Abstract
Although many resting state fMRI human studies have been published, the number of such rodent studies is considerably less. The reason for this is the severe technical challenge of high magnetic field small rodent imaging. Local magnetic field susceptibility changes at air tissue boundaries cause image distortion and signal losses. The current study reports measures of functional connectivity in mice using only isoflurane for the anesthetic. Because all anesthetic agents will alter cerebral blood flow and cerebral metabolism, the impact these changes have on neuronal connectivity has yet to be fully understood, however this work reports for the first time that reliable functional connectivity measures in mouse brain can be obtained with isoflurane.
Keywords: Resting state fMRI, Default Mode Network, Isoflurane, Functional Connectivity
1. Introduction
Resting state functional Magnetic Resonance Imaging (rsfMRI) has generated a great deal of interest among neuroscientists, as reflected in the marked increase of publications on the topic in recent years. Unlike standard fMRI, the subject is not required to perform any specific task during the scan, the subject remains at ‘rest’. This is particularly advantageous in studies of patients who have difficulty performing such tasks. Resting state fMRI typically examines low frequency oscillations (typically less than 0.1Hz) using Blood Oxygen Level Dependent (BOLD) perturbations of the MR signal. These low frequency oscillations can be highly correlated across regions and thus allow mapping of neuronal networks. The temporal and regional correlation of brain activity is often referred to as functional connectivity (FC) and provides a modality to measure neuronal “network” connectivity, while the subject is at rest. Following the initial rsfMRI demonstration of resting state networks for motor regions (Biswal, Yetkin et al., 1995), other networks have been shown that relate to sensory regions, attentional regions, salience regions, and the like (De Luca M., Beckmann et al., 2006). These networks have also been demonstrated in positron emission tomography (PET) scanning (Raichle, MacLeod et al., 2001), suggesting that they are general across scanning modalities. A useful review of rsfMRI findings in neuropsychiatric disorders can be found in the work of Greicius (Greicius, 2008).
In humans, a set of regions including the medial prefrontal cortex, posterior cingulate, lateral parietal regions, and parahippocampus have been shown to form a network that has been very reliably demonstrated across studies. This network, termed the default mode network (DMN), has been shown to be anti-correlated with a task related network (TRN), which includes dorsal anterior cingulate and inferior parietal regions (Fox, Snyder et al., 2005). These networks show consistency across individuals (Damoiseaux, Rombouts et al., 2006) and across time (Shehzad, Kelly et al., 2009). Recent work has shown that the DMN can be detected in other species. For instance, in anesthetized monkeys, Vincent et al. (Vincent, Patel et al., 2007) showed a network that closely resembles that in humans. Similarly, Lu et al. (Lu, Zou et al., 2012) was able to demonstrate a default mode network in the rat. These animal studies have all used BOLD or PET methods to examine the DMN. A recent study used optical imaging to detect a DMN in mice (White, Bauer et al., 2011).
Several resting state studies in rats have used a continuous infusion of dexmedetomidine (Lu, Zou et al., 2012) or medetomidine (Zhao, Zhao et al., 2008). These anesthetic strategies act as analgesic muscle relaxant, and so the animal is much less sedated than with isoflurane. The impact of the administered anesthetic on the resting state neuronal network remains unclear and much further investigation is required. The present study demonstrates that reliable functional connectivity measures in mice can be detected using a regime of isoflurane alone and thus simplifies the anesthetic administration procedure.
fMRI measures of murine brain are also technically challenging because inhomogeneities and discontinuities in bulk magnetic susceptibility cause local magnetic field gradients, which results in image distortion and signal losses. This is particularly problematic for mouse brain as the ratio of air space to tissue volume is very high. Because the brain surface represents a major source of the locally generated field gradients, a significantly larger percentage of a mouse brain is affected as compared to human brain. Echo Planar Imaging (EPI) is the imaging modality typically used for fMRI studies. Unfortunately, EPI is very sensitive to the susceptibility-induced distortions, making mouse brain fMRI extremely difficult. In this study an interleaved EPI acquisition was used, which breaks down the acquired data into several segments. The advantage of this method is the reduction in the sensitivity to the susceptibility- induced distortions, but without any loss of temporal resolution. Here we report the first demonstration of functional connectivity in mice using a robust interleaved EPI acquisition using only isoflurane as the anesthetic agent. We also report data on the stability of this measure.
