Abstract
Ketogenic diets are high-fat, low-carbohydrate formulations effective in treating medically-refractory epilepsy, and recently we demonstrated lowered sensitivity to thermal pain in rats fed a ketogenic diet for 3–4 weeks. Regarding anticonvulsant and hypoalgesic mechanisms, theories are divided as to direct effects of increased ketones and/or decreased glucose, metabolic hallmarks of these diets. To address this point, we characterized the time course of ketogenic diet-induced thermal hypoalgesia, ketosis, and lowered glucose in young male rats fed ad libitum on normal chow or ketogenic diets. A strict 6.6:1 (fat:(carbohydrates + protein), by weight), ketogenic diet increased blood ketones and reduced blood glucose by two days of feeding, but thermal hypoalgesia did not appear until 10 days. Thus, ketosis and decreased glucose are not sufficient for hypoalgesia. After feeding a 6.6:1 ketogenic diet for 19 days, decreased thermal pain sensitivity and changes in blood chemistry reversed one day after return to normal chow. Effects on were consistent between two different diet formulations: a more moderate and clinically-relevant ketogenic diet formula (3.0:1) produced hypoalgesia and similar changes in blood chemistry as the 6.6:1 diet, thus increasing translational potential. Furthermore, feeding the 3.0:1 diet throughout an extended protocol (10–11 weeks) revealed that significant hypoalgesia and increased ketones persisted whereas low glucose did not, demonstrating that ketogenic diet-induced hypoalgesia does not depend on reduced glucose. In separate experiments we determined that effects on thermal pain responses were not secondary to motor or cognitive changes. Together, these findings dissociate diet-related changes in nociception from direct actions of elevated ketones or decreased glucose, and suggest mechanisms with a slower onset in this paradigm. Overall, our data indicate that metabolic approaches can relieve pain.
Indexing Words: β-hydroxybutyrate, epilepsy, glucose, metabolic therapy, short-term memory, thermal nociception
Introduction
The ketogenic diet (KD) is a low-carbohydrate, high-fat diet protocol prescribed to treat epilepsy.8,25,49 The KD minimizes glucose metabolism and promotes ketones (β-hydroxybutyrate, acetoacetate, acetone) as an alternate energy source. This metabolic shift is thought to augment inhibition and/or limit excitation, but specific mechanisms are ill-defined. Indeed, the KD reduces central excitability,5,7 but theories are divided as to whether these effects are produced directly by ketones and/or low glucose, fatty acids, or downstream metabolic effects.18,22,23,29,30,33,58
Reduced excitability would be expected to have effects beyond treating seizures. Notably, a number of inhibitory mechanisms hypothesized to underlie the efficacy of the ketogenic diet – e.g. activation of K+ channels, adenosine A1 receptors or γ-aminobutyric acid (GABA) receptors - can cause hypoalgesia,27,47,51,57 and anticonvulsant drugs are used to treat neuropathic pain.2,21,55 These drugs typically act by decreasing neuronal activity and/or altering membrane potential.39,42
Given its success in reducing seizures - and hypothesized anticonvulsant mechanisms - the KD might be expected to influence pain.31,44 Consistent with this prediction, recently we found that feeding with a KD for 3–4 weeks reduced sensitivity to thermal pain in rats.43 Establishing broader applicability and identifying key mechanisms of the KD in vivo are essential to uncover novel targets for pain relief. Thus, it is critical to test if hypoalgesia relates directly to ketosis or reduced glucose, two primary metabolic actions of a KD. For example, in vitro reports have described direct effects of ketones on K+ channels and vesicular neurotransmitter uptake sites,22,29 and low glucose can inhibit synaptic transmission3 via activation of adenosine A1 receptors and K+ channels.23,48
In addition to the importance of revealing any causal relationships among metabolic changes and analgesic effects, it is critical to understand the parameters of effective ketogenic diet ratios; dietary compliance and palatability is a major issue with ketogenic diets, particularly in adults. Our initial work used a research formulation KD considerably more restrictive (>6:1 (fat:(carbohydrates + protein), by weight) than diets prescribed to treat pediatric or adult epilepsy (ranging from 4:1 to 1:1),24 thus limiting broader clinical interpretations.
Here we compared two KD formulations: a strict research formulation (6.6:1 ratio) as well as a moderate clinical-strength diet (3.0:1 ratio), and tested longer time points (up to 11 weeks of diet treatment). Over a days-to-weeks time scale we quantified the evolution of thermal pain sensitivity, blood ketones, and blood glucose. We found the onset of observed hypoalgesia was delayed significantly as compared to the onset of ketosis or decreased glucose. Furthermore, at later time points glucose was no longer low but hypoalgesia was still observed. Therefore, the general ability of the KD to reduce thermal pain is supported, and it does not appear to rely directly on primary metabolic consequences of a KD.
Materials and Methods
Animals
Subjects were male Sprague-Dawley rats, bred in the Trinity College Animal Care Facility. All experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Trinity College Animal Care and Use Committee. Animals were switched to the experimental diets (without prior fasting) at or just after weaning (3–3.5 weeks of age), or remained on the standard control diet (CD). All food and water access was ad libitum, and KD was changed daily. The KDs had ketogenic ratios (fat:(protein + carbohydrates)) of 6.6:1 (F3666; Bio-Serv, Frenchtown, New Jersey) or 3.0:1 (F5140; Bio-Serv); the CD (LabDiet 5001; PharmaServ, Framingham, Massachusetts) had a corresponding ratio of < 0.1:1. Diet constituents are listed in Table 1. Animals remained on the diets for varying times before behavioral testing began.
Table 1.
