Abstract
Atrioventricular (AV) endocardium transforms into the cushion mesenchyme, the primordia of the valves and membranous septa, through epithelial-mesenchymal-transformation (EMT). While bone morphogenetic protein (BMP)-2 is known to be critical for AV EMT, the role of BMP-2 in post-EMT AV valvulogenesis remains to be elucidated. To find BMP signaling loops, we first localized Type I BMP receptors (BMPRs), BMPR-1A (ALK3), -1B (ALK6) and ALK2 in AV cushion mesenchyme in stage-24 chick embryos. Based on the BMP receptor expression pattern, we examined the functional roles of BMP-2 and BMP signaling in post-EMT valvulogenesis by using stage-24 AV cushion mesenchymal cell aggregates cultured on 3D-collagen gels. Exogenous BMP-2 or constitutively active (ca) BMPR-1B (ALK6)–virus treatments induced migration of the mesenchymal cells into the collagen gels, whereas noggin, an antagonist of BMPs, or dominant-negative (dn) BMPR-1 B (ALK6)-virus treatments reduced cell migration from the mesenchymal cell aggregates. Exogenous BMP-2 or caBMPR-1B (ALK6) treatments significantly promoted expression of an extracellular matrix (ECM) protein, periostin, a known valvulogenic matrix maturation mediator, at both mRNA and protein levels, whereas periostin expression was repressed by adding noggin or dnBMPR-1B (ALK6)-virus to the culture. Moreover, transcripts of Twist and Id1, which have been implicated in cell migration in embryogenesis and activation of the periostin promoter, were induced by BMP-2 but repressed by noggin in cushion mesenchymal cell cultures. These data provide evidence that BMP-2 and BMP signaling induce biological processes involved in early AV valvulogenesis, i. e. mesenchymal cell migration and expression of periostin, indicating critical roles for BMP signaling in post-EMT AV cushion tissue maturation and differentiation.
Keywords: Bone morphogenetic protein (BMP), Cardiac cushions, Viral gene transfer, Periostin, Extracellular matrix protein, Cell migration, Cell proliferation, Valvulogenesis, Heart, Chicken
Introduction
Cardiac valvuloseptal morphogenesis is one of the key morphogenic events during four-chambered heart formation. Defects in valvuloseptal morphogenesis are among the most common and serious of all congenital hearts defects (Hoffman and Kaplan, 2002; Hoffman et al., 2004). Two segments of the endocardium-–atrioventricular (AV) and outflow tract (OT) endocardium--transform into cushion mesenchyme, primordia of the valves and membranous septa through an epithelial-mesenchymal transformation (EMT) (reviews, Eisenberg and Markwald et al, 1995; Nakajima et al., 2000; Armstrong and Bishop, 2004; Person et al., 2005). This transformation occurs at around Hamburger and Hamilton (HH) stage 15 in the AV canal of the chick heart (Hamburger and Hamilton, 1951). Transformed endocardial cells subsequently migrate into the underlying extracellular matrix (ECM) referred to as “cardiac jelly” and remodel the cardiac jelly into mesenchymalized swellings, called “ cardiac cushions” by HH stage 24/25. Distal outgrowth and maturation of the cardiac cushions are the initial and critical morphogenetic events during post-EMT valvulogenesis. These morphogenetic events, which begins at HH stage 26, involves i) migration of post-EMT endocardial cells into acellular cardiac jelly; ii) proliferation of post-EMT cells and iii) expression of valvulogenic molecules which include ECM proteins involved in maturation of the cushion mesenchyme (de la Cruz and Markwald, 1998; Oosthoek et al., 1998). However, overall mechanisms of these biological processes during distal outgrowth and maturation of post-EMT cardiac cushion mesenchyme are unknown.
