Abstract
Recent guidelines on cancer screening have given not only more screening options but also conflicting recommendations. Thus, patients, with their clinicians’ support, must decide whether to get screened or not, which modality to use, and how often to get screened. Decision aids could potentially lead to better shared decision making regarding screening between the patient and the clinician. We reviewed 73 decision aids on screening for breast, cervical, colorectal, and prostate cancers. The goal of this review was to assess the effectiveness of such decision aids, examine areas in need for more research, and determine how the decision aids can be currently applied in the real world setting. Most studies used sound study design. Significant variation existed in setting, theoretical framework, and measured outcomes. Just over a third of the decision aids included an explicit values clarification. Other than knowledge, little consistency was noted in which patient attributes were measured as outcomes. Few studies actually measured shared decision making. Little information was available on the feasibility and outcomes of integrating decision aids into practice. We discuss the implications for future research, as well as what the clinicians can do now to incorporate decision aids into their practice.
Keywords: Mass Screening, Decision Aid, Neoplasms, Decision Making
Introduction
In recent years, screening strategies for many conditions have become increasingly complex. Guidelines now recommend more options for cancer screening. Also, some guidelines have conflicting recommendations. Thus, patients, with their clinicians’ support, must decide whether to get screened or not, which modality to choose, and how often to get screened. These considerations are foundational to informing patients’ preferences, and make these decisions “preference-sensitive.” Decision aids could be an ideal tool to help the patients understand their risks of getting a particular cancer, screening options available (including a possible option of not getting screened), recommended screening time intervals, and their own values and preferences for a particular option and outcome. Consequently, decision aids have proliferated in recent years. They usually include information on the disease/condition and the associated tests/treatments, probabilities of outcomes (benefits and harms) for each test/treatment option, and some form of values clarification exercise to help the patients determine which option would best match their values. They may also include guidance or coaching in the process of decision making [1]. They are not meant to replace the discussion between the patient and his/her clinician, but rather to complement it.
Cancer screening decisions are increasingly recognized as being preference-sensitive, due to the increased recognition of harms from sequelae of screening, the need to tailor screening recommendations to the patient’s risk, multiple options available in some screening tests, and conflicting recommendations from the guidelines. The potential of harm from screening was highlighted recently when the United States Preventive Services Task Force (USPSTF) recommended against routine prostate cancer screening [2]. They made this recommendation while other guidelines had similarly weighed the benefits and harms of prostate cancer screening and instead of discouraging screening, stressed shared decision making between the patient and the clinician to decide whether to get screened or not [3]. Other cancer screenings involve preference-sensitive decisions as well, such as colorectal cancer screening, where options include stool blood test, flexible sigmoidoscopy, colonoscopy, and other modalities [4, 5]. Even screening for cancers traditionally without many options has become more complex, with some recent guidelines recommending shared decision making between the patient and clinician to determine whether to get screened for breast cancer or not for women between the ages 40 and 49 years [6], consideration of magnetic resonance imaging for women with high risk of breast cancer [7], and options of cytological testing every 3 years or cytological testing plus Human Papillomavirus testing every 5 years to screen for cervical cancer [8]. The purpose of this review is to summarize what is known about the effect of decision aids on cancer screening, and to explore areas where more information is needed to fully understand the impact of decision aids on the process and outcomes of shared decision making between the patient and the clinician.
Methods
Screened Cancers
We focused our attention on cancers for which the national guidelines recommend screening the general population [4–8]. Thus, we selected the decision aids for screening of cancers for breast, cervical, and colon/rectum. In addition, we looked at genetic testing for women considered to be at high risk for breast cancer, since it was felt to be an important option for selected high-risk women desiring further screening evaluation for breast cancer. Finally, we included prostate cancer screening in our search, since all guidelines except those by the USPSTF recommend that at least a discussion occur between the patient and clinician to decide whether screening for prostate cancer would be warranted in the particular patient [2, 3].
Study Identification
The literature search was conducted for English language articles in five databases: MEDLINE (January 1980–May 2012); Cumulative Index to Nursing and Allied Health Literature (CINAHL; January 1980–May 2012); EMBASE (1980–May 2012); Cochrane Central Register of Controlled Trials (CCRCT; updated May 2012) and Science Citation Index (SCI; January 1980–May 2012). The MEDLINE, CINAHL, EMBASE and CCRCT searches were conducted via the Ovid interface. The majority of the topical search retrieval was obtained via MEDLINE using Medical Subject Headings, including Breast Neoplasms; Colorectal Neoplasms; Uterine Cervical Neoplasms; Prostatic Neoplasms; Mass Screening; Decision Support Techniques; Decision Making; Decision Making, Computer-Assisted; and Decision Support Systems, Clinical. In addition, limited text word searching was utilized. Corresponding key word searches with Boolean syntax were conducted in CCRCT and SCI.
Theoretical Framework
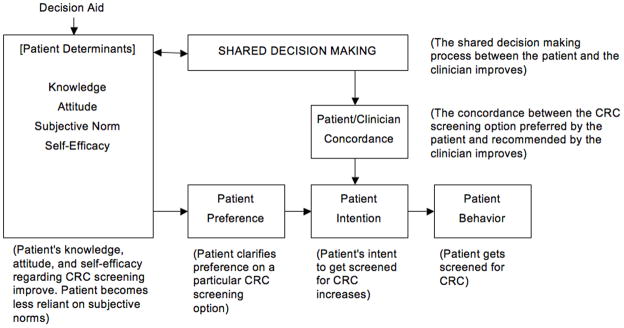
In order to provide a theoretical organizing framework for our evaluation of studies we have adapted the Integrative Model of Behavior by Frosch, which combines four theories most frequently applied in health behavior research in the past 30 years (Theory of Reasoned Action, Theory of Planned Behavior, Health Belief Model, Social Cognitive Theory) [9]. This theory combines measurable constructs of behavior (attitudes, perceived social norms, self-efficacy, behavioral intention) to the actual behavior. Because an important aspect of decision aids is the clarification of preferences and values, we have added that component, as well as how the subsequent patient/clinician discussion ensues in terms of shared decision making and patient/clinician concordance (match between the patient’s preferred screening option and the clinician’s recommended option) [10, 11]. Figure 1 illustrates our adapted framework with relevant examples. Applied to the topic of this review, use of decision aids to affect the patient’s behavior on cancer screening, this theoretical framework provides a helpful structure for understanding where decision aids intervene and exert their influence on screening behavior. It influenced the selection of decision attributes evaluated. Our analysis focused on understanding the impact of the decision aid on patient’s attributes, shared decision making, and patient/clinician concordance. Our model suggests that all of these are important to understanding the impact on patient screening behavior and determined the questions posed in the review.
Figure 1.
Procedure
Two evaluators (MJ and MR) reviewed each of the identified articles independently to determine if the study was relevant to the topic. Publications were excluded if the article was a review, an opinion article, an abstract, descriptive of a new decision aid without an intervention/trial component, or measured for usability but not for effects on patient knowledge, attitude or behavior. Also, they were excluded if the study patients had an established cancer diagnosis, since our focus was on cancer screening. For the same reason, we excluded the study if it included treatment (e.g., prophylactic mastectomy) as an option. We selected the studies if the decision aid contained information on the disease/condition and the associated tests/treatments and probabilities of outcomes (benefits and harms) for each test/treatment option [1]. We excluded studies where the intervention was provided solely through a healthcare professional (e.g., a script), since decision aids by definition are separate tools that complement the patient/clinician discussion and aid in decision making. We did not directly contact the study authors but thoroughly reviewed the relevant articles for the original and detailed description of the decision aid intervention, and when necessary, reviewed the references for the original description of the intervention. Also, we directly accessed the decision aids if available. We included pre-/post-intervention and other nonrandomized designs as well as randomized controlled design, because we wanted to be as inclusive as possible to capture innovative decision aids. For that reason, we also included pilot studies as long as they had intervention and evaluation components. A number of publications were found that were duplicate reports of a single study or serial reports from the same study. In these cases, the study was counted as one, although all pertinent publications were reviewed. The cited literature referenced in relevant studies were also examined for possible additional studies.
Study Questions/Measures
We categorized the measures into the following:
-
Does the decision aid utilized in the study address the issues important to be addressed in a screening decision aid [1]?
We determined whether the decision aid included the information on the cancer and the screening test options involved, probabilities of outcomes including benefits and harms for each option, explicit value clarifications exercise to help the patient determine which option would best match his/her values, and guidance or coaching in the process of decision making. For values clarification, we specifically looked for existence of a process (e.g., exercise) that would actively engage the patient in clarifying his/her values. For guidance or coaching, we looked for the specific ways in which the decision aid or the study addressed discussion with the clinician.
-
Does the study measure the effect of the decision aid on the patient attributes established in the theories of behavioral research?
We determined whether the study measured patient’s knowledge, attitude, perceived normative pressure, self-efficacy, preference clarification, and intent regarding the cancer and cancer screening test in question. Here, preference is different from values in that “preference” is defined as an actual preference for a certain option.
-
Does the study address the impact of the decision aid on the patient behavior in question?
We determined whether the decision aid increased or decreased the patient uptake of the particular cancer screening test, and whether it was by subjective (e.g., patient self-report) or objective (e.g., chart review) report. Also, we determined whether the completed cancer screening test was the option that the patient had originally chosen at the time of the decision aid use.
-
Does the study address the effect of the decision aid on the subsequent discussion between the patient and his/her clinician?
We determined whether the study addressed the subsequent discussion between the patient and his/her clinician (i.e., shared decision making) and whether it was affected by the decision aid use. We also determined whether there was concordance between the patient and the clinician, that is, whether they agreed on a particular cancer screening test option [11]. Additionally, we determined whether any other factors after the patient/clinician encounter (e.g., family members, media) were considered in having influenced patient’s screening behavior.
-
Does the decision aid appear to be applicable in real-world practices?
We determined the feasibility of applying the decision aid in real-world practice through the study’s setting, patient selection, and whether the decision aid was stand-alone or done in conjunction with other clinical activities. We also determined whether the study addressed the ability of the decision aid to be used in repeat screening and whether cost analysis was performed.
Determination of Outcomes
For the outcomes, only the outcomes based on intention to treat were considered when the intention to treat numbers were available. Also, where p was available, only the outcomes that were statistically significant at p<0.05 or less were considered to be meaningfully different. Outcomes were compared between groups for the studies that were randomized or factorial in design. Outcomes were compared before and after the intervention for the studies with pre-/post-intervention design.
Specific Areas Addressed Based on the Five Questions
The two evaluators independently read each publication to determine each of the following areas.
Primary Author and Year
Target Cancer for Screening
Cancer Screening Options Addressed
Target Population and Characteristics (e.g., patient, clinician or both)
Study Design
Setting (e.g., community or academic)
Follow-up Duration
-
Content of the Decision Aid
Theoretical framework
Provision of information
Risks and benefits
Values clarification exercise
Guidance on decision making and communication
Provision of a no-screening option
Discussion of when to stop screening
-
Patient Outcomes Assessed for the Decision Aid
Knowledge
Attitude
Subjective norm
Self-efficacy
Preference clarification
Intention
Screening behavior (also: whether it was self-report or observational review)
-
Patient/clinician Outcomes Assessed for the Decision Aid
Shared decision making
Concordance
-
Practice Outcomes Assessed for the Decision Aid
Post-visit factors (e.g., effect of media, family and friends)
Incorporation of the decision aid into practice (e.g., meaningful use)
Effect of the decision aid on repeated screening
Cost analysis
The evaluators met as a group to review their classifications, discussed any disagreements, and arrived at a consensus of opinion for all studies.
Results
Seventy-nine studies were identified that evaluated 73 decision aids meeting the criteria outlined above. Only two decision aids dealt with cervical cancer screening [12, 13]. Eighteen decision aids dealt with breast cancer screening, of which nine concerned mammography for the general population [14–23] and nine concerned genetic testing for those considered to be at high risk for breast cancer based on family history and other information [24–33]. Twenty-one decision aids dealt with colorectal cancer screening [34–54]. Twenty-nine decision aids dealt with prostate cancer screening [55–87]. Two decision aids dealt with both colorectal and prostate cancer screening [88, 89], and one decision aid dealt with all four cancer screenings: breast, cervical, colorectal, and prostate [90].
Characteristics of the Identified Studies
Table 1 summarizes the characteristics of the identified studies. For breast and prostate cancers, screening options were a single test, namely mammogram [14–23] or genetic (BRCA) testing [24–33] and prostate specific antigen (PSA) [55–87], respectively. The breast self-examination and clinical breast examination in one decision aid on mammogram screening were not considered options but rather came as a set with the mammogram [14]. Similarly, digital rectal examination and PSA in twelve of the 29 decision aids on prostate cancer screening were not options but considered as a set [58, 59, 61, 65, 69, 73, 75, 77–79, 82, 87]. The two decision aids on cervical cancer screening also dealt with a single test, cervical cytology (Papanicolaou smear) [12, 13]. Decision aids for colorectal cancer screening were the only ones that considered choosing among multiple screening test options. In these cases, options varied from just one (n=4 of 21 decision aids) [37, 45, 47, 49] to two (n=4) [39, 43, 48, 53] to three or more (n=13) [34–36, 38, 40–42, 44, 46, 50–52, 54]. One study explicitly compared a two-option decision aid, namely stool blood test and colonoscopy, to a five-option decision aid, which options included stool blood test, flexible sigmoidoscopy, stool blood test and flexible sigmoidoscopy, barium enema, and colonoscopy [41]. Of note, the newer screening tests of magnetic resonance imaging for women at high risk for breast cancer, human papilloma virus testing for cervical cancer screening, and computer tomography colonography and stool DNA testing for colorectal cancer screening have not yet been incorporated in the published decision aids.
