomly chosen cells from 12 images obtained from three independent experiments.
Intralysosomal accumulation of liposomal VPRIV by FACS analysis
The intralysosomal delivery of VPRIV by Rh-modified liposomes in MDMs and Gaucher’s fibroblasts was evaluated by flow cytometry. MDMs and Gaucher’s fibroblasts were treated with VPRIV-free Rh-modified liposomes (Rh-Lip), VPRIV-loaded plain liposomes (Lip-E) or VPRIV-loaded Rh-modified liposomes (Rh-Lip-E), or with free VPRIV (4.2 mU/ml) for 4 h. The liposome-supplemented medium was then removed and the cells were incubated for 20 h at 37°C in liposome-free medium. The cells were harvested by incubation with lidocaine/EDTA for 5 min at 37°C and then washed twice with RPMI medium by centrifugation at 1000 g for 5 min. To evaluate the activity of intracellular GlcCerase, the cell suspension was mixed with an equal volume of medium supplemented with PFB-FDGlu (at a 75 μM final concentration) and incubated for 3 h at 37°C. The cell fluorescence was analyzed by flow cytometry.
Statistical analysis
The data were tested for statistical significance using the two-tailed homoscedastic Student’s t-test. Calculated p-values were considered significant at p < 0.05.
Results
Preparation & characterization of liposomal formulations
All liposomal formulations were characterized in terms of size, ζ-potential and loading of FITC-dextran and VPRIV (Table 1) . The presence of Rh did not affect the liposome size. However, Rh-modification of the liposomal surface increased both FITC-dextran and VPRIV content. Since nonloaded empty Rh-Lip had an increased membrane charge (Table 1), the observed increase in the FITC-dextran and VPRIV loading can be explained by their increased association with the liposomal surface.
Table 1.
Characterization of liposomes.
| Formulation | Composition (mol%) |
Size ± SD (nm) | ζ-potential ± SD (mV) |
FITC-dextran/ lipids (mg/mg) |
Enzyme loading (U/ml) |
||
|---|---|---|---|---|---|---|---|
| ePC | Chol | Rh | |||||
| Lip-FD | 70 | 30 | – | 170±23 (n = 2) | −0.42±0.6 (n = 2) | 7.0 (n = 3) | – |
| Rh-Lip-FD | 69 | 30 | 1 | 185±11 (n = 2) | −2.38±1.9 (n = 2) | 11.2 (n = 3) | – |
| Rh-Lip | 69 | 30 | 1 | 207±16 (n = 2) | 8.26±3.4 (n = 2) | – | – |
| Lip-E | 70 | 30 | – | 189±15 (n = 4) | −4.42±0.5 (n = 4) | – | 0.272±0.07 (n = 6) |
| Rh-Lip-E | 69 | 30 | 1 | 197±16 (n = 4) | −1.38±0.9 (n = 4) | – | 0.5±0.10 (n = 6) |
Chol: Cholesterol; ePC: Egg phosphatidylcholine; FITC: Fluorescein isothiocyanate; Lip-E: Velaglucerase alfa-loaded plain liposomes; Lip-FD: Fluorescein isothiocyanate-dextran-loaded plain liposomes; Rh: Octadecyl-rhodamine B; Rh-Lip: Octadecyl-rhodamine B-modified liposomes; Rh-Lip-E: Velaglucerase alfa-loaded rhodamine B-modified liposomes; Rh-Lip-FD: Fluorescein isothiocyanate-dextran-loaded octadecyl-rhodamine B-modified liposomes; SD: Standard deviation.
Previously, using liposomes loaded with self-quenching concentrations of calcein, we demonstrated the high stability of Rh-modified liposomal formulation after 24-h incubation in the presence of serum proteins [18]. Additionally, Rh-Lip did not demonstrate any significant effect on the cell viability at the concentrations below 1 mg/ml of lipids (data not shown).
Evaluation of GlcCerase recovery in MDMs treated with conduritol B epoxide
To develop a Gaucher’s cell model, we differentiated U937 monocytes to macrophages by treatment with PMA and then inhibited intracellular GlcCerase with CBE. CBE, a specific irreversible inhibitor of GlcCerase activity, is commonly used for simulating Gaucher’s phenotype in vitro [19].