Constituents of diets.
| LabDiet 5001 | Bio-Serv F5140 (3.0:1) | Bio-Serv F3666 (6.6:1) | |
|---|---|---|---|
| Fats | 5.7 | 69.0 | 76.7 |
| Proteins | 23.9 | 18.1 | 8.5 |
| Carbohydrates from vitamin mix | NS | 1.95 | 2.04 |
| Carbohydrates from mineral mix | NS | 0.77 | 0.41 |
| Other carbohydrates | 48.7 | 2.5 | 0.76 |
Values are % by weight of total. AIN vitamin mix is 97.7% carbohydrate by weight. AIN mineral mixes are 22.1% (5140) or 11.8% (3666) carbohydrate by weight. NS = not specified. Protein percentages are calculated from casein, which is typically 89% protein, plus methionine. Added values of percentages do not reach 100% as there are unlisted constituents. Ketogenic ratios (in parentheses) are listed for the two ketogenic diets. Information retrieved from product information sheets.
Nocifensive testing
Animals were placed on a hotplate (Columbus Instruments, Columbus, Ohio) and latency to hindpaw-associated nocifensive behavior was measured. Most rats demonstrated paw lifting followed rapidly by licking, although lifting without licking was also counted. Rats were kept on the hotplate surface with a Plexiglas box (27 x 30 x 18 cm). Animals were removed from the plate immediately upon display of nocifensive behavior, and all tests were limited to a 60 s ceiling to prevent tissue damage. Because we expected that dietary effects on thermal pain sensitivity would be mild, low temperatures (i.e., <50°C) were included.1 The lowest temperature was established as one that would allow the 60 s ceiling would be reached. The highest temperature was established as one that yielded a response in control rats in the 10–15 s range, allowing time for accurate scoring of the response and also to prevent tissue damage. Testing occurred once-daily on six consecutive days, with plate temperature increasing 1°C daily, from 46 to 51°C; no rat went through the 6-day hotplate testing regimen more than once. Daily hotplate testing influences latency to nocifensive behavior minimally or not at all.13,16 Rats remained on their respective diets throughout testing except for a subset of strict KD-fed animals that were reverted to the CD for 24 h before a repeated, final 51°C test.
Motor/cognitive testing
Separate groups of rats were tested for changes in motor activity and short-term memory in a non-rewarded, spontaneous alternation test based on exploratory drive. The Y-maze consisted of three angularly equidistant 45 × 10 cm arms with 21 cm-high walls surrounding a triangular center. Testing began once the animal was placed into the start arm under red light and continued for 5 min; the order of arm entries was recorded. Locomotor activity was measured as total number of arm entries, and spatial working memory was measured by the percentage of possible spontaneous alternations (sequential entry into all three arms). Testing was repeated over the course of two weeks of dietary treatment to match nocifensive testing. Data from rats that had two or fewer arm entries were excluded from working memory analysis.
Blood chemistry
To avoid the possibility of the biochemical or behavioral results being affected by handling, separate groups of rats were devoted to blood chemistry. Tail blood was taken either as a repeated measure several times over the course of dietary treatment (isoflurane-anesthetized rats) or at an individual time point (waking rats). Glucose and β-hydroxybutyrate were quantified from whole blood using a Precision Xtra meter with glucose and ketone strips (Abbott Laboratories, Abbott Park, Illinois).
Analysis
Behavior and blood chemistry data were analyzed with t-tests, one-way, or two-way repeated measures analysis of variance as appropriate. Neuman-Keuls tests were used for post hoc comparisons. Data are presented as mean ± standard error.
Results
As outlined in detail below, we found that two different KD formulations produced similar significant hypoalgesia to thermal pain. In both cases we found a differential time course of behavioral versus metabolic effects of KDs.
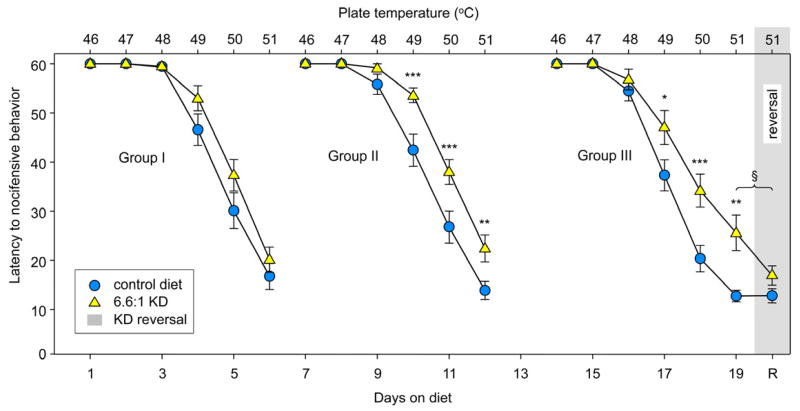
To establish time courses of hypoalgesia, ketosis, and reduced glucose, male rats were fed with the 6.6:1 KD (or were kept on the CD) for 1, 7 or 14 days before the behavioral testing series (1 temperature per day for 6 days) to explore the evolution of KD-induced hypoalgesia. The KD had no significant effects on nocifensive behavior in animals fed a KD for 1–6 days (Fig. 1, Group I, left). When a separate group of rats underwent the same testing after being fed this KD for 7–12 days, however, significant effects became clear, with the earliest significant comparison found at 10 days of treatment (at 49°C; Fig. 1, Group II, middle). Similar significant effects of this diet were quantified in rats fed for 14–19 d (Fig. 1, Group III, right).
Figure 1.