Bone morphogenetic protein (BMP) is a member of the TGF-β superfamily proteins, and it is one among many molecules implicated in AV EMT (Armstrong and Bishop, 2004; Person et al., 2005). BMP signaling was found to be essential for AV EMT in studies with explant cultures in mice (Sugi et al., 2004) and chicks (Okagawa et al., 2007), and in BMP-2 conditional knockout (CKO) experiments in mice (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006). However, BMP-2 CKO at the EMT stage causes subsequent lethality, which prevents investigation of the role of BMP-2 during post-EMT valvulogenesis. BMPs exert their biological function by interacting with cell surface receptors. BMP receptors (BMPRs) consist of Type I and Type II receptors and Type II receptors transphosphorylate the glycine-serine rich domain (GS domain) of Type-I receptors and transduce signals (Hogan, 1996; Yamashita et al., 1996; de Caestecker, 2004). The Type II BMP receptor, BMPRII is reported to be expressed ubiquitously in the entire embryo at least up to the mid-gestation stages (Roelen et al., 1997). Therefore, to find the potential BMP signaling loops during post-EMT valvulogenesis, we first explored the expression patterns of Type I BMPRs by localizing BMPR-1A (ALK3), BMPR-1B (ALK6) and ALK2 in the HH stage-24 post-EMT AV cushion mesenchyme in the chick. Basing our investigation on the BMP receptor expression patterns, in this work we examined whether BMP signaling regulated the biological processes necessary for distal outgrowth and maturation of post-EMT cushion mesenchyme during early valvulogenesis.
Periostin is a 90-kDa secreted ECM protein, related to the midline fasciclin-1 gene in Drosophila (Horiuchi et al., 1999). It has also been identified as a heart-enriched gene in embryonic day (ED) 10.5 mouse embryos by a subtractive cDNA microarray approach (Kruzynska-Freitag et al., 2001). Periostin is highly expressed in the maturation zone of post-EMT cardiac cushions in mice (Kruzynska-Freitag et al., 2001; Norris et al., 2005) and in chicks (Norris et al., 2004; Kern et al., 2005). Periostin is found to regulate collagen I fibrillogenesis and contribute to the biomechanical properties of connective tissues in skin and AV valves (Norris et al., 2007). Recent data using chick primary culture assays demonstrated that periostin enhanced cell invasion/migration and collagen condensation by AV cushion mesenchyme, indicating that periostin mediates matrix maturation, a crucial process in early valvulogenesis (Butcher et al. 2007). Regarding the regulation of periostin expression, periostin is known to be induced by BMP-2 in MC3T3 cells (Ji et al., 2000) and by TGFβ-2 in primary osteoblast cells (Horiuchi et al., 1999). BMP inducible helix-loop-helix (HLH) proteins, Twist1 and Id1 (Valdimarsdottir et al., 2002; Komaki et al., 2007), are implicated in regulating the activation of the periostin promoter in osteogenesis (Oshima et al., 2002; Connerney et al., 2006). However, little is known about regulation of periostin expression in cardiac cushion mesenchymal cells during post-EMT valvulogenesis. Therefore, we have chosen periostin as a post-EMT differentiation and maturation target protein whose expression can be regulated by BMP signaling during early AV valvulogenesis.
To study the regulation of post-EMT cushion mesenchyme differentiation and maturation, which begins at HH stage 26 (de la Cruz and Markwald, 1996; Oosthoek et a., 1998) as mentioned above, we developed and established a primary culture assay system using HH stage-24 chick AV cushion mesenchymal cell aggregates with three-dimensional (3D) collagen gels in which we are able to evaluate i) post-EMT mesenchymal cell migration, ii) post-EMT mesenchymal cell proliferation and iii) post-EMT valvulogenic protein expression. Using this culture assay system, we show that BMP-2 and BMP signaling through BMP receptors induce mesenchymal cell migration and periostin expression at both the mRNA and protein levels as well as Id1 and Twist mRNA expression but do not induce proliferation of post-EMT AV cushion mesenchymal cells.
Materials and Methods
Chick Embryos
Viral-free fertilized eggs of White Leghorn (Gallus gallus domesticus) chicken were purchased from Spafas Inc. (Norwich, CT) and incubated in a humid atmosphere at 37°C. Stages of embryonic development were determined by using the criteria of Hamburger and Hamilton (HH) (1951).
