Abstract
Posttraumatic stress disorder (PTSD) is associated with problems in intimate relationships, partly due to deficits in social cognition. In this study, the role of arginine vasopressin (AVP) in the link between PTSD and partner-specific social cognition was examined. Participants were 24 individuals from 12 heterosexual couples in which at least one partner exhibited clinically significant PTSD symptoms. Attention to partner expressions of anger was examined as an indicator of distress and need for affiliative behaviors to repair the relationship bond. AVP administration improved the speed of men’s attentional engagement with their partners’ expressions of anger and alleviated the negative impact of PTSD on this social cognitive process. Further, men’s morning urinary AVP levels were negatively correlated with their PTSD severity. No such effects were found among women or for attention to unfamiliar men’s or women’s anger expressions. Thus, the AVP system may function in the relationship problems associated with PTSD.
Keywords: trauma, PTSD, social information processing, relationship satisfaction
Posttraumatic stress disorder (PTSD) is a psychiatric condition marked by dysregulated stress reactivity following a traumatic experience. Symptoms include fluctuation between re-experiencing and avoidance of trauma reminders and emotions, as well as emotional numbing and physiological hyperarousal. PTSD leads to widespread problems in intimate relationships (e.g., distress, divorce, violence), particularly for men (Monson, Taft, & Fredman, 2009). Social cognition plays an important role in the relationship problems associated with PTSD (Charuvastra & Cloitre, 2008). For example, the link between PTSD and perpetration of intimate partner violence appears to be a function of a range of social cognitive deficits (Taft, Schumm, Marshall, Panuzio, & Holtzworth-Munroe, 2008) and, given the influence on later-stage processes, early-stage social cognitive processes are especially important to the association between PTSD and such relationship problems (Sippel & Marshall, 2011).
The nonapeptide arginine vasopressin (AVP) may play a role in the link between PTSD and social cognitive processes that lead to relationship problems. In male rodents, trauma (i.e., repeated social subjugation) leads to lower central AVP levels (Ferris, 2000). Although divergent findings exist, other forms of severe stress (e.g., maternal separation) also lead to diminished central AVP functioning among male rodents (see Veenema, 2009). Similarly, traumatized children have low morning urinary AVP levels (Wismer Fries, Ziegler, Kurian, Jacoris, & Pollak, 2005). In contrast, men with PTSD have been found to exhibit elevated plasma AVP levels (de Kloet, Vermetten, Geuze, Wiegant, & Westenberg, 2008). Also suggesting a role of AVP in trauma and PTSD are findings that intranasal administration of AVP increases electromyographic responses to personal combat imagery, but not other stressful or neutral stimuli, among male combat veterans with PTSD (Pitman, Orr, & Lasko, 1993).
An abundance of evidence among rodents documents the role of AVP in social cognition (e.g., social recognition) and social behavior (e.g., pair bonding, aggression; see Albers, 2011 and Young & Wang, 2004). Context appears to be quite important in determining the valence of AVP’s effect (Goodson & Thompson, 2010). For example, particularly among male rodents, AVP facilitates affiliation with select mates as well as aggression toward male conspecifics, presumably to decrease reproductive competition (Young & Wang, 2004). Context may be especially salient following trauma, as indicated by work demonstrating that AVP plays a role in the impact of adolescent trauma on aggressive behavior during a resident:intruder model based on the size and age of the intruder conspecific (Delville, Melloni, & Ferris, 1998). Emerging evidence among humans largely mirrors the research among rodents. That is, AVP affects social cognition such that intranasal AVP administration 1) increases the speed with which men recognize sexual words (Guastella, Kenyon, Unkelbach, Alvares, & Hickie, 2011), 2) increases men’s familiarity memory for facial expressions of emotions (Guastella, Kenyon, Alvares, Carson, & Hickie, 2010), and 3) alters activation of the medial prefrontal cortex-amygdala circuit, which is implicated in emotion regulation, when men view emotional expressions (Zink, Stein, Kempf, Hakimi, & Meyer-Lindenberg, 2010). Similarly, polymorphisms of the AVPR1a gene are associated with amygdala activation in response to angry and fearful facial expressions (Meyer-Lindenberg et al., 2009). At the same time, AVP selectively decreases men’s ability to recognize facial expressions of negative emotions displayed by other men, but not women (Uzefovsky, Shalev, Israel, Knafo, & Ebstein, 2012). AVP administration also enhances men’s facial EMG response to viewing other men’s neutral facial expressions, making such responses similar to those that occur when viewing men’s angry expressions (Thompson, Gupta, Miller, Mills, & Orr, 2004). This effect may be sex-specific: AVP administration stimulates men’s agonistic facial expressions and decreases men’s perceptions of friendliness in response to photographs of other men, but stimulates women’s affiliative facial expressions and increases women’s perceptions of friendliness in response to photographs of other women (Thompson, George, Walton, Orr, Benson, 2006).
AVP also plays a role in social behavior and the relationship problems frequently observed among individuals with PTSD. For example, cerebrospinal fluid AVP levels are elevated among men (but not women) with personality disorders and a history of aggression (Coccaro, Kavoussi, Hauger, Cooper, & Ferris, 1998). AVP is also important in human affiliative behaviors, even sometimes among same-sex male dyads. When playing a game against either a computer or an unfamiliar man, intranasal administration of AVP increases men’s reciprocated cooperation and, in response, activates regions of the bed nucleus of the stria terminalis, lateral septum, and stria terminalis, which include AVP circuitry implicated in affiliation (Rilling et al., 2012). Similarly, in terms of intimate relationships, elevated plasma AVP is associated with a lower frequency of couples’ negative communication behaviors (Gouin et al., 2010). AVP may also increase the salience of intimate relationships and function as a marker of relationship distress. Plasma AVP has been found to be elevated among men (but not women) experiencing relationship distress (Taylor, Saphire-Bernstein, & Seeman, 2009). Also among men but not women, the RS3 repeat polymorphism of the AVP receptor 1a gene (AVPR1a) is associated with less partner bonding, but also fewer marital crises or threats of divorce (Walum et al., 2008). Interestingly, Maher and colleagues (2011) found that a particular single nucleotide polymorphism, rs11174811, of the AVPR1a gene is associated with men’s (but not women’s) greater relationship distress in a clinical sample recruited for severe drug use disorders, but the same SNP was associated with men’s greater perceived marital warmth in a population-based sample. Thus, sample characteristics, and potentially other individual or contextual/relationship factors, may play a role in the function of AVP’s influence on complex social behaviors.