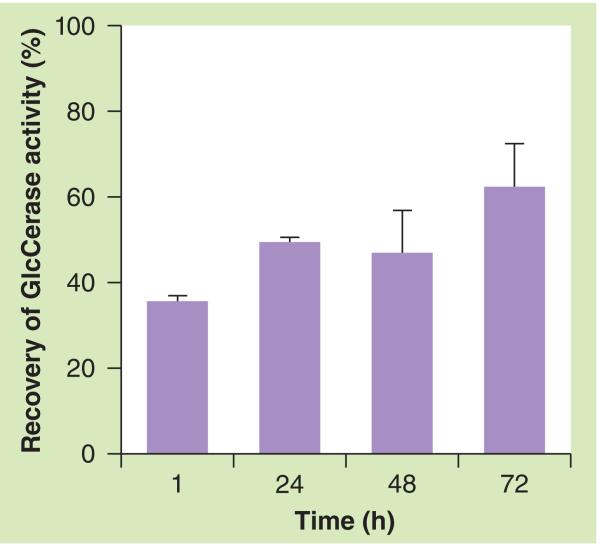
We evaluated the reappearance of the activity of the intracellular GlcCerase by comparing the fluorescence intensities produced by incubating the MDMs with PFB-FDGlu, a fluorogenic GlcCerase substrate. The incubation of cells with PFB-FDGlu leads to its accumulation in the lysosomes followed by its conversion into the green fluorescent PFB-F dye (ex/em: ~492/516 nm) by GlcCerase. Figure 1 shows that after the removal of the inhibitor, the activity of GlcCerase rose to 35.5 ± 1.24% within 1 h, 49.2 ± 0.95% after 24 h, remained at 46.8 ± 9.7% even after 48 h and rose to 62.1 ± 10.1% after 72 h. The results are consistent with previous research in which a different method showed that the recovery of enzyme activity after 48 h was approximately 50% [20]. Yatziv et al. found that the enzyme activity in human blood-derived macrophages treated with CBE recovered almost fully to normal levels after 5 days [21]. Based on the fact that the postincubation of CBE-treated monocytes for 24 h leads to the lysosomal accumulation of the GlcCerase substrate glucocerebroside [20], we chose the 24-h time point to achieve a more accurate Gaucher’s phenotype, despite the increased enzyme activity at 24 h compared with the 1 h time point.
Figure 1. Post-conduritol B epoxide recovery of glucocerebrosidase activity in monocyte-derived macrophages.
Monocyte-derived macrophages preincubated with conduritol B epoxide (CBE) for 72 h were post-incubated at 1, 24, 48 and 72 h with CBE-free medium. The recovery of the enzyme activity was measured by flow cytometry using 5-(pentafluorobenzoylamino)fluorescein diglucoside, a specific fluorogenic GlcCerase-specific substrate, and represented as a percentage of the enzyme activity in the control (CBE-untreated) cells. The data represent the mean of three different experiments ± standard deviation. GlcCerase: Glucocerebrosidase.
Cell–liposome interaction by flow cytometry
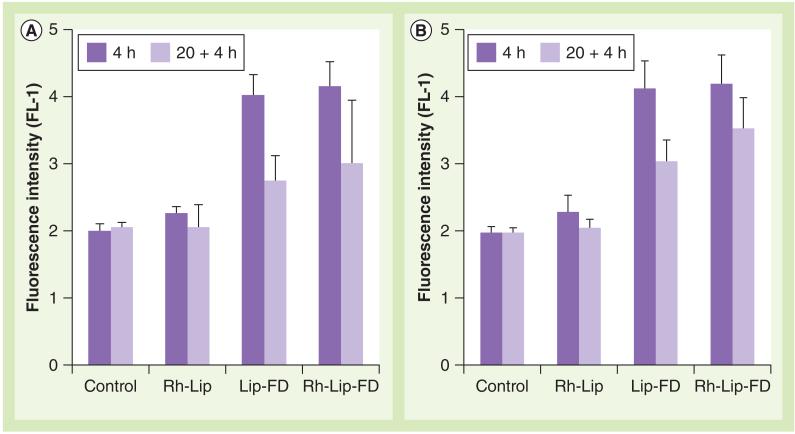
The interaction of Rh-Lip-FD and plain Lip-FD with MDMs (treated or untreated with CBE) was evaluated by flow cytometry (Figure 2) . Both CBE-treated and untreated MDMs showed a similar level of FITC fluorescence irrespective of its distribution in the cells (Figure 2). A decrease in fluorescence was observed after an additional incubation for 20 h in the liposome-free medium. This decrease may be attributed to the partial quenching of FITC fluorescence. Despite this decrease in fluorescence, the fluorescence of the 24-h incubation group was significantly higher than in the control cell groups.
Figure 2. Interaction of liposomes with monocyte-derived macrophages.