Detection of the Type I BMP receptor expression from HH stage-24 chick AV canal by reverse transcription-polymerase chain reaction (RT- PCR)
HH stage-24 AV cushion mesenchyme was carefully separated from the associated myocardium. RT-PCR analysis of BMPR-1A, BMPR-1B and ALK2 was performed as described previously (Sugi and Markwald, 2003; Okagawa et al., 2007) with minor modifications. Briefly, total RNA was extracted and purified from HH stage-24 AV cushion mesenchyme or myocardium using RNA STAT-60 (Tel-Test Inc). Complementary DNA was prepared with an iScript cDNA synthesis kit (BIO RAD) according to the manufacturer’s instructions. PCR primer pairs were designed for BMPR-1A (L49204, forward, 5′-CTGAGAGTTGAGCGATTG-3′, reverse, 5′-CAGCCAGAAGCAAGTGTTGG-3′), BMPR-1B (D13432, forward, 5′-GGAGAGCAGAAAAGAAGATAGTGAGG-3′, reverse, 5′-TGGTGTGGAATAGGAGTGTCC-3′) and ALK2 (U38622, forward, 5′-CTGTGTTGGGGTCACTG-3′, reverse, 5′-TGGAAGCAGCCTTTCTGG-3′). PCR was performed with these primer pairs and Taq polymerase on a iCycler iQ Real-Time PCR machine (BIO-RAD) using 30 cycles of 94°C for 30 sec, 56°C for 30 sec and 72°C for 2 min. That the PCR products were not amplified from genomic DNA was verified by treating samples with RNase-free DNase-1 (Stratagene) before RT. As a negative control, the RT step was omitted. The PCR products were verified via the thermal cycle sequencing using TagDNA polymerase and fluorescent dye-labeled termination (Medical University of South Carolina (MUSC), Biotechnology Resources Laboratory).
Whole-mount and section in situ hybridization (ISH) for BMP receptors
HH stage-25 chick heart RNA was isolated using RNeasy Column (Qiagen) and reverse-transcribed into cDNA (Stratagene) (Norris et al., 2004). For whole-mount ISH, a 200–250 bp sequence was used for better penetration and rinsing of the probes to reduce the background binding. The following sequences were amplified for type I BMP receptors using HH stage-25 chick heart cDNA: BMPR-1A (L49204; nt 351–640), BMPR-1B (D13432; nt 387–720) and ALK2 (U38622; nt 21–299) for the whole mount ISH probes. For section ISH, longer sequences were used for the better detection of the mRNA expression for BMPR-1A (L49204; nt 366–996) and BMPR-1B (D13432, nt 387–838). Amplified sequences were verified by sequencing (MUSC, Biotechnology Resources Laboratory). Database sequence homology searches confirmed that the sequences for BMPR-1A, BMPR-1B and ALK-2 used in the ISH are not anticipated to cross-react with other family members and are highly specific. Riboprobes were generated by DIG RNA labeling (SP6/T7, Roche). Riboprobes were purified by RNeasy column (Qiagen) and quantified using a UV spectrophotometer. ISH was performed as previously described with slight modifications (Norris, et al., 2004; Somi et al., 2004). ISH experiments were carried out on stage-24 chick embryos. A specific signal was only observed when whole hearts or sections were hybridized with the antisense riboprobe. No signal was detected when using the control sense riboprobe (Fig. 1).
Fig. 1.