Sex differences exist in the neuroanatomy of the rodent AVP system, with gonadal hormones influencing AVP production and organizational effects on AVP expression (de Vries, 2008). Consequently, AVP’s role in social recognition, pair bonding, and offensive aggression is largely specific to males (Albers, 2011; Young & Wang, 2004), although notable exceptions exist in which AVP is similarly associated with high levels of pair bonding for both sexes (e.g., Cho, de Vries, Williams, & Carter, 1999). Only one known study to date reports on sex differences in the neuroanatomy of the human AVP system. Ishunina and Swaab (1999) found AVP neurons in the hypothalamic supraoptic and paraventricular nuclei to be larger in men than women, suggesting greater AVP expression in men. In addition, as reviewed above, many of the effects of AVP among humans have only been reported among male samples; when sex differences are examined, the results are often specific to men or differ in valence between the sexes. Therefore, AVP may be more important to men’s than women’s PTSD and associated social cognitive deficits. Because sex differences in AVP expression do not necessarily directly translate into sex differences in AVP function (as in, for example, the prairie vole, the animal with the largest sex difference in AVP innervation, but the smallest sex difference in social behavior; deVries, 2008), this hypothesis requires testing. Such work can serve to inform model building aimed at explaining the differential impact of PTSD on men’s and women’s relationship problems.
Despite preclinical research suggesting that AVP facilitates male rodents’ affiliative responses towards their mates but aggressive responses towards others (particularly competing males), the impact of AVP on humans’ social cognition has not been examined relative to one’s intimate partner, thus impeding theoretical extensions to humans’ pair bonding and relationship functioning. Further, although therapeutic effects of AVP have been proposed for PTSD (de Kloet et al., 2008), its role in the social problems central to PTSD has not been tested. The primary aim of this pilot study is to examine whether AVP modulates the link between PTSD and early-stage partner-specific social cognition. Anger expressed by an intimate partner may signal relationship distress and a need for affiliative behaviors to repair the relationship bond; vigilance for a partner’s angry expressions may be key to the initiation of reparative behaviors. Thus, AVP should facilitate attentional engagement with partner expressions of anger. AVP should also alleviate the expected negative impact of PTSD severity on vigilance for partner expressions of anger. These effects should be stronger for men than women. Examination of the effect of AVP on attentional engagement with unfamiliar men’s and women’s expressions of anger is designed to provide insight into whether the proposed effects of AVP are specific to the perception of one’s partner, thus specific hypotheses are not made beyond generally expecting the proposed effects to be weaker in reference to unfamiliar men and women compared to one’s partner.
Methods
Participants
Participants included 24 individuals from 12 cohabitating, community-recruited heterosexual couples in which at least one partner exhibited clinically significant PTSD symptoms. Couples were ineligible if either partner had cardiovascular disease, high blood pressure, a seizure disorder, asthma, endocrine disturbance, or abused alcohol. Women were naturally ovulating, not using hormonal contraceptives, not peri-menopausal, and under age 50.
Participants averaged 32.2 (SD = 9.3) years of age with an individual monthly income of $1644 (SD = $1903) and 17.3 (SD = 3.2) years of education; most (79%) were Caucasian. All participants experienced a DSM-IV defined trauma. Participants’ index traumas varied greatly, with the most frequent traumas including the sudden death of a friend or loved one, abortion, childhood violence, physical assaults, combat, and life-threatening illness (including drug overdose). Five men and six women met diagnostic criteria for PTSD. The wife was PTSD-positive and the husband was PTSD-negative in five couples, the husband was PTSD-positive and the wife was PTSD-negative in four couples, both partners were PTSD-negative in two couples, and both partners were PTSD-positive in one couple.
Procedures
Participants’ first void morning urine samples were collected the day prior to, and day of, their laboratory sessions, then assayed for AVP using published procedures (Wismer Fries et al., 2005). Hormone levels were calculated per mg creatinine to account for fluid variability.
Laboratory sessions began at 8:30am in the early follicular phase of the female partner’s menstrual cycle. Following informed consent procedures, participants completed self-report measures and were photographed posing facial expressions of emotions. In a double-blind manner, participants then self-administered via nasal spray 1 ml solution containing 20 IU AVP or placebo. Ten minutes later, participants began a computer task designed to measure attention to anger expressions. This length of time between treatment and task was chosen because intranasal AVP administration leads to substantial increases in AVP measured in CSF and plasma within ten minutes of administration (Born et al., 2002). Approximately 45 minutes later, PTSD symptom severity was measured via clinical interview.
Measures
PTSD Symptom Severity
The Clinician Administered PTSD Scale (CAPS; Blake, Weathers, Nagy, & Kaloupek, 1995) is a structured interview that assesses the frequency and intensity of each PTSD symptom using standard prompt questions and explicit, behaviorally-anchored rating scales. The CAPS is commonly considered the gold standard in PTSD assessment. The CAPS has demonstrated high interrater reliability (.92 - .99) and convergent validity with other PTSD measures (Weathers, Ruscio, & Keane, 2001), including excellent sensitivity (90%) and specificity (95%; Hyer, Summers, Boyd, Litaker, & Boudeywns, 1996). In the current sample, the internal consistency reliability coefficient was .94.
Traumatic Events
The Traumatic Life Events Questionnaire (TLEQ; Kubany et al., 2000) was used to assist in the determination of the index trauma to be assessed during the CAPS interview. The TLEQ lists 22 types of potentially traumatic events and asks respondents to indicate if they experienced each event, and if so, how many times. The TLEQ also includes queries about the experience of fear, helplessness, and horror, and which trauma currently causes the most distress. The TLEQ has demonstrated adequate levels of test-retest reliability and good content validity (Kubany et al., 2000).