A 6.6:1 KD produces hypoalgesia which develops over time. Three groups of rats were challenged with an incremental 6-temperature test (one temperature per day) after varying times on the 6.6:1 KD. Top and bottom X-axes indicate hotplate temperature and days on diet, respectively. Gray shading indicates one day reversal of the KD in Group III. Left: Group I was tested from 1–6 days of KD feeding. Latency to behavioral response decreased with increased temperature, as expected, but there was no effect of the diet at any temperature at these early time points: Diet F=2.8, n.s.; Temperature F=160.3, p<0.001; Interaction F=1.5, n.s; n=20, both groups. Middle: Group II was tested across the same temperature range from 7–12 days, and a significant effect of diet was found; latency to behavioral response was increased in the KD group at 49, 50 and 51°C: Diet F=14.5, p<0.001; Temperature F=187.9, p<0.001; Interaction F=4.4, p<0.001; n=19–20. Right: Group III was tested from 14–19 days of KD feeding, and, similar to Group II, diet-induced hypoalgesia was also found at 49, 50 and 51°C: Diet F=5.7, p=0.19; Temperature F=158.4, p<0.001; Interaction F=4.0, p<0.001; n=18–20. Far right: After the last day of the temperature series, Group III KD-fed rats were reversed to the CD for 1 day (Day 20) and all rats were retested at 51°C. There was no difference between the behavioral response of this diet-reversal group and the CD-fed animals. *p<0.05, **p<0.01, ***p<0.001 compared to CD at corresponding temperatures. §p<0.05, t-test, comparing pre- and post-diet reversal values at 51°C in the 6.6:1 KD group.
After the 6-trial test sequence in the group fed for 14–19 days, animals on the 6.6:1 KD were switched back to the CD for 1 day and were retested at the highest temperature (51°C): hypoalgesia was no longer present (Fig. 1, far right, reversal); behavioral responses of rats reverted to the CD became similar to those found in CD-fed animals at the same time point. As expected, CD-fed animals tested twice at 51°C showed similar sensitivity on both days (Fig. 1, Group III, far right, 19 days versus R).
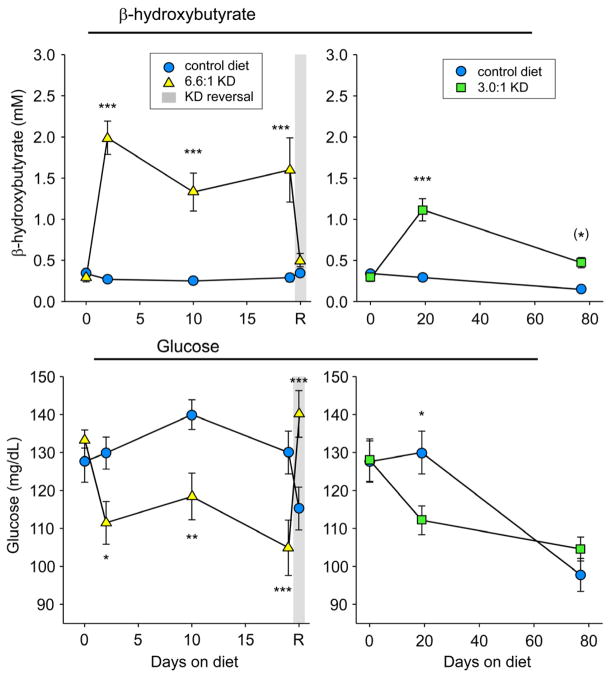
In contrast to the gradual onset of hypoalgesia to thermal pain, increased blood ketones and decreased glucose were measured at the earliest time point tested (2 days of 6.6:1 KD feeding) and remained significant for at least 19 days (Fig. 2, left panels). Thus, hallmark KD-induced changes in blood chemistry became significant days prior to any KD-induced changes in thermal nociception. Consistent with behavioral effects, these changes in blood chemistry reversed after 1 day of CD-feeding (Fig. 2, left panels).
Figure 2.
Two different KD formulations (6.6:1 and 3.0:1) produce ketosis (increased β-hydroxybutyrate, top panels) and lowered glucose (bottom panels). Starting at 2 days of 6.6:1 KD feeding, ketones and glucose were significantly elevated and reduced, respectively, and remained so for 19 days (switch to CD led to reversal of these effects; gray shading indicates one day reversal of the KD). With the 3.0:1 KD, these effects were present at 19 days, but only ketosis remained at 74 days. The decrease in blood glucose in the control group from 3 to 13 weeks of age is an effect we consistently find and appears to be developmental. For brevity, only Diet-x-Time interactions are listed here - Top left panel: F=10.8, p<0.001. Top right panel: F=21.7, p<.001. Bottom left panel: F=8.7, p<0.001. Bottom right panel: F=4.4, p=0.016. Number of subjects was 12–14. (*)p=0.055, *p<0.05, **p<0.01, ***p<0.001 compared to CD at corresponding times.
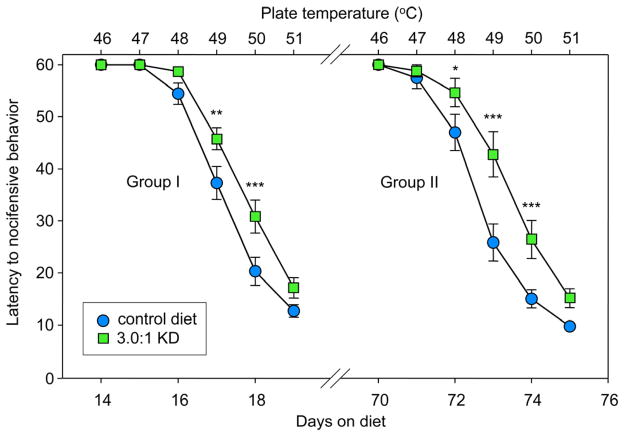
To explore the effects of a diet formula more similar to those used clinically (typically for pediatric epilepsy), we tested a less strict KD and longer treatment times (up to 11 weeks). Similar to the 6.6:1 KD, we found significant hypoalgesia at 14–19 days of feeding on a 3.0:1 KD (49 and 50°C; Fig. 3, Group I, left). In addition, we tested the longevity of these effects using the 3.0:1 formula. After extended feeding (70–75 days), hypoalgesia was still present, and became significant at three temperatures (48, 49, and 50°C), suggesting that this effect could be evolving at a timescale of weeks (Fig. 3, Group II, right).