Macrophages had (A) active or (B) inhibited lysosomal glucocerebrosidase. Activity of glucocerebrosidase in monocyte-derived macrophages was inhibited by treatment of the cells with conduritol B epoxide (200 μM). The cells were treated with equal amounts of plain Lip-FD, Rh-Lip or Rh-Lip-FD for 4 h (dark bars) followed by washing and additional incubation for 20 h in liposome-free medium (pale bars). The cells were then treated with 5-(pentafluorobenzoylamino)fluorescein diglucoside and analyzed by flow cytometry. The data represent the mean of three different experiments ± standard deviation.
Lip-FD: Fluorescein isothiocyanate-dextran-loaded plain liposomes;
Rh-Lip: Octadecyl-rhodamine B-modified liposomes; Rh-Lip-FD: Fluorescein isothiocyanate-dextran-loaded octadecyl-rhodamine B-modified liposomes.
Intracellular localization of Rh-Lip-FD by confocal laser scanning microscopy
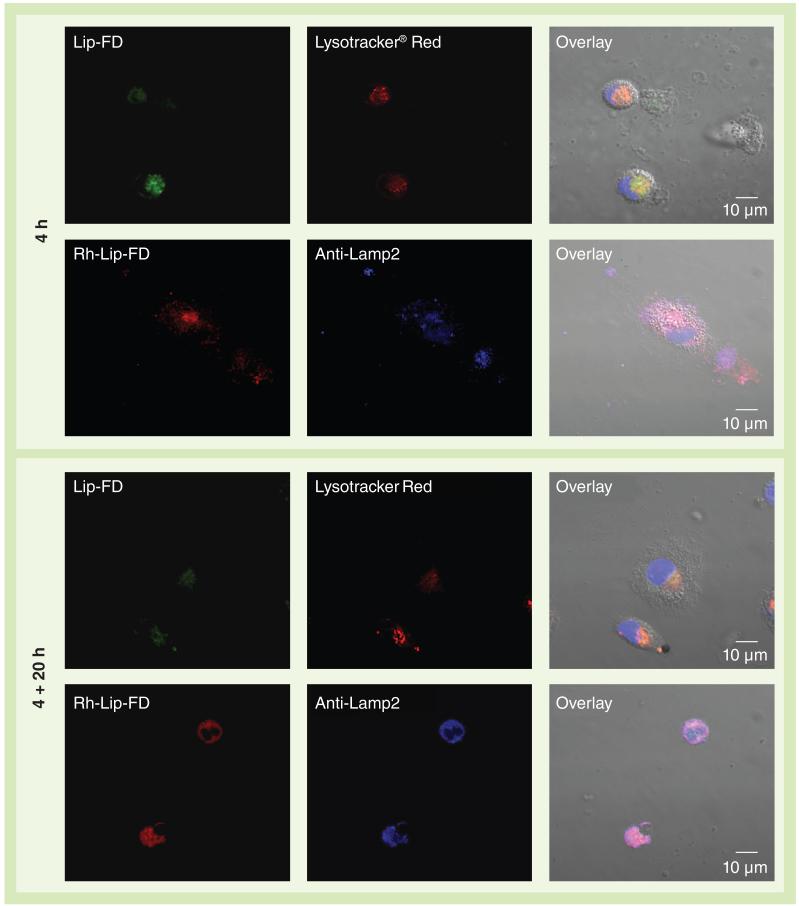
The lysosomal localization of plain Lip-FD or Rh-Lip-FD was evaluated by confocal laser scanning microscopy. Figure 3 shows representative confocal fluorescence micrographs of MDMs treated with plain Lip-FD or Rh-Lip-FD for 4 h, and for 4 + 20 h. The co-localization of FITC-dextran (green channel) and Lysotracker Red (red channel) was followed for the plain Lip-FD-treated cells, while the co-localization of Rh (red channel) and anti-Lamp2 mAb (blue channel) was used in the case of the Rh-Lip-FD-treated cells. The results of confocal microscopy showed that a 4-h incubation of the cells with Rh-Lip-FD led to the localization of Rh, mostly in the lysosomes with a high degree of co-localization with Lamp2 mAb (PCC = 0.8 ± 0.08; MOP = 0.9 ± 0.11). The cells treated with the same concentration of plain Lip-FD (50 μg/ml) showed much lower localization of FITC-dextran in the lysosomes (PCC = 0.2 ± 0.13; MOP = 0.3 ± 0.06). Most of the plain Lip-FD-treated cells were not internalized after 4 h incubation with cells and remained on the cell surface while the Rh-Lip-FD-treated cells were found in the perinuclear area. An additional incubation of Lip-FD-treated cells for 20 h led to an increased co-localization of FITC-dextran with Lysotracker Red-labeled lysosomes (PCC = 0.30 ± 0.13; MOC = 0.4 ± 0.17). However, it was still far less than the co-localization of Rh-Lip-FD and anti-Lamp2 mAb-labeled lysosomes (PCC = 0.7 ± 0.10; MOC = 0.8 ± 0.09). Thus, we have demonstrated that Rh modification of the liposomal surface leads to significantly better lysosomal accumulation compared with plain, non-modified liposomes.