Relationship Satisfaction
The Dyadic Adjustment Scale (Spanier, 1979) is a psychometrically sound (Heyman, Sayers, & Bellack, 1994; Kurdek, 1992) 32-item self-report measure of perceptions of relationship consensus, cohesion, affectional expression, and satisfaction. Scores can range from 1 to 151, with scores less than 97 indicative of low marital satisfaction or distressed relationships. In the present sample, coefficient alpha was .94.
Relationship Commitment
The Personal Dedication subscale of the Commitment Inventory (Stanley & Markman, 1992) includes a 36-items assessing dedication and commitment to one’s intimate relationship. This subscale exhibits strong reliability and construct validity (Stanley & Markman, 1992). In the present sample, coefficient alpha was .87.
Attentional Engagement with Anger
The Exogenous Cueing Paradigm, Emotional Modification (Fox, Russo, Bowles, & Dutton, 2001) is an adaptation of Posner’s (1980) spatial cueing paradigm, designed to measure selective attention to emotions. In the current study, each trial began with a computer display of a central fixation cross and two white rectangles to the left and right of the cross, all presented against a black background for 500ms. The fixation cross and rectangles were then replaced by a photographic stimulus (anger or neutral) displayed on the left or right side of the screen for 1250ms. The stimulus was then followed by a target (a “3” or an “8”) presented on the left or right side of the screen that remained on the screen until the participant responded. The inter-trial interval was 500ms. Participants were instructed to indicate whether the target was a 3 or an 8 by pressing the corresponding key on the keyboard as quickly and accurately as possible. All trials were fully randomized; 50% of the trials were validly cued (i.e., photograph and target on the same side) and 50% were invalidly cued (i.e., photograph and target on different sides). Stimuli were each displayed eight times, such that the target (a “3” or an “8”), target location (left or right), and cue location (left or right), were fully crossed and balanced. Enhanced attentional engagement by anger was operationalized as faster identification of the target following anger stimuli, compared to neutral stimuli, on validly cued trials. Positive scores indicate faster engagement with angry expressions.
Emotional Expressions
Using procedures developed by Marshall and Holtzworth-Munroe (2010), participants were trained to produce facial expressions of anger according to instructions derived from the Facial Affect Coding System (FACS; Ekman & Friesen, 1978) and the minimum muscular movements recommended by Parke and Waters (1996). Participants were informed of the target emotion and the expression was demonstrated; then they were coached to move each muscle included in the expression. To make the stimuli representative of naturalistic expressions, expressions were obtained at both high and low intensity levels. This process was repeated until one photograph at each intensity level was obtained, initially judged by the author to be an accurate depiction of the emotion. Participants typically repeated the process 2-3 times with approximately 15-20 photographs taken. One photograph of each participant displaying a neutral expression (i.e., no muscular movements) was also chosen. Photographs were transformed to gray-scale, cropped around the face, and increased in brightness, if necessary.
Four men and four women, unfamiliar to the participants, were recruited from the community using flyers. Their photographs were developed using the same method of expression elicitation used with the participants. These men and women varied in age and all were Caucasian.
AVP and Placebo Preparation
Commercially available (American Regent, Inc., Shirley, NY) AVP included 1 ml solution containing 20 IU AVP and a 0.5% chlorobutanol. The placebo included 1 ml solution containing saline and 0.5% chlorobutanol. Solutions were placed in semitransparent vials with attached nasal applicators.
Data Preparation and Statistical Analyses
On the exogenous cueing paradigm, trials with reaction times greater than 2000ms were excluded to decrease error associated with delayed responding. In addition, due to a technical error, one participant did not complete any trials for her partner’s anger expressions. Her missing data were estimated using expectation-maximization (EM) based upon her remaining data. EM provides less biased estimates than listwise deletion, pairwise deletion, or regression substitution (Schafer & Graham, 2002).
Continuous PTSD severity scores were used for the analyses because they improve statistical power, are more stable over time, display higher levels of reliability and validity, and yield a greater amount of information than categorical measures of psychopathology (Cohen, 1992; Watson, 2005; Widiger & Clark, 2000). Furthermore, taxometric analyses suggest that PTSD is a dimensional, rather than a categorical, disorder and PTSD may be more accurately conceptualized as an extreme reaction to trauma rather than as a discrete clinical syndrome (e.g., Broman-Fulks et al., 2006).
Scores from two members of a dyad (e.g., intimate partners) tend to be correlated, which can bias significance testing (Kenny, 1995). Therefore, Kenny, Kashy, and Cook’s (2006) guidelines for distinguishable members were used to test whether male and female partners exhibited similarity (i.e., within-group dependency) in the dependent variable. A statistically nonsignificant correlation indicates that data can be analyzed by individual rather than by couple.
Primary data analyses included hierarchical linear regression models predicting attentional engagement with anger displayed by one’s partner, unfamiliar women, and unfamiliar men. Covariates included basal AVP level averaged across the two measurement days, tobacco use, and psychotropic medication use. Independent variables included treatment (AVP or placebo), sex, PTSD symptom severity, and all two- and three-way interactions among the independent variables.
Due to the current directional hypotheses (Jones, 1952) and well-cited criticisms of null hypothesis testing (e.g., Cohen, 1994), particularly within small samples in which power is limited, one-tailed tests were used to maximize power to detect small effects (Cohen, 1992).
Results
Stimuli Validation
Two male and two female independent raters naïve to study goals rated all photographs in terms of categorical emotion displayed and intensity of emotions displayed. Photographs of anger expressions were rated as displaying a significantly higher intensity of anger than photographs of neutral expressions (t = -6.23, p < .01). Husbands and wives did not differ in the intensity of anger expressed (t = -1.20, ns) and intensity of anger expressed did not correlate with PTSD severity (r = .17, ns). Rates of percent correct were as follows: Wives’ anger (.80) and neutral (.70), husbands’ anger (.77) and neutral (.83), unfamiliar women’s anger (.63) and neutral (.73), unfamiliar men’s anger (.73) and neutral (1.00). This level of percent correct is similar to that obtained for other standardized emotional expression stimuli when raters make forced-choice categorical judgments (e.g., Ekman & Friesen, 1976). The relatively lower ratings for unfamiliar women’s anger appeared to be largely due to two photos frequently being viewed as expressions of fear.