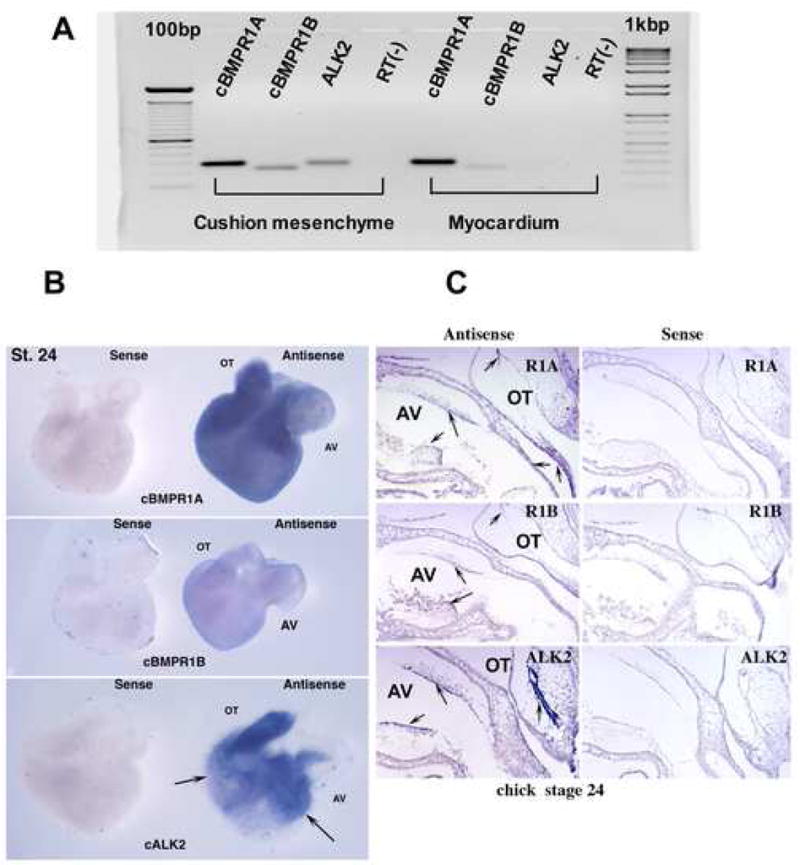
A. RT-PCR analysis revealed mRNA expression of type I BMP receptors in HH stage-24 chick AV canal. BMPR-1A and BMPR-1B were detected in AV cushion mesenchyme and myocardium. ALK2 was detected in the AV cushion mesenchyme; however, little ALK2 expression was seen in the AV myocardium. B. Whole-mount in situ hybridization revealed mRNA expression of Type I BMP receptors in HHstage-24 chick AV hearts. Abundant BMPR1A expression was found in the AV and OT cushion mesenchyme and in the myocardium. ALK2 was localized in the AV and OT cushion mesenchyme and ventricular endocardium but not in the myocardium (arrows show endocardium). Lower levels of BMPR-1B expression were detected in HH stage-24 whole hearts. C. Section in situ hybridization revealed BMPR-1A expression in the AV and OT endocardium and cushion mesenchyme. BMPR-1A expression was also detected in the myocardium (arrows) in the HH stage-24 chick heart. Intense expression of ALK2 was detected in the AV and OT endocardium and cushion mesenchyme but not in the myocardium. Low levels of BMPR-1B expression were detected ubiquitously in stage-24 whole hearts. No specific binding was detected with the sense probes.
Generation and preparation of retrovirus
Two mutant constructs of chick BMPR-1B cloned into the replication-competent avian retroviral vector RCAS- (A) (Hughes et al., 1987) were kindly provided by Dr. L. Niswander. The dominant-negative form (dnBMPR-1B) blocks BMP signaling pathways by a single amino acid substitution (Lys-231 to Arg) within the adenosine triphosphate binding site in the kinase domain. Because dnBMPR-1B lacks the activity of an intracellular kinase domain, dnBMPR-1B expressed at the cell surface can bind BMPs but does not transmit signals. The constitutively active form (caBMPR-1B) has a single amino acid substitution (Gln-203 to Asp) within the GS activation domain. caBMPR-1B is activated constitutively and transmits signals without BMP binding. dnBMPR-1B and caBMPR-1B viruses were previously constructed and verified in avian embryonic tissue culture as being capable of blocking and activating BMP signaling, respectively (Zou and Niswander, 1996; Zou et al., 1997).