2. Materials and Methods
2.1 Image acquisition strategy
The imaging approach used in this study consists of an interleaved snapshot EPI module. This method splits the conventional EPI sequence into a series of excitation–acquisition blocks applied in immediate succession within a single repetition period. The full details of this acquisition strategy have been described previously (Guilfoyle and Hrabe, 2006). Because there are virtually no delays between the acquisition blocks, there is almost no loss of temporal resolution in comparison with conventional EPI. The susceptibility distortions are minimized owing to shorter sampling intervals after each excitation, in much the same way as parallel acquisitions methods. Briefly, there are four key elements: (1) Variable flip angles , s=0, 1, 2, …, n−1, are employed to equalize the transverse magnetization among all n segments. (2) Polarity of the read gradient is reversed between the segments to preserve the k-space structure of a traditional EPI acquisition in the dataset combined from all interleaved segments. (3) Onset of acquisition in a segment’s is delayed by (s/n) TRL, where TRL = readout length, to ensure smooth T2* decay over the k-space data set, free of a step-wise modulation and the associated ghosting in the reconstructed image. (4) There are no other delays between the acquisition blocks to avoid any loss of temporal resolution in comparison with the conventional EPI.
2.2 Resting State Measurements
All data were obtained on a 7.0 Tesla Agilent (Santa Clara, CA) 40 cm bore system. The gradient coil insert had an internal diameter of 12 cm with a maximum gradient strength of 600 mT/m and minimum rise time of 200 μs. A Rapid (Rimpar, Germany) volume transmit coil (72 mm ID) and a 2 channel receive-only surface coil were used for RF transmission and reception. All resting state fMRI acquisitions were acquired with the following parameters: matrix size 64 × 64, field of view = 30 mm, slice thickness = 1 mm, number of slices = 14 with a 0.1 mm gap and interleaved, echo time = 20 ms, repetition time = 2s, number of volumes = 360, 3 segments with variable flip angles = 35°, 45° and 90°.
Anatomical images with the same field of view, slice thickness, and number of slices as the rsfMRI data were also acquired. These images are used for image registration. The anatomical acquisition was a Fast Spin Echo sequence with an echo train length of 8, matrix size of 128 × 128, repetition time of 2 s and effective echo time of 6.5 ms. Total acquisition times: resting state fMRI =12 minutes and anatomical scan = 2 minutes. All first and second order shims were automatically adjusted with FASTMAP (Gruetter, 1993) from a voxel placed over the volume of interest.
2.3 Animals
Twelve male C57BL/6 mice with an average age of 5 months and average weight of 28 g were used in this study. An SA Instruments, Inc. animal monitoring unit (model 1025, Stony Brook, NY) was used for monitoring respiration, heart rate and rectal temperature. Respiration was measured with a pressure transducer placed under the abdomen just below the ribcage. An infra-red pulse oximeter sensor, placed on the tail, or hind foot, enabled heart rate monitoring. Body temperature was maintained using forced warm air, controlled by a feedback circuit between the heater and thermistor. All animals were anesthetized using an isoflurane vaporizer set at the following: 3–4% for induction, 2% during piloting, and 1.5% during resting state scanning. After induction, subjects were placed on the RF coil tray and restrained by the head using a bite bar and ear bars placed half way into the aural canal. Oxygen was used as the carrier gas and delivered at low flow rate (</= 0.5 L/min.) to a cone positioned before the bite bar, where gases mixed with air were passed over the rodents nose. All animals were maintained at 37+/−0.2 °C and respiration ranged between 50 to 70 breaths per minute with a median heart rate of 500 beats per minute during scanning.