Participants’ reaction times did not differ between low- and high-intensity anger expressions displayed by their partners (t = 0.69, ns), unfamiliar women (t = 0.59, ns), or unfamiliar men (t = 0.41, ns). Low- and high-intensity photos were combined in subsequent analyses.
Among participants who received placebo (given AVP’s expected impact on attention), attentional engagement with partner anger was positively correlated with marital satisfaction (r = .67, p < .01) and commitment (r = .69, p < .01), thus supporting interpretation of the measure of attentional engagement with partner anger. Sex did not moderate these relations (satisfaction: b = .01, t = .04, ns; commitment: b = .001, t = .003, ns).
Descriptive Statistics and Bivariate Correlations
Scores between partners within couples did not correlate for the dependent variables of attentional engagement with expressions of anger displayed by one’s partner (r = -.09, ns), unfamiliar women (r = -.02, ns), or unfamiliar men (r = .15, ns), thus allowing individual-based analyses.
Descriptive statistics for all continuous study variables are displayed in Table 1. Measures of skew and kurtosis were not excessive, although distributions of scores for attentional engagement with unfamiliar women’s and men’s anger expressions were somewhat leptokurtic, indicating a possibility of extreme score influence. The distribution of scores for PTSD severity was somewhat platykurtic, indicating a low probability of extreme score influence. PTSD severity scores ranged from 2 to 75, with no significant sex differences (men: M = 31.0, SD = 23.6; women: M = 40.7, SD = 26.8), t(22) = -0.94, ns. On average, participants reported an average degree of marital satisfaction, with substantial range in scores such that a meaningful portion of participants (33%) was martially dissatisfied. Ten participants (42%) reported using tobacco and eight participants (33%) reported using psychotropic medications. Basal AVP levels did not differ between men (M = 42.63, SD = 20.66) and women (M = 42.64, SD = 26.14), t(22) = -.001, ns.
Table 1.
Descriptive Statistics and Intercorrelations Among Study Variables
| Variable | M | SD | Range | Skew | Kurtosis |
|---|---|---|---|---|---|
| Attentional engage: partner anger | -13.81 | 80.20 | -174.75 - 91.75 | -0.54 | -0.66 |
| Attentional engage: unfamiliar women’s anger | -1.70 | 72.68 | -181.25 - 153.00 | -0.60 | 1.89 |
| Attentional engage: unfamiliar men’s anger | 4.74 | 70.05 | -185.00 - 138.94 | -0.43 | 1.30 |
| PTSD severity | 35.83 | 25.16 | 2 - 75 | 0.24 | -1.56 |
| Basal AVP | 42.63 | 23.04 | 13.61 - 87.03 | 0.57 | -0.53 |
| Relationship satisfaction | 101.00 | 19.38 | 47 - 133 | -0.76 | 1.26 |
| Relationship commitment | 201.96 | 23.14 | 141 - 240 | -0.80 | 1.15 |
Overall, PTSD severity was not significantly correlated with basal AVP levels (r = -.13), but a sex difference existed (b = .38, t = 1.86, p < .05) such that PTSD severity was significantly negatively correlated with basal AVP levels among men (r = -.60, p < .05), but not women (r = .19, ns). PTSD severity was not significantly correlated with relationship satisfaction (r = .12, ns) or commitment (r = .01, ns) and no sex difference existed, b = .18, t = 0.82, ns and b = -.09, t = -0.41, ns, respectively. Similarly, basal AVP was not significantly correlated with relationship satisfaction (r = -.01, ns) or commitment (r = -.17, ns) and no sex difference existed, b = -.06, t =-0.13, ns and b = .17, t = 0.36, ns, respectively.
Prediction of Attentional Engagement with Anger Expressions
Results of hierarchical regression analyses predicting attentional engagement with partner, unfamiliar women’s, and unfamiliar men’s expressions of anger are presented in Table 2. When predicting attentional engagement with partner expressions of anger, no covariates (i.e., basal AVP, tobacco use, psychotropic medication use) were predictive. The main effect of treatment group was significant: those who received AVP engaged with partner expressions of anger more quickly than those who received placebo. The other main effects were not significant. Each two-way interaction was significant. The interaction between sex and treatment indicates that men who received AVP engaged more quickly with partner expressions of anger than men who received placebo, while there was no treatment effect for women (Figure 1). The interaction between sex and PTSD severity indicates that greater PTSD severity was associated with faster engagement with partner expressions of anger among women, but not among men. The interaction between treatment and PTSD severity indicates that greater PTSD severity was associated with faster engagement with partner expressions of anger among those who received AVP, but not those who received placebo. Finally, the three-way interaction was significant: Greater PTSD severity was associated with slower attentional engagement with partner expressions of anger among men who received placebo, but faster attentional engagement among men who received AVP and women in both treatment groups (Figure 2).
Table 2.