Figure 3.
A moderate 3.0:1 KD formulation produces hypoalgesia which persists through 10–11 weeks of feeding. Separate groups of rats were challenged with a daily incremental 6-temperature test after 2 or 10 weeks on the 3.0:1 KD. Top and bottom X-axes indicate hotplate temperature and days on diet, respectively. Group I was tested from 14–19 days, and the diet had significant effects at 49 and 50°C: Diet F=9.4, p=0.005; Temperature F=205.5, p<0.001; Interaction F=3.0, p=0.013; n=18–20. Group II was tested from 70–75 days, and the diet had significant effects at 48, 49 and 50°C: Diet F=10.5, p=0.003; Temperature F=172.6, p<0.001; Interaction F=4.4, p<0.001; n=17–19. *p<0.05, **p<0.01, ***p<0.001 compared to CD at corresponding temperatures.
Upon exploring the relationship between the behavioral and metabolic effects of the 3.0:1 KD, we found that at 19 days the effects were similar; the 3.0:1 KD caused ketosis, decreased glucose and hypoalgesia similar to the stricter KD. However, after 76 days, when behavioral effects were still significant, ketosis remained but glucose was no longer decreased by this KD (Fig. 2, right panels). Increased ketones and the lack of a significant difference in blood glucose from CD-fed and 3.0:1 KD-fed at 76 days of diet treatment was similar between anesthetized rats having had multiple blood sampling (Fig. 2, right panels) and unanesthetized rats sampled only once (β-hydroxybutyrate: CD - 0.16±0.03 mM, KD - 0.49±0.10 mM, p<0.01; glucose: CD - 96±5 mg/dL, KD - 99±3 mg/dL, n.s.). Thus, KD-induced thermal hypoalgesia (Fig. 3, Group II, right) can occur without a significant reduction in blood glucose.
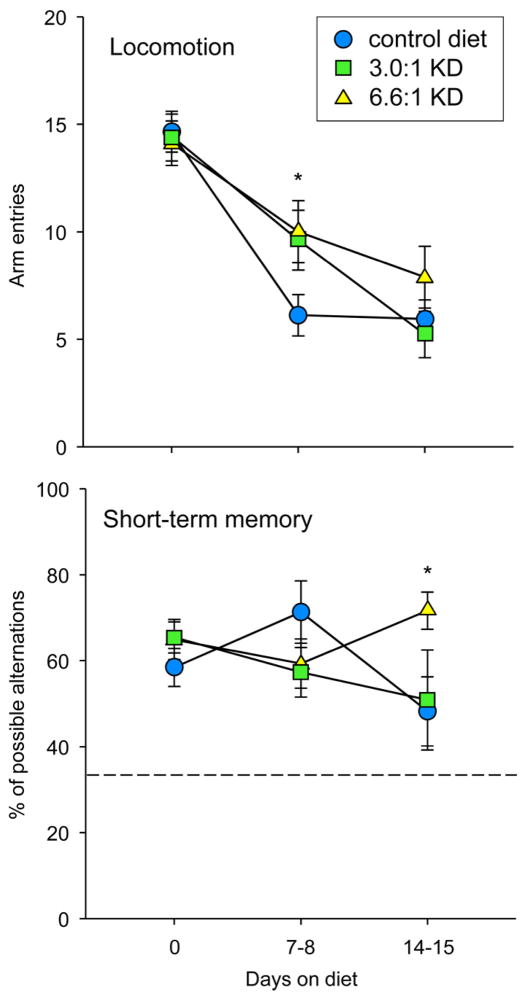
Diet effects on motor activity were assessed with repeated explorations of a Y-maze before and during treatment. In all groups, locomotion decreased over repeated testing. However, after 7–8 days, locomotion was significantly higher in both KD groups compared to the control group; this difference was not apparent one week later (Fig. 4, top). Thus, at two weeks of KD treatment, when we found significant differences in thermal pain responses, we found neither locomotor hyperactivity nor motor impairment. Consistent with this finding, in previous work we determined that the 6.6:1 KD does not impair motor coordination after longer feeding (10 weeks) in mice.45
Figure 4.
KDs transiently and minimally affected locomotion and spatial memory in the Y-maze. Top: Locomotion decreased over repeated testing in all groups, but was increased with both KDs at one week of feeding compared to controls; this difference was no longer present at two weeks. Bottom: The accuracy of spontaneous alternations significantly increased at two weeks of feeding with the 6.6:1 KD; this effect was not present with the 3.0:1 KD. Dashed line indicates level for random arm entries. Locomotion: Time F=60.7, p<0.001; Interaction F=2.6, p=0.041. Memory: Interaction F=2.5, p=0.047; n=17–18. *p<0.05 compared to CD at corresponding time.
In conjunction with locomotor activity, the Y-maze protocol assessed short-term memory by quantifying spontaneous alternation behavior. Level of alternation behavior varied, but the only significant difference was that scores were higher at two weeks for the 6.6:1 KD compared to control diet. We have previously found mild beneficial effects of this 6.6:1 diet on spontaneous alternation accuracy in mice.45 Together, these data suggest effects of this diet on spontaneous alternation are either neutral or positive.