Figure 3. Co-localization of liposomal formulations with lysosomal markers.
Monocyte-derived macrophages were treated with Lip-FD (green) or Rh-Lip-FD (red) for 4 h, followed by incubation for 20 h in the liposome-free medium. The treated cells were stained with lysosomal markers: Lysotracker® Red (Invitrogen/Molecular Probes Inc., OR, USA), shown here in red, or Lamp2 monoclonal antibodies (blue) in the case of Lip-FD-or Rh-Lip-FD-treated cells, respectively. In Lysotracker Red-stained cells, the nuclei were visualized by Hoechst 33342 (blue). Co-localization of liposomal formulations with respective lysosomal markers was analyzed by confocal microscopy. Scale bar = 10 μm. Lip-FD: Fluorescein isothiocyanate-dextran-loaded plain liposomes; Rh-Lip-FD: Fluorescein isothiocyanate-dextran-loaded octadecyl-rhodamine B-modified liposomes.
Determination of GlcCerase activity in Gaucher’s fibroblasts by flow cytometry
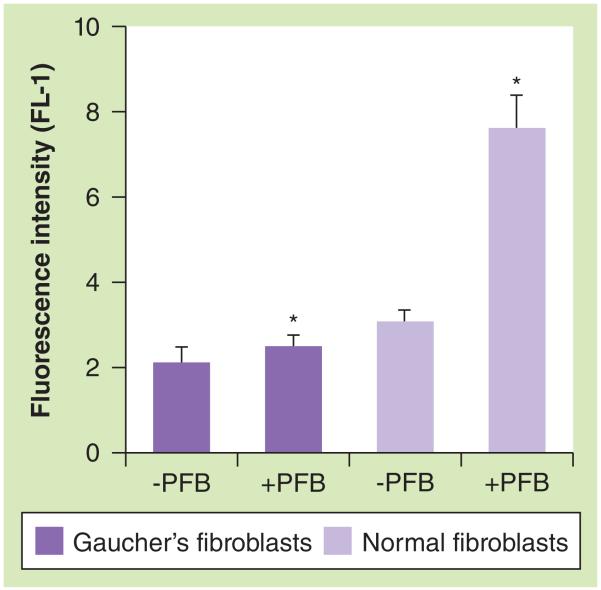
The levels of GlcCerase activity in Gaucher’s fibroblasts and normal human fibroblasts were determined by measuring the fluorescence of the cells treated with PFB-FDGlu by flow cytometry (Figure 4). As expected, the fluorescence intensity measured in normal fibroblasts was fourfold higher than in Gaucher’s fibroblasts, which indicates the absence/lower level of the enzyme in the Gaucher’s fibroblasts. Thus, these Gaucher’s fibroblasts are a suitable cell model to estimate the efficiency of enzyme delivery of lysosomotropic liposomes.
Figure 4. Glucocerebrosidase activity in Gaucher’s fibroblasts by flow cytometry.
Gaucher’s and normal human fibroblasts were treated with PFB diglucoside and resultant fluorescence intensity (channel FL-1) was determined by flow cytometry. Each value is the mean ± standard deviation of three different experiments.
*p < 0.0001.
PFB: 5-(Pentafluorobenzoylamino)fluorescein.