Hierarchical multiple regression analyses predicting speed of attentional engagement with partner, unfamiliar women’s, and unfamiliar men’s expressions of anger.
| Partner | Unfamiliar Women | Unfamiliar Men | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| b | t | ΔR2 | b | t | ΔR2 | b | t | ΔR2 | |
| Step 1: Covariates | .06 | .39 | .14 | ||||||
| Basal AVP | -.17 | -0.79 | -.62 | -3.49** | -.19 | -0.90 | |||
| Tobacco use | -.16 | -0.74 | .12 | 0.71 | -.28 | -1.34 | |||
| Psychotropic medication | .11 | 0.51 | .02 | 0.12 | .20 | 0.96 | |||
| Step 2: Main effects | .34 | .16 | .19 | ||||||
| Treatment | .46 | 1.75* | -.16 | -0.72 | .01 | 0.02 | |||
| Sex | .27 | 1.43 | .16 | 0.97 | .07 | 0.34 | |||
| PTSD severity | .32 | 1.49 | .35 | 1.90* | .45 | 2.00* | |||
| Step 3: 2-way interactions | .24 | .10 | .11 | ||||||
| Sex × Treatment | -.45 | -2.29** | -.07 | -0.35 | -.12 | -0.47 | |||
| Sex × PTSD | .44 | 2.40* | -.26 | -1.43 | .33 | 1.42 | |||
| Treatment × PTSD | .38 | 1.75* | -.22 | -0.99 | .25 | 0.91 | |||
| Step 4: 3-way interaction | .13 | .05 | .00 | ||||||
| Sex × Treatment × PTSD | -.40 | -2.78** | .24 | 1.43 | .06 | 0.26 | |||
p < .05,
p < .01, one-tailed.
Figure 1.
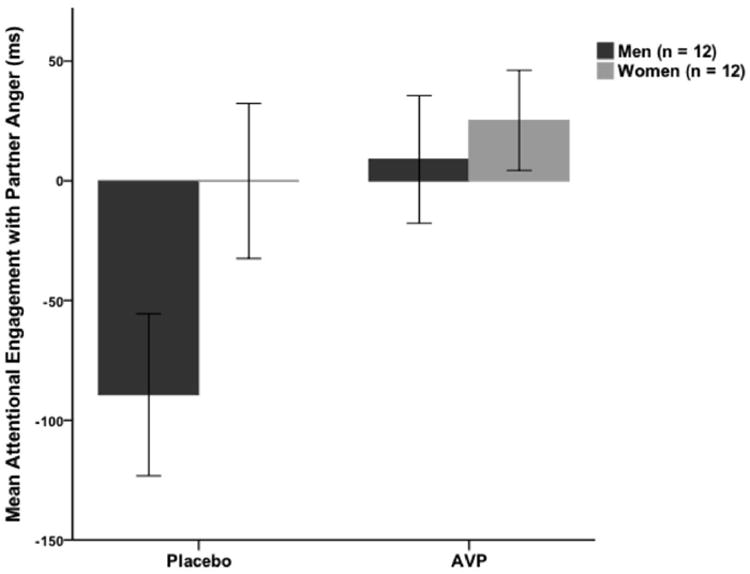
Mean speed of engagement with partner expressions of anger (relative to partner neutral expressions) predicted by treatment group and sex. Positive scores represent faster engagement with anger expressions than neutral expressions.
Figure 2.
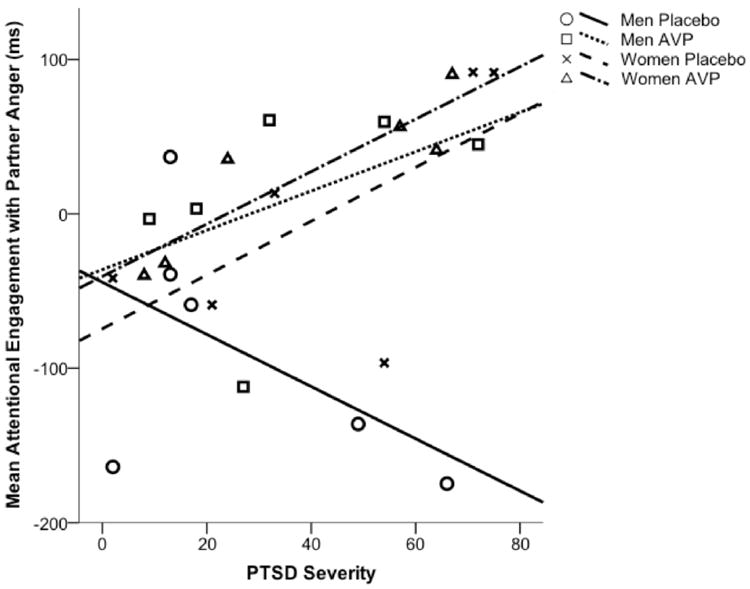
Mean speed of engagement with partner expressions of anger (relative to partner neutral expressions) predicted by treatment group, sex, and PTSD severity. Positive scores represent faster engagement with anger expressions than neutral expressions.
When predicting attentional engagement with unfamiliar women’s expressions of anger, basal AVP was predictive such that higher levels of basal AVP were associated with slower engagement with unfamiliar women’s anger expressions. No other covariates were predictive. The main effect of PTSD severity was significant such that greater PTSD severity was associated with faster engagement with unfamiliar women’s expressions of anger. No other main effects were significant. Additionally, no interaction effects were statistically significant. Further exploration of the finding of a relation between basal AVP and attentional engagement with unfamiliar women’s expressions of anger indicated that this relation was significantly moderated by sex such that the negative relation was stronger for men than women, b = 1.09, t = 3.89, p < .001. Treatment (AVP or placebo) and PTSD severity did not play a significant role in this relation.
When predicting attentional engagement with unfamiliar men’s expressions of anger, no covariates were predictive. The main effect of PTSD severity was significant such that greater PTSD severity was associated with faster engagement with unfamiliar men’s expressions of anger. No other main effects were significant. Additionally, no interaction effects were statistically significant.
For all regression models, when excluding the covariates of tobacco and psychotropic medication use, the pattern of results remained the same, although some effects became slightly weaker. Similarly, exclusion of the two photos of unfamiliar women expressing anger that were not consistently categorized as anger by the independent raters did not change the pattern of results.