Table 1.
Characteristics of Identified Studies on Decision Aids
| Author & Year* | Screening Options | Target Population** | Design | Setting | Follow-up Duration |
|---|---|---|---|---|---|
| Breast Cancer Mammogram Screening (n=9) | |||||
| Kadison 1998 [14] | BSE, CBE, MMG | Women aged 22–75 years | Longitudinal uncontrolled study: Interactive voice response risk assessment (initially n=343; follow-up n=189) | 2 companies in US | 8 months |
| Street 1998 [15] | MMG | Women aged 40–75 years | RCT: Computer-based multimedia DA (n=54) vs. print DA (n=54) | 2 primary care clinics in US | Immediate |
| Lawrence 2000 [16] | MMG | Women aged 49–89 years | One-time uncontrolled intervention: Print DA (n=103) | 1 medical school, 1primary care clinic, & 1 community center in US | Immediate |
| Valdez 2001 [17] | MMG | Hispanic women aged ≥40 years | Parallel-group randomized experimental design (pre- vs. post): Computer kiosk-based DA (n=269) | 5 clinics and 1 community-based organization in US | 4 months |
| Rimer 2001 [18], 2002 [19] | MMG | Women aged 40–44 and 50–54 years | 3-arm RCT: Tailored print newsletter + telephone counseling (n=339) + tailored print newsletter (n=374) + usual care (n=378) | 1 state-based health insurance membership in US | 24 months |
| Lewis 2003 [20] | MMG | Women aged 35–49 years | 3-arm RCT: Positive video (n=64) vs. neutral video (n=54) vs. negative video (n=60) | University-based general medicine clinic in US | Immediate |
| Mathieu 2007 [21] | MMG | Women aged 70–71 years | RCT: Print DA (n=367) vs. Usual care (n=367) | Communities in Australia | 1 month |
| Vernon 2008 [22] | MMG | Women aged ≥52 years | 3-arm RCT: Tailored print + targeted print intervention (n=1803) + targeted print intervention only (n=1857) + usual care (n=1840) | National veteran registry in US | 2 years |
| Mathieu 2010 [23] | MMG | Women aged 38–45 years | RCT: Immediate web-based DA (n=189) vs. delayed web-based DA (n=223) | Online recruitment in Australia | Immediate |
| Breast Cancer Genetic Testing (n=9) | |||||
| Lerman 1997 [24] | BRCA testing | Women aged 18–75 years with family history of breast or ovarian cancer | 3-arm RCT: Print DA + counseling (n=122) + print DA (n=114) only vs. waiting list control (n=164) | 2 cancer centers in US | 1 month |
| Green 2001 [25] | BRCA testing | Women aged 19–59 years with family history of breast cancer | 3-arm RCT: Interactive, multi-media CD-ROM DA + counseling (n=29) vs. counseling (n=29) vs. usual care (n=14) | 1 federal research facility in US | Immediate |
| Schwartz 2001 [26] | BRCA testing | Ashkenazi Jewish women aged 18–83 years | RCT: Print DA (n=191) vs. Usual care (n=190) | Religious organization in | 1 month |
| Green 2004 [27], 2005 [28] | BRCA testing | Women aged 24–77 years with personal or family history of breast cancer | RCT: Interactive, multi-media CD-ROM DA + counseling (n=106) vs. counseling(n=105) | 5 university hospitals & 1 community hospital in US | 6 months |
| Miller 2005 [29] | BRCA testing | Women aged ≥18 years | RCT: Print DA vs. usual care (total n=279) | 1 federal research facility in US | 6 months |
| Wang 2005 [30] | BRCA testing | Women aged 22–76 years | 2×2 factorial design: CD-ROM DA + counselor feedback (n=50) vs. CD-ROM DA only (n=50) vs. counselor feedback only (n=49) vs. usual care (n=48) | 1 university-based cancer clinic in US | Immediate |
| Wakefield 2008a [31] | BRCA testing | Women aged ≥18 years with family history of breast/ovarian cancer | RCT: Print DA (n=73) vs. Control pamphlet (n=72) | 5 cancer clinics in Australia | 6 months |
| Wakefield 2008b [32] | BRCA testing | Women aged ≥18 years with family history of breast/ovarian cancer | RCT: Detailed print DA (n=73) vs. Contorl pamphlet (n=75) | 5 cancer clinics in Australia | 6 months |
| Gray 2009 [33] | BRCA testing | Women aged 18–70 years with personal/family history of breast or ovarian cancer | 3-arm RCT: Website with risk information on BRCA testing attributed to experts (n=98) vs. not attributed (n=93) vs. no risk information (n=93) | 1 university-based research facility in US | Immediate |
| Cervical Cancer Screening (n=2) | |||||
| Adab 2003 [12] | Cervical cytology | Women aged 20–64 years | RCT: Leaflet with risks & uncertainties (n=155) vs. standard leaflet (n=145) | 3 general practices in United Kingdom | Immediate |
| Park 2005 [13] | Cervical cytology | Women of unknown ages | Nonequivalent, control group, post-test only design: DA (n=48) vs. usual care (n=48) | 1 church in Korea | Immediate |
| Colorectal Cancer Screening (n=21) | |||||
| Pignone 2000 [34] | SBT, FS, SBT+FS | Men & women aged 50–75 years | RCT: Video DA (n=125) vs. usual care (n=124) | 3 community primary care practices in US | 3–6 months |
| Wolf 2000 [35] | SBT, FS, SBT+FS | Men & women ≥65 years | 3-arm RCT: Absolute risk script (n=136) vs. relative risk script (n=130) vs. control script (n=133) | 4 general internal medicine practices (1 university, 3 community) in US | Immediate |
| Dolan 2002 [36] | SBT, FS, SBT+FS, BE, COL | Men & women aged 50–83 years | RCT: Print DA (n=50) vs. Usual care (n=47) | 1 community and 1 university-based internal medicine clinc in US | Immediate |
| Zapka 2004 [37] | FS | Men & women aged 50–74 years | RCT: Educational video (n=450) + mailing vs. no video (n=488) | 5 primary care practices in US | 6 months |
| Jerant 2007 [38] | SBT, FS, COL | Men & women aged ≥50 years | RCT: Tailored multimedia computer program (n=24) vs. non-tailored program (n=25) | 6 community family practices in US | Immediate |
| Myers 2007 [39] | SBT, SBT+FS | Men & women aged 50–74 years | 4-arm RCT: Tailored print + phone counseling (n=386) vs. tailored print (n=386) vs. non-tailored print (n=387) vs. usual care (n=387) | 1 university-based family practice in US | 24 months |
| Ruffin 2007 [40] | SBT, FS, SBT+FS, BE, COL | Men & women never screened for CRC, aged 50–70 years | RCT: Interactive website (n=87)vs. standard website (n=87) | 3 communities (urban, suburban, rural) in US | 24 weeks |
| Griffith 2008a [41] | SBT, FS, SBT+FS, BE, COL (SBT, COL in 2-option) | Men & women aged 48–75 years | RCT: 5-option DVD DA (n=25) vs. 2-option DVD DA (n=37) | 1 university-based research facility in US | Immediate |
| Griffith 2008b [42] | SBT, FS, SBT+FS, BE, COL | Men & women aged 50–85 years | RCT: 5-option + no screening option video DA (n=57) vs. 5-option video DA (n=49) | 3 communities in US | Immediate |
| Katsumura 2008 [43] | SBT, COL | Men & women aged 40–59 years | RCT: Internet-based information + risk information (n=146) vs. internet-based information only (n=139) | 1 internet community in Japan | Immediate |
| Lewis 2008 [44] | SBT, FS, COL | Men & women aged 50–75 years | RCT: Mailed print DA (n=137) + waiting list control (n=100) | University-based general medicine clinic in US | 5 months |
| Trevena 2008 [45] | SBT | Men & women aged 50–74 years | RCT Print DA (n=157) vs. government guidelines (n=157) | 6 community family practices in Australia | 1 month |
| Makoul 2009 [46] | SBT, FS, COL | Hispanic men & women aged 50–80 years | Pre-test/post-test design: Computer kiosk DA (n=270) | 2 community clinics in US | Immediate |
| Manne 2009 [47] | COL | Men & women with family history of CRC | 3-arm RCT: Tailored print + telephone counseling (n=112) vs. tailored print (n=161) vs. standard print (n=139) | 26 medical centers in US | 6 months |
| Lewis 2010 [48] | SBT, COL | Men & women aged 75–95 years | One-time uncontrolled intervention: Print DA (n=46) | 1 senior center in US | Immediate |
| Smith 2010 [49] | SBT | Men & women with low educational attainment, aged 55–64 years | 3-arm RCT: Print & DVD DA + question prompt list (n=196) vs. print & DVD DA only (n=188) vs. standard information (n=188) | Community in Australia | 3 months |
| Miller 2011 [50] | SBT, FS, COL | Men & women aged 50–74 years | RCT: Web-based DA (n=132) vs. usual care (n=132) | 1 community internal medicine clinic in US | 24 weeks |
| Pignone 2011 [51] | SBT, FS, SBT+FS, BE, COL | Men & women aged 52–80 years | Clustered RCT: DVD/VHS DA (n=211) + academic detailing of practices vs. usual care (n=232) | 32 primary care practices participating in a single health insurance plan in US | 12 months |
| Schroy 2011 [52] | SBT, FS, SBT+FS, BE, COL | Men & women aged 50–75 years | 3-arm RCT: DVD DA + personalized risk assessment (n=223) vs. DVD DA (n=212) vs. usual care (n=231) | 1 university-based internal medicine clinic & 1 community health center in US | Immediate |
| Steckelberg 2011 [53] | SBT, COL | Men & women aged 50–75 years | RCT: Print DA with risk information (n=785) vs. print standard information (n=792) | Health insurance membership in Germany | 6 months |
| Vernon 2011 [54] | SBT, FS, BE, COL | Men & women aged 50–70 years | 3-arm RCT: Tailored website (n=413) vs. non-tailored website (n=398) vs. usual care (n=413) | 1 university-based clinic in US | 24 months |
| Prostate Cancer Screening (n=29) | |||||
| Flood 1996 [55] | PSA | Men aged ≥50 years | RCT: Educational videotape (n=184) vs. control videotape (n=188) | 1 university hospital in US (free screening program and clinic) | Immediate |
| Wolf 1996 [56], 1998 [57] | PSA | Men aged ≥50 years | RCT: Scripted DA (n=103) vs. usual care (n=102) | 4 university-affiliated primary care practices in US | Immediate |
| Myers 1999 [58] | PSA, DRE | Men aged 40–70 years | RCT: Print information + tailored information (n=192) vs. print information (n=221) only | 1 university-based clinic in US | 1 year |
| Schapira 2000 [59] | PSA, DRE | Men aged 50–80 years | RCT: Print DA with detailed risk description (n=122) vs. print information without (n=135) | 1 VA clinic in US | 2 weeks |
| Frosch 2001 [60] | PSA | Men aged ≥50 years | 2×2 factorial design: Shared decision making video + discussion on risks and benefits (n=42) vs. video only (n=46) vs. discussion only (n=45) vs. usual care (n=43) | 1 community hospital in US | Immediate |
| Wilt 2001 [61] | PSA, DRE | Men aged ≥50 years | RCT: Mailed print DA (n=180) + survey vs. usual care (n=195) | 1 VA primary care clinic in US | 1 year |
| Volk 1999 [62], 2003 [63] | PSA | Men aged 45–70 years | RCT: Video DA before doctor visit (n=80) vs. information booklet 2 weeks after doctor visit (n=80) | 1 university-based family medicine clinic | 1 year |
| Frosch 2003 [64] | PSA | Men aged ≥50 years | RCT: Web-based DA (n=114) vs. video DA (n=112) | 1 community hospital in US | Immediate |
| Gattellari 2003 [65] | PSA, DRE | Men aged 40–70 years | RCT: Print DA (n=126) vs. conventional pamphlet (n=122) | 13 general practices in Australia | 3 days |
| Ruthman 2004 [66] | PSA | Men aged 50–80 years | Staged 2-group pre-/post-test quasi-experimental design: Video DA (n=52) vs. usual care (n=52) | 1 VA clinic in US | Immediate |
| Sheridan 2004 [67] | PSA | Men aged 45–85 years | One-time uncontrolled intervention with pre-/post-tests: print DA (n=188) | 1 university-based internal medicine clinic in US | Immediate |
| Gattellari 2005 [68] | PSA | Men aged 50–70 years | 3-arm RCT: Video DA (n=141) vs. print DA (n=140) vs. conventional leaflet (n=140) | 1 large community in Australia | ≥7 days |
| Myers 2005 [69] | PSA, DRE | African American aged 40–69 years | RCT: Print DA + educational session (n=121) vs. print DA only (n=121) | 3 community primary care practices in US | 6–11 months |
| Partin 2004 [70], 2006 [71] | PSA | Men aged ≥50 years | 3-arm RCT: Video DA (n=308) vs. print DA (n=295) vs. usual care (n=290) | 4 VA clinics in US | 1 year |
| Watson 2006 [72] | PSA | Men aged 40–75 years | RCT: Print DA + survey (n=980) vs. usual care (n=980) | 11 general practices in United Kingdom | Immediate |
| Kripalani 2007 [73] | PSA, DRE | Men aged 45–70 years | 3-arm RCT: Detailed educational print (n=101) vs. simple educational print (n=101) vs. usual care (n=101) | 1 academic teaching hospital in US | Immediate |
| Krist 2007 [74] | PSA | Men aged 50–70 years | 3-arm RCT: Web-based DA (n=226) vs. print DA (n=196) vs. usual care (n=76) | 1 community family practice in US | 2 weeks |
| Ellison 2008 [75] | PSA, DRE | African American men aged 40–65 years | RCT: Web-based DA tailored to family history of prostate cancer (n=46) vs. web-based non-tailored DA (n=41) | 1 annual mason convention in US | Immediate |
| Ilic 2008 [76] | PSA | Men never screened for prostate cancer, aged >45 years | 3-arm RCT: Web-based education (n=56) vs. video-based education (n=55) vs. print-based education (n=50) | 5 states in Australia | 1 week |