Primary fibroblasts were collected from day-10 chick embryos and transfected with retroviral constructs using FuGENE 6-transfection reagent (Roche). Supernatant from the transfected cultures was collected and concentrated by ultracentrifugation. Concentrated virus was resuspended in M199 medium (Invitrogen) and stored at −80 °C. The viral titer was measured with immunohistochemical detection of viral protein p19 with monoclonal antibody, AMV3c2 (Developmental Studies Hybridoma Bank) using virus-infected chick fibroblast cultures. A viral titer (2 X 109 to 5 X1010 active viron/ml) was routinely achieved after ultracentrifugation of the viral supernatant.
AV cushion mesenchymal cell aggregate culture
AV cushion mesenchyme was dissected out from HH stage-24 chick AV cushions by carefully removing the associated myocardium. Cushion mesenchyme was dispersed by trypsinization as described previously (Sugi et al., 2003). Following the procedure that was described for culturing cell aggregates from chick blastoderm (Eisenberg and Markwald, 1997), 40,000 cells were cultured as a hanging drop in 20μl of serum-free medium 199 (Invitrogen) supplemented with ITS (5μg/ml insulin, 5μg/ml transferrin and 5 ng/ml selenium, BD Sciences) and antibiotics (100units/ml penicillin and 100μg/ml streptomycin, Invitrogen) overnight. Resultant mesenchymal cell aggregates were placed on hydrated collagen gels (1mg/ml, rat tail tendon, BD Sciences) and cultured with medium 199 supplemented with 1% chick serum (Sigma), ITS and antibiotics as described above. Two hrs later, recombinant human BMP-2 (kindly provided by the Genentics Institute, Cambridge, MA) at final concentrations ranging from 20 – 200 ng/ml, recombinant human noggin (kindly provided by Regeneron Pharmaceuticals) at final concentration of 500 ng/ml or dnBMPR1B- or caBMPR1B-RCAS -virus (final concentration, 1.5X108 active viron/ml) was added alone or in combination with BMP-2 to the culture. Cultures were observed daily under an inverted microscope with Hoffman optics (Olympus, IMT-2). Cell migration, expression of periostin protein and mRNA, and cell proliferation were evaluated at 72 hrs.
Cell migration assay
Seventy-two hrs after placing the AV cushion mesenchymal cell aggregates on collagen gels, cell migration was assessed under an inverted microscope with Hoffman optics (Olympus, IMT-2). Cell numbers were counted in a series of focal planes from the surface to the bottom of the collagen gels at 40 μm intervals.
BrdU incorporation assay
BrdU incorporation was used as an index of cell proliferation. The BrdU incorporation assay for cushion mesenchymal cells was performed as previously described (Sugi et al., 2003). Briefly, BrdU was added (final concentration = 50 μM) for 2 hrs before the termination of the culture. After rinsing with phosphate buffered saline (PBS), pH. 7.4, cultured cushion mesenchymal cells were incubated in 2N HCl containing 0.5% Tween20 and processed for immunohistochemical detection of BrdU in the nuclei with anti-BrdU (BD Sciences). All nuclei were stained with propidium iodide. Stained samples were observed under a Leica TCS SP2 AOMS confocal microscope.
Immunostaining and Immunointensity analysis
Immunostaining and immunointensity analysis for cushion mesenchymal cell aggregates cultured on collagen gels were performed as described before (Sugi et al., 2004) with minor modifications. Briefly, cultured cushion mesenchymal cell aggregates were fixed in 100% methanol at 20°C and processed for immunostaining with anti-phospho-Smad1/5/8 (Cell signaling), anti-chick periostin, or an RCAS virus marker (AMV3c2, Hybridoma Bank) depending on the experimental necessity. Anti-chick periostin antibodies were produced against a peptide corresponding to amino acids 122–140 of chick periostin and characterized by Western analysis and immunohistochemistry (Kern et al., 2005). Immunostained samples were observed under a confocal microscope (Leica TCS SP2 AOBS). The immunointensity of periostin and AMV3c2 were evaluated by measuring the intensity of the immunofluorescence of the cell aggregates on photographs using computer software, Adobe Photoshop 8.0.