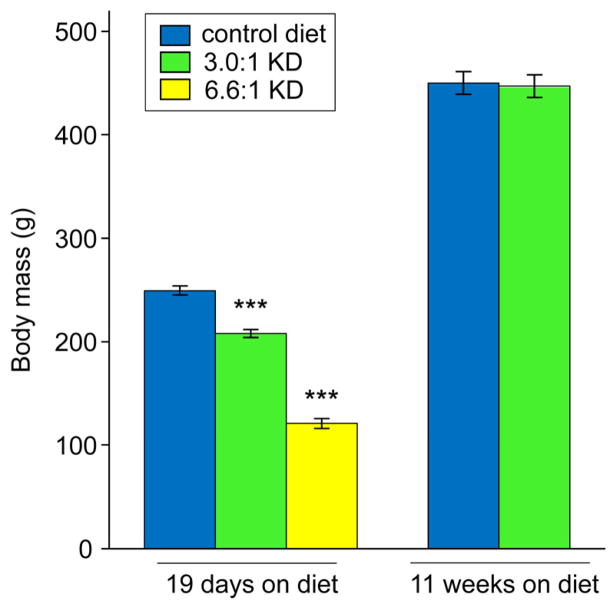
After 19 d of feeding with either KD, body weights were reduced, although less so for the 3.0:1 KD (Fig. 5). Reduced body weight was no longer present at 76 days of feeding with the 3.0:1 KD (Fig. 5), a time point when elevated ketones and reduced thermal pain sensitivity were demonstrated.
Figure 5.
Varying KD effects on body weight. At 19 days of feeding, KDs led to lower body weight, although the effect was smaller for the moderate 3.0:1 KD (F=232.5, p<0.001; n=12, all groups). At 75 days, weights of rats fed the 3.0:1 KD did not differ from CD-fed rats (t-test, n.s., n=17–19). ***p<0.001 compared to CD.
Discussion
This study demonstrates that reduced sensitivity to thermal pain is produced consistently by KDs – at both a very high-fat research-strength (6.6:1) and a more moderate clinical-strength (3.0:1). Importantly the hypoalgesic effect is consistent across a range of diet treatments lasting from 1.5 to 11 weeks, the longest time point tested here. In addition, for the first time, we establish a clear temporal distinction between the initial appearance of two hallmark metabolic changes observed with a KD – increased blood ketones and decreased blood glucose – and the onset of diet-related hypoalgesia. Furthermore, we determined that the altered sensitivity to thermal pain was not due to negative general effects on locomotor activity or working memory.
Our data dissociate thermal hypoalgesia from ketosis and lowered glucose in at least two ways. First, we found both metabolic effects but no behavioral effects at time points of less than 6 days. Specifically, rats fed the strongest KD (6.6:1) for 1–6 days demonstrated reduced glucose and increased ketones with no change in sensitivity to thermal pain. Second, we found behavioral effects with only one metabolic effect: significant hypoalgesia, with ketosis - but without low glucose - was found in rats fed the 3.0:1 KD for 10–11 weeks. It is notable that all hypoalgesic states quantified here were accompanied by ketosis, and ketosis might be necessary but not sufficient: at 2 d we found significant ketosis but no change in thermal nociception. We conclude that KD-related thermal hypoalgesia is not produced immediately by decreased glucose or elevated ketones acting directly on molecular targets, and hypothesize that the effect stems from central biochemical or metabolic adaptations to a KD.
The KD is meant to mimic fasting. Fasting has many parallels to the KD, including metabolic consequences and anticonvulsant activity. Fasting also produces thermal hypoalgesia.10 While fasting is necessarily linked to loss of body mass, we found that KD-induced hypoalgesia (like KD-related antiepileptic effects in children17) is not related directly to weight loss. We make this conclusion based on our present results with rats tested at 10–11 weeks of diet treatment and previously published work with adult rats fed diets for 3–4 weeks.43
This study and our previous study reporting thermal hypoalgesia43 contrast with Ziegler et al.,61 which describes thermal hyperalgesia in a tail flick assay after extended KD feeding. Here our length of diet treatment (10–11 weeks) and diet formulation (3.0:1 KD, containing 18% protein) were similar those used by Ziegler et al., and thus these factors are unlikely to underlie the apparently disparate results. The nature of the behavioral responses could be relevant, as withdrawal in the tail flick assay is a spinally-mediated reflex whereas paw withdrawal/licking is a voluntary motor behavior involving the brain. An overall difference in thermal pain sensitivity between glabrous and hairy skin might also be involved.52
The KD was developed originally to treat epilepsy, and there are a number of striking parallels between its known anticonvulsant effects and the hypoalgesic effects observed here: (1) both effects are delayed compared to the onset of ketosis and low glucose;6,11,40,46 (2) both appear to depend on ketosis - though ketosis alone is not sufficient for either;4,19,50,53 and (3) breaking the diet by eating carbohydrates (or by injecting glucose) reverses the effects of the diet.20,32,34,54 This pattern suggests a common central mechanism involving reduced excitability or augmented inhibition, and argues against mediation by direct actions of ketones or glucose on targets such as ion channels or neurotransmitter transporters. Possible mechanisms “downstream” of immediate KD effects include more efficient production of ATP12,36 leading to better maintenance of membrane potential through the ATPase Na+/K+ pump,15,56 increased levels of the inhibitory neuromodulator adenosine (and consequent KATP activation) as a metabolite of augmented ATP and/or due to downregulation of the adenosine-metabolizing enzyme adenosine kinase,23,30,32 or modified GABA and glutamate levels secondary to shifted equilibria of the enzyme aspartate transaminase and the mitochondrial malate/α-ketoglutarate antiporter.28,60
Alternatively, levels of some dietary constituents of a ketogenic diet, notably fatty acids, rise in the body with a time course that more closely parallels that of hypoalgesia.9,11 In vitro data show that polyunsaturated fatty acids can open K+ channels,26,58 and it has been suggested that polyunsaturated fats are thus a key component in the antiseizure effect of the KD.14 The 6.6:1 KD used presently is higher in polyunsaturated fats than the CD (12.2% versus 1.3% by weight), although it is not specifically enriched in these fats. Notably, rats made obese through a high-fat/high-carbohydrate diet also demonstrate thermal hypoalgesia,37 whereas genetically obese rats are thermally hyperalgesic,41 suggesting a role for high fat in the diet. The present and prior work shows, however, that obesity is not necessary for thermal hypoalgesia,43 and the KD and other low-carbohydrate diets may represent a comparatively healthier means for high fat intake compared to a high-fat/high-carbohydrate diet.