| Stephens 2008 [77] | PSA, DRE | African American men aged 40–70 years and non-African American men aged 50–70 years | Solomon 4-group design: Pre-test + print DA + post-DA process measures + post-test (n=50) vs. DA booklet + post-DA process measures + post-test (n=50) vs. pre-test + post-test (n=50) vs. post-test only (n=50) | 10 urban professional research facilities in US | Immediate |
| Volk 2008 [78] | PSA, DRE | African American men aged 40–70 years, non-African American men aged 50–70 years | RCT: Computer-based interactive DA (n=224) vs. print DA + CD (n=226) | 2 primary care clinics in US | 2 weeks |
| Weinrich 2008 [79] | PSA, DRE | African American men aged 40–70 years, Caucasian men aged 50–70 years | Post-intervention, quasi-experimental design: Enhanced DA (print DA + physician and peer pictures and statements; n=120) vs. print DA only (n=110) | 4 urban communities in US | Immediate |
| Frosch 2008 [80], (Bhatnagar 2009 [81]) | PSA | Men aged ≥50 years | 2×2 factorial design: Didactic DA + chronic disease trajectory (n=152) vs. DA only (n=155) vs. chronic disease trajectory only (n=153) vs. control (public websites on prostate cancer; n=151) | 1 community hospital in US | 2–3 weeks |
| Allen 2009 [82] | PSA, DRE | African American men ≥50 years | Pre-/post-test quasi-experimental design: Computerized-tailored DA (n=108) | Multiple community settings in US | Immediate |
| Allen 2010 [83] | PSA | Men aged ≥45 years | RCT: Computerized-tailored DA (n=398) vs. no intervention (n=414) | 12 work sites in US | 3 months |
| Joseph-Williams 2010 [84] | PSA | Men never screened with PSA, aged 50–75 years | 4-arm RCT: Web-based DA (n=129) vs. print DA (n=126) vs. surveys only (n=127) vs. usual care (n=132) | 1 community in United Kingdom | 6 months |
| Rubel 2010 [85] | PSA | Non-African American men aged 50–70 years | Solomon 4-group design: Pre-test + print DA + post-test (n=50) vs. print DA + post-test (n=50) vs. pre-test + post-test (n=50) vs. post-test only (n=50) | 5 professional research facilities in US | Immediate |
| Van Vugt 2010 [86] | PSA | Men never screened with PSA, aged 55–65 years | One-time uncontrolled intervention with pre-/post-tests: print DA (n=729) | 1 city in Netherlands | Immediate |
| Capik 2012 [87] | PSA, DRE | Turkish men aged 41–65 years | Pre-/post-test longitudinal study: web-assisted education and reminders (n=110) | 2 public institutions in Turkey | 6 months |
| Multiple Cancer Screening (n=3) | |||||
| Frosch 2008 [88] | Prostate: PSA; colorectal: not specified | Men and women aged ≥50 years except African American men, who were aged ≥45 years | Sequential distribution of information brochure and video DA: video DA (n=100) vs. information brochure (n=107) | 13 community-based primary care practices in US | Immediate |
| Brackett 2010 [89] | Prostate: PSA; colorectal: not specified | Men aged 50–75 years for prostate cancer; men & women aged 50–75 years for CRC | 4 video DA distribution methods: Automatic pre-visit DA mailing (n=1625), pre-visit video DA mailing upon request (n=84), post-visit video DA offered by medical assistant (n=724), post-visit video DA offered by physician (n=52) | 1 rural university hospital and 1 rural VA hospital in US | Immediate |
| Krist 2012 [90] | Prostate: PSA; breast, cervical, colorectal: not specified | Men & women aged 18–75 years | RCT: Interactive preventive health record that includes DAs pertinent to the patient’s indicated cancer screening (n=2250) vs. usual care (n=2250) | 8 primary care practices in US | 16 months |
Abbreviations:
BE: Barium Enema
BSE: Breast Self-Examination
CBE: Clinical Breast Examination
COL: Colonoscopy
CRC: Colorectal Cancer
DA: Decision Aid
DRE: Digital Rectal Examination
FS: Flexible Sigmoidoscopy
MMG: Mammogram
PSA: Prostate Specific Antigen
RCT: Randomized Controlled Trial
SBT: Stool Blood Test
US: United States
VA: Veterans Administration
Listed in ascending order of the year of publication for each cancer.
All target populations were patients, not clinicians.
Content of Decision Aids
Tables 2 summarizes the content of the decision aids used in the studies. Most decision aids utilized a theoretical framework (n=41 of 73 decision aids), particularly the transtheoretical model (n=11) [15, 18, 19, 22, 34, 38, 41, 42, 47, 50, 51, 54] and the Ottawa decision support framework (n=8) [21, 23, 31, 32, 48, 52, 82, 83]. Interestingly, most decision aids on prostate cancer screening (n=19 of 29 decision aids) [55–57, 60–68, 72–78, 84–86] and all decision aids on multiple cancer screening (n=3) [89–90] did not incorporate a theoretical framework. A few had adopted a formative approach (e.g., interviews, focus groups, and expert feedback; n=24 of 73 decision aids) [16, 17, 22, 29, 31, 32, 34, 36, 40, 42, 44, 46, 48, 52, 53, 55, 59, 60, 62, 63, 70, 71, 83, 88, 89], but others seemed to have moved relatively quickly from literature and expert review to creation of the tool and pre-testing.
Table 2.
Content of Decision Aids
| Author & Year* | Theoretical Framework | Description of Development | Provision of Information | Risks & Benefits | Values Clarification Exercise | Guidance on Decision Making & Communication | Addresses Option of No Screening | Addresses When to Stop Screening |
|---|---|---|---|---|---|---|---|---|
| Breast Cancer Mammogram Screening (n=9) | ||||||||
| Kadison 1998 [14] | None | Literature review, peer-review, pre-test | Yes | No | No | Yes | No | No |
| Street 1998 [15] | Transtheoretical model, elaboration likelihood model | Pilot test | Yes | No | No | No | No | No |
| Lawrence 2000 [16] | None | Content development by multidisciplinary team and lay women; reliability & validity testing | Yes | Yes | No | No | No | No |
| Valdez 2001 [17] | Social learning theory | Expert consultation, key informant interviews, focus groups | Yes | No | No | Yes | No | No |
| Rimer 2001 [18], 2002 [19] | Transtheoretical model, precaution adoption process model | Tailored messages based on baseline survey findings | Yes | Yes | Yes | Yes | Yes | No |
| Lewis 2003 [20] | None | Literature review, pre-test | Yes | Yes | No | No | Yes | No |
| Mathieu 2007 [21] | Ottawa decision support framework | Markov model, pilot test | Yes | Yes | Yes | Yes | Yes | Yes |
| Vernon 2008 [22] | Transtheoretical model, health belief model, social cognitive theory, theory of planned behavior | Targeted: Focus groups Tailored: Tailored messages based on baseline survey findings |
Yes | Yes | Yes | Yes | No | No |
| Mathieu 2010 [23] | Ottawa decision support framework | Markov model, pilot test | Yes | Yes | Yes | Yes | Yes | No |
| Breast Cancer Genetic Testing (n=9) | ||||||||
| Lerman 1997 [24] | Behavioral models of decision-making | Structural protocol | Yes | Yes | No | No | Yes | No |
| Green 2001 [25] | None | Provides same information as the genetic counselors | Yes | Yes | Yes | Yes | Yes | No |
| Schwartz 2001 [26] | None | N/A | Yes | Yes | No | No | Yes | No |
| Green 2004 [27], 2005 [28] | None | Provides same information as the genetic counselors | Yes | Yes | Yes | No | Yes | No |
| Miller 2005 [29] | Cognitive-social health processing model | Formative evaluation: Interviews, focus groups | Yes | Yes | No | Yes | Yes | No |
| Wang 2005 [30] | None | Collaboration between content experts and health-related media experts | Yes | Yes | No | Yes | Yes | No |
| Wakefield 2008a [31] | Ottawa decision support framework | Content analysis, pilot test | Yes | Yes | Yes | Yes | Yes | No |
| Wakefield 2008b [32] | Ottawa decision support framework | Content analysis, pilot test | Yes | Yes | Yes | Yes | Yes | No |
| Gray 2009 [33] | Cognitive-social theory | Literature review, expert consultation, pre-test | Yes | Yes | No | No | Yes | No |
| Cervical Cancer Screening (n=2) | ||||||||
| Adab 2003 [12] | None | Added information on risks and uncertainties to the National Health Service Cervical Screening Programme leaflet | Yes | Yes | No | No | No | No |
| Park 2005 [13] | Health belief model, theory of reasoned action, self-efficacy theory | Developed from focus groups | Yes | Yes | No | No | No | No |
| Colorectal Cancer Screening (n=21) | ||||||||
| Pignone 2000 [34] | Transtheoretical model | Patient preference checking, focus groups | Yes | Yes | No | Yes | No | No |
| Wolf 2000 [35] | None | Physician panel, pilot testing | Yes | Yes | No | No | Yes | No |
| Dolan 2002 [36] | Analytic hierarchy process | Structured interviews, feasibility testing | Yes | Yes | Yes | Yes | Yes | No |
| Zapka 2004 [37] | PRECEDE/PROCEED model, social cognitive theory | Literature review | Yes | Yes | No | No | No | No |
| Jerant 2007 [38] | Transtheoretical model | Personally tailored feedback messages | Yes | Yes | Yes | No | No | No |
| Myers 2007 [39] | Preventive health model | Tailored messages based on baseline survey findings | Yes | Yes | Yes | No | No | No |
| Ruffin 2007 [40] | Elaboration likelihood model | Developed empirically from 10 focus groups and 30 patient interviews | Yes | Yes | Yes | Yes | No | No |
| Griffith 2008a [41] | Transtheoretical model | Previous DA, literature review, usability testing | Yes | Yes | No | Yes | No | No |
| Griffith 2008b [42] | Transtheoretical model | Previous DA, literature review, focus groups, expert/patient review | Yes | Yes | No | Yes | Yes | No |
| Katsumura 2008 [43] | Analytic hierarchy process | Construction of decision model | Yes | Yes | Yes | No | No | No |
| Lewis 2008 [44] | None Decision Aid Standard | Literature review, patient focus groups, patient & expert review | Yes | Yes | No | Yes | No | No |
| Trevena 2008 [45] | Theory of planned behavior | Incorporated research-derived expert & lay beliefs | Yes | Yes | Yes | Yes | No | No |
| Makoul 2009 [46] | Extended parallel process model | Patient interviews & focus groups, usability testing | Yes | Yes | No | Yes | No | No |
| Manne 2009 [47] | Health belief model, transtheoretical model, dual process theory | Construction of tailored messages | Yes | Yes | No | No | No | No |
| Lewis 2010 [48] | Ottawa decision support framework | Literature review, patient interviews, cognitive testing | Yes | Yes | Yes | No | Yes | No |
| Smith 2010 [49] | None | Specific design for adults with low literacy skills, using plain language and basic design, focus groups | Yes | Yes | Yes | Yes | Yes | No |
| Miller 2011 [50] | Transtheoretical model | Previous DA, literature review, usability testing | Yes | Yes | No | Yes | No | No |
| Pignone 2011 [51] | Transtheoretical model | Previous DA, literature review, usability testing | Yes | Yes | No | Yes | No | No |
| Schroy 2011 [52] | Ottawa decision support framework | Literature review, existing DA review, expert opinion, focus groups, usability testing | Yes | Yes | Yes | Yes | No | No |
| Steckelberg 2011 [53] | United Kingdom Medical Research Council framework for complex intervention | Literature review, focus groups, expert review | Yes | Yes | No | No | Yes | No |
| Vernon 2011 [54] | Transtheoretical model | Intervention mapping, incorporating theory and empiric evidence | Yes | Yes | Yes | No | No | No |
| Prostate Cancer Screening (n=29) | ||||||||
| Flood 1996 [55] | None | Literature review, patient focus groups, patient & expert review | Yes | Yes | No | Yes | Yes | No |
| Wolf 1996 [56], 1998 [57] | None | Physician panel review, pilot testing | Yes | Yes | No | No | Yes | Yes |
| Myers 1999 [58] | Preventive health model | Tailored messages based on baseline survey findings | Yes | Yes | Yes | Yes | Yes | No |
| Schapira 2000 [59] | Health belief model | Focus groups | Yes | Yes | No | No | Yes | No |
| Frosch 2001 [60] | None | Literature review, patient focus groups, patient & expert review | Yes | Yes | No | Yes | Yes | No |
| Wilt 2001 [61] | None | Expert review, content validity check, readability pre-testing | Yes | Yes | No | No | Yes | No |
| Volk 1999 [62], 2003 [63] | None | Literature review, patient focus groups, patient & expert review | Yes | Yes | No | Yes | Yes | No |
| Frosch 2003 [64] | None | Conversion of a video DA (Frosch 2001) to web-based DA | Yes | Yes | No | Yes | Yes | No |
| Gattellari 2003 [65] | None | Literature review, pilot testing | Yes | Yes | Yes | Yes | Yes | No |
| Ruthman 2004 [66] | None | Literature review, patient focus groups, patient & expert review | Yes | Yes | No | Yes | Yes | No |
| Sheridan 2004 [67] | None | Literature review, cognitive interviewing and feedback | Yes | Yes | No | No | Yes | No |
| Gattellari 2005 [68] | None | Literature review, pilot testing | Yes | Yes | Yes | Yes | Yes | No |
| Myers 2005 [69] | Preventive health model | Tailored messages based on baseline survey findings | Yes | Yes | Yes | Yes | Yes | No |