Quantitative RT-PCR for periostin, Id1 and Twist
Total RNA was extracted and purified using RNA STAT-60 (Tel-Test Inc) from cushion mesenchymal- cell aggregates that had been cultured for 72 hrs on the collagen gels. Obtained mRNA was further purified by PicoPure RNA Isolation kit (Arcturus). Complementary DNA was prepared using the iScriptTM cDNA synthesis kit (BIO RAD) according to the manufacturer’s instruction. The following PCR primer pairs were designed to specifically amplify chick periostin (AY65700, Forward, 5′-TCGGTGGAAAACTGCTAAGAG-3′; Reverse 5′-TCTGCTGGCTTGATGATTTG -3′), chick Id1 (AY040527, Forward 5′-CTCCCAACTCCACTTTCCAG-3′; Reverse 5′AGCGGCGACCAATAGCAG-3′), chick Twist (AY126449, Forward, 5′-GGGATAGGGGTGTGTGTGTG-3′; Reverse 5′-GAGAGGAGGGACTTTTGCTG-3′). Real-time PCR reactions were performed using 50 cycles of 94°C for 30 sec, 56°C for 30 sec and 72°C for 2 min. Chick periostin amplification was normalized to chick β-actin amplification for each experimental treatment. QRT-PCR results represent at least three independent experiments with reactions performed in triplicate.
Statistical analyses
Analysis of variance (ANOVA) was used to determine significant differences between groups. When differences were found among groups, the Turkey Kramer’s t-test was used to determine if the difference was statistically significant. Differences were considered significant if p< 0.05.
Results
mRNA expression of type I BMP receptors by HH stage-24 chick AV canal
RT-PCR analysis revealed mRNA expression of BMPR-1A and BMPR-1B in both AV cushion mesenchyme and myocardium from HH stage-24 chick embryos (Fig. 1A). ALK2 was detected in the AV cushion mesenchyme; however, ALK2 expression was not detectable in the myocardium (Fig.1A).
By whole-mount ISH BMPR-1A was localized in the AV and OT cushion mesenchyme from HH stage 24 chick embryos. BMPR-1A expression was also detected in the myocardium by whole mount (Fig, 1B, cBMPR1A) and by section ISH (arrows in Fig.1C, R1A). ALK2 was localized in the AV and OT endocardium and cushion mesenchyme (Fig.1B and arrows Fig.1C, ALK2) and in the ventricular endocardium (arrows in Fig. 1B, cALK2) but not in the myocardium. A lower level of BMPR-1B expression was detected ubiquitously by the HH stage-24 whole hearts (Fig.1B, cBMPR1B) and in the sections from HH stage-24 chick embryos (Fig. 1C, R1B). Sense probes used as negative controls for the ISH did not produce any detectable signals (sense panels in Fig 1. B and C).
Development of AV cushion mesenchymal cell aggregate culture
To study the regulation of post-EMT cushion mesenchyme maturation and differentiation, we developed a primary culture assay system, in which we were able to evaluate post-EMT mesenchymal cell migration, cell proliferation and post-EMT valvulogenic protein expression. This culture assay system was developed using dissociated HH stage-24 chick AV cushion mesenchymal cells. Cushion mesenchymal cells were cultured as a hanging drop overnight with serum-free medium. Resultant mesenchymal cell aggregates were cultured on the surface of 3D collagen gels with 1% chick serum and observed daily for 72 hrs (Fig. 2A–C). The advantages of this culture assay system are that the mesenchymal cells are initially trypsinized and possess minimal ECM proteins before BMP or virus treatments are initiated (Fig. 2A), and that the original cell number is easily quantified for subsequent monitoring in response to BMP signaling.
Fig. 2.