All animal procedures were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the Nathan Kline Institute for psychiatric research.
2.4. Statistical Analysis
Scripts from Biswal et al. (Biswal, Mennes et al., 2010) (available at: http://www.nitrc.org/forum/forum.php?forum_id=1383) were used to preprocess the data as in our previous work in rats (Wilson, Hoptman et al., 2011). Briefly, the first 10 volumes were discarded to eliminate T1 relaxation effects. Thereafter, images were motion-corrected using AFNI (Cox, 1996). Time series were smoothed using a 2 mm FWHM Gaussian kernel using FSL, and grand mean scaled to a value of 10000 using FSL. Subsequently, the data were bandpass filtered (0.005Hz–0.1 Hz) and linear and quadratic trends were removed using AFNI. A high resolution anatomical template was chosen from the median volume of a larger set of 36 mice scanned to date.
Seed regions were chosen from an ICA-based analysis that demonstrated a DMN in the rat (Lu, Zou et al., 2012). Seed regions were traced on coronal sections in left and right posterior lateral cerebral cortex, medial cerebral cortex, insula, and prelimbic cortex. Their FC was computed on a voxel-wise basis. Correlations were converted to Z-scores using Fisher’s r-to-z transformation.
Group-level analyses were conducted using FSL’s ordinary least squares (OLS) model implemented in FLAME. A one-sample t-test on RSFC maps was performed to examine regions showing significant FC across the 12 mice. This statistical procedure produced threshold z-statistic maps of clusters defined by a threshold of Z = 2.3 and a corrected cluster threshold of p = .05 using Gaussian Random Field theory (Worsley, 2001).
3. Results
3.1 System stability
In any fMRI experiment it is important to establish that the system hardware is not introducing systematic noise into the analysis and that any system fluctuations are much less than the variations in signal to be measured. The Biomedical Informatics Research Network website offers publically available software for system stability measures (http://www.birncommunity.org/resources/tools/). This analysis results in a detailed output of the system stability.
Three major metrics can be used as a reliable measure of system stability. They are percentage signal fluctuation, signal drift and Radius of Decorrelation (RDC). A gel phantom was used for these measurements. The exact formula for the phantom is provided on the BIRN website. The percentage fluctuation is calculated as the standard deviations of the mean signal intensity of a time series divided by the mean signal intensity. The signal drift is the percentage signal variation of the time series (the percentage fluctuation is corrected for signal drift and is therefore a measure of shot to shot stability). The radius of decorrelation is a metric resulting from the Weisskoff stability test (Weisskoff, 1996). As a region of interest (ROI) gets larger, the standard deviation gets smaller through averaging of an increasing number of voxels. If the neighboring voxels are truly independent, then the standard deviation of a time series divided by the mean of the time series should be inversely proportional to the square root of the number of voxels in the ROI. The RDC is the size at which the statistical independence of the voxels is lost (Weisskoff, 1996). A 15 ml tube filled with gel as specified by the BIRN website was used in these experiments with the same acquisition parameters as the in vivo experiments. The percent fluctuation was 0.09% with a 1% signal drift and a RDC of 18 using a 20×20 pixel ROI. The BOLD activation is typically 1–2 % so the hardware fluctuations are significantly less than the neuronal blood flow modulations of the MR signal.