| Partin 2004 [70], 2006 [71] | Social cognitive theory | Literature review, patient focus groups, patient & expert review | Yes | Yes | No | Yes | Yes | No |
| Watson 2006 [72] | None | Expert review, field testing | Yes | Yes | No | Yes | Yes | No |
| Kripalani 2007 [73] | None | Multi-disciplinary team design, pilot testing | Yes | Yes | No | Yes | Yes | No |
| Krist 2007 [74] | None | Expert review | Yes | Yes | No | No | No | No |
| Ellison 2008 [75] | None | Based on Cochrane Review’s definition of DA | Yes | Yes | No | No | Yes | No |
| Ilic 2008 [76] | None | N/A | Yes | Yes | No | No | Yes | No |
| Stephens 2008 [77] | Prostate cancer screening decisional conflict model | Created by the Centers for Disease Control & Prevention | Yes | Yes | No | Yes | Yes | No |
| Volk 2008 [78] | None | Integration of didactic soap-opera episodes with interactive learning modules | Yes | Yes | Yes | No | Yes | No |
| Weinrich 2008 [79] | Social learning theory | Previous research findings, community feedback | Yes | Yes | No | Yes | Yes | No |
| Frosch 2008 [80], (Bhatnagar 2009 [81]) | Chronic disease model | Literature review, healthcare professional feedback, patient usability testing | Yes | Yes | Yes | No | Yes | No |
| Allen 2009 [82] | Ottawa decision support framework | Expert opinion & published research findings | Yes | Yes | Yes | Yes | Yes | No |
| Allen 2010 [83] | Ottawa decision support framework | Expert opinion, International Patient Decision Aid Standards, focus groups, usability testing | Yes | Yes | Yes | Yes | Yes | No |
| Joseph-Williams 2010 [84] | None | Expert review, field testing | Yes | Yes | Yes | Yes | Yes | No |
| Rubel 2010 [85] | None | Created by the Centers for Disease Control & Prevention | Yes | Yes | No | No | Yes | No |
| Van Vugt 2010 [86] | None | Based on screening results of 6288 men | Yes | Yes | No | No | Yes | No |
| Capik 2012 [87] | Health belief model | Literature review | Yes | No | No | Yes | No | No |
| Multiple Cancer Screening (n=3) | ||||||||
| Frosch 2008 [88] | None | Literature review, patient focus groups, patient & expert review | Yes | Yes | No | Yes | Yes (for prostate cancer screening) | No |
| Brackett 2010 [89] | None | Literature review, patient focus groups, patient & expert review | Yes | Yes | No | Yes | Yes (for prostate cancer screening) | No |
| Krist 2012 [90] | None | Efficacy, adoption and dissemination trials | Yes | Yes | No | Yes | Yes (for prostate cancer screening) | No |
Listed in ascending order of the year of publication for each cancer.
Variation was seen in the methods of values clarification exercise, where the patient actively engages in a process where his or her values regarding the screening test(s) are clarified. The patient had to be actively involved in an exercise, such as writing out the pros and cons (in cases of paper-based decision aids) or clicking on choices (in case of web-based and other interactive decision aids). Less than half of the decision aids utilized these exercises (n=27 of 73 decision aids) [18, 19, 21–23, 25, 27, 28, 31, 32, 36, 38–40, 43, 45, 48, 49, 52, 54, 58, 65, 68, 69, 78, 80–84]. None of the two decision aids that addressed cervical cancer screening [12, 13] or the three decision aids that addressed multiple cancer screenings [88–90] incorporated them.
Variation was also seen in the methods of providing guidance on making decisions and communicating with the clinician. Some were simply a statement of recommendation to speak to a clinician [14, 18, 19, 21, 36, 40, 46, 52, 55, 58, 62–64, 66, 70–73, 77]. Others provided a list of questions to ask the clinician [17, 49, 84]; some of these provided a list customized to the patient [22, 31, 82]. Other studies attempted to facilitate communication through practice-based interventions, such as having the patient use the decision aid immediately before or during genetic counseling [30–32] or providing color codes in the chart to let the clinicians know of the patient’s readiness for colorectal cancer screening [34, 41, 50, 51]. In all, 43 of 73 decision aids, including all three decision aids on multiple cancer screening [88–90], provided the guidance on communicating with clinicians. Neither of the two decision aids on cervical cancer screening provided it [12, 13].
Regarding provision of a “no screening” option, all decision aids involving breast cancer genetic testing [24–33] and all but two decision aids involving prostate cancer screening (including those targeting multiple cancers) [55–73, 75–86, 88–90] provided them. This is understandable, since the choice in these cases is to undergo the screening test or not. This option was also available in four of nine breast cancer mammogram screening [18–21, 23] and six of 21 colorectal cancer screening decision aids [35, 36, 42, 48, 49, 53]. Neither of the two cervical cancer screening decision aids provided it [12, 13]. Out of all decision aids, only one on mammogram screening [21] and one on prostate cancer screening [56, 57] dealt with when to stop screening.
Patient Outcomes Assessed for the Decision Aids
Knowledge was assessed in a majority of the decision aids (n=52 of 73). Twelve decision aids had no effect on knowledge [15, 20, 29, 38, 41, 42, 47, 64, 70, 71, 76, 78, 87]. Attitude was assessed in 35 of 73 decision aids. There was no impact on attitude in four breast cancer mammogram decision aids [15, 18–20, 23]. Of the seven breast cancer genetic testing studies, one showed an increase [26] and two showed a decrease in perceived personal risk [24, 27, 28], one showed a decrease in positive belief [33], and one showed a decrease in worry [30]. The remaining two showed no difference [31, 32]. One decision aid on cervical cancer screening showed decreased perception of procedural and cognitive barriers and increased perceived benefit of Papanicolaou test [13]. Of the eight colorectal cancer screening decision aids for which attitude was measured [38, 42, 43, 45, 47, 49, 53, 54] there was little impact. Studies including a “no screening” option resulted in less clarity on perceived benefits [42] and less positive attitude overall to colorectal cancer screening [49]. The fourteen prostate cancer screening decision aids in which the attitude was measured, all but one containing a “no screening” option, showed overall a more negative attitude toward prostate cancer screening [59–61, 65, 68, 72, 76, 77, 80–82, 84–87]. Subjective norm, or perceived social pressure to engage or not to engage in a behavior, was addressed in just five of 73 decision aids [31, 32, 47, 54, 88]. All but one of them --subjective norm decreased with one decision aid on multiple cancer screening [88]-- showed no effect. Self-efficacy was addressed in ten of 73 decision aids. It was increased with five decision aids [13, 38, 54, 65, 82], decreased in two [60, 88], and not different in three [45, 65, 83].
Preference clarification was assessed in 31 of 73 decision aids [16, 21, 23, 31–33, 36, 40–43, 45, 48–50, 52, 53, 55, 60, 61, 64, 65, 68, 74, 76–78, 80–84]. In nineteen of these, preference clarification was assessed through decreased decisional conflict, greater values clarity (e.g., subscale of decisional conflict scale), or greater informed choice (combination of knowledge, values clarity, and intent) [21, 23, 31, 32, 36, 41, 42, 45, 48, 49, 53, 74–84].
Intention was measured in 40 of 73 decision aids. Nine decision aids led to increased intention to get screened; seven of these were on colorectal cancer screening [34, 38, 46, 48, 50, 52, 54], and one each was on cervical cancer screening [13] and prostate cancer screening [86]. Decreased intention to get screened was noted in thirteen decision aids: eight of these were on prostate cancer screening [55–57, 60, 62, 63, 66, 70, 71, 80, 81, 84], two on breast cancer genetic testing [29, 33], and one each on mammogram screening [23], cervical cancer screening [12], and multiple cancer screening [88]. No difference in intention was noted in eighteen decision aids [21, 24–28, 35, 41, 42, 45, 47, 65, 67, 68, 72, 76, 82, 83, 87].
Screening behavior was assessed in 36 of 73 decision aids. Thirteen decision aids led to an increase in screening (seven colorectal [34, 39, 40, 44, 47, 50, 51], three prostate [58, 73, 87], two mammogram [14, 17], one cervical [13]), while five decision aids led to a decrease (three prostate [55, 62, 63, 84], one breast cancer genetic [30], one colorectal [49]). Eighteen decision aids showed no difference in screening.
Patient/clinician and Practice Outcomes Assessed for the Decision Aid
Many of the studies on decision aids did not address any of the issues related to shared decision making, concordance, post-visit factors, incorporation into practice, impact of the decision aid, and cost analysis. Eighteen of 73 decision aids were assessed for their effect on shared decision making. No trend for increased degree of shared decision making was noted in these eighteen studies. All studies utilized patient self-report and did not use observational measures, such as audio-recorded data, to assess shared decision making. Of note, none of the decision aids on mammogram or cervical cancer screening addressed shared decision making as a measure [12–23]. Concordance, or whether the patient and clinician agreed on a particular cancer screening test option, was addressed in just five of 73 decision aids [31, 32, 40, 52, 68]. It was high in the two decision aids on breast cancer genetic testing [31, 32], but only modest in the two decision aids on colorectal cancer screening [40, 52]. The single decision aid study on prostate cancer screening that addressed concordance noted that it was affected by the format of the decision aid [68].
Only two of 73 decision aid studies, both dealing with breast cancer genetic testing, considered post-visit factors as potential mediators of screening behavior [31, 32]. In both studies, patient’s sharing of received materials with family was assessed, with one noting an increase [31] and the other noting no difference [32]. None of the decision aids were assessed for the effect of media, referral process for testing, and other factors that may have also affected screening. Eleven of 73 decision aids were also assessed for incorporation into practice: five on breast cancer genetic testing [25, 29–32], four on colorectal cancer screening [34, 41, 50, 51], and two on multiple cancer screening [89, 90]. The studies generally attempted to link a clinician visit to the decision aid through timing [30] or modifications to the patient’s chart [31, 41, 50]. Four studies specifically dealt with how to incorporate the decision aid into usual practice [32, 51, 89, 90]. Only four of 73 decision aids were evaluated for cost of administration [22, 44, 54, 70, 71]. It varied considerably, from $2 per decision aid intervention administered for prostate cancer screening [70, 71], $53 per participant for colorectal cancer screening [54], $94 per additional patient screened for colorectal cancer [44], to $1116 or more per additional patient screened for breast cancer [22].
Discussion
Only 73 decision aids were found to have published data using our search strategies. This is a rather modest number given the many recommendations to use such tools [91, 92]. Most decision aids were evaluated with a sound research design, such as randomized controlled, 2×2 factorial, and Solomon four-group designs. The use of theoretical framework and the description of how the decision aid was developed were more variable. Our finding that just 41 of 73 decision aids (56%) used a theoretical framework is better than the findings from Durand’s review, where seventeen of 50 studies (34%) were shown to have used a theoretical framework in the development of decision aids for screening and treatment [93]. However, the difference is likely due to the inclusion of other diseases and treatments in their review. When the studies in their review are limited to decision aids on cancer screening, eight of fifteen studies (53%) utilized a theoretical framework, a figure similar to ours. Having a theoretical framework is important to determine how and why a particular decision aid is effective, since it is from this framework that the measurable outcomes are derived. The presence of a framework, however, did not necessarily mean that the development of the decision aid was well described. In particular, less than a third of the studies contained enough information for the reviewers to be able to determine that a formative approach had been adopted.