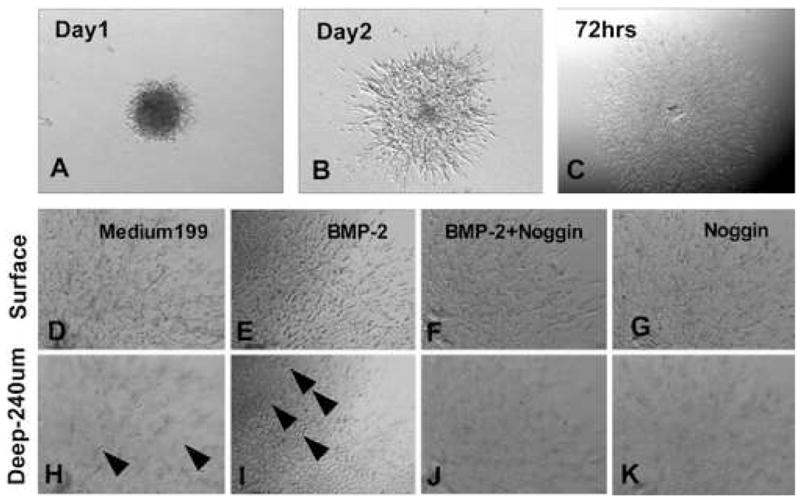
A–C. Representative pictures from cultured AV cushion mesenchymal cell aggregates. These pictures show how cushion mesenchymal cell aggregates grow on collagen gels with control M199 medium. AV cushion mesenchymal cells from HH stage-24 chick embryos were cultured as a hanging drop overnight. Resultant mesenchymal cell aggregates were placed on collagen gels and cultured for an additional 72 hrs. D-K. Higher magnification view of AV cushion mesenchymal cell aggregate culure. Upper panels show the surface view, and lower panels show a deeper view in collagen gels (240μm from the surface). Mesenchymal cells treated with BMP-2 (200 ng/ml) migrated deeper into the collagen gels (arrowheads in I) than the control culture (arrow heads in H). BMP-2-promoted mesenchymal cell migration was abolished when noggin (500 ng/ml) was added to the medium (F and J). Noggin treatments reduced mesenchymal cell migration below the level of control Medium 199 treatments (G and K).
BMP-2 induces AV cushion mesenchymal cell migration
AV cushion mesenchymal cells treated with BMP-2 (200ng/ml) migrated deeper into the collagen gels (Fig. 2, arrowheads in I) than the control (Fig. 2, arrowheads in H). Effects of BMP-2 were abolished when noggin (500 ng/ml) was added to the BMP-2-containing medium (Fig. 2J). Noggin alone with the Medium 199 also repressed migration of cushion mesenchymal cells (Fig. 2K). No significant differences were seen in surface migration area between treatments (data not shown). Quantitative analysis indicated the dose-dependent effects of BMP-2 in mesenchymal cell migration into collagen gels (Fig. 3). As indicated in Fig. 3A, as little as 50 ng/ml of BMP-2 significantly promoted mesenchymal cell migration. BMP-2-promoted cell migration was abolished by adding noggin to the BMP-2-treated mesenchymal cell culture (Fig. 3B). Moreover, the endogenous level of mesenchymal cell migration was repressed in cultures treated with noggin alone compared to the control (Fig. 3B). These results indicate that BMP-2 induces cell migration by cultured AV cushion mesenchymal cells.
Fig. 3.