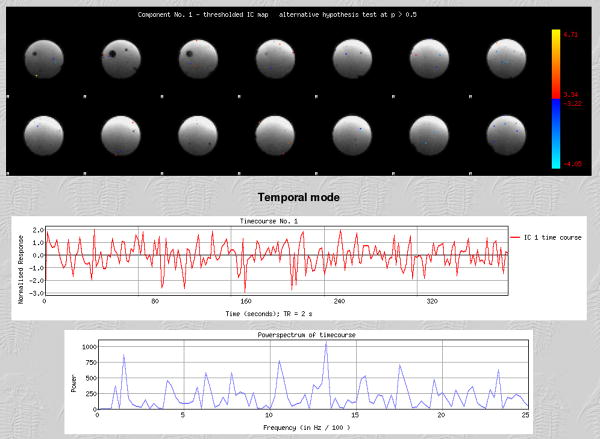
Figure 1 shows the results of the Independent Component Analysis (ICA) using the FSL’s MELODIC software (www.fmrib.ox.ac.uk/fsl/) from the gel phantom with the in vivo acquisition parameters. Only one component was detected which explained 0.64 % of the total variance. Also seen from Figure 1, the temporal and spatial distributions were fairly uniform and did not indicate any clear region or frequency range as representative of the phantom scan.
Figure 1.
Independent component analysis (ICA) of the BIRN phantom scan. Top panel shows spatial map of component (red = positive values, blue = negative values). Middle panel shows time course of component. Bottom panel shows frequency power spectrum for component.
3.2 In Vivo Results
We found evidence of a DMN for lateral cortical and medial cortical seeds (see Figure 2 and Table 1). Functional connectivity for these seeds included cortical regions, and, in the case of the right lateral and medial seed, subcortical regions such as the hippocampus, that are often included in the DMN. Evidence was less clear-cut for insular and prelimbic seeds (Figure 3), which showed more circumscribed regional FC.
Figure 2.
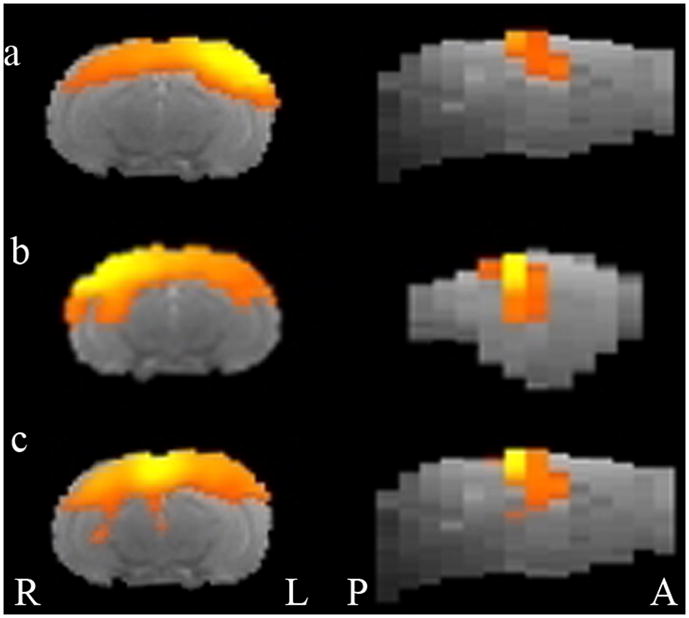
Functional connectivity maps for seeds in Default Mode Network (DMN) derived from Lu et al. (2012) in the rat superimposed on an anatomical template image. Left panel is coronal view (radiological orientation), right panel shows sagital view. a) Left lateral cortical seed, b) Right lateral cortical seed, c) Medial cortical seed. L=left, R=right, A=anterior, P=posterior.
Table 1.
Resting state network peak data
| ROI | Cluster Size | Z-max | p-value |
|---|---|---|---|
| Left lateral | 965 | 4.39 | 4.09×10−5 |
| Right lateral | 741 | 3.81 | .000155 |
| Medial cortex | 687 | 3.68 | .000211 |
Figure 3.