The reviewed decision aids uniformly provided information about the cancer and screening tests and the benefits and risks of each screening option. In contrast, just over a third of the decision aids provided explicit values clarification exercises. Values clarification may be explicit, in that patient actions is required through an exercise, such as writing down pros and cons, answering surveys to create tailored messages, and analytical hierarchy process. It could also be implicit, such as when comparing the options in a table. It is currently unclear whether the explicit method is superior to the implicit method, although there is emerging evidence that the former may be better [94]. Regarding the provision of guidance on making decisions and communicating with the clinician, only few decision aids provided recommendations tailored to the patient. This may be better provided as part of a practice-based intervention, where the decision aid is just one of interventions, rather than attempting to put everything into a stand-alone tool [90].
Other than the decision aids on breast cancer genetic testing and prostate cancer screening, where the decision in question is to be screened or not, few studies provided the option of “no screening.” For the decision aids on cancer screening established to be effective (e.g., cervical cancer screening, colorectal cancer screening), whether to include the option of no testing may be a delicate balance between patient autonomy and beneficence [95]. Interestingly, of the six colorectal cancer screening decision aids that had included this option, only one showed a clear decrease in screening uptake [49]. This may have occurred, because the decision aid in question provided a choice of getting a stool blood test vs. not getting one, whereas in the other five studies, the “no screening” option was listed along with two or more screening options. Thus, the study by Smith is similar to the studies of decision aids on breast cancer genetic testing and prostate cancer screening, which have been shown to decrease the test uptake. It is of interest to note that the decision aid that specifically addressed how the inclusion of a “no screening” option along with multiple screening options for colorectal cancer affected patient intent showed no difference, but also showed that the patients presented with a “no screening” option felt less clarity in making a decision [42].
Few studies included information on when to stop screening. For breast cancer genetic testing, this is understandable, since it is a one-time only test. For others, recommendations on what age to stop screening did not become available until the recent guidelines. Also, since most studies focused on a single decision making event and not multiple decisions over time, and typically had a cap on maximum age for inclusion, the issue may not have been relevant. From the perspective of incorporating a decision aid into daily practice, it may be more feasible to have a separate discussion on when to stop screening prompted through a clinician reminder system [96].
Other than knowledge, there was little consistency in the patient attributes measured as outcomes in the studies. The attributes in the Health Belief Model (e.g., perceived benefit, perceived risk) were used most frequently when assessing the positive or negative attitudes toward screening. Subjective norm and self-efficacy were rarely measured. These measures would be important in determining the contribution of the decision aids in the decision autonomy of the patients after their use. For example, patients who perceive greater social pressure (either through their family, peers, or clinician) may still be affected by others’ advice after using the decision aid.
Preference clarification was most commonly assessed by the Decisional Conflict Scale [97]. This scale includes subscales of “informed” and “values clarity,” which may be particularly relevant when measuring the effects of decision aids. Some studies have adopted an informed choice measurement, which is felt to be a better measure of decision quality and combines the scores of knowledge, values clarity, and intent or behavior [98]. This measurement may be increasingly adopted in the future studies on decision aids.
Just half of the studies actually measured screening uptake as an outcome. Of these, over half were by patient self-report after a variable period of time. This may be problematic, since patients tend to over-report screening behavior [99, 100]. Other studies used patient intention rather than screening uptake as the final outcome, which has even lower correlation with actual behavior than self-report [101]. Of note, ten of 73 decision aids had neither intention nor behavior as the outcome [15, 16, 20, 43, 64, 75, 77, 78, 85, 89]. There is little justification not to measure one or both of these outcomes at this time.
Few studies have actually addressed the patient/clinician communication subsequent to the decision aid use. Since the decision aids are purported to improve shared decision making, it is surprising that there are few objective data to support such claims [1, 102]. The studies that did measure some component of shared decision making based their measurements on patient self-report. Unfortunately, they are not considered to be sufficiently objective measures compared to third-observer instruments such as OPTION, Decision Support Assessment Tool, or the Informed and Shared Decision Making instrument [103–107]. The use of these measures would require recording of the patient/clinician encounter, which would also make them available for qualitative and mixed-methods analyses, enriching the findings. Intriguing questions that may be answered through these processes include: How is patient/physician communication affected by the use of decision aids? Is sharing decision making between the patient and physician always positively impacted by decision aids? Are there instances where patient activation by decision aids may be deleterious (e.g., patient strongly inclined to use stool blood test for colorectal cancer screening but the physician strongly recommends colonoscopy; frustrated, the patient decides not to get screened)?
Even rarer than the measurement of shared decision making was the measurement of concordance. Since shared decision making allows for a decision to be deferred when an agreement is not met, it would be important to assess whether the decision aid led to increase in agreement between the patient and the clinician. Current cancer screening literature, particularly colorectal cancer screening, reveals a potential negative impact on shared decision making as the clinicians increasingly prefer colonoscopy as the test of choice, to the exclusion of considering patient preference [108]. Thus, whether patients activated through decision aids could steer the clinicians toward a more shared decision making approach and increased concordance would be an important outcome measure. Post-visit factors, such as the influence of media and family and the ease of the referral process for subsequent testing, were addressed so rarely as to be inconsequential.
The study settings and populations in which the decision aids were utilized were sufficiently variable. Thus, these decision aids would likely lead to similar results in other settings. More problematic was that the decision aids tended to be stand-alone and not integrated into the daily practice routine. This would likely limit the practical use of these decision aids. When intention to treat analysis is adopted, many studies show a very small to negligible effect by the decision aids, due to the low usage by the patients. Additionally, studies have shown that although clinicians like the concept of decision aids, they actually rarely use them in settings that are conducive to shared decision making and where publicly accessible decision aids are available [109]. Unless the decision aids incorporate risk assessment and tailor their values clarification exercises accordingly (e.g., moving from multiple options in average risk to recommending colonoscopy in increased risk patients in colorectal cancer screening), or a reminder system exists that could link patients to decision aids based on their profile, clinicians may perceive the decision aids to be too cumbersome. These barriers may not be overcome unless a more comprehensive, practice-based approach is adopted [110–113]. An excellent example of using a practice-based approach in a real-world setting is a recent publication from a large health system in Washington State. Their organizational effort to implement decision aids for hip and knee arthritis and joint replacement surgery was associated with 26 percent fewer hip replacement surgeries, 38 percent fewer knee replacements, and 12–21 percent lower costs over six months [114]. Currently, only one study has attempted at an improvement in cancer screening through practice-wide intervention, including use of decision aids [90].
What Can Practicing Physicians Do?
First, physicians need to accept that cancer screening has elements that are sensitive to patient preferences and choice. Second, it would be helpful for the physicians to know how to access useful decision aids. An example is the repository of decision aids available from the Ottawa Hospital Research Institute in Canada. Their website (http://decisionaid.ohri.ca/AZlist.html) contains links to high quality decision aids in various topics, including screening for all of the cancers discussed in this review. Third, many organizations offer free information to the patients in a way that may still provide them with desired information on how the cancer screening tests work and their risks and benefits. An example would be the American Cancer Society website (http://www.cancer.org), which provides the latest information on screening for breast, cervical, colorectal, and prostate cancers, among others. The barrier is how and when one uses these existing tools in a busy day. It will likely be either before or after the visit, thus unloading time and effort from the visit itself. Fourth, the state-of-the-art interactive decision aid may not be feasible in a real world practice setting at this time. This reality may be reflected in the fact that many of even the more recent studies use print rather than web-based decision aids.
With the increasing use of electronic medical records linked to patient portals, as well as advances in mobile phones and their apps, decision aids that are accessible and easily understandable may become more available in a timely manner to the patients in the near future. The features used to evaluate the studies in this review would serve as an excellent checklist of physicians to use when examining such tools.
Limitations
First, despite an exhaustive attempt to identify all published English language studies on this topic, due to the differences in current indexing practices in and among the electronic databases, we cannot ensure that we have examined all of the published English language works in this area. Some studies were published in abstracts only and could not be included due to lack of detail. Second, there are likely to be unpublished studies relevant to this area. It is unknown if the results of these studies would sway the assessment given that most unpublished studies contain negative findings. Third, the published data lacked significant details of how the decision aids work. We searched for relevant articles on their development and accessed the original tools if available, but this was possible in only a minority of the cases. It may be that some decision aids actually possess the features that we had concluded as lacking. Fourth, the published data lacked detailed information on how the decision aids were developed and how the outcomes measures were determined. Because of this, we did not rigidly apply the International Patient Decision Aids Collaboration (IPDAS) criteria to the decision aids. Of note, IPDAS is an internationally recognized scoring system of decision aid quality [115]. It measures the quality of the decision aids in ten dimensions, including information provided, description of probabilities, and availability of decision guidance. It is increasingly influential in determining how decision aids should be developed. Finally, our approach to evaluating these studies highlights the vast array of complex data that needs to be gathered and analyzed to adequately address the topics that were considered. For many investigators, collecting such quantity and diversity of data may have been beyond their funding, resources, or skill set. It also may not be well reviewed at study sections that place a priority on focused research questions. In addition, many investigators’ research team lacks expertise in certain areas not addressed. The collection of adequate data also may create too much burden on the study participants, which would limit accrual and follow-up. Thus, the ideal studies would be a series of studies expanding the focus and further refining the intervention, which we did not find.
Unique Features of Our Review
Many high quality reviews are available on decision aids [1, 116, 117]. Our review is unique, because it focused on cancer screening and measurable outcomes based on a theoretical framework. In particular, our review elucidated that few decision aids on cancer screening actually evaluated their effect on patient’s entire decision making process, including shared decision making, and reaching concordance with their clinician. Our review showed where further research is needed, as we detail below. Among the most important would be: having a theoretical framework so that appropriate outcomes are measured, objective assessment of shared decision making, and attention to applicability in other settings.
Suggestions for Future Research
A strong theoretical framework should support the decision aid and guide its development as well as measurement outcomes. There should be a clear correlation between the theoretical framework and the measured outcomes.
There should be more studies that critically compare explicit vs. implicit values clarification.
An objective measure of screening uptake (e.g., paper chart review, extraction of electronic health record data) should be adopted to assess the effectiveness of the decision aid.
Shared decision making between the patient and the clinician should be recorded and objectively measured by validated tools.
Other potential mediators that temporally occur after the patient’s decision aid use, such as media and family influence, should be considered.
How decision aids would fare as part of meaningful use in primary care practices should be assessed through better integration into practice and a broader, practice-based approach to measure effectiveness.
To address applicability in real-world settings, studies should continue to be performed in heterogeneous community practice settings, utilizing practice-based research networks.
Long-term effectiveness and viability should be addressed, including the effect on repeated screening and cost-effectiveness and cost-benefit analyses.
With the advent of more options in breast and cervical cancer screening and the need for even better informed and shared decision making in prostate cancer screening with the advent of conflicting guidelines, there are even more opportunities for decision aids to be useful in the setting of cancer screening.
Conclusion
Decision aids are here to stay. Although much research needs to be done to determine what really makes for an effective decision aid, practical applications are already occurring. Many decision aids are now available free of charge. Clinicians are encouraged to explore them, select those that fit best with their current understanding of the topic in question, and apply them to their practice workflow in a creative way.
Table 3.