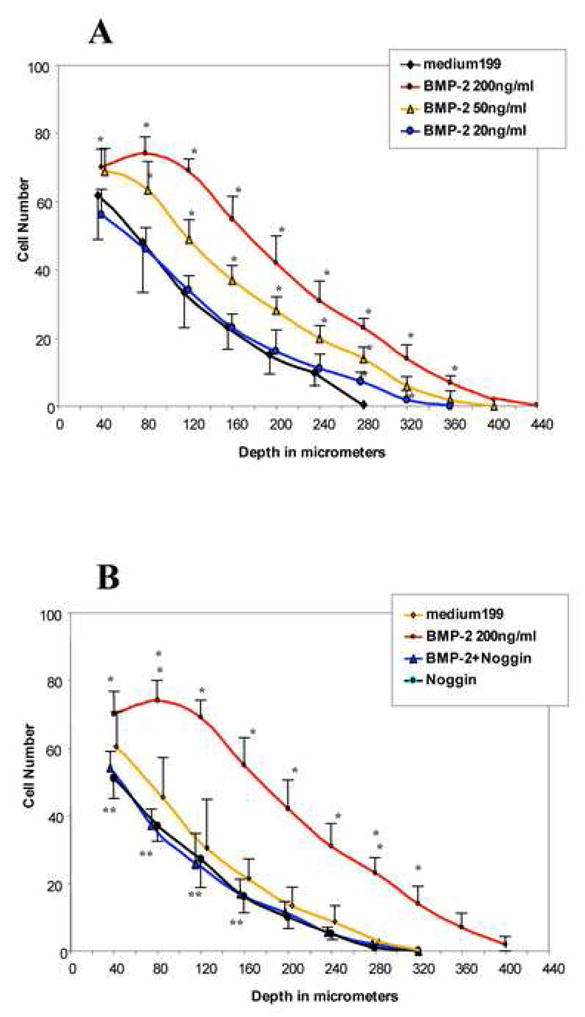
Quantitative evaluation of cell migration by HH stage-24 AV cushion mesenchymal cell aggregate culture. A. BMP-2 (as low as 50 ng/ml) promoted cell migration (*p<0.001). B. BMP-2-promoted cell migration was abolished by adding noggin (500 ng/ml) to the mesenchymal cell-aggregtes culture (*p<0.001). Mesenchymal cell migration was reduced in cultures treated with noggin alone or noggin with BMP-2 compared to the control (**p<0.05).
BMP treatment enhances phospho-Smad 1/5/8 expression in cushion mesenchymal cells
To test whether exogenous BMP-2 treatment induces intracellular BMP signaling in cultured AV cushion mesenchymal cells, activation of Smad 1/5/8 phosphorylation was examined by detecting phospho-Smad 1/5/8 expression. AV cushion mesenchymal cells treated with BMP-2 (200 ng/ml) and cultured on collagen gels showed significantly elevated phospho-Smad 1/5/8 expression (Fig. 4B and D). Noggin treatment repressed endogenous phopho-Smad1/5/8 expression in the AV cushion mesenchymal cells compared to control culture (Fig. 4C and D). These results indicate that BMP-2 treatment activates intracellular BMP signaling in cultured AV cushion mesenchymal cells.
Fig. 4.
A–C. Phospho-Smad 1/5/8 expression was detected in cultured AV cushion mesenchymal cells. BMP-2 treated mesenchymal cells showed more phopho-Smad1/5/8 immunostaining (B) than control Medium 199 treated cushion mesenchymal cells (A). Noggin treatments reduced phospho-Smad 1/5/8 staining in the cells (C). D. Quantitative evaluation of phospho-smad1/5/8 immunostaining revealed that cushion mesenchymal cells treated with BMP-2 expressed elevated phopho-Smad1/5/8 staining (p<0.01), while noggin treatments significantly reduced phospho-Smad1/5/8 staining (D).
BMP-2 does not stimulate mesenchymal cell proliferation
To determine whether BMP-2 regulates AV cushion mesenchymal cell proliferation, we assessed BrdU incorporation as an index of cell proliferation in AV cushion mesenchymal cells. Neither exogenously added BMP-2 nor noggin treatment altered the BrdU incorporation by AV cushion mesenchymal cells compared to the control Medium 199 treatment (Supplemental Fig. 1A-H and Fig. 1I). These results indicate that BMP-2 does not alter the proliferation ratio of HH stage-24 post-EMT AV cushion mesenchymal cells.
BMP-2 treatment increases periostin expression by AV cushion mesenchymal cells