Clinical work with the KD for diseases and conditions other than epilepsy is overdue yet only beginning. This is particularly true for pain, which shares pharmaceutical approaches with epilepsy, and remains a prevalent chronic problem without good pharmaceutical options for many patients. In the one extant study regarding clinical pain and a KD, the beneficial effect of a KD on self-reported pain was at the threshold of statistical significance.59 However, this study was not a dedicated study of pain but rather one that included self-reported pain as part of assessment of quality of life (several physical and mental aspects of quality of life were improved significantly by the KD), and the subject population did not consist of pain sufferers. In chronic pain patients, fasting - which similarly forces ketone-based metabolism – offers well-established pain relief (reviewed by Michalsen35) suggesting that metabolic approaches have untapped potential for treating pain.
In summary, we provide clear evidence that altered metabolism can reduce pain: KDs consistently produce hypoalgesia to thermal pain in rats. Compared to acute metabolic effects, hypoalgesia has a slow onset – although it can reverse rapidly upon terminating the diet. Alongside the present results, multiple lines of evidence support the potential efficacy of metabolic strategies in relieving pain. Foremost, the KD has been proven to be anticonvulsant, and anticonvulsant medications are often prescribed for chronic pain. Also, the KD mimics the metabolic consequences of fasting, and fasting reduces pain. Ongoing research into the mechanisms of the KD and analogous metabolic approaches might lead to the coveted “diet in the pill”.38 Here we demonstrated that the KD produces hypoalgesia in rats, whereas clinical studies of KD treatment in chronic pain sufferers might demonstrate that the KD (more sustainable and tolerable than fasting), can be an effective non-pharmacological tool available immediately for controlling pain.
Perspective.
Chronic pain is a common and debilitating condition. We show that a ketogenic diet, a high fat, very low carbohydrate diet well-known for treating epilepsy, lowers sensitivity to thermal pain in rats. This reduced pain is not temporally-correlated with hallmark diet-induced changes in blood glucose and ketones.
Acknowledgments
We thank Joshua H. Altschuler for assistance in data collection, and Jenny Nord for animal care.
Footnotes
Disclosures
Funded by National Institute of Neurological Disorders and Stroke (DNR, NS065446; SAM, NS066392 & NS065957), National Science Foundation (SAM, IOS-0843585), and Trinity College. The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ankier SI. New hot plate tests to quantify antinociceptive and narcotic antagonist activities. Eur J Pharmacol. 1974;27:1–4. doi: 10.1016/0014-2999(74)90195-2. [DOI] [PubMed] [Google Scholar]
- 2.Attal N. Drug treatment for neuropathic pain. Presse Med. 2008;37:346–353. doi: 10.1016/j.lpm.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Bachelard HS, Cox DWG, Drower J. Sensitivity of guinea-pig hippocampal granule cell field potentials to hexoses in vitro: an effect on cell excitability? J Physiol. 1984;352:91–102. doi: 10.1113/jphysiol.1984.sp015279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bough KJ, Chen RS, Eagles DA. Path analysis shows that increasing ketogenic ratio, but not β-hydroxybutyrate, elevates seizure threshold in the rat. Dev Neurosci. 1999;21:400–406. doi: 10.1159/000017390. [DOI] [PubMed] [Google Scholar]
- 5.Bough KJ, Schwartzkroin PA, Rho JM. Caloric restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia. 2003;44:752–760. doi: 10.1046/j.1528-1157.2003.55502.x. [DOI] [PubMed] [Google Scholar]
- 6.Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 7.Cantello R, Varrasi C, Tarletti R, Cecchin M, D’Andrea F, Veggiotti P, Bellomo G, Monaco F. Ketogenic diet: electrophysiological effects on the normal human cortex. Epilepsia. 2007;48:1756–1763. doi: 10.1111/j.1528-1167.2007.01156.x. [DOI] [PubMed] [Google Scholar]
- 8.Cross JH, Neal EG. The ketogenic diet - update on recent clinical trials. Epilepsia. 2008;49 (Suppl 8):6–10. doi: 10.1111/j.1528-1167.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 9.Dahlin M, Hjelte L, Nilsson S, Åmark P. Plasma phospholipid fatty acids are influenced by a ketogenic diet enriched with n-3 fatty acids in children with epilepsy. Epilepsy Res. 2007;73:199–207. doi: 10.1016/j.eplepsyres.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 10.de los Santos-Arteaga M, Sierra-Domínguez SA, Fontanella GH, Delgado-García JM, Carríon AM. Analgesia induced by dietary restriction is mediated by the κ-opioid system. J Neurosci. 2003;23:11120–11126. doi: 10.1523/JNEUROSCI.23-35-11120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekaban AS. Plasma lipids in epileptic children treated with the high fat diet. Arch Neurol. 1966;15:177–184. doi: 10.1001/archneur.1966.00470140067009. [DOI] [PubMed] [Google Scholar]