Patient Outcomes Assessed for the Decision Aids
| Author & Year** | Knowledge | Attitude | Subjective Norm | Self-Efficacy | Preference Clarification | Intention | Screening Behavior |
|---|---|---|---|---|---|---|---|
| Breast Cancer Mammogram Screening (n=9) | |||||||
| Kadison 1998 [14] | N/A | N/A | N/A | N/A | N/A | N/A | Self-report: Increased BSE, CBE, but MMG not increased |
| Street 1998 [15] | No difference | No difference in personal importance or anxiety | N/A | N/A | N/A | N/A | N/A |
| Lawrence 2000 [16] | N/A | N/A | N/A | N/A | Less preference on MMG, weaker feeling towards their own decision regarding MMG | N/A | N/A |
| Valdez 2001 [17] | N/A | N/A | N/A | N/A | N/A | N/A | Self-report: 51% had completed or scheduled MMG |
| Rimer 2001 [18], 2002 [19] | Increased for tailored print + telephone counseling vs. usual care; not increased for tailored print vs. usual care | Increased risk perception | N/A | N/A | N/A | N/A | Self-report: No difference |
| Lewis 2003 [20] | No difference | No change or difference in perception of benefits & harms | N/A | N/A | N/A | N/A | N/A |
| Mathieu 2007 [21] | Increased | N/A | N/A | N/A | Greater values clarity and informed choice | No difference | Self-report: No difference |
| Vernon 2008 [22] | N/A | N/A | N/A | N/A | N/A | N/A | Self-report: No difference |
| Mathieu 2010 [23] | Increased | No difference in perceived benefits & harms or anxiety | N/A | N/A | No difference in informed choice | Decreased | N/A |
| Breast Cancer Genetic Testing (n=9) | |||||||
| Lerman 1997 [24] | Increased | Decreased perceived personal risk; increased perceived limitations; no difference in perceived benefits | N/A | N/A | N/A | No difference | N/A |
| Green 2001 [25] | Increased | N/A | N/A | N/A | N/A | No difference | N/A |
| Schwartz 2001 [26] | Increased | No difference in perceived benefits; increased perceived risk | N/A | N/A | N/A | No difference | N/A |
| Green 2004 [27], 2005 [28] | Increased in low risk group; no difference in high risk group | Greater decrease in absolute risk perception in low risk group; no difference in high risk group; no difference in decrease in relative risk perception; greater decrease in anxiety | N/A | N/A | N/A | No difference | Self-report: No difference |
| Miller 2005 [29] | No difference | N/A | N/A | N/A | N/A | Decreased in average risk, increased in high risk | N/A |
| Wang 2005 [30] | Increased | Worry declined | N/A | N/A | N/A | N/A | Chart audit: Decreased |
| Wakefield 2008a [31] | Increased | No difference in distress (e.g., intrusive & avoidant thoughts, anxiety, depression) | No difference in perceived family involvement in decision-making | N/A | No difference in Decisional Conflict Scale except for Informed subscale (more informed); no difference in informed choice or decisional regret | N/A | Self-report: No difference |
| Wakefield 2008b [32] | Increased | No difference in distress (e.g., intrusive & avoidant thoughts, anxiety, depression) | No difference in perceived family involvement in decision-making | N/A | No difference in Decisional Conflict Scale except for Informed subscale (more informed) and Clear Values subscale (clearer values); no difference in informed choice or decisional regret | N/A | Self-report: No difference |
| Gray 2009 [33] | N/A | Decrease in positive beliefs; no difference in trust in internet testing or belief that internet testing is wise | N/A | N/A | Increased preference for clinic testing rather than direct-to-consumer testing | Decreased | N/A |
| Cervical Cancer Screening (n=2) | |||||||
| Adab 2003 [12] | N/A | N/A | N/A | N/A | N/A | Decreased | N/A |
| Park 2005 [13] | Increased | Decreased perception of procedural barriers & cognitive barriers; increased perceived benefit of Pap test; no difference in perceived susceptibility & seriousness | N/A | Increased | N/A | Increased | Self-report: Increased |
| Colorectal Cancer Screening (n=21) | |||||||
| Pignone 2000 [34] | N/A | N/A | N/A | N/A | N/A | Increased | Chart review: Screening ordering & completion increased |
| Wolf 2000 [35] | Increased | N/A | N/A | N/A | N/A | No difference | N/A |
| Dolan 2002 [36] | N/A | N/A | N/A | N/A | Decisional conflict decreased | N/A | Chart review: No difference |
| Zapka 2004 [37] | N/A | N/A | N/A | N/A | N/A | N/A | Self-report: No difference |
| Jerant 2007 [38] | No difference | No difference in perceived benefits & barriers | N/A | Increased | N/A | Increased | N/A |
| Myers 2007 [39] | N/A | N/A | N/A | N/A | N/A | N/A | Review of chart, billing, laboratory database: Increased |
| Ruffin 2007 [40] | N/A | N/A | N/A | N/A | Increased | N/A | Self-report: Increased |
| Griffith 2008a [41] | No difference | N/A | N/A | N/A | No difference in decisional conlict & satisfaction | No difference | N/A |
| Griffith 2008b [42] | No difference | Less clarity on benefits & downsides with DA that included “no screen” option | N/A | N/A | Less clarity on help in making a decision with DA that included “no screen” option | No difference | N/A |
| Katsumura 2008 [43] | N/A | Higher priority on “avoiding disadvantage” in Internet-based information plus risk | N/A | N/A | Less preference for COL | N/A | N/A |
| Lewis 2008 [44] | N/A | N/A | N/A | N/A | N/A | N/A | Chart review: Increased |
| Trevena 2008 [45] | Increased | No difference in anxiety | N/A | No difference in perceived behavioral control | Increase in decisions that were informed and had clear values | No difference | Self-report: No difference |
| Makoul 2009 [46] | Increased | N/A | N/A | N/A | N/A | Increased | N/A |
| Manne 2009 [47] | Not a mediator | Not mediators: perceived risk, severity, preventability | Not mediators: physician & family support | N/A | N/A | Partial mediator: decisional balance | Self-report with clinician confirmation: Increased |
| Lewis 2010 [48] | Increased | N/A | N/A | N/A | Decisional conflict decreased | Increased | N/A |
| Smith 2010 [49] | Increased | Less positive; No difference in worry about CRC | N/A | N/A | Increased informed choice; decreased decisional conflict; no difference in decisional satisfaction or confidence | N/A | Laboratory database: Decreased |
| Miller 2011 [50] | N/A | N/A | N/A | N/A | Report of test preference increased | Increased | Chart review: Screening ordering & completion increased |
| Pignone 2011 [51] | N/A | N/A | N/A | N/A | N/A | N/A | Self-report: Increased |
| Schroy 2011 [52] | Increased | N/A | N/A | N/A | No difference between 2 DA groups | Increased | N/A |
| Steckelberg 2011 [53] | Increased | Less positive | N/A | N/A | Increased informed choice | N/A | Self-report: No difference |
| Vernon 2011 [54] | Increased | Increased pros; decreased cons; no difference in worry | No difference in social influence | Increased | N/A | Increased | Self-report: No difference |
| Prostate Cancer Screening (n=29) | |||||||
| Flood 1996 [55] | Increased | N/A | N/A | N/A | Greater preference for conservative treatment | Decreased | Chart review: Decreased |
| Wolf 1996 [56], 1998 [57] | N/A | N/A | N/A | N/A | N/A | Decreased | N/A |
| Myers 1999 [58] | N/A | N/A | N/A | N/A | N/A | N/A | Review of chart & billing data: Increased screening adherence |
| Schapira 2000 [59] | Increased | Decrease in perceived benefit | N/A | N/A | N/A | N/A | Chart review: No difference |
| Frosch 2001 [60] | Increased | Less concern about prostate cancer | N/A | Less confidence about their decision | Greater preference for conservative treatment | Decreased | N/A |
| Wilt 2001 [61] | Increased | No difference in screening belief | N/A | N/A | No difference in preference for conservative treatment | N/A | Laboratory database: No difference |
| Volk 1999 [62], 2003 [63] | Increased at 2 weeks and at 1 year | N/A | N/A | N/A | N/A | Decreased | Self-report: Decreased |
| Frosch 2003 [64] | No difference | N/A | N/A | N/A | Less preference for conservative treatment | N/A | N/A |
| Gattellari 2003 [65] | Increased | No difference in worry about dying from prostate cancer | N/A | More likely to report ability of making informed choice | No difference in overall decisional uncertainty | No difference | N/A |
| Ruthman 2004 [66] | Increased | N/A | N/A | N/A | N/A | Less likely to prefer PSA screening | N/A |
| Sheridan 2004 [67] | Increased | N/A | N/A | N/A | N/A | No change in interest in prostate cancer screening | N/A |
| Gattellari 2005 [68] | Increased in print DA vs. video DA and leaflet | No difference in worry about developing prostate cancer | N/A | No difference in perceived ability to make informed choice | No difference in overall decisional uncertainty Less interest in PSA screening | No difference | N/A |
| Myers 2005 [69] | N/A | N/A | N/A | N/A | N/A | N/A | Chart review: No difference |
| Partin 2004 [70], 2006 [71] | No difference | N/A | N/A | N/A | N/A | Decreased | Chart review: No difference |
| Watson 2006 [72] | Increased | More negative attitude toward PSA screening | N/A | N/A | N/A | No difference | N/A |
| Kripalani 2007 [73] | N/A | N/A | N/A | N/A | N/A | N/A | Chart review: Increased PSA ordering |
| Krist 2007 [74] | Increased | N/A | N/A | N/A | No difference in Decisional Conflict Scale | N/A | Self-report & clinician report: No difference |
| Ellison 2008 [75] | Increased | N/A | N/A | N/A | N/A | N/A | N/A |
| Ilic 2008 [76] | No difference | No difference in anxiety | N/A | N/A | No difference in decisional conflict | No difference | N/A |
| Stephens 2008 [77] | Increased | Increased risk perception | N/A | N/A | Feeling more informed, to have clearer values, and to make more effective decision | N/A | N/A |
| Volk 2008 [78] | No difference | N/A | N/A | N/A | Decreased decisional conflict | N/A | N/A |
| Weinrich 2008 [79] | Increased | N/A | N/A | N/A | N/A | N/A | Chart review: No difference |
| Frosch 2008 [80], (Bhatnagar 2009 [81]) | Increased for DA but not chronic disease trajectory | No difference in concern | N/A | N/A | Greater decrease in decisional conflict; no difference in preference for conservative treatment | Greater decrease in DA only and chronic disease trajectory only groups to get PSA screening but not combined vs. control | N/A |
| Allen 2009 [82] | Increased | No difference in risk perception | N/A | Increased | Decreased decisional conflict | No change in decisional stage | N/A |
| Allen 2010 [83] | Increased | N/A | N/A | No difference | No difference in decisional conflict; no change in decisional consistency (match between values and preference for screening) | Increased decisional stage; no change in desire for screening | N/A |
| Joseph-Williams 2010 [84] | Increased | More negative attitude toward PSA screening; no difference in anxiety | N/A | N/A | Decreased decisional conflict | Decreased | Chart review: Decreased |
| Rubel 2010 [85] | Increased | No difference in positive or negative schema; no difference in risk perception | N/A | N/A | N/A | N/A | N/A |
| Van Vugt 2010 [86] | Increased | Decreased in perceived risk; more negative attitude toward PSA test | N/A | N/A | N/A | Increased | N/A |
| Capik 2012 [87] | No difference | Increased susceptibility; decreased barrier; no change in benefit or seriousness | N/A | N/A | N/A | No change | Self-report: Increased |
| Multiple Cancer Screening (n=3) | |||||||
| Frosch 2008 [88] | Increased | No difference | Decreased | Decreased | N/A | Decreased | N/A |
| Brackett 2010 [89] | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Krist 2012 [90] | N/A | N/A | N/A | N/A | N/A | N/A | Self-report: No difference in individual cancer screenings; increased for overall delivery of preventive services |
Abbreviations:
COL: Colonoscopy
CRC: Colorectal Cancer
DA: Decision Aid
N/A: Not Addressed
PSA: Prostate Specific Antigen
Listed in ascending order of the year of publication.
Acknowledgments
Dr. Jimbo receives funding from the National Cancer Institute: R01CA152413.
Contributor Information
Masahito Jimbo, Email: mjimbo@med.umich.edu, Departments of Family Medicine and Urology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48109-0708, Phone: (734) 998-7120 Ext 334, Fax: (734) 998-7335.
Gurpreet K. Rana, Email: preet@umich.edu, Taubman Health Sciences Library, University of Michigan, 1135 E. Catherine, Ann Arbor, MI 48109-0726, Phone: (734) 936-1399, Fax: (734) 763-1473.
Sarah Hawley, Email: sarahawl@med.umich.edu, Departments of Internal Medicine and Health Management and Policy, University of Michigan, NCRC 2800 Plymouth Road Building, 16/406E, Ann Arbor, MI 48109-2800, Phone: (734) 936-8816.
Margaret Holmes-Rovner, Email: mholmes@msu.edu, Health Services Research, Center for Ethics and Department of Medicine, Michigan State University College of Human Medicine, 965 Fee Road Rm C203, East Lansing, MI, 48824-1316, Phone: (517) 353-5197.
Karen Kelly-Blake, Email: kellyka1@msu.edu, Center for Ethics and Humanities in the Life Sciences, Michigan State University College of Human Medicine, East Fee Hall, 965 Fee Road Room C215, East Lansing, MI 48824, Phone: (517) 353-8582, Fax: (517) 353-3289.
Donald E. Nease, Jr., Email: donald.nease@ucdenver.edu, Department of Family Medicine and Colorado Health Outcomes Program, University of Colorado – Denver, 13199 E. Montview Blvd, Suite 300, Mail Stop F443, Aurora, CO 80045, Phone: (303) 724-6270, Fax: (303) 724-1839.
Mack T. Ruffin, IV, Email: mruffin@umich.edu, Associate Chair for Research Programs, Department of Family Medicine, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48109-0708, Phone: (734) 998-7120 Ext 310, Fax: (734) 998-7335.