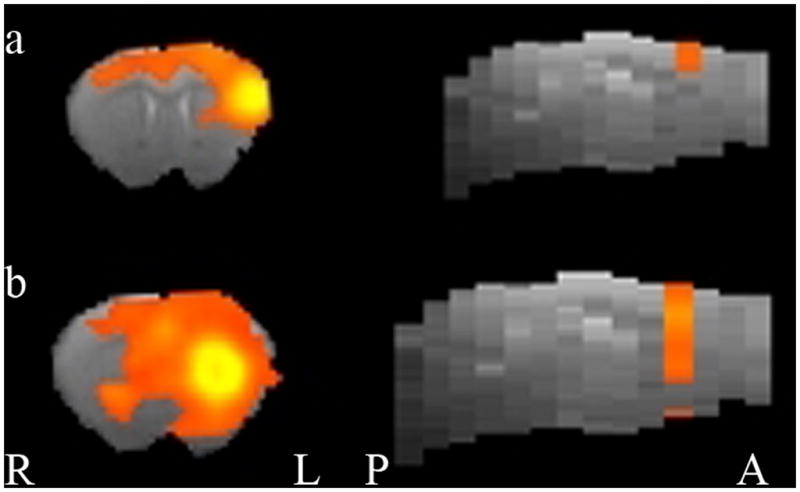
Functional connectivity maps for seeds showing less clear representation of DMN. a) Left Insula Seed, b) Left prelimbic seed. Other details are as in Figure 2.
4. Discussion
The present study demonstrates that functional connectivity measures of mouse brain can be reliably made despite the challenges of large magnetic field susceptibility changes present and with isoflurane alone as the anesthetic agent. This extends prior studies showing DMN in the mouse using optical imaging (White, Bauer et al., 2011), and extends previous fMRI resting state studies demonstrating this network in rats and mice (Jonckers, Van et al., 2011), monkeys (Vincent, Patel et al., 2007), and humans (Raichle, MacLeod et al., 2001). Although much further investigation is required on the effects of the anesthetic regime on resting state FC, this is the first study, as far as we are aware, to demonstrate that robust resting state measurements in the mouse brain can be achieved with the administration of isoflurane alone as the anesthetic agent.
Anesthetic agents are known to effect cerebral blood flow. In a recent study (Ciobanu, Reynaud et al., 2012), a T2*sensitive MR imaging method was used to illustrate how different anesthetics yield changes in image contrast. This work showed that at ultra high magnetic field (17.2T) the T2* contrast was very sensitive to the anesthetic used. Medetomidine and ketamine-xylazine agents both resulted in much larger hemodynamic MR signal changes compared to isoflurane. These differences were much less pronounced at 7T. Another study (Hoffman et al., 1991) showed that the effect of cerebral blood flow was relatively small, although regional changes in cerebral blood flow were observed. These studies suggest that resting state analysis of neuronal networks when using any anesthetic should be interpreted with care. The anesthetics influence blood flow and metabolism and thus may have an impact on interpretation of functional connectivity measures that rely on the hemodynamic response. Clearly further research is required to determine the nature of the anesthetic hemodynamic response and its effect on the functional connectivity hemodynamic response.
As in all resting state studies, animal or human, the image resolution is somewhat a limiting factor. Future technical developments may improve spatial and temporal resolution to allow more specificity in FC measurements. One such innovation is the use of multiband sequences (Feinberg, Moeller et al., 2010). This technique uses multiple coils for simultaneous acquisitions from several slices and thus increases the efficiency of acquisition. This approach may hold promise for future rodent FC mapping.
The existence of the DMN in mice has important implications both from an evolutionary perspective, such that similar networks exist at different levels across species, and from a research perspective. First, it highlights the technical feasibility of detecting such networks even in very small mammals. Second, the DMN has proven to be a sensitive indicator of brain function across a number of different psychiatric disorders, including ADHD (Uddin, Kelly et al., 2008), schizophrenia (Whitfield-Gabrieli and Ford, 2011), and depression (Sheline, Barch et al., 2009; Alexopoulos, Hoptman et al., 2012). Because transgenic mice can be developed with any number of mutations or their combinations, the demonstration of the feasibility of using MRI to detect the mouse DMN opens up a valuable set of avenues to further examine the pharmacogenetic basis of psychiatric and neurological disorders.