- 12.DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB., Jr Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3:331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- 13.Espejo EF, Mir D. Differential effects of weekly and daily exposure to the hot plate on the rat’s behavior. Physiol Behav. 1994;55:1157–1162. doi: 10.1016/0031-9384(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 14.Fraser DD, Whiting S, Andrew RD, Macdonald EA, Musa-Veloso K, Cunnane SC. Elevated polyunsaturated fatty acids in blood serum obtained from children on the ketogenic diet. Neurology. 2003;60:1026–1029. doi: 10.1212/01.wnl.0000049974.74242.c6. [DOI] [PubMed] [Google Scholar]
- 15.Greene AE, Todorova MT, Seyfried TN. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J Neurochem. 2003;86:529–537. doi: 10.1046/j.1471-4159.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 16.Gunn A, Bobeck EN, Weber C, Morgan MM. The influence of non-nociceptive factors on hot-plate latency in rats. J Pain. 2011;12:222–227. doi: 10.1016/j.jpain.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamdy RF, Turner Z, Pyzik PL, Kossof EH. Lack of influence of body mass index on the efficacy of the ketogenic diet. J Child Neurol. 2007;22:1167–1171. doi: 10.1177/0883073807306255. [DOI] [PubMed] [Google Scholar]
- 18.Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–292. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hori A, Tandon P, Holmes GL, Stafstrom CE. Ketogenic diet: effects on expression of kindled seizures and behavior in adult rats. Epilepsia. 1997;38:750–758. doi: 10.1111/j.1528-1157.1997.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 20.Huttenlocher PR. Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res. 1976;10:536–540. doi: 10.1203/00006450-197605000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Johannessen Landmark C. Antiepileptic drugs in non-epileptic disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22:27–47. doi: 10.2165/00023210-200822010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawamura M, Jr, Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors and KATP channels. J Neurosci. 2010;30:3886–3895. doi: 10.1523/JNEUROSCI.0055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kossoff EH, Zupec-Kania BA, Åmark PE, Ballaban-Gil KR, Bergqvist AGC, Blackford R, Buchhalter JR, Caraballo RH, Cross JH, Dahlin MG, Donner EJ, Klepper J, Jehle RS, Kim HD, Liu YMC, Nation J, Nordli DR, Jr, Pfeifer HH, Rho JM, Stafstrom CE, Thiele EA, Turner Z, Wirrell EC, Wheless JW, Veggiotti P, Vining EPG the Charlie Foundation, the Practice Committee of the Child Neurology Society: Optimal clinical management of children receiving the ketogenic diet: Recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50:304–317. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 25.Kossoff EH, Zupec-Kania BA, Rho JM. Ketogenic diets: an update for child neurologists. J Child Neurol. 2009;24:979–988. doi: 10.1177/0883073809337162. [DOI] [PubMed] [Google Scholar]
- 26.Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J. 2000;19:1784–1793. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy RA, Proudfit HK. The analgesic action of baclofen [β-(4-chlorophenyl)-γ-aminobutyric acid] J Pharmacol Exp Ther. 1977;202:437–445. [PubMed] [Google Scholar]
- 28.Lund TM, Risa Ø, Sonnewald U, Schousboe A, Waagepetersen HS. Availability of neurotransmitter glutamate is diminished when β-hydroxybutyrate replaces glucose in cultured neurons. J Neurochem. 2009;110:80–91. doi: 10.1111/j.1471-4159.2009.06115.x. [DOI] [PubMed] [Google Scholar]
- 29.Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. J Neurosci. 2007;27:3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masino SA, Geiger JD. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends Neurosci. 2008;31:273–278. doi: 10.1016/j.tins.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masino SA, Kawamura M, Jr, Wasser CD, Pomeroy LT, Ruskin DN. Adenosine, ketogenic diet and epilepsy: the emerging therapeutic relationship between metabolism and brain activity. Curr Neuropharmacol. 2009;7:257–268. doi: 10.2174/157015909789152164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masino SA, Li T, Theofilas P, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, Boison D. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Invest. 2011;121:2679–2683. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masino SA, Rho JM. Mechanisms of Ketogenic Diet Action. In: Rogawski MA, Delgado-Escueta AV, Noebels JL, Avoli M, Olsen RW, editors. Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information; Bethesda, MD: 2011. pp. 1001–1022. [PubMed] [Google Scholar]
- 34.McQuarrie I, Keith HM. Epilepsy in children: relationship of variations in the degree of ketonuria to occurrence of convulsions in epileptic children on ketogenic diets. Am J Dis Child. 1927;34:1013–1029. [Google Scholar]
- 35.Michalsen A. Prolonged fasting as a method of mood enhancement in chronic pain syndromes: a review of clinical evidence and mechanisms. Curr Pain Headache Rep. 2010;14:80–87. doi: 10.1007/s11916-010-0104-z. [DOI] [PubMed] [Google Scholar]
- 36.Nakazawa M, Kodama S, Matsuo T. Effects of ketogenic diet on electroconvulsive threshold and brain contents of adenosine nucleotides. Brain Dev. 1983;5:375–380. doi: 10.1016/s0387-7604(83)80042-4. [DOI] [PubMed] [Google Scholar]