Lysosomal delivery of GlcCerase by Rh-modified lysosomotropic liposomes
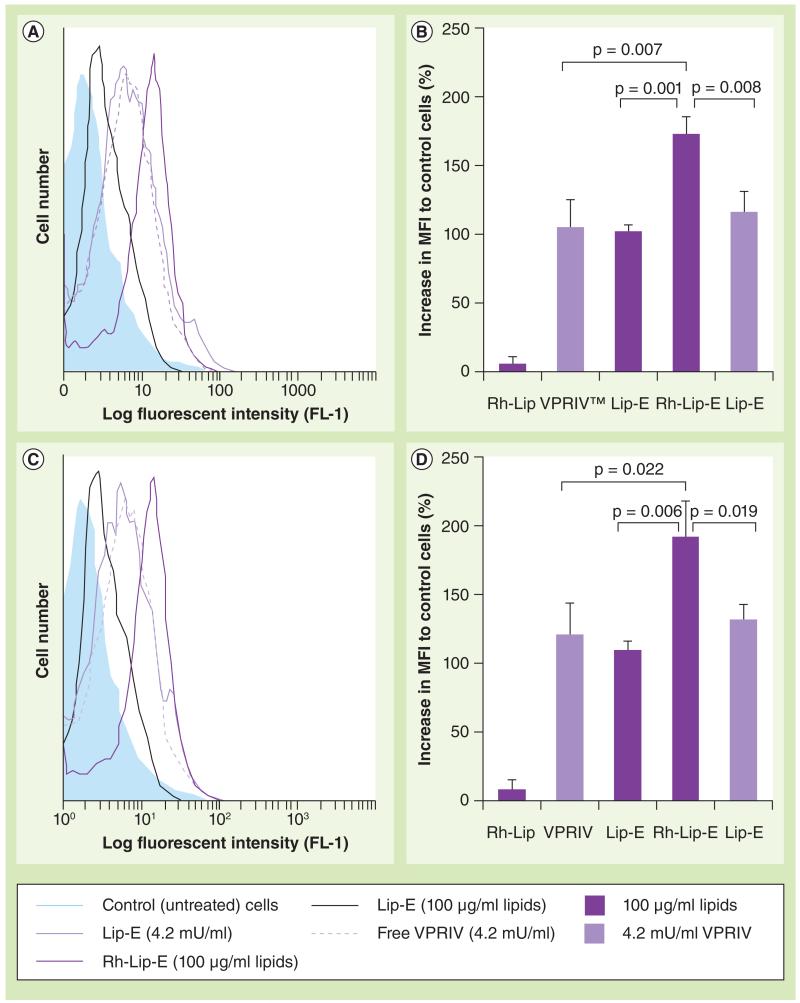
To evaluate the efficiency of GlcCerase delivery into lysosomes by Rh-modified lysosomotropic liposomes, we compared the fluorescence intensity of the cells after liposomal treatment and subsequent incubation with PFB-FDGlu. Gaucher’s fibroblasts or MDMs were treated with Lip-E or Rh-Lip-E for 4 h, then incubated for 20 h with liposome-free medium. The treatment with Rh-Lip and free nonliposomal VPRIV (at a concentration equal to the liposomal formulations) were used as controls. Since the Rh-Lip-E had increased VPRIV content relative to the Lip-E, we used two different concentrations of Lip-E as controls. One concentration was equal to the concentration of the lipids in Rh-Lip-E (100 μg/ml) and the second was equal to VPRIV content in Rh-Lip-E (4.2 mU/ml). The liposome-treated cells were then incubated with PFB-FDGlu and the lysosomal accumulation of the liposomal VPRIV was estimated by monitoring the fluorescence intensity measured by flow cytometry. The results were represented as a percentage of increase in mean fluorescent intensity normalized to control (untreated) cells.
As shown in Figure 5a & b, the efficiency of enzyme delivery into lysosomes provided by Rh-Lip-E was significantly higher when compared with Lip-E. The fluorescence intensity from the Rh-Lip-E-treated group, in both cell models, was higher than the plain Lip-E-treated group (100 μg/ml lipid concentration). To demonstrate that this difference is not due to lower enzyme loading in Lip-E than in Rh-Lip-E, an additional control with Lip-E at a concentration that provides the same amount of VPRIV as in the 100 μg/ml lipid concentration of Rh-Lip-E was used. The fluorescence intensity of this group was also significantly lower than in the Rh-Lip-E group. The free VPRIV-treated group showed slightly higher fluorescence intensity than the plain Lip-E-treated group. Despite this, the fluorescence intensity of the free VPRIV-treated group (4.2 mU/ml) was lower than in the Rh-Lip-E-treated group. Thus, the efficiency of enzyme delivery into lysosomes by Rh-Lip-E was significantly higher (up to 68%) than for Lip-E or free VPRIV.
Figure 5. Lysosomal delivery of velaglucerase alfa by octadecyl-rhodamine B-modified liposomes.
Monocyte-derived macrophages with inhibited glucocerebrosidase (A & B) or Gaucher’s fibroblasts (C & D) were treated with Rh-Lip, Lip-E or Rh-Lip-E, or with free VPRIV for 4 + 20 h. The level of the intralysosomal glucocerebrosidase was measured by flow cytometry (channel FL-1) of the cells incubated with 5-(pentafluorobenzoylamino)fluorescein diglucoside. (A & C) Typical histogram analysis. (B & D) A percentage increase in MFI normalized to control (untreated) cells. Each value is the mean ± standard deviation of three different experiments (each in triplicate).
