Abstract
A major quandary in memory is how hippocampal place cells, widely recognized as elements of a spatial map, contribute to episodic memory, our capacity to remember unique experiences that depends on hippocampal function. Here we recorded from hippocampal neurons as rats performed a T-maze alternation task in which they were required to remember a preceding experience over a delay in order to make a subsequent spatial choice. As has been reported previously in other variations of this task, we observed differential firing that predicted correct subsequent choices, even as the animal traversed identical locations prior to the choice. Here we also observed that most place cells also fired differently on correct as compared to error trials. Among these cells, a large majority fired strongly before the delay or during the retrieval phase but were less active or failed to activate when the animal subsequently made an error. These findings join the place cell phenomenon with episodic memory performance dependent on the hippocampus, revealing that memory accuracy can be predicted by the activation of single place cells in the hippocampus.
Keywords: hippocampus, place cells, subsequent memory effect
Several recent studies have characterized place cells in rats performing maze tasks where they are required to remember the preceding episode in order to select a correct path on the next trial (Frank et al., 2000; Wood et al., 2000; Ferbinteanu & Shapiro, 2003; Bower et al., 2005; Lee et al., 2006; Ainge et al., 2007a; Griffin et al., 2007). These studies have shown that ensembles of hippocampal neurons distinguish different routes animals take as they pass through the same places, and therefore predict past and future spatial choices. In addition, in some of these studies place cells similarly predicted routes associated with occasional mistakes (Ferbinteanu & Shapiro, 2003; Pastalkova et al., 2008). These findings are consistent with the observation that place cells can maintain their spatial firing patterns, and do so associated with behavioral choices, when critical maze cues are removed (O’Keefe & Speakman, 1987). The combined findings from these studies suggest that errors are not due to “forgetfulness”, i.e., failure to retrieve a memory representation, but rather to retrieval of the incorrect spatial representation. However, this conclusion is not consistent with the results of functional imaging studies on humans showing that a high level of hippocampal activation during encoding or retrieval predicts accurate subsequent memory, whereas subsequent errors are predicted by a low level of hippocampal activity (Brewer et al., 1998; Wagner et al., 1998). These studies suggest that errors are associated with a failure to encode or retrieve a hippocampal memory representation. Here we examined whether hippocampal activity at the level of individual neurons also predicts subsequent memory in rats performing a memory task that depends on hippocampal function.
METHODS
Subjects
The subjects were seven male Long-Evans rats weighing between 350–400g at the time of electrode implantation. The rats were allowed ad libitum access to food for the duration of the experiment, but were restricted to 30 minutes of water per day on the day before each training, testing, and recording session. If no testing or recording was to take place the following day, water was available ad libitum for 24 hrs. The rats were housed singly and kept on a 12hr/12hr light/dark cycle. Recording and testing were carried out during the light phase of the cycle, and rats were tested approximately five days per week. The experiment was conducted in accordance with guidelines set forth by the National Institutes of Health, and protocols were approved by the Boston University Charles River Campus Institutional Care and Use of Animals Committee.
Apparatus
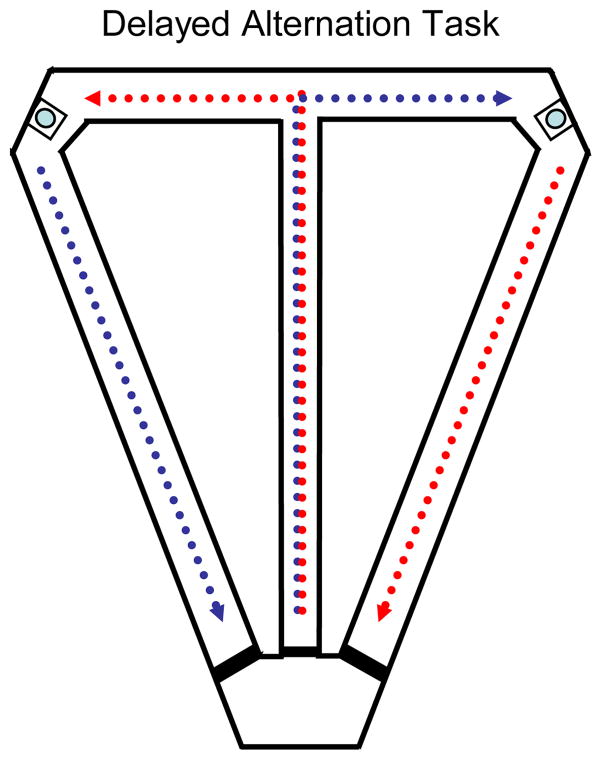
The modified T-maze apparatus used (Figure 1) was constructed of wooden runways 8 cm wide with wooden walls 2 cm high. Both the walls and floor were painted black. The central runway that comprised the stem of the T was 130 cm long, and additional wall strips were added to this portion of the maze to narrow its’ width to 7.5 cm. A crosspiece 94 cm long formed the choice arms. The distal ends of the choice arms were connected to the base of the stem by additional runways. Small Plexiglas wells (6.35 x3 6.35 cm square plaques with circular depressions of radius of 1 cm and maximum depth of 0.5 cm) were recessed into the floor at the end of each choice arm at the points marked by circles in Figure 1. Water was delivered to the wells via an 18 gauge cannula hooked up to a reservoir via tubing and under the control of solenoid valves activated by hand-operated switches. The T-maze was elevated 80 cm from the ground on pillars that had inside reverse guillotine doors for controlling the rat’s access to the arms of the maze. The maze was surrounded by black curtains on three sides (the fourth side was partially open to the remainder of the room), and several large, high-contrast, distinctive visual cues were attached to the curtains. The platform and cues remained at the same location relative to each other and to the remainder of the environment throughout the experiment.