Discussion
The current study provides preliminary evidence that AVP may influence men’s partner-specific social cognition. That is, men who received intranasal AVP exhibited faster attentional engagement with partner expressions of anger than men who received placebo. In contrast, AVP administration had no apparent influence on the speed of attentional engagement with unfamiliar women’s and men’s expressions of anger. Thus, as appears to be the case for the structurally, and often functionally, similar nonapeptide, oxytocin (Bartz, Zaki, Bolger, & Ochsner, 2011), AVP does not simply increase the salience of social cues; instead, context and individual factors are also important. Additionally, assuming that faster attentional engagement with partner expressions of anger represents an affiliative response to a distress cue that is central to relationship functioning, these findings may be used to help extend to humans preclinical research indicating that AVP facilitates pair bonding, likely via social cognitive mechanisms and often specifically among males (Albers, 2011; Young & Wang, 2004). Further, if the observed impact on social cognition translates into pair bond behaviors, the current results suggest that AVP may be important to the maintenance of pair bonds. Research with rodents indicates that AVP is important for initial pair bond formation (Albers, 2011; Young & Wang, 2004); however, the current study and all prior studies examining AVP in human relationships were conducted among individuals in established relationships. It will be important for future research to contrast the possible role of AVP in human pair bond formation versus maintenance.
The current study additionally provides preliminary evidence that AVP may alter the impact of PTSD on partner-specific social cognition, particularly among men. That is, among men who received placebo, greater PTSD severity was associated with slower engagement with partner expressions of anger, whereas among men who received AVP (and women in both treatment groups), greater PTSD severity was associated with faster engagement with partner expressions of anger. Furthermore, basal AVP levels were lower among men with more severe PTSD symptoms. These findings 1) extend to humans preclinical research indicating that trauma diminishes AVP functioning (Ferris, 2000; Veenema, 2009), 2) extend to the clinical phenomenon of adult PTSD findings of lower AVP levels among children with a history of neglect (Wismer Fries et al., 2005), and 3) provide evidence of specificity to men. Together, these findings suggest that men with more severe PTSD symptoms may not consistently produce adequate levels of AVP, but receptor binding may remain intact, allowing them to experience the apparent social-cognitive consequences of AVP administration. Future research designed to more directly examine such neural mechanisms is needed.
The current finding of lower urinary AVP among men with more severe PTSD symptoms diverges from results of de Kloet and colleagues (2008) in which men with PTSD exhibited elevated plasma AVP levels. Their findings may be due to systematically biased AVP reactivity to the stress of venipuncture and/or participation in a study of PTSD among those with PTSD. Additionally, the difference between studies may be a function of first-void morning urinary AVP reflecting peripheral AVP levels over a longer, and different, time period than AVP in plasma during a laboratory session. Additional work is needed to better understand how AVP production fluctuates over time, across contexts, and with the experience of different PTSD symptoms. It is possible that men with more severe PTSD symptoms experience substantial variability in AVP production aligning with fluctuations in specific PTSD symptoms. For example, the elevated AVP observed by de Kloet and colleagues (2008) may be a function of reexperiencing and/or hyperarousal symptoms in a potentially stressful situation, whereas the diminished AVP observed in the current study may be a function of avoidance and emotional numbing symptoms used to enable and sustain sleep. Moreover, differences across measures of peripheral AVP, as well as systematic ways in which central and peripheral release of AVP diverge, are not well understood at this time (Heinrichs, 2009; Wotjak, Ganster, Kohl, Holsboer, Landgraf, & Engelmann, 1998). Further knowledge regarding such issues would help answer these questions, in addition to shedding light on why basal AVP levels were not associated with attentional engagement with partner expressions of anger, but were associated with men’s attentional engagement with unfamiliar women’s expressions of anger. Without such answers, a theoretical interpretation of these findings would be highly speculative.
The observed sex differences in the current study are consistent with those of many animal studies and existing knowledge of the structure and function of the AVP system (de Vries et al., 2008; Ishunina & Swaab, 1999; Young & Wang, 2004). The current findings also support those of Thompson and colleagues (2006) in which sex differences in the effect of AVP on human social cognition were found. However, unlike Thompson and colleagues’ study in which the valence of the effect of AVP among women was opposite that of men, in the current study, no significant effect of AVP on social cognition was observed among women. Additionally, no effect of AVP among either sex was observed for attention to unfamiliar women’s and men’s anger expressions, whereas Thompson and colleagues observed effects of AVP on evaluations of, and EMG responses to, photographs of same-sex individuals. Thus, sex differences in the effect of AVP on social cognition may vary according to the stage of social cognition examined, with AVP being more likely to impact women’s social cognition at later stages of processing.
Caution is warranted regarding the interpretation that enhanced attentional engagement with partner expressions of anger represents a socially desirable pair-bonding process and, consequently, that AVP may enhance pair-bonding behaviors via social cognition. Instead, it is possible that partner expressions of anger may be perceived as threatening and AVP may increase attention to threat via its effect on stress reactivity under conditions of evaluative stress (Shalev et al., 2011), potentially resulting in adversarial behaviors such as aggression. Indeed, slower attentional disengagement from expressions of anger displayed by unfamiliar persons has been associated with individual factors such as dominance and testosterone (Terburg, Aarts, & von Honk, 2012; Terburg, Hooiveld, Aarts, Kenemans, & van Honk, 2011), and AVP has been frequently examined as modulator of aggression (see Veenema, 2009 and Veenema & Neumann, 2008). However, several reasons exist to question this alternative interpretation of the meaning of attentional engagement with partner anger expressions. First, strong positive correlations were observed among attentional engagement with partner expressions of anger and relationship satisfaction and commitment. Second, attention to expressions of anger displayed specifically by one’s partner has not previously been examined and, therefore, may function in a manner distinct from that of attention to expressions of anger displayed by unfamiliar people. Third, PTSD symptom severity was associated with faster attentional engagement with partner anger more so among women than men and women with PTSD display more intimacy behaviors in their relationships than men with PTSD (Hanley, Leifker, Blandon, & Marshall, in press). Fourth, the sum of the research on the impact of AVP on aggression indicates that, at a simple level, AVP can both increase and decrease aggression (Veenema, 2009; Veenema & Neumann, 2008). Variations in the function of AVP on aggression are likely due to cross-species anatomical differences (Veenema & Neumann, 2008), as well as individual and contextual differences (Ferris, 2000). Furthermore, studies using intracerebral microdialysis during displays of intermale aggression indicate that AVP release may be a consequence of aggression, rather than a cause of aggression (Veenema & Neumann, 2008). Therefore, although it appears that enhanced attentional engagement with partner expressions of anger represents a socially desirable pair-bonding process, this interpretation may be specific to characteristics of the current, relatively small sample. Further research among humans is needed to better understand variability in the effects of AVP based on context (e.g., social history, relationship characteristics, task demands) and individual characteristics (e.g., genotype, trauma timing, impulsive or aggressive tendencies).