Lip-E: Velaglucerase alfa-loaded plain liposomes; MFI: Mean fluorescent intensity;
Rh-Lip: Octadecyl-rhodamine B-modified liposomes; Rh-Lip-E: Velaglucerase alfa-loaded rhodamine B-modified liposomes; VPRIV: Velaglucerase alfa.
Discussion
In our previous studies [18,22], we have demonstrated that liposomes modified with Rh, a lysosomotropic targeting ligand, acquire the ability to target lysosomes specifically and allow for an increased delivery of liposome-entrapped material (FITC-dextran) to lysosomes in HeLa cells.
In this study, we attempted to demonstrate that Rh-modified lysosomotropic liposomes can improve delivery of VPRIV into lysosomes of Gaucher’s fibroblasts and MDMs, a representative model of Gaucher’s macrophages.
Our previous experiments carried out in HeLa cells, although conclusive, do not entirely apply to lysosomal storage disease conditions. Studies performed with lysosomal storage disease cell models were, therefore, required to demonstrate that this delivery system has potential for therapy of lysosomal storage diseases. Since macrophages are the main cell type affected in Gaucher’s disease, we used U937 monocytes after maturation into macrophages by treatment with PMA. As reported previously, PMA stimulation followed by resting in the absence of PMA increases the number of lysosomes and other organelles in THP-1 cells [23]. Based on previous research, we applied CBE treatment to inhibit the GlcCerase activity in the developed MDMs [19].
To measure the activity of the intralysosomal GlcCerase, we used PFB-FDGlu, a fluorogenic GlcCerase-specific substrate, that accumulates in lysosomes and becomes fluorescent when hydrolyzed by GlcCerase. Thus, using flow cytometry analysis of the intact cells incubated with PFB-FDGlu, we evaluated the activity of the intralysosomal GlcCerase in Gaucher’s fibroblasts and MDMs.
After inhibition of GlcCerase with CBE in MDMs, the enzyme activity returned to 35% (Figure 1) within an hour of CBE removal. This closely resembles GlcCerase activity in Gaucher’s macrophages, which is typically 5–25% compared with normal cells. Thus, CBE-treated MDMs are a suitable cell model of Gaucher’s macrophages and can be useful to test the lysosomal delivery of a therapeutical enzyme by Rh-modified lysosomotropic liposomes.
Using the Gaucher’s cell model, we carried out flow cytometry and confocal microscopy experiments to check further whether the developed cell model could be used for studies with a liposomal formulation containing VPRIV. With this in mind, the Rh-Lip-FD and Lip-FD (40 kDa) were prepared because the molecular weight of FITC-dextran is closer to the VPRIV (63 kDa). The interaction of Rh-Lip-FD and Lip-FD with MDMs was evaluated by flow cytometry (Figure> 2), and the accumulation of liposomal FITC-dextran in the lysosomal compartment was estimated by confocal microscopy (Figure 3). The results confirmed that Rh modification of the liposome surface did not increase cell–liposome interactions, but led to preferential localization in the lysosomal compartment of the MDMs compared with non-modified Lip-FD. Thus, the cell model developed proved to be robust and was, therefore, used for further studies with VPRIV-loaded liposomes.
Finally, using flow cytometric analysis of the cells treated with PFB-FDGlu, we estimated the ability of the Rh-modified lysosomotropic liposomes to deliver liposomal VPRIV to lysosomes in Gaucher’s fibroblasts and MDMs (Figure 5). Comparing cell fluorescence (which appeared as a result of PFB-FDGlu cleavage by the intralysosomal GlcCerase), we clearly demonstrated that the intralysosomal accumulation of VPRIV in the cells treated with Rh-Lip-E was significantly increased (up to 68%) relative to the cells treated with Lip-E or free VPRIV. Thus, Rh-modified lysosomotropic liposomes improved the lysosome accumulation of liposome-entrapped enzymes both in nonphagocytic Gaucher’s fibroblasts and in phagocytic MDMs, and such an improvement can shift the balance from pathological to normal cell phenotypes.
The ability of lysosomes to fuse with different cellular compartments is well known [24]. It is also established that cells can internalize liposomes by different endocytotic mechanisms, such as caveolae- and clathrin-mediated endocytosis [25]. We assumed that modification of the liposomal surface with the lysosomotropic ligand Rh could improve the lysosomal targeting due to more effective fusion of the endocytosed liposomes with lysosomes. Taking into account the fact that one of the main limitations of ERT is the inability to administer GlcCerase to target bone marrow and brain tissues affected by the disease, we believe that a multifunctional lysosomotropic liposome, which can be engineered to target the respective tissues [26,27], may overcome such limitations of the current therapeutic approach.