Figure 1.
The delayed spatial alternation task. Blue: path on left to right (LR) trials; Red: path on right to left (RL) trials.
Continuous Alternation Training
Before implantation of the recording electrodes, the rats were shaped in multiple stages to perform a continuous spatial alternation task on the modified T-maze (Jung et al., 1998; Wood et al., 2000). In the first stage of training, each rat was placed at the base of the central stem of the apparatus, facing the choice arms. Clear Plexiglas barriers were placed such that the rat was forced to traverse the central stem and enter one of the choice arms. After it entered one of the arms, a small drop of water was delivered to the well in that arm. The rat was prevented from retracing its’ route on the choice arm, and so then traversed the connecting arm back to the base of the T. At this point a barrier blocked the entrance to the opposite connecting arm, forcing the animal to traverse the stem of the T again. Another barrier blocked the entrance to the previously entered arm, so the rat was then forced to enter the other choice arm, and water was delivered to the well in this arm. This procedure was repeated using barriers to direct the animal’s traversals over the stem and to alternate entries into the choice arms, until the animals ran the pattern consistently. In the second stage of training, the use of barriers at the choice point was phased out; each time the rat reached the end of the stem it could enter either arm, but it was rewarded only for alternating arm entries and was not allowed to retrace its steps. In the third stage of training, the barrier forcing the rat into the stem after returning along the connecting arms was phased out. The animals continued to run in a “figure 8”–like pattern despite no barriers, but they were prevented from retracing their steps at any point using reverse guillotine doors built into the maze.
During each subsequent training and testing session, the rats were placed on the central stem with no barriers and allowed to run 15–20 continuous trials. The experimenter remained outside the curtained enclosure throughout the session. The animal’s behavior was observed via a video monitor connected to a tracking system. On each trial when the rat made a correct (alternating) arm choice, a drop of water was delivered to the well in that arm after the arm entry. On trials when the animal made the incorrect choice, no reward was provided. Furthermore, no reward was provided even if the rat retraced its steps back to the choice point and entered the other choice arm. Instead, following mistakes the rat was required to continue along the connecting arm, reenter the stem, and make the correct choice on the following trial.
Delayed Alternation Testing
To examine hippocampal neuronal firing patterns during performance with an increased memory demand, the rats were tested on a delayed alternation version of the task immediately following the completion of 15–20 continuous alternation trials on each recording session. The delay was imposed by retaining the rat in the start area of the maze using the built-in reverse guillotine doors for 30 sec, a period much longer than what has been shown to result in deficits in rats with hippocampal lesions (Ainge and Wood, 2003). The doors were not raised until the rat reached the start area of the maze, ensuring that any differential firing during the delayed alternation task on the return arms was not a result of a visual cue provided by the raising of the doors. No food or water reward was given during the delay period. At the end of the delay, the doors were lowered, allowing the rat to leave the start area and make a free choice, with reward given only for alternating the response performed on the previous trial. If the rat made more than two sequential errors, reverse guillotine doors were raised when the rat reached the choice point, forcing a correct response. The delayed alternation trial block contained the same number of trials as was performed in the continuous trials block.
Surgery
When performance on the continuous alternation reached asymptote, a microdrive array of six 13μm tetrodes (Recce & O’Keefe, 1989; Gray et al., 1995) was implanted, aimed at area CA1 of the dorsal hippocampus. Rats were anesthetized using isoflurane delivered with 100% oxygen and placed in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA). The skull was exposed, and bregma and lambda were made level. A small hole (~1.5 mm diameter) was drilled over the hippocampus on one side of the skull for the placement of the electrode array, and six additional holes were drilled for the placement of skull screws used for electrical grounds and for securing the microdrive to the skull. The electrode array was implanted just above the dorsal hippocampus at 3.6 mm posterior to bregma, 2.4 mm lateral to bregma, and 1–1.5 mm below the surface of the brain. The cannula was coated with sterile petroleum jelly. Grip cement (Henry Schein Inc., Melville, NY) was used to secure the microdrive to the skull, and to cover the exposed skull.
Data Acquisition
Following a 7-day recovery period, daily screening for unit activity was conducted while the rats were in an opaque rectangular box (61.6 cm long × 43.8 cm wide × 40.0 cm high) that was outside of the T-maze apparatus. If pyramidal cell activity was identified (see Data Analysis), the animal was placed on the T-maze, and unit activity was recorded while the animal performed first the continuous spatial alternation task, followed by the delayed alternation task as described in the section on behavioral training. If no pyramidal cell activity was identified during screening, the rats were allowed to run a session of 20 continuous alternation trials without recording units. The electrode was advanced 40–80 μm after the session and allowed to settle overnight (at least 16 hrs) before the next recording session.
Neural activity was first passed through a multi-channel unity gain source follower field effect transistor (jFET) that was connected via a fine wire cable to the animal’s headstage. It was then passed through an overhead commutator (Biela Development Inc., Gaithersburg, MD), differentially amplified between 5,000x–10,000x (Neuralynx Inc., Tuscon, AZ), band-pass filtered from 600–6000 Hz, and digitized at 28kHz (Data Translation DT2821, Data Translation Inc., Marlboro MA) using Enhanced Discovery software (Datawave Technologies, Longmont, CO) on a Pentium-based personal computer. One wire from each tetrode was filtered from 1–400 Hz and sampled at 1 kHz to record the local field potential. For each recording session, a dedicated electrode that was driven to the corpus callosum served as a reference for differential recording. In some rats, an additional electrode was placed at the hippocampal fissure to record the electroencephalogram, and was filtered and sampled as described above.