References
- 1.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane DB Syst Rev. 2011;(10):Art. No.: CD001431. doi: 10.1002/14651858.CD001431.pub3. a0.1002/14651858.CD001431. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 3.Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer update 2010. CA Cancer J Clin. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 4.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 5.Whitlock EP, Lin JS, Liles E, Bell TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the US Preventive Services Task Force. Ann Intern Med. 2008;149:638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 6.Nelson HD, Yne TK, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saslow D, Boetes C, Burke W. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 8.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frosch DL, Légaré F, Fishbein M, Elwyn G. Adjuncts or adversaries to shared decision making? Applying the Integrative Model of behavior to the role and design of decision support interventions in healthcare interactions. Implement Sci. 2009;4:73–82. doi: 10.1186/1748-5908-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan JL, Ellis SJ, Chambers R. Defining shared decision making and concordance: are they one and the same? Postgrad Med J. 2002;78:383–384. doi: 10.1136/pmj.78.921.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sepucha K, Ozanne EM. How to define and measure concordance between patients’ preferences and medical treatments: a systematic review of approaches and recommendations for standardization. Patient Educ Couns. 2010;78:12–23. doi: 10.1016/j.pec.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Adab P, Marshall T, Rouse A, Randhawa B, Sangha H, Bhangoo N. Randomised controlled trial of the effect of evidence based information on women’s willingness to participate in cervical cancer screening. J Epidemiol Commun H. 2003;57:589–593. doi: 10.1136/jech.57.8.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SM, Chang SB, CCW Effects of a cognition-emotion focused program to increase public participation in Papanicolaou smear screening. Public Health Nurs. 2005;22:289–298. doi: 10.1111/j.0737-1209.2005.220404.x. [DOI] [PubMed] [Google Scholar]
- 14.Kadison P, Pelletier EM, Mounib EL, Oppedisano P, Poteat HT. Improved screening for breast cancer associated with a telephone-based risk assessment. Prev Med. 1998;27:493–501. doi: 10.1006/pmed.1998.0313. [DOI] [PubMed] [Google Scholar]
- 15.Street RL, Jr, Van Order A, Bramson R, Manning T. Preconsultation education promoting breast cancer screening: does the choice of media make a difference? Journal Cancer Educ. 1998;1:152–161. doi: 10.1080/08858199809528537. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence VA, Streiner D, Hazuda HP, et al. A cross-cultural consumer-based decision aid for screening mammography. Prev Med. 2000;30:200–208. doi: 10.1006/pmed.1999.0620. [DOI] [PubMed] [Google Scholar]
- 17.Valdez A, Banerjee K, Fernandez M, Ackerson L. Impact of a multimedia breast cancer education intervention on use of mammography by low-income Latinas. J Cancer Educ. 2001;16:221–224. doi: 10.1080/08858190109528777. [DOI] [PubMed] [Google Scholar]
- 18.Rimer BK, Halabi S, Sugg Skinner C, et al. The short-term impact of tailored mammography decision-making interventions. Patient Educ Couns. 2001;43:269–285. doi: 10.1016/s0738-3991(00)00172-5. [DOI] [PubMed] [Google Scholar]
- 19.Rimer BK, Halabi S, Sugg Skinner C, et al. Effects of a mammography decision-making intervention at 12 and 24 months. Am J Prev Med. 2002;22:247–257. doi: 10.1016/s0749-3797(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 20.Lewis CL, Pignone MP, Sheridan SL, Downs SM, Kinsinger LS. A Randomized trial of three videos that differ in the framing of information about mammography in women 40 to 49 years old. J Gen Intern Med. 2003;18:875–883. doi: 10.1046/j.1525-1497.2003.21152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathieu E, Barratt A, Davey HM, et al. Informed choice in mammography screening: a randomized trial of a decision aid for 70-year-old women. Arch Intern Med. 2007;167:2039–2046. doi: 10.1001/archinte.167.19.2039. [DOI] [PubMed] [Google Scholar]
- 22.Vernon SW, del Junco DJ, Tiro JA, et al. Promoting regular mammography screening II. results from a randomized controlled trial in US women veterans. J Natl Cancer I. 2008;100:347–358. doi: 10.1093/jnci/djn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathieu E, Barratt AL, McGeechan K, et al. Helping women make choices about mammography screening: An online randomized trial of a decision aid for 40-year-old women. Patient Educ Couns. 2010;81:63–72. doi: 10.1016/j.pec.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Lerman C, Biesecker B, Benkendorf JL, et al. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. J Natl Cancer I. 1997;89:148–157. doi: 10.1093/jnci/89.2.148. [DOI] [PubMed] [Google Scholar]
- 25.Green MJ, Biesecker BB, McInerney AM, Mauger D, Fost N. An interactive computer program can effectively educate patients about genetic testing for breast cancer susceptibility. Am J Med Genet. 2001;103:16–23. doi: 10.1002/ajmg.1500. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz MD, Benkendorf J, Lerman C, et al. Impact of educational print materials on knowledge, attitudes, and interest in BRCA1/BRCA2: testing among Ashkenazi Jewish women. Cancer. 2001;92:932–940. doi: 10.1002/1097-0142(20010815)92:4<932::aid-cncr1403>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 27.Green MJ, Peterson SK, Baker MW, et al. Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: A randomized controlled trial. J Amer Med Assoc. 2004;292:442–452. doi: 10.1001/jama.292.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green MJ, Peterson SK, Baker MW, et al. Use of an educational computer program before genetic counseling for breast cancer susceptibility: effects on duration and content of counseling sessions. Genet Med. 2005;7:221–229. doi: 10.1097/01.GIM.0000159905.13125.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller SM, Fleisher L, Roussi P, et al. Facilitating informed decision making about breast cancer risk and genetic counseling among women calling the NCI’s cancer information service. J Health Commun. 2005;10(Suppl1):119–336. doi: 10.1080/07366290500265335. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Gonzalez R, Milliron KJ, Strecher VJ, Merajver SD. Genetic counseling for BRCA1/2: a randomized controlled trial of two strategies to facilitate the education and counseling process. Am J Med Genet. 2005;134:66–73. doi: 10.1002/ajmg.a.30577. [DOI] [PubMed] [Google Scholar]
- 31.Wakefield CE, Meiser B, Homewood J, et al. A randomized controlled trial of a decision aid for women considering genetic testing for breast and ovarian cancer risk. Breast Cancer Res Tr. 2008;107:289–301. doi: 10.1007/s10549-007-9539-2. [DOI] [PubMed] [Google Scholar]
- 32.Wakefield CE, Meiser B, Homewood J, et al. A randomized trial of a breast/ovarian cancer genetic testing decision aid used as a communication aid during genetic counseling. Psycho-Oncol. 2008;17:844–854. doi: 10.1002/pon.1353. [DOI] [PubMed] [Google Scholar]
- 33.Gray SW, O’Grady C, Karp L, et al. Risk information exposure and direct-to-consumer genetic testing for BRCA mutations among women with a personal or family history of breast or ovarian cancer. Cancer Epidem Biomar. 2009;18:1303–1311. doi: 10.1158/1055-9965.EPI-08-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening. A randomized, controlled trial. Ann Intern Med. 2000;133:761–769. doi: 10.7326/0003-4819-133-10-200011210-00008. [DOI] [PubMed] [Google Scholar]
- 35.Wolf AM, Schorling JB. Does informed consent alter elderly patients’ preferences for colorectal cancer screening? Results of a randomized trial. J Gen Intern Med. 2000;15:24–30. doi: 10.1046/j.1525-1497.2000.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolan JG, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Med Decis Making. 2002;22:125–139. doi: 10.1177/0272989X0202200210. [DOI] [PubMed] [Google Scholar]
- 37.Zapka JG, Lemon SC, Puleo E, et al. Patient education for colon cancer screening: a randomized trial of a video mailed before a physical examination. Ann Intern Med. 2004;141:683–692. doi: 10.7326/0003-4819-141-9-200411020-00009. [DOI] [PubMed] [Google Scholar]
- 38.Jerant A, Kravitz RL, Rooney M, et al. Effects of a tailored interactive multimedia computer program on determinants of colorectal cancer screening: a randomized controlled pilot study in physician offices. Patient Educ Couns. 2007;66:67–74. doi: 10.1016/j.pec.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110:2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 40.Ruffin MT, Fetters MD, Jimbo M. Preference-based electronic decision aid to promote colorectal cancer screening: results of a randomized controlled trial. Prev Med. 2007;45:267–273. doi: 10.1016/j.ypmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Griffith JM, Lewis CL, Brenner AR, Pignone MP. The effect of offering different numbers of colorectal cancer screening test options in a decision aid: a pilot randomized trial. BMC Med Inform Decis. 2008;8:4. doi: 10.1186/1472-6947-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffith JM, Fichter M, Fowler FJ, Lewis C, Pignone MP. Should a colon cancer screening decision aid include the option of no testing? A comparative trial of two decision aids. BMC Med Inform Decis. 2008;8:10. doi: 10.1186/1472-6947-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katsumura Y, Yasunaga H, Imamura T, Ohe K, Oyama H. Relationship between risk information on total colonoscopy and patient preferences for colorectal cancer screening options: Analysis using the Analytic Hierarchy Process. BMC Health Serv Res. 2008;8:106. doi: 10.1186/1472-6963-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis CL, Brenner AT, Griffith JM, Pignone M. The uptake and effect of a mailed multi-modal colon cancer screening intervention: A pilot controlled trial. Implement Sci. 2008;3:32. doi: 10.1186/1748-5908-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trevena LJ, Irwig L, Barratt A. Randomized trial of a self-administered decision aid for colorectal cancer screening. J Med Screen. 2008;15:76–82. doi: 10.1258/jms.2008.007110. [DOI] [PubMed] [Google Scholar]
- 46.Makoul G, Cameron KA, Baker DW, et al. A multimedia patient education program on colorectal cancer screening increases knowledge and willingness to consider screening among Hispanic/Latino patient. Patient Educ Couns. 2009;76:220–226. doi: 10.1016/j.pec.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Manne SL, Coups EJ, Markowitz A, et al. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Ann Behav Med. 2009;37:207–217. doi: 10.1007/s12160-009-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis C, Golin CE, DeLeon C, et al. A targeted decision aid for the elderly to decide whether to undergo colorectal cancer screening: Development and results of an uncontrolled trial. BMC Med Inform Decis. 2010;10:54. doi: 10.1186/1472-6947-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith SK, Trevena L, Simpson JM, et al. A decision aid to support informed choices about bowel cancer screening among adults with low education: randomised controlled trial. Brit Med J. 2010;341:5370. doi: 10.1136/bmj.c5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller DP, Jr, Spangler JG, Case LD, et al. Effectiveness of a web-based colorectal cancer screening patient decision aid: A randomized controlled trial in a mixed-literacy population. Am J Prev Med. 2011;40:608–615. doi: 10.1016/j.amepre.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pignone M, Winquist A, Schild LA, et al. Effectiveness of a patient and practice-level colorectal cancer screening intervention in health plan members: the CHOICE trial. Cancer. 2011;117:3352–3362. doi: 10.1002/cncr.25924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroy PC, 3rd, Emmons K, Peters E, et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Med Decis Making. 2011;31:93–107. doi: 10.1177/0272989X10369007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steckelberg A, Huelfenhaus C, Haastert B, Muehlhauser I. Effect of evidence based risk information on “informed choice” in colorectal cancer screening: randomised controlled trial. Brit Med J. 2011;342:d3193. doi: 10.1136/bmj.d3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vernon SW, Bartholomew LK, McQueen A, et al. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: Sometimes more is just the same. Ann Behav Med. 2011;41:284–299. doi: 10.1007/s12160-010-9258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flood AB, Wennberg JE, Nease RF, Jr, et al. The importance of patient preference in the decision to screen for prostate cancer. Prostate Patient Outcomes Research Team. J Gen Intern Med. 1996;11:342–349. doi: 10.1007/BF02600045. [DOI] [PubMed] [Google Scholar]
- 56.Wolf AM, Nasser JF, Wolf AM, Schorling JB. The impact of informed consent on patient interest in prostate-specific antigen screening. Arch Intern Med. 1996;156:1333–1336. [PubMed] [Google Scholar]
- 57.Wolf AM, Schorling JB. Preferences of elderly men for prostate-specific antigen screening and the impact of informed consent. The Journals of Gerontology Series A, Biological Sciences & Medical Sciences. 1998;53:M195–200. doi: 10.1093/gerona/53a.3.m195. [DOI] [PubMed] [Google Scholar]
- 58.Myers RE, Chodak GW, Wolf TA, et al. Adherence by African American men to prostate cancer education and early education. Cancer. 1999;86:88–104. doi: 10.1002/(sici)1097-0142(19990701)86:1<88::aid-cncr14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 59.Schapira MM, Van Ruiswyk J. The effect of an illustrated pamphlet decision-aid on the use of prostate cancer screening tests. J Fam Practice. 2000;49:418–424. [PubMed] [Google Scholar]