Conclusion
Modification of the liposome surface with the Rh significantly increases the delivery of VPRIV to lysosomes in both Gaucher’s fibroblasts and macrophages, representing a cell model of Gaucher’s macrophages.
Future perspective
LSDs are associated with the deficiency of certain lysosomal enzymes, which leads to accumulation of corresponding substrates in lysosomes. Taken together, these diseases pose a serious medical problem. The main approach for the treatment of LSDs is ERT based on the administration of exogenous enzymes. This procedure still remains limited and expensive owing to poor delivery and low stability of therapeutic enzymes.
The use of liposome-immobilized enzymes opened new opportunities for ERT, which have been known for a long time especially in the treatment of diseases localized to liver cells, which are natural targets for liposomes. Still, liposome-based preparations are not in general clinical use for ERT, and there is a clear need to sharply increase the efficiency of the delivery of liposomal enzymes to lysosomes inside cells and, thus, achieve lysosomal targeting.
We believe that the use of lysosome-targeted liposomes may significantly improve delivery of therapeutic enzymes into lysosomes for the treatment of lysosome-associated diseases.
Executive summary.
-
■
Lysosome-targeted liposomes loaded with the therapeutic glucocerebroside velaglucerase alfa were prepared by modification of the liposomal surface with lysosomotropic octadecyl-rhodamine B (Rh).
-
■
The pattern of glucocerebrosidase (GlcCerase) activity in monocyte-derived macrophages (MDMs) treated with conduritol B epoxide resembles the pattern of GlcCerase activity in Gaucher’s macrophages.
-
■
To obtain model Gaucher’s macrophages, U937 monocytes were maturated to macrophages by treatment with phorbol myristate acetate followed by inhibition of the intracellular GlcCerase with conduritol B epoxide treatment.
-
■
The remaining GlcCerase activity (25–30%) in MDMs resembles the level of GlcCerase activity in Gaucher’s macrophages
-
■
Rh-modified liposomes were localized mostly in the lysosomes, as demonstrated by the confocal microscopy.
-
■
Rh-modified lysosomotropic liposomes strongly improve the lysosomal accumulation of liposomal glucocerebroside velaglucerase alfa both in nonphagocytic Gaucher’s fibroblasts and phagocytic MDMs.
Acknowledgments
This research was funded by the NIH grant RO1 CA128486 to VP Torchilin.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the princi ples outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372(9645):1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 2.Van Der Ploeg AT, Reuser AJ. Pompe’s disease. Lancet. 2008;372(9646):1342–1353. doi: 10.1016/S0140-6736(08)61555-X. [DOI] [PubMed] [Google Scholar]
- 3.Zarate YA, Hopkin RJ. Fabry’s disease. Lancet. 2008;372(9647):1427–1435. doi: 10.1016/S0140-6736(08)61589-5. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski GA, Hopkin RJ. Enzyme therapy for lysosomal storage disease: principles, practice, and prospects. Ann. Rev. Genomics Hum. Genet. 2003;4:403–436. doi: 10.1146/annurev.genom.4.070802.110415. [DOI] [PubMed] [Google Scholar]
- 5.Jmoudiak M, Futerman AH. Gaucher disease: pathological mechanisms and modern management. Br. J. Haematol. 2005;129(2):178–188. doi: 10.1111/j.1365-2141.2004.05351.x. [DOI] [PubMed] [Google Scholar]
- 6.Naito M, Takahashi K, Hojo H. An ultrastructural and experimental study on the development of tubular structures in the lysosomes of Gaucher cells. Lab. Invest. 1988;58(5):590–598. [PubMed] [Google Scholar]
- 7.Bussink AP, Van Eijk M, Renkema GH, Aerts JM, Boot RG. The biology of the Gaucher cell: the cradle of human chitinases. In: Kwang WJ, editor. International Review of Cytology. Academic Press; TN, USA: 2006. pp. 71–128. [DOI] [PubMed] [Google Scholar]
- 8.Piran S, Amato D. Gaucher disease: a systematic review and meta-analysis of bone complications and their response to treatment. J. Inherit. Metab. Dis. 2010;33(3):271–279. doi: 10.1007/s10545-010-9071-0. [DOI] [PubMed] [Google Scholar]