The rats’ location was recorded using a video camera system (Datawave Technologies) by tracking two incandescent bulbs on the rats’ head, with one made brighter than the other by putting a resistor in series between the two bulbs. Position data for each light was sampled at 60 Hz and recorded as x–y coordinates. The coordinates and timestamps were saved to disk with the unit data.
Data Analysis
Unit isolation was performed off-line using Offline Sorter (Plexon, Dallas, TX). Only cells that had duration of at least 300 ms from peak to valley and a mean firing rate (total spikes divided by recording session time) of less than 2.5 Hz were analyzed. Cells with these characteristics were considered to fit the criteria for being hippocampal pyramidal neurons (Ranck, 1973). Cells that reappeared across daily sessions (that is, cells that appeared on the same wires of a tetrode, possessed similar waveforms, and had a similar place field) were included in subsequent analyses only once. Each well-isolated unit’s firing pattern in the maze was characterized using Neuroexplorer (Plexon, Dallas, TX). The maze was divided into 2.0 × 2.0 cm pixels, and firing rate within each pixel was calculated as the total number of spikes divided by the total time spent in that pixel across the entire session. Firing rate was calculated only for periods when the rat was moving at a speed greater than 2 cm/sec. Place fields were characterized as a minimum of eight contiguous pixels, meeting either at the corner or edge, with each cell’s place field having a firing rate at least three times the cell’s mean rate to be considered for further analysis. Following separation of the recording session based on experimental condition and trial type, place fields were recreated for all cells to enable visualization of the experimental manipulations.
To identify differences in firing patterns associated with continuous and delayed alternation trial blocks, correct and error trials, and disambiguation of left and right turn trials, the entire maze was segmented into 7.5 cm long sectors, with 35 sectors comprising each trial episode from one water port to the other (left to right and right to left) using a script written in MATLAB (The Mathworks, Natick, MA). A section at the top and bottom of the stem measuring 27.5 cm long was omitted from all data analysis, as the rats were turning at these points, often taking non-overlapping trajectories. All data from the entire recording session was parsed into left to right and right to left trials, error trials (left to left and right to right), and was then further separated into the appropriate condition (continuous/delay; correct/error) using time-stamped behavioral flags that were inserted via a button box into the file by the experimenter during the recording session.
Statistics
For each cell, the firing rate was calculated as the number of spikes in each bin of the maze divided by the amount of time spent in that bin. Repeated measures ANCOVA, using an equal number of trials between conditions, was used to identify arm segments associated with increased firing and to compare activity patterns between left and right turn trials for cells that fired when the rat was on the stem on correct trials, between correctly performed and error trials in the delay condition, and between the continuous and delay conditions on correct trials. A place cell was identified by a main effect of arm segment on any arm. Main effects for left-right discrimination on the stem, or trial accuracy, task, or interactions involving these conditions with arm segment, identified cells that distinguished these conditions at p < 0.05. ANCOVA, using running speed, head direction, and lateral position as covariates with firing rate in each segment, was used to confirm that any observed differences in firing patterns across all maze arms were not due to any of the covariate parameters (Wood et al., 2000). Post-hoc comparisons used paired t-tests or Welch’s two-sample t-test where applicable. Also, standard ANOVAs were used to compare firing rates across arm segments between the two behavioral tasks.
In addition, to compare the robustness with which cells distinguished the correct trials and errors, we calculated the log-likelihood ratio (Dayan and Abbott, 2001), a value that indicates the difference in firing patterns between correct trials and errors, for every neuron. The log-likelihood ratio was calculated as:
where p[r|C, x] is the probability density function of correct trials at position x, evaluated at the observed firing rate r; p[r|E, x] is the equivalent function for errors (Dayan and Abbott, 2001). To find p[r|C, x], we divided data sets from correct trials into bins and estimated the rate of each cell on each trial in each bin. We calculated the mean and variance over all trials at each value of x, and assumed that p[r|C, x] was normally distributed with this mean and variance. The function p[r|E, x] was calculated the same way from errors. For each cell, log-likelihood ratios ln {p[r|C, x]/p[r|E, x]}were summed over all segment bins for each trial. In cases where the log-likelihood sum is greater than zero, maximum likelihood analysis predicts that the data came from a correct trial; otherwise, an error is predicted. Here we report the average absolute value of the summed log-likelihood ratio; larger values of this term indicate firing-rate patterns that are statistically more distinct. A similar analysis was performed to measure the strength of differentiation of firing on the stem in left-turn and right-turn trials in the continuous and delay conditions.
In separate analyses, power in the theta band recorded from the pyramidal cell layer was computed for the period when the animal traversed an arm on each trial and compared across correct versus error conditions in the delayed alternation task using a t-test.
RESULTS
Place cells predict subsequent memory
An examination of firing patterns predicting subsequent memory success was performed on the data from delayed alternation trials. A total of 213 putative CA1 pyramidal cells were isolated, 101 of which had place fields on one or more of the straight segments of maze arms during the delayed alternation tests (Figure 1). From these cells a total of 145 place fields were distributed throughout locations on the maze, including the return arms, stem, and goal arms; our quantitative analyses focus on these place fields as independent spatial representations even when they are derived from the same cells. Notably, as previously observed (Wood et al., 2000), some cells that were active when the rat traversed the stem of the maze fired differentially depending on whether the rat came from the left side of the maze and subsequently turned right (LR trials) or came from the right and subsequently turned left (RL trials; see examples in Figure 2). Of the 31 place fields observed on the maze stem, 18 (58%) differed on correct LR versus RL trials as determined by ANCOVAs that also ruled out differences in running speed, head direction, or lateral position on the stem as potential confounds in explaining the trajectory specific activity (see below).