- 37.Ramzan I, Wong BK, Corcoran GB. Pain sensitivity in dietary-induced obese rats. Physiol Behav. 1993;54:433–435. doi: 10.1016/0031-9384(93)90231-4. [DOI] [PubMed] [Google Scholar]
- 38.Rho J, Sankar R. The ketogenic diet in a pill: is this possible? Epilepsia. 2008;49 (Suppl 8):127–133. doi: 10.1111/j.1528-1167.2008.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rho JM. How does altered metabolism lead to seizure control? Partially filling the knowledge gap. Epilepsy Curr. 2010;10:159–161. doi: 10.1111/j.1535-7511.2010.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rho JM, Kim DW, Robbins CA, Anderson GD, Schwartzkroin PA. Age-dependent differences in flurothyl seizure sensitivity in mice treated with a ketogenic diet. Epilepsy Res. 1999;37:233–240. doi: 10.1016/s0920-1211(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 41.Roane DS, Porter JR. Nociception and opioid-induced analgesia in lean (Fa/−) and obese (fa/fa) Zucker rats. Physiol Behav. 1986;38:215–218. doi: 10.1016/0031-9384(86)90156-3. [DOI] [PubMed] [Google Scholar]
- 42.Rogawski MA. Diverse mechanisms of antiepileptic drugs in the development pipeline. Epilepsy Res. 2006;69:273–294. doi: 10.1016/j.eplepsyres.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruskin DN, Kawamura M, Jr, Masino SA. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One. 2009;4:e8349. doi: 10.1371/journal.pone.0008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruskin DN, Masino SA. The nervous system and metabolic dysregulation: emerging evidence converges on ketogenic diet therapy. Front Neurosci. 2012;6:33. doi: 10.3389/fnins.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruskin DN, Ross JL, Kawamura M, Jr, Ruiz TL, Geiger JD, Masino SA. A ketogenic diet delays weight loss and does not impair working memory or motor function in the R6/2 1J mouse model of Huntington’s disease. Physiol Behav. 2011;103:501–507. doi: 10.1016/j.physbeh.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samala R, Willis S, Borges K. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008;81:119–127. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998;347:1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 48.Scholfield CN, Steel L. Presynaptic K-channel blockade counteracts the depressant effect of adenosine in olfactory cortex. Neuroscience. 1988;24:81–91. doi: 10.1016/0306-4522(88)90313-2. [DOI] [PubMed] [Google Scholar]
- 49.Sharma S, Gulati S, Kalra V, Agarwala A, Kabra M. Seizure control and biochemical profile on the ketogenic diet in young children with refractory epilepsy - Indian experience. Seizure. 2009;18:446–449. doi: 10.1016/j.seizure.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Sirven J, Whedon B, Caplan D, Liporace J, Glosser D, O’Dwyer J, Sperling MR. The ketogenic diet for intractable epilepsy in adults: preliminary results. Epilepsia. 1999;40:1721–1726. doi: 10.1111/j.1528-1157.1999.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 51.Sollevi A. Adenosine for pain control. Acta Anaesthesiol Scand Suppl. 1997;110:135–136. doi: 10.1111/j.1399-6576.1997.tb05532.x. [DOI] [PubMed] [Google Scholar]
- 52.Taylor DJ, McGillis SL, Greenspan JD. Body site variation of heat pain sensitivity. Somatosens Mot Res. 1993;10:455–465. doi: 10.3109/08990229309028850. [DOI] [PubMed] [Google Scholar]
- 53.Todorova MT, Tandon P, Madore RA, Stafstrom CE, Seyfried TN. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia. 2000;41:933–940. doi: 10.1111/j.1528-1157.2000.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 54.Uhlemann ER, Neims AH. Anticonvulsant properties of the ketogenic diet in mice. J Pharmacol Exp Ther. 1972;180:231–238. [PubMed] [Google Scholar]
- 55.Vanotti A, Osio M, Mailland E, Nascimbene C, Capiluppi E, Mariani C. Overview of pathophysiology and newer approaches to treatment of peripheral neuropathies. CNS Drugs. 2007;21 (Suppl 1):3–12. doi: 10.2165/00023210-200721001-00002. [DOI] [PubMed] [Google Scholar]
- 56.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Welch SP, Dunlow LD. Antinociception activity of intrathecally administered potassium channel openers and opioid agonists: a common mechanism of action? J Pharmacol Exp Ther. 1993;267:390–399. [PubMed] [Google Scholar]
- 58.Xu X-p, Erichsen D, Börjesson SI, Dahlin M, Åmark P, Elinder F. Polyunsaturated fatty acids and cerebrospinal fluid from children on the ketogenic diet open a voltage-gated K channel: A putative mechanism of antiseizure action. Epilepsy Res. 2008;80:57–66. doi: 10.1016/j.eplepsyres.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Yancy WS, Jr, Almirall D, Maciejewski ML, Kolotkin RL, McDuffie JR, Westman EC. Effects of two weight-loss diets on health-related quality of life. Qual Life Res. 2009;18:281–289. doi: 10.1007/s11136-009-9444-8. [DOI] [PubMed] [Google Scholar]
- 60.Yudkoff M, Daikhin Y, Melø TM, Nissim I, Sonnewald U, Nissim I. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Annu Rev Nutr. 2007;27:415–430. doi: 10.1146/annurev.nutr.27.061406.093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziegler DR, Gamaro GD, Araújo E, Bassani MG, Perry MLS, Dalmaz C, Gonçalves C-A. Nociception and locomotor activity are increased in ketogenic diet fed rats. Physiol Behav. 2005;84:421–427. doi: 10.1016/j.physbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]