We report fMRI functional connectivity measures in mouse brain.
Reliable functional connectivity measures can be obtained using isoflurane as the anesthetic agent.
We found evidence of a default mode network for lateral cortical and medial cortical seeds.
Acknowledgments
This work was supported in part by NIH grants from NCRR 1S10RR023534-01awarded to C. Branch, NIMH R01 DA03383 awarded to DC Javitt, NIMH P50 MH086385 awarded to DC Javitt and NIMH R21 MH084031 awarded to MJ Hoptman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Mennes M, Zuo XN, Gohel S, Kelly C, Smith S, Beckmann C, Bucker R, Colcombe S, Dogonowski A-M, Ernst M, Hyde JS, Kotter R, McMahon K, Maddon D, Madsen K, Butler PD, Hampson M, Hoptman MJ, Kiviniemi V, Li S-J, Lin C-P, Lowe M, Mayberg H, Peltier S, Petersen S, Raichle M, Rombouts S, Rypma B, Schlagger B, Schmidt S, Siegle GJ, Sorg C, Teng G-J, Villringer A, Walter M, Wang L-H, Whitfield-Gabrieli S, Windishchberger C, Zhang H-Y, Zang Y-F, Castellanos FX, Milham MP. Towards Discovery Science of Human Brain Function: The ‘1000 Connectomes’ Project. Proceedings of the National Academy of Sciences, USA. 2010;107:4734–9. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Ciobanu L, Reynaud O, Uhrig L, Jarraya B, Le BD. Effects of anesthetic agents on brain blood oxygenation level revealed with ultra-high field MRI. PLoS One. 2012;7:e32645. doi: 10.1371/journal.pone.0032645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29:1359–67. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, Glasser MF, Miller KL, Ugurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One. 2010;5:e15710. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van E, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–11. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Guilfoyle DN, Hrabe J. Interleaved snapshot echo planar imaging of mouse brain at 7.0 T. NMR Biomed. 2006;19:108–15. doi: 10.1002/nbm.1009. [DOI] [PubMed] [Google Scholar]
- Hoffman WE, Edelman G, Kochs E, Werner C, Segil L, Albrecht RF. Cerebral autoregulation in awake versus isoflurane-anesthetized rats. Anesth Analg. 1991;73:753–757. doi: 10.1213/00000539-199112000-00013. [DOI] [PubMed] [Google Scholar]
- Jonckers E, Van AJ, De VG, Van der LA, Verhoye M. Functional connectivity fMRI of the rodent brain: comparison of functional connectivity networks in rat and mouse. PLoS One. 2011;6:e18876. doi: 10.1371/journal.pone.0018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proc Natl Acad Sci U S A. 2012;109:3979–84. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The Resting Brain: Unconstrained yet Reliable. Cereb Cortex. 2009;19:2209–29. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–54. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van E, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Weisskoff RM. Simple measurement of scanner stability for functional NMR imaging of activation in the brain. Magn Reson Med. 1996;36:643–5. doi: 10.1002/mrm.1910360422. [DOI] [PubMed] [Google Scholar]
- White BR, Bauer AQ, Snyder AZ, Schlaggar BL, Lee JM, Culver JP. Imaging of functional connectivity in the mouse brain. PLoS One. 2011;6:e16322. doi: 10.1371/journal.pone.0016322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default Mode Network Activity and Connectivity in Psychopathology. Annu Rev Clin Psychol. 2011 doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Hoptman MJ, Gerum SV, Guilfoyle DN. State-dependent functional connectivity of rat olfactory system assessed by fMRI. Neurosci Lett. 2011;497:69–73. doi: 10.1016/j.neulet.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An introduction to methods. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- Zhao F, Zhao T, Zhou L, Wu Q, Hu X. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage. 2008;39:248–60. doi: 10.1016/j.neuroimage.2007.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]