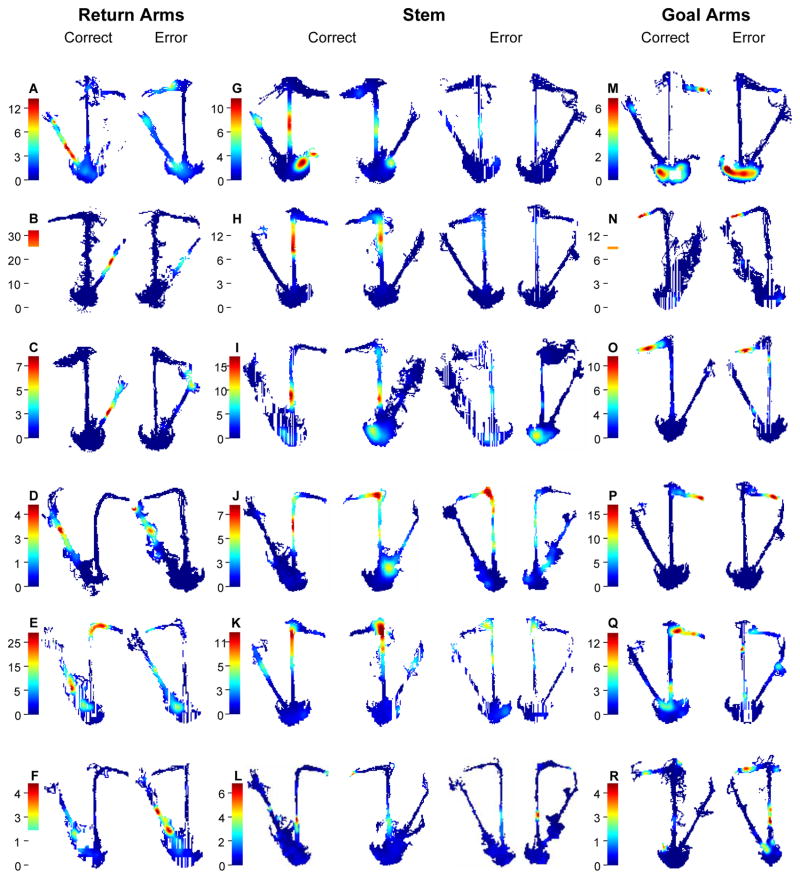
Figure 2. Spatial distributions of firing rates comparing the pattern of firing on correct trials versus errors in the delayed alternation task.
A–F. Cells that fired differentially as rats traversed different parts of a return arm. A–B. Detailed descriptions are provided in the text. For other cells, correct vs. error: C. F(1,125) = 6.15, p = 0.014; D. F(1,189) = 6.425, p = 0.01; E. F(1,71) = 14.31, p = 0.0003). F. This cell fired strongly only on errors and hardly on correct trials (F(1,179) = 8.21, p = .004).
G–L. Cells that fired differentially as rats traversed different parts of the maze stem. G–H. Detailed descriptions are provided in the text. I–L. Cells that fired as rats traversed different parts of the stem. I. This cell fired robustly on both LR and RL correct trials and hardly fired on errors (correct vs. error X segment: LR F(1,359) = 18.38, p < 0.0001; RL F(1,99) = 4.44, p = 0.00009) J. This cell fired strongly on LR but not RL correct trials and hardly fired on errors (LR correct vs. error X segment: F(1,79) = 6.323, p = .000003; RL correct vs. error X segment: F(1,39) = 2.8008, p = 0.026). K. This cell fired strongly as the rat approached the end of the stem on LR and RL correct trials and hardly fired on errors (LR correct vs. errors X segment: F(9,179) = 2.469, p = 0.011; RL correct vs. errors X segment: F(9,119) = 3.09, p = 0.00256). L. This cell had a higher firing rate when traversing the stem on LR than RL trials during correct trials, and the reverse pattern on errors (correct trials LR vs. RL X segment: F(9,119) = 2.197, p = 0.02; LR correct vs. error: F(1,79) = 16.62, p = 0.0001; RL correct vs. error X segment: F(1,39) = 4.622, p = 0.002).
M–R. Cells that fired differentially as rats traversed different parts of a goal arm. M–N. Detailed descriptions are provided in the text. O–P. Cells that fired similarly on correct and error trials as rats traversed the goal arm (correct vs. error: O. F(1,99) = 0.06, p = 0.79; P. F(1,89) = 0.06, p = 0.79). Q. A cell that fired strongly on correct trials but not errors (correct vs. errors: F(1,59) = 5.53, p = 0.022). R. This cell had a unique firing pattern on errors and hardly fired on correct trials (correct vs. error: F(1,59) = 7.43, p = 0.008).