Specific study limitations bear note. First and foremost, the sample size is quite small. Although the sample was recruited to avoid many potential confounds, replication is necessary. Second, tobacco and psychotropic medication use may impact the AVP system, yet, to support generalizability, participant exclusion criteria did not contain these factors. Because these factors were not significant model covariates, substantial impact is unlikely. Third, the small within-couple associations could have impacted results. Fourth, the current results may not be specific to PTSD. AVP is associated with other related psychological conditions (e.g., panic disorder, Keck et al., 2008; but inconsistently with depression, see Heinrichs, 2009 and Parker et al., 2010), likely due to AVP’s function in the stress response system and the centrality of stress in multiple psychological disorders. Finally, the greatest challenge to recruitment was the exclusion of women using hormonal contraceptives. Generalizability to women using hormonal contraceptives or women in different menstrual cycle phases is unknown.
In sum, the current study adds to emerging research on how hormones impact complex social behaviors based upon both individual and contextual factors. Although preliminary, it appears that men with more severe PTSD symptoms may not consistently receive the positive effects of AVP, yet, when AVP is produced, it promotes partner-specific social cognition that is impaired among men with more severe PTSD symptoms. It is possible that such processes may initiate affiliative behaviors to maintain or repair relationship bonds. Furthermore, given the reciprocal influence of social support on PTSD symptoms (Kaniasty & Norris, 2008), healthy relationship functioning may serve to alleviate stress and anxiety, thus decreasing PTSD symptom severity. It is hoped that the current study serves to promote continued investigation into potential neuropeptide mechanisms of PTSD and relationship problems, ultimately leading to the design and testing of novel therapeutic interventions.
Highlights.
Morning urinary AVP levels were lower among men with more severe PTSD
Intranasal AVP administration improved men’s partner-specific social cognition
AVP lessened the negative impact of PTSD on men’s partner-specific social cognition
No effect of AVP was found among women
No effect of AVP was found for social cognition relative to unfamiliar men or women
Acknowledgments
This project was supported by award number K12 HD055882 (Penn State BIRCWH Program) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). This research was also supported by the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health, through Grant UL1 RR033184. Assays were conducted by the Wisconsin National Primate Research Center (WNPRC) at the University of Wisconsin, Madison (UWM), which was made possible in part by grant number P51 RR000167 from the NCRR to the UWM WNPRC and conducted at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This publication’s content is solely the responsibility of the author and does not necessarily represent the official views of the NICHD, NCRR, NCATS, or NIH.
I thank Feea R. Leifker, Lauren M. Sippel, and Kelly S. Parker-Guilbert for their assistance with study administration and data collection, as well as Sheri A. Berenbaum and anonymous reviewers for their helpful comments on a previous version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers HE. The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Hormones and Behavior. 2011;61:283–292. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends in Cognitive Sciences. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: A transnasal approach to the human brain. Nature Neuroscience. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Broman-Fulks JJ, Ruggiero KJ, Green BA, Kilpatrick DG, Danielson CK, Resnick HS, Saunders BE. Taxometric investigation of PTSD: Data from two nationally representative samples. Behavior Therapy. 2006;37:364–380. doi: 10.1016/j.beth.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Charuvastra A, Cloitre M. Social bonds and posttraumatic stress disorder. Annual Review of Psychology. 2008;59:301–328. doi: 10.1146/annurev.psych.58.110405.085650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MM, de Vries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: Correlates with aggression and serotonin function in personality-disordered subjects. Archives of General Psychiatry. 1998;55:708–714. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- Cohen J. The earth is round (p < .05) American Psychologist. 1994;49:997–1003. [Google Scholar]
- Cohen J. Statistical power analysis. Current Directions in Psychological Science. 1992;1:98–101. [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Wiegant VM, Westenberg HGM. Elevated plasma arginine vasopressin levels in veterans with posttraumatic stress disorder. Journal of Psychiatry Research. 2008;42:192–198. doi: 10.1016/j.jpsychires.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Delville Y, Melloni RH, Jr, Ferris C. Behavioral and neurobiological consequences of social subjugation during puberty in golden hamsters. The Journal of Neuroscience. 1998;18:2667–2672. doi: 10.1523/JNEUROSCI.18-07-02667.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. In: Neumann ID, Landgraf R, editors. Progress in Brain Research. Elsevier; Netherlands: 2008. pp. 17–27. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Facial Affect Coding System: A technique for the measurement of facial movement. Palo Alto, CA: Consulting Psychologists Press; 1978. [Google Scholar]
- Ferris CF. Adolescent stress and neural plasticity in hamsters: A vasopressin-serotonin model of inappropriate aggressive behaviour. Experimental Physiology. 2000;85S:85S–90S. doi: 10.1111/j.1469-445x.2000.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Current Opinion in Neurobiology. 2010;20:784–794. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Gouin J, Carter CS, Pournajafi-Nazarloo H, Glasser R, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35:1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB. Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biological Psychiatry. 2010;67:1220–1222. doi: 10.1016/j.biopsych.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Unkelbach C, Alvares GA, Hickie IB. Arginine vasopressin selectively enhances recognition of sexual cues in male humans. Psychoneuroendocrinology. 2011;36:294–297. doi: 10.1016/j.psyneuen.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Hanley K, Leifker FR, Blandon AY, Marshall AD. Gender differences in the impact of posttraumatic stress disorder symptoms on community couples’ intimacy behaviors. Journal of Family Psychology. doi: 10.1037/a0032890. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman RE, Sayers SL, Bellack AS. Global marital satisfaction versus marital adjustment: An empirical comparison of three measures. Journal of Family Psychology. 1994;8:432–446. [Google Scholar]