- 60.Frosch DL, Kaplan RM, Felitti V. Evaluation of two methods to facilitate shared decision making for men considering the prostate-specific antigen test. J Gen Intern Med. 2001;16:391–398. doi: 10.1046/j.1525-1497.2001.016006391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilt TJ, Paul J, Murdoch M, et al. Educating men about prostate cancer screening: a randomized trial of a mailed pamphlet. Eff Clin Pract. 2001;4:112–120. [PubMed] [Google Scholar]
- 62.Volk RJ, Cass AR, Spann SJ. A randomized controlled trial of shared decision making for prostate cancer screening. Arch Fam Med. 1999;8:333–340. doi: 10.1001/archfami.8.4.333. [DOI] [PubMed] [Google Scholar]
- 63.Volk RJ, Spann SJ, Cass AR, Hawley ST. Patient education for informed decision making about prostate cancer screening: a randomized controlled trial with 1-year follow-up. Ann Fam Med. 2003;1:22–28. doi: 10.1370/afm.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frosch DL, Kaplan RM, Felitti VJ. A randomized controlled trial comparing internet and video to facilitate patient education for men considering the prostate specific antigen test. J Gen Intern Med. 2003;18:781–787. doi: 10.1046/j.1525-1497.2003.20911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gattellari M, Ward JE. Does evidence-based information about screening for prostate cancer enhance consumer decision-making? A randomised controlled trial. J Med Screen. 2003;10:27–39. doi: 10.1258/096914103321610789. [DOI] [PubMed] [Google Scholar]
- 66.Ruthman JL, Ferrans CE. Efficacy of a video for teaching patients about prostate cancer screening and treatment. Am J Health Promot. 2004;18:292–295. doi: 10.4278/0890-1171-18.4.292. [DOI] [PubMed] [Google Scholar]
- 67.Sheridan SL, Felix K, Pignone MP, Lewis CL. Information needs of men regarding prostate cancer screening and the effect of a brief decision aid. Patient Educ Couns. 2004;54:345–351. doi: 10.1016/j.pec.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Gattellari M, Ward JE. A community-based randomised controlled trial of three different educational resources for men about prostate cancer screening. Patient Educ Couns. 2005;57:168–182. doi: 10.1016/j.pec.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 69.Myers RE, Daskalakis C, Cocroft J, et al. Preparing African-American men in community primary care practices to decide whether or not to have prostate cancer screening. J Natl Med Assoc. 2005;97:1143–1154. [PMC free article] [PubMed] [Google Scholar]
- 70.Partin MR, Nelson D, Radosevich D, et al. Randomized trial examining the effect of two prostate cancer screening educational interventions on patient knowledge, preferences, and behaviors. J Gen Intern Med. 2004;19:835–842. doi: 10.1111/j.1525-1497.2004.30047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Partin MR, Nelson D, Flood AB, Friedemann-Sanchez G, Wilt TJ. Who uses decision aids? Subgroup analyses from a randomized controlled effectiveness trial of two prostate cancer screening decision support interventions. Health Expect. 2006;9:285–295. doi: 10.1111/j.1369-7625.2006.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watson E, Hewitson P, Brett J, et al. Informed decision making and prostate specific antigen (PSA) testing for prostate cancer: a randomized controlled trial exploring the impact of a brief patient decision aid on men’s knowledge, attitudes and intention to be tested. Patient Educ Couns. 2006;63:367–379. doi: 10.1016/j.pec.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 73.Kripalani S, Sharma J, Justice E, et al. Low-literacy interventions to promote discussion of prostate cancer: a randomized controlled trial. Am J Prev Med. 2007;33:83–90. doi: 10.1016/j.amepre.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krist AH, Woolf SH, Johnson RE, Kerns JW. Patient education on prostate cancer screening and involvement in decision making. Ann Fam Med. 2007;5:112–119. doi: 10.1370/afm.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ellison GL, Weinrich SP. A randomized trial comparing web-based decision aids on prostate cancer knowledge for African-American men. J Natl Med Assoc. 2008;100:1139–1145. doi: 10.1016/s0027-9684(15)31481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ilic D, Egberts K, McKenzie JE, Risgridger G, Green S. Informing men about prostate cancer screening: A randomized controlled trial of patient education materials. J Gen Intern Med. 2008;23:466–471. doi: 10.1007/s11606-007-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stephens RL, Xu Y, Volk RJ, et al. Influence of a patient decision aid on decisional conflict related to PSA testing: a Structural Equation Model. Health Psychol. 2008;27:711–721. doi: 10.1037/0278-6133.27.6.711. [DOI] [PubMed] [Google Scholar]
- 78.Volk RJ, Jibaja-Weiss ML, Hawley ST, et al. Entertainment education for prostate cancer screening: a randomized trial among primary care patients with low health literacy. Patient Educ Couns. 2008;73:482–489. doi: 10.1016/j.pec.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weinrich SP, Seger RE, Rao GS, et al. A decision aid for teaching limitations of prostate cancer screening. J Natl Black Nurses Assoc. 2008;19:1–11. [PubMed] [Google Scholar]
- 80.Frosch DL, Bhatnagar V. Internet patient decision support: a randomized controlled trial comparing alternative approaches for men considering prostate cancer screening. Arch Intern Med. 2008;168:363–369. doi: 10.1001/archinternmed.2007.111. [DOI] [PubMed] [Google Scholar]
- 81.Bhatnagar V, Frosch DL, Tally SR, et al. Evaluation of an internet-based disease trajectory decision tool for prostate cancer screening. Value Health. 2009;12:101–108. doi: 10.1111/j.1524-4733.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 82.Allen JD, Mohllajee AP, Shelton RC, Drake BF, Mars DR. A computer-tailored intervention to promote informed decision making for prostate cancer screening among African American men. Am J Mens Health. 2009;3:340–351. doi: 10.1177/1557988308325460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allen JD, Othus MK, Hart A, et al. A randomized trial of a computer-tailored decision aid to improve prostate cancer screening decisions: Results from the take the wheel trial. Cancer Epidem Biomar. 2010;19:2172–2186. doi: 10.1158/1055-9965.EPI-09-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joseph-Williams N, Evans R, Edwards A, et al. Supporting informed decision making online in 20 minutes: An observational web-log study of a PSA test decision aid. J Med Internet Res. 2010;12:e15. doi: 10.2196/jmir.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubel SK, Miller JW, Stephens RL, et al. Testing the effects of a decision aid for prostate cancer screening. J Health Commun. 2010;15:307–321. doi: 10.1080/10810731003686614. [DOI] [PubMed] [Google Scholar]
- 86.van Vugt HA, Roobol MJ, Venderbos LDF, et al. Informed decision making on PSA testing for the detection of prostate cancer: An evaluation of a leaflet with risk indicator. Eur J Cancer. 2010;46:669–677. doi: 10.1016/j.ejca.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 87.Capik C, Gozum S. The effect of web-assisted education and reminders on health belief, level of knowledge and early diagnosis behaviors regarding prostate cancer screening. Eur J Oncol Nurs. 2012;16:71–77. doi: 10.1016/j.ejon.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Frosch DL, Légaré F, Mangione CM. Using decision aids in community-based primary care: a theory-driven evaluation with ethnically diverse patients. Patient Educ Couns. 2008;73:490–496. doi: 10.1016/j.pec.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brackett C, Kearing S, Cochran N, Tosteson AN, Blair Brooks W. Strategies for distributing cancer screening decision aids in primary care. Patient Educ Couns. 2010;78:166–168. doi: 10.1016/j.pec.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 90.Krist AH, Woolf SH, Rothermich SF, et al. Interactive preventive health record to enhance delivery of recommended care: a randomized trial. Ann Fam Med. 2012;10:312–319. doi: 10.1370/afm.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Edwards A, Elwyn G. The potential benefits of decision aids in clinical medicine. J Amer Med Assoc. 1999;282:779–780. doi: 10.1001/jama.282.8.779. [DOI] [PubMed] [Google Scholar]
- 92.Barry MJ. Health decision aids to facilitate shared decision making in office practice. Ann Intern Med. 2002;136:127–135. doi: 10.7326/0003-4819-136-2-200201150-00010. [DOI] [PubMed] [Google Scholar]
- 93.Durand M-A, Stiel M, Boivon J, Elwyn G. Where is the theory? Evaluating the theoretical frameworks described in decision support technologies. Patient Educ Couns. 2008;71:125–135. doi: 10.1016/j.pec.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 94.Feldman-Stewart D, Tong C, Siemnes R, et al. The impact of explicit values clarification exercises in a patient decision aid emerges after the decision is actually made: evidence from a randomized controlled trial. Med Decis Making. 2012;32:616–626. doi: 10.1177/0272989X11434601. [DOI] [PubMed] [Google Scholar]
- 95.Bekker HL. Decision aids and uptake of screening. Brit Med J. 2010;341:c5407. doi: 10.1136/bmj.c5407. [DOI] [PubMed] [Google Scholar]
- 96.Jimbo M, Nease DE, Jr, Ruffin MT, IV, Rana GK. Information technology and cancer prevention. CA Cancer J Clin. 2006;56:26–36. doi: 10.3322/canjclin.56.1.26. [DOI] [PubMed] [Google Scholar]
- 97.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. (Updated manual available at: http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf.) [DOI] [PubMed] [Google Scholar]
- 98.Sepucha KR, Fowler FJ, Jr, Mulley AG. Policy support for patient-centered care: the need for measurable improvements in decision quality. Health Affair. 2004 doi: 10.1377/hlthaff.var.54. 10.1377/hlthaff.var.54–62. [DOI] [PubMed] [Google Scholar]
- 99.Gordon NP, Hiatt RA, Lampert DI. Concordance of self-reported data and medical record audit for six cancer screening procedures. J Natl Cancer Inst. 1993;85:566–570. doi: 10.1093/jnci/85.7.566. [DOI] [PubMed] [Google Scholar]
- 100.McGovern PG, Lurie N, Margolis KL, Slater JS. Accuracy of self-report of mammography and Pap smear in a low-income urban population. Am J Prev Med. 1998;14:201–208. doi: 10.1016/s0749-3797(97)00076-7. [DOI] [PubMed] [Google Scholar]
- 101.Sheeran P. Intention-behavior relations: a conceptual and empirical review. Eur Rev Soc Psychol. 2002;12:1–36. [Google Scholar]
- 102.O’Connor AM, Llewellyn-Thomas HA, Flood AB. Patient decision aids: a review of the evidence base for shared decision making. Health Aff. 2004 doi: 10.1377/hlthaff.var.63. 10.1377/hlthaff.var.63. [DOI] [PubMed] [Google Scholar]
- 103.Légaré F, Ratté S, Stacey D, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews. 2010;(5):Art. No.: CD006732. doi: 10.1002/14651858.CD006732.pub2. 10.1002/14651858.CD006732.pub2. [DOI] [PubMed] [Google Scholar]
- 104.Wunderlich T, Cooper G, Divine G, et al. Inconsistencies in patient perceptions and observer ratings of shared decision making: the case of colorectal cancer screening. Patient Educ Couns. 2010;80:358–363. doi: 10.1016/j.pec.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elwyn G, Edwards A, Wensing M, et al. Shared decision making: developing the OPTION scale for measuring patient involvement. Qual Saf Health Care. 2003;12:93–99. doi: 10.1136/qhc.12.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guimond P, Bunn H, O’Connor AM, et al. Validation of a tool to assess health practitioners’ decision support and communication skills. Patient Educ Couns. 2003;50:235–245. doi: 10.1016/s0738-3991(03)00043-0. [DOI] [PubMed] [Google Scholar]
- 107.Braddock CH, 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. J Amer Med Assoc. 1999;282:2313–2320. doi: 10.1001/jama.282.24.2313. [DOI] [PubMed] [Google Scholar]
- 108.McQueen A, Bartholomew LK, Greisinger AJ, et al. Behind closed doors: physician-patient discussions about colorectal cancer screening. J Gen Intern Med. 2009;4:1228–1235. doi: 10.1007/s11606-009-1108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hill L, Mueller M-R, Roussos S, et al. Opportunities for the use of decision aids in primary care. Fam Med. 2009;41:350–355. [PubMed] [Google Scholar]
- 110.Carney PA, Dietrich AJ, Keller A, et al. Tools, teamwork, and tenacity: an office system for cancer prevention. J Fam Pract. 1992;35:388–394. [PubMed] [Google Scholar]
- 111.Carpiano R, Flocke S, Frank S, Stange K. Tools, teamwork, and tenacity: an examination of family practice office system influences on preventive service delivery. Prev Med. 2003;36:131–140. doi: 10.1016/s0091-7435(02)00024-5. [DOI] [PubMed] [Google Scholar]
- 112.Miller WL, Crabtree BF, McDaniel R, Stange KC. Understanding change in primary care practice using complexity theory. J Fam Pract. 1998;46:369–376. [PubMed] [Google Scholar]
- 113.Miller WL, McDaniel RR, Jr, Crabtree BF, Stange KC. Practice jazz: understanding variation in family practices using complexity science. J Fam Pract. 2001;50:872–878. [PubMed] [Google Scholar]
- 114.Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids At Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Affairs. 2012;31:2094–2104. doi: 10.1377/hlthaff.2011.0686. [DOI] [PubMed] [Google Scholar]
- 115.Elwyn G, O’Connor A, Stacey D, et al. International Patient Decision Aids Standards (IPDAS) Collaboration. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. Brit Med J. 2006;333:417–422. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stacey D, Samant R, Bennett C. Decision making in oncology: A review of patient decision aids to support patient participation. Cancer J Clin. 2008;58:293–304. doi: 10.3322/CA.2008.0006. [DOI] [PubMed] [Google Scholar]
- 117.O’Connor AM, Bennett C, Stacey D, et al. Do patient decision aids meet effectiveness criteria of the International Patient Decision Aid Standards collaboration? A systematic review and meta-analysis. Med Decis Making. 2007;27:554–574. doi: 10.1177/0272989X07307319. [DOI] [PubMed] [Google Scholar]