In addition, 115 (79.3%) of the 145 place fields differed on correct trials versus errors. Nearly all (29 of 31) place fields on the stem distinguished correct versus error trials, whereas somewhat fewer (68 out of 80) place fields on the return arms and just over half (18 of 34) of the place fields on the goal arms distinguished correct trials and errors (χ2(2) = 19.82; p < 0.0001). To measure the strength of discrimination between correct trials and errors, we estimated for each place field the log-likelihood ratio, a metric whose value indicates the magnitude of the difference in firing patterns between the two conditions (see Methods). The log likelihood ratio was higher for place fields on the stem (median = 5.13; 95% CI [4.29, 8.01]) and return arms (median = 4.1; 95% CI [3.43, 5.34]) than those on the goal arms (median = 3.29; 95% CI [1.83, 3.8]; Kruskal-Wallis H(2) = 9.79, p = 0.007; Figure 3A). Post-hoc comparisons revealed the average log-likelihood ratio was significantly higher for place fields on the stem than both the return (Mann-Whitney U = 1380.0; p = .026) and goal arms (Mann-Whitney U = 361.0; p = .002), and the higher log-likelihood ratio on the return arms relative to the goal approached significance (Mann-Whitney U = 1743.0; p = .053). Thus, the activity of hippocampal place cells predicted memory success, and the predictions of accuracy were most numerous and most robust just before or after the memory delay.
Figure 3.
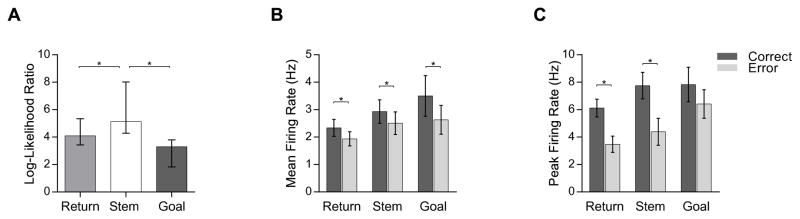
The firing patterns of hippocampal place cells distinguish correct vs. error trials. A. Median log-likelihood ratios (upper and lower 95% CI) for place fields in different maze arms measuring strength of discrimination for correct trials versus errors. Average mean (B) and average peak (C) firing rates (± 1 S.E.) for place fields in different maze arms on correct versus error trials.
The predominant pattern was that cells fired robustly on correct trials and fired at a much lower rate or were inactive on errors. Mean firing rates were significantly lower following error trials across all maze segments (Figure 3B; Return Arms: t(79) = 2.69; p = 0.008; Stem: t(56) = 3.21; p = 0.002; Goal Arms: t(33) = 2.78; p = 0.009). Similarly, peak firing rates also showed a significant reduction on error trials relative to correct trials on the return arms (t(79) = 2.09; p = 0.039) and stem (t(56) = 3.46; p = 0.001), but not the goal arms (t(33) = 2.02; p = 0.051; Figure 3C).
Of the 115 place fields that differentiated correct trials and errors, 92 (80%) involved a reduced firing rate on errors. To illustrate this phenomenon, several examples of place fields on return, stem, and goal arms are provided in Figure 2. The cell in Figure 2A fires robustly as the rat approached the end of the left return arm on correct but not error trials (correct vs. error: F(1,161) = 8.58, p = 0.004) and the cell in Figure 2B fired strongly as the rat is in the midst of the right return arm on correct trials, and much less on errors (correct vs. error: F(1,125) = 4.55, p = 0.03). Other examples of place fields on the return arm that predict accuracy of subsequent memory are shown in Figure 2, panels C–F. The cell in Figure 2G fired robustly as the animal traversed the stem on correct LRtrials, much less so on RL trials (LR vs. RL: F(1,559) = 11.40, p = 0.0007), and hardly fired on errors (correct vs. error X segment: LR F(9, 179) = 4.18, p = 0.00007; RL F(9,139) = 2.35; p = .02 ). Figure 2H shows a cell that fired strongly on both correct LR and correct RL trials (F(1,259) = 3.02, p = 0.08), but did not fire on either type of error (correct vs. error X segment: LR F(9,179) = 3.72, p = 0.0002; RL F(9,119) = 3.57, p = 0.0007). Other examples of place fields on the maze stem that distinguished correct trials and errors are provided in Figure 2, panels I–L. Figure 2M shows a cell that fired as the animal approached the right goal on correct trials but not errors (correct vs. error: F(1,69) = 6.18, p = 0.02). As noted above, many of the cells that fired when the rat traversed the goal arm do not distinguish correct trials and errors. For example, Figure 2N shows a cell that fired as the animal approached the left goal similarly on correct and error trials (correct vs. error: F(1,79) = 0.12, p = 0.73). Additional examples of place fields on the goal arm that did or did not distinguish correct trials and errors are shown in Figure 2, panels N–R. In a minority (20%) of the place fields, the spatial pattern was qualitatively different on error trials, typically involving strong spatially specific activity at a location where the activity was low or absent on correct trials (e.g., Figure 2F & R). Only one cell had a pattern of activity on error trials that was opposite to that on correct trials (Figure 2L). Thus almost no place cells fired on errors consistent with the incorrect spatial choice. Rather, in the large majority of place cells, errors were predicted by a reduced level of activity, as compared to robust activation on correct trials.