- Hyer LA, Summers MN, Boyd S, Litaker M, Boudewyns PA. Assessment of older combat veterans with the Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1996;9:587–593. doi: 10.1007/BF02103667. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus; size changes in relation to age and sex. The Journal of Clinical Endocrinology and Metabolism. 1999;84:4637–4644. doi: 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- Jones LV. Test of hypothesis: One-sided vs. two-sided alternatives. Psychological Bulletin. 1952;49:43–46. doi: 10.1037/h0056832. [DOI] [PubMed] [Google Scholar]
- Keck ME, Kern N, Erhardt A, Unschuld PG, Ising M, Binder EB, et al. Combined effects of exonic polymorphisms in CRHR1 and AVPR1B genes in a case/control study for panic disorder. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2008;147B:1196–1204. doi: 10.1002/ajmg.b.30750. [DOI] [PubMed] [Google Scholar]
- Kenny DA. The effect of nonindependence on significance testing in dyadic research. Personal Relationships. 1995;2:67–75. [Google Scholar]
- Kenny DA, Kashy DA, Cook DL. Dyadic Data Analysis. New York: Guilford; 2006. [Google Scholar]
- Kubany ES, Leisen MB, Kaplan AS, Watson SB, Haynes SN, Owens JA. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychological Assessment. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Kurdek LA. Dimensionality of the Dyadic Adjustment Scale: Evidence from heterosexual and homosexual couples. Journal of Family Psychology. 1992;6:22–35. [Google Scholar]
- Maher BS, Vladimirov VI, Latendresse SJ, Thiselton DL, McNamee R, Vanyokov MM, et al. The AVPR1A gene and substance use disorders: Association, replication, and functional evidence. Biological Psychiatry. 2011;70:519–527. doi: 10.1016/j.biopsych.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AD, Holtzworth-Munroe A. Recognition of wives’ emotional expressions: A mechanism in the relationship between psychopathology and intimate partner violence perpetration. Journal of Family Psychology. 2010;24:21–30. doi: 10.1037/a0017952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Dean M, Weinberger DR. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Molecular Psychiatry. 2009;14:968–975. doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson CM, Taft CT, Fredman SJ. Military-related PTSD and intimate relationships: From description to theory-driven research and intervention development. Clinical Psychology Review. 2009;29:707–714. doi: 10.1016/j.cpr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke FI, Waters K. Computer Facial Animation. Wellesley, MA: A K Peters; 1996. [Google Scholar]
- Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, Schatzberg AF. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Research. 2010;178:359–362. doi: 10.1016/j.psychres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Lasko NB. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Research. 1993;48:107–117. doi: 10.1016/0165-1781(93)90035-f. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. The 7th Sir F.C Bartlett Lecture. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson RR, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Shalev I, Israel S, Uzefovsky F, Gritsenko I, Kaitz M, Ebstein RP. Vasopressin needs an audience: Neuropeptide elicited stress responses are contingent upon perceived social evaluative threats. Hormones and Behavior. 2011;60:121–127. doi: 10.1016/j.yhbeh.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Sippel LM, Marshall AD. Posttraumatic stress disorder symptoms, intimate partner violence perpetration, and the mediating role of shame processing bias. Journal of Anxiety Disorders. 2011;25:903–910. doi: 10.1016/j.janxdis.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. Journal of Marriage and Family. 1976;38:15–28. [Google Scholar]
- Stanley SM, Markman HJ. Assessing commitment in personal relationships. Journal of Marriage and Family. 1992;54:595–608. [Google Scholar]
- Taft CT, Schumm JA, Marshall AD, Panuzio J, Holtzworth-Munroe A. Family-of-origin maltreatment, posttraumatic stress disorder symptoms, social information processing deficits, and relationship abuse perpetration. Journal of Abnormal Psychology. 2008;117:637–646. doi: 10.1037/0021-843X.117.3.637. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science. 2009;21:3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Terburg D, Aarts H, van Honk J. Testosterone affects gaze aversion from angry faces outside of conscious awareness. Psychological Science. 2012;23:459–463. doi: 10.1177/0956797611433336. [DOI] [PubMed] [Google Scholar]
- Terburg D, Hooiveld N, Aarts H, Kenemans JL, van Honk J. Eye tracking unconscious face-to-face confrontations: Dominance motives prolong gaze to masked angry faces. Psychological Science. 2011;22:314–319. doi: 10.1177/0956797611398492. [DOI] [PubMed] [Google Scholar]
- Thompson R, Gupta S, Miller K, Mills S, Orr S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29:35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proceedings of the National Academy of Sciences USA. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzefovsky F, Shalev I, Israel S, Knafo A, Ebstein RP. Vasopressin selectively impairs emotion recognition in men. Psychoneuroendocrinology. 2012;37:576–580. doi: 10.1016/j.psyneuen.2011.07.018. [DOI] [PubMed] [Google Scholar]
- van Londen L, Goekoop JG, van Kempen GMJ, Frankhuijzen-Seirevogel AC, Wiegant VM, van der Velde E, De Wied D. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology. 2007;17:284–292. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- Veenema A. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: What can we learn from animal models? Frontiers in Neuroendocrinology. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin: Regulation of complex social behaviours. In: Newman ID, Landgraf R, editors. Progress in Brain Research. Vol. 170. Elsevier; Netherlands: 2008. pp. 261–276. [DOI] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Lichtenstein P, et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proceedings of the National Academy of Sciences USA. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM–V. Journal of Abnormal Psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychological Assessment. 1999;11:124–133. [Google Scholar]
- Widiger TA, Clark LA. Toward DSM–V and the classification of psychopathology. Psychological Bulletin. 2000;126:946–963. doi: 10.1037/0033-2909.126.6.946. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian J, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences USA. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: New insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zink CF, Stein JL, Kempf L, Hakimi S, Meyer-Lindenberg A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. The Journal of Neuroscience. 2010;30:7017–7022. doi: 10.1523/JNEUROSCI.4899-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