- 9.Elstein D, Cohn GM, Wang N, Djordjevic M, Brutaru C, Zimran A. Early achievement and maintenance of the therapeutic goals using velaglucerase alfa in type 1 Gaucher disease. Blood Cells Mol. Dis. 2011;46(1):119–123. doi: 10.1016/j.bcmd.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Lachmann RH. Enzyme replacement therapy for lysosomal storage diseases. Curr. Opin. Pediatr. 2011;23(6):588–593. doi: 10.1097/MOP.0b013e32834c20d9. [DOI] [PubMed] [Google Scholar]
- 11.Connock M, Burls A, Frew E, et al. The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review. Health Technol. Assess. 2006;10(24):iii–iv. ix–136. doi: 10.3310/hta10240. [DOI] [PubMed] [Google Scholar]
- 12.Gregoriadis G. Liposomes in the therapy of lysosomal storage diseases. Nature. 1978;275(5682):695–696. doi: 10.1038/275695a0. [DOI] [PubMed] [Google Scholar]
- 13.Gregoriadis D, Louis L, Neerunjun D. Comparative effect and fate of non-entrapped and liposome-entrapped neuraminidase injected into rats. Biochem. J. 1974;140(2):323–330. doi: 10.1042/bj1400323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel HM, Ryman BE. Alpha-mannosidase in zinc-deficient rats. Possibility of liposomal therapy in mannosidosis. Bioch. Soc. Trans. 1974;2:1014–1017. [Google Scholar]
- 15.Steger LD, Desnick RJ. Enzyme therapy. VI: comparative in vivo fates and effects on lysosomal integrity of enzyme entrapped in negatively and positively charged liposomes. Biochim. Biophys. Acta. 1977;464(3):530–546. doi: 10.1016/0005-2736(77)90028-1. [DOI] [PubMed] [Google Scholar]
- 16.Braidman IP, Gregoriadis G. Rapid partial-purification of placental glucocerebroside β-glucosidase and its entrapment in liposomes. Biochem. J. 1977;164(2):439–445. doi: 10.1042/bj1640439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umezawa F, Eto Y, Tokoro T, Ito F, Maekawa K. Enzyme replacement with liposomes containing beta-galactosidase from Charonia lumpas in murine globoid cell leukodystrophy (twitcher) Biochem. Biophys. Res. Commun. 1985;127(2):663–667. doi: 10.1016/s0006-291x(85)80212-6. [DOI] [PubMed] [Google Scholar]
- 18.Koshkaryev A, Thekkedath R, Pagano C, Meerovich I, Torchilin VP. Targeting of lysosomes by liposomes modified with octadecyl-rhodamine B. J. Drug Target. 2011;19(8):606–614. doi: 10.3109/1061186X.2010.550921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newburg DS, Shea TB, Yatziv S, Raghavan SS, McCluer RH. Macrophages exposed in vitro to conduritol B epoxide resemble Gaucher cells. Exp. Mol. Pathol. 1988;48:317–323. doi: 10.1016/0014-4800(88)90068-8. [DOI] [PubMed] [Google Scholar]
- 20.Das PK, Murray GJ, Gal AE, Barranger JA. Glucocerebrosidase deficiency and lysosomal storage of glucocerebroside induced in cultured macrophages. Exp. Cell. Res. 1987;168(2):463–474. doi: 10.1016/0014-4827(87)90019-x. [DOI] [PubMed] [Google Scholar]
- 21.Yatziv S, Newburg DS, Livni N, Barfi G, Kolodny EH. Gaucher-like changes in human blood-derived macrophages induced by beta-glucocerebrosidase inhibition. J. Lab. Clin. Med. 1988;111(4):416–420. [PubMed] [Google Scholar]
- 22.Meerovich I, Koshkaryev A, Thekkedath R, Torchilin VP. Screening and optimization of ligand conjugates for lysosomal targeting. Bioconjug. Chem. 2011;22(11):2271–2282. doi: 10.1021/bc200336j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE. 2010;5(1):e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 2007;8(8):622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 25.Huth U, Wieschollek A, Garini Y, Schubert R, Peschka-Suss R. Fourier transformed spectral bio-imaging for studying the intracellular fate of liposomes. Cytometry A. 2004;57(1):10–21. doi: 10.1002/cyto.a.10105. [DOI] [PubMed] [Google Scholar]
- 26.Schnyder A, Huwyler J. Drug transport to brain with targeted liposomes. NeuroRx. 2005;2(1):99–107. doi: 10.1602/neurorx.2.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sou K, Goins B, Oyajobi BO, Travi BL, Phillips WT. Bone marrow-targeted liposomal carriers. Expert Opin. Drug Deliv. 2011;8(3):317–328. doi: 10.1517/17425247.2011.553218. [DOI] [PMC free article] [PubMed] [Google Scholar]