The two versions of the task involve partially overlapping spatial representations, and place cells more strongly differentiated correct trial types when a memory delay is imposed
Our protocol also allowed us to compare the activity patterns of place cells when animals were required to retain memories over a delay between trials, wherein performance depends on hippocampal function, with that when animals can continuously alternate, wherein performance is unimpaired in animals with hippocampal damage (Ainge et al., 2007b). Here we compared the firing patterns of neurons on correct trials in the two versions of the task. The number of neurons that had place fields in the delay condition (101) was not significantly different from that in the continuous condition (91; χ2(1) = 2.67, p = 0.11), and the distribution of place fields among maze arms did not significantly differ between the two conditions (χ2(2) = 0.23, p = 0.89). However, ANOVAs revealed that 75% of the place fields involved distinct firing patterns between the two conditions. These cells fired exclusively or more strongly in either the continuous or delay condition, or had qualitatively different spatial firing patterns in the two conditions (see examples in Figure 4). Differences in firing patterns between the two conditions might be due to the differences in memory demands, differences in the animals’ behavior associated with continuous running versus the requirement to stop in the delay condition, or differences in the appearance of retractable walls in the delay condition and not in the continuous condition. Regardless of the source, the striking differences indicate that the hippocampal network carries information about both the commonalities (25%) and the differences (75%) in the two testing conditions.
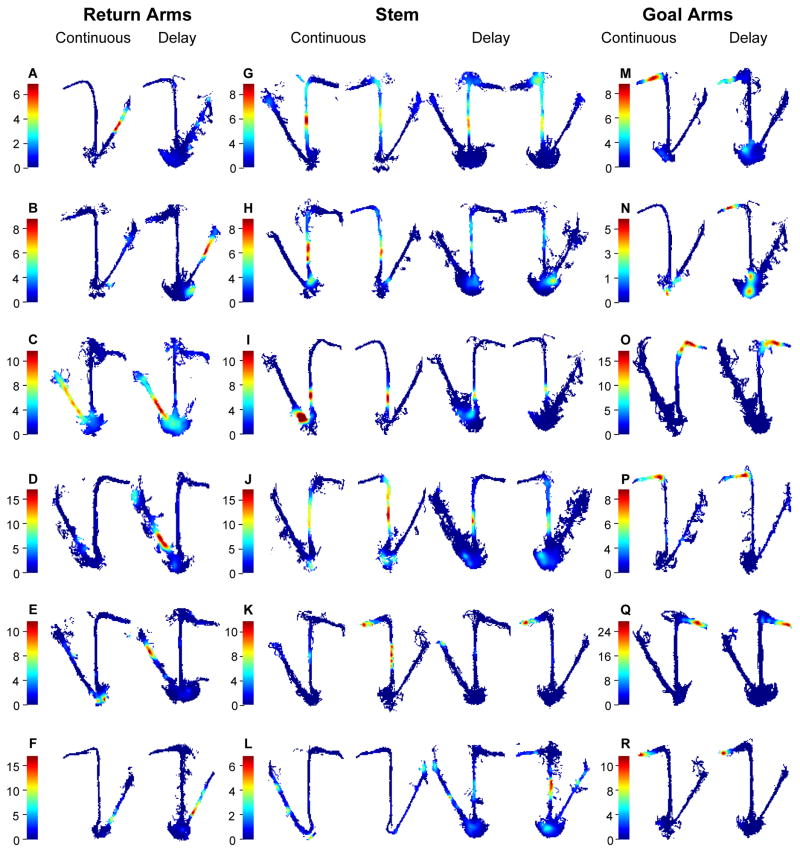
Figure 4. Examples of firing patterns observed on continuous alternation and delayed alternation (correct trials only).
A–F. Cells that fired differentially as rats traversed different parts of a return arms. A. A cell that fired as the rats was in the midst of the right return arm in the continuous task but not the delay task (F(1,269) = 16.82, p < 0.0001) B. A cell that fired as the rats was in the midst of the right return arm in the delay task but not the continuous task (F(1,251) = 60.33, p < 0.0001) C. A cell that fired strongly as the rats reached the end of the left return arm in the delay task and less so in the continuous task (F(1,233) = 14.65, p < 0.0001) D. A cell that fired strongly as the rats reached the end of the left return arm in the delay task and not in the continuous task (F(1,305) = 322.77, p < 0.0001; E. A cell that fires strongly when the rat is in the midst of the left return arm in the delay but not the continuous task (continuous vs. delay: F(1,215) = 77.28, p < 0.0001). F. Stronger firing at the end of the right return arm in the delay task but not in the continuous task (continuous vs. delay: F(1,251) = 42.95, p < 0.0001).
G–L. Cells that fired as rats traversed different parts of the stem. G. This cell had a higher firing rate when traversing the stem on LR trials in the continuous task, but the difference between continuous and delay tasks was not reliable (F(1,279) = 2.3, p = 0.13). H. This cell fired robustly in the continuous task on LR trials and less so on RL trials, and fired much less in the delay task (continuous vs. delay: F(1,779) =57.33, p < 0.0001; LR vs. RL: F(1,779) = 8.64, p = 0.003). I. This cell fired strongly on both LR and RL trials in the continuous task but did not fire in the delay task (continuous vs. delay: F(1,599) = 75.3, p < 0.0001). J. In the continuous task, this cell fired more strongly on RL than LR trials and fired less in the delay task (continuous vs. delay: F(1,1139) = 14.91, p < 0.0001; LR vs. RL X continuous vs. delay: F(2,1139) = 3.3, p = 0.04). K. A cell that fires more strongly when the rat traverses the stem on RL than LR trials in the continuous task (LR vs. RL: F(1,279) = 10.39, p = .001), and does not fire in the stem during the delay task (continuous vs. delay F(1,559) = 19.35, p < 0.0001). Note also a place field of the same cell that fires on the left goal arm similarly on the continuous and delay tasks. L. A cell that fires as the rat traverses the stem in the delay task, more so during RL than LR trials (LR vs. RL: F(1,259) = 10.252, p = .001), and does not fire in the continuous task (continuous vs. delay: F(1,519) = 5.06, p = 0.03).
M–R. Cells that fired as rats traversed different parts of the goal arms. M–O. Cells that fired differentially in the continuous and delay tasks (M. continuous vs. delay X segment F(9,129) = 3.38, p = 0.01; N. continuous vs. delay: F(1,149) = 9.15, p = 0.003;. O. continuous vs. delay: F(1,149) = 12.65, p < 0.0001). P–R. Cells that fired similarly in the two tasks (P. continuous vs. delay: F(1,159) = 1.51, p = 0.22; Q. continuous vs. delay: F(1,129) = 1.0, p = 0.32; R. continuous vs. delay F1,139 = 0.98, p = 0.32).
In addition, the log-likelihood ratios that measure the strength of discrimination between LR and RL trials for cells that fire when the rat traverses the stem were significantly higher in the delayed alternation task (median = 2.97; 95% CI [2.63, 3.53]) than the continuous alternation task (median = 1.72; 95% CI [1.09, 2.01]; Mann-Whitney U = 2.12, p < 0.0001). Thus, hippocampal neurons more strongly differentiate the two paths in the version of the task that requires hippocampal function. This result cannot be explained by differences in the demand for alternating, which was the same in both conditions, or other variables (see below), and therefore likely reflects the stronger demand for hippocampal memory function when a delay is imposed.
Prediction of subsequent memory is not attributable to differences in behavior or theta
The observed differences in hippocampal firing patterns associated with memory are not attributable to differences in overt behavior or prominence of the theta rhythm as animals traverse the maze arms. None of the cells included in these analyses showed differences in firing patterns on correct versus error trials or on continuous versus delayed alternation trials that could be attributed to differences in running speed, head direction, or lateral position on the arms, as indicated by ANCOVAs that were applied to adjust the proportion of error accounted for by all of the included factors in the analysis of each unit’s firing. A total of 6 cells that fired as the animal traversed the stem and 4 that fired as the animal traversed a return arm were rejected from analysis by the ANCOVA analyses after previously being found significant by standard ANOVA and are not considered in the data presented here. In addition, there were no consistent differences in theta (7–12Hz) power as animals traversed any of the arms on correct versus error trials in the delayed alternation task (all p values > 0.3).
Discussion
Previous studies have shown that hippocampal neuronal activity patterns and strength of population coding of study cues predict subsequent success in memory for bar presses in rats (Hampson & Deadwyler, 1996), or for words in humans (Cameron et al., 2001). Other studies specifically examining place cells have reported diminished spatial specificity in a condition of degraded spatial cues associated with diminished memory performance (Puryear et al., 2006), different firing rates in an environment where familiar cues are partially altered (Leutgeb et al., 2005), and diminished firing rates of place cells during errors when spatial strategies are switched in a spatial memory task (Bahar et al., 2011). Additional studies have shown that place cells fire in register with spatial choices (O’Keefe & Speakman, 1987; Ferbinteanu & Shapiro, 2003; Kubie et al., 2007; Pastalkova et al., 2008), can fire in anticipation of an expected reward at a goal site (Hok et al., 2007), and can acquire responses to tones associated with shock in an environment (Moita et al., 2003). However, none of these studies related the engagement of place representations to memory success or failure on specific episodes of a spatial task where performance is dependent on hippocampal function. Here we observed for the first time that the level of activation of individual place cells predicts the accuracy of memory judgments during the performance on a spatial task.
Our observation of robust differential firing of place cells associated with subsequent left and right choices as rats perform a delayed alternation, more so than in continuous alternation, contrasts with a report of almost no differential firing associated with subsequent spatial choices in a delayed alternation (Ainge et al., 2007b). The difference in findings may be due to distinctions between the training protocols and leading to different strategies used in solving the delayed alternation task. In the Ainge et al. study (2007b), recordings were taken from rats trained only in the delay task. This might have encouraged a strategy where the animals did not plan their choices until they arrived at the T-maze choice point, consistent with classic views about the use of vicarious trial and error at choice points in spatial memory tasks (Tolman, 1948; Johnson & Redish, 2007). Such a strategy would predict the absence of differential firing prior to arriving at the choice point. In contrast, in the present study rats were trained on continuous alternation then tested with and without a delay. Training on the continuous alternation might have encouraged animals to decide on their trajectories before or when they reach the base of the maze stem, leading to distinct representations of the trajectories during the entire route. The results of other studies have also varied on the extent to which hippocampal neurons distinguish paths in different T-maze tasks (Frank et al., 2000; Wood et al., 2000; Ferbinteanu & Shapiro, 2003; Bower et al., 2005; Lee et al., 2006; Ainge et al., 2007b; Griffin et al., 2007) or fire similarly regardless of subsequent choices (Lenck-Santini et al., 2001; Bower et al., 2005). While it is not fully clear what behavioral parameters result in trajectory specific firing, clearly the task conditions used here promoted the use of spatial representations that reflect the entire route on each trial type.
The gold standard in functional imaging studies on human memory is the “subsequent memory effect” - increases in activation of a brain region during encoding that predict success on subsequent memory judgments. Several studies have reported subsequent memory effects in the hippocampus and perirhinal cortex for humans performing verbal and pictoral memory tests (reviewed in Eichenbaum et al., 2007). Furthermore, other recent work has shown a strong relation between level of hippocampal activation and strength of subsequent memory (reviewed in Squire et al., 2004). Hippocampal activation in the subsequent memory effect in these studies is measured by the BOLD signal in fMRI, has been correlated with single neuron responses (Rees et al., 2000) but is best predicted by local field potentials that are dominated by dendritic activation in large neural populations (Logothetis, 2008). The present results confirm the findings from functional imaging studies at the level of the principle cells that perform the critical information processing. Furthermore, the present findings indicate that hippocampal neurons distinguish memory conditions that do and do not depend on hippocampal function, and provide insights about the nature of neural representations that underlie memory success and failure at the single cell level. Place cell activity when the rat is on the return arm may reflect encoding of where the rat is coming from prior to the memory delay, and the present findings suggest that failure to encode this information appropriately is a likely cause of subsequent memory failure. Activity during traversal of the stem just after the memory delay may reflect retrieval of the correct route, and conversely, the failure to activate these representations is another likely cause of subsequent memory failure. Activity during traversal of the goal arm was more often the same on correct and error trials, suggesting that more of the neural activity during this phase of the trial reflects the route leading to expected reward independent of memory. These observations suggest that spatial information encoded by hippocampal neurons is utilized both during the encoding and retrieval phases of memory performance and less so during the approach towards an expected reward.
Highlights.
Rats performed both continuous and delayed spatial alternation tasks
Many but not all hippocampal neurons had distinct firing patterns in the two tasks
In the delay task, place cells more strongly differentiated left and right turn trials
In the delay task, place cell activation level predicted subsequent memory accuracy
This “subsequent memory effect” complements findings in fMRI studies in humans
Acknowledgments
Supported by NIMH MH51570 & NIA AG09973
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainge JA, Tamosiunaite M, Woergoetter F, Dudchenko PA. Hippocampal CA1 cells encode intended destination on a maze with multiple choice points. J Neurosci. 2007a;27:9769–9779. doi: 10.1523/JNEUROSCI.2011-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainge JA, Matthijs van der Meer AA, Langston RF, Wood ER. Exploring the role of context-dependent hippocampal activity in spatial alternation behavior. Hippocampus. 2007b;17:988–1002. doi: 10.1002/hipo.20301. [DOI] [PubMed] [Google Scholar]
- Bahar AS, Shirvalkar PR, Shapiro ML. Memory-guided learning: CA1 and CA3 neuronal ensembles differentially encode the commonalities and differences between situations. J Neurosci. 2011;31:12270–12281. doi: 10.1523/JNEUROSCI.1671-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower MR, Euston DR, McNaughton BL. Sequential-context-dependent hippocampal activity is not necessary to learn sequences with repeated elements. J Neurosci. 2005;25:1313–1323. doi: 10.1523/JNEUROSCI.2901-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JDE. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Cameron KA, Yashaer S, Wilson CL, Fried I. Human hippocampal neurons predict how well word pairs will be remembered. Neuron. 2001;30:289–298. doi: 10.1016/s0896-6273(01)00280-x. [DOI] [PubMed] [Google Scholar]
- Dayan P, Abbott LF. Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Ann Rev Neurosci. 2007;20:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Gray CM, Maldonado PE, Wilson M, McNaughton BL. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex. J Neurosci Meth. 1995;63:43–54. doi: 10.1016/0165-0270(95)00085-2. [DOI] [PubMed] [Google Scholar]
- Griffin AL, Eichenbaum H, Hasselmo ME. Spatial representations of hippocampal CA1 neurons are modulated by behavioral context in a hippocampus-dependent memory task. J Neurosci. 2007;27:2416–2423. doi: 10.1523/JNEUROSCI.4083-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Ensemble codes involving hippocampal neurons are at risk during delayed performance tests. Proc Natl Acad Sci USA. 1996;93:13487–13493. doi: 10.1073/pnas.93.24.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok V, Lenck-Santini PP, Roux S, Save E, Muller RU, Poucet B. Goal related activity in hippocampal place cells. J Neurosci. 2007;27:472–482. doi: 10.1523/JNEUROSCI.2864-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neuronal ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubie JL, Fenton A, Novikov N, Touretzsky D, Muller RU. Changes in goal selection induced by cue conflicts are in register with predictions from changes in place cell field locations. Behav Neurosci. 2007;121:751–763. doi: 10.1037/0735-7044.121.4.751. [DOI] [PubMed] [Google Scholar]
- Lee I, Griffin AL, Zilli EA, Eichenbaum H, Hasselmo ME. Gradual translocation of spatial correlates of neuronal firing in the hippocampus toward prospective reward locations. Neuron. 2006;51:639–650. doi: 10.1016/j.neuron.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini PP, Save E, Poucet B. Place cell firing does not depend on the direction of turn in a Y-maze alternation task. Eur J Neurosci. 2001;13:1055–1058. doi: 10.1046/j.0953-816x.2001.01481.x. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in the hippocampus. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Logothetis N. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Moita MAP, Moisis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location specific location specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res. 1987;68:1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puryear CB, King M, Mizumori SJY. Specific changes in hippocampal spatial codes predict working memory performance. Behav Brain Res. 2006;169:168–175. doi: 10.1016/j.bbr.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I Behavioral correlates and firing repertoires. Experimental Neurology. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Recce ML, O’Keefe J. The tetrode: an improved technique for multi-unit extracellular recording. Soc Neurosci Abstr. 1989;15:1250. [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nature Neurosci. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Ann Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Tolman EC. Cognitive maps in rats and men. Psych Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rottee M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building Memories: Remembering and Forgetting of Verbal Experiences as Predicted by Brain Activity. Science. 1998;281:1188–119. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;2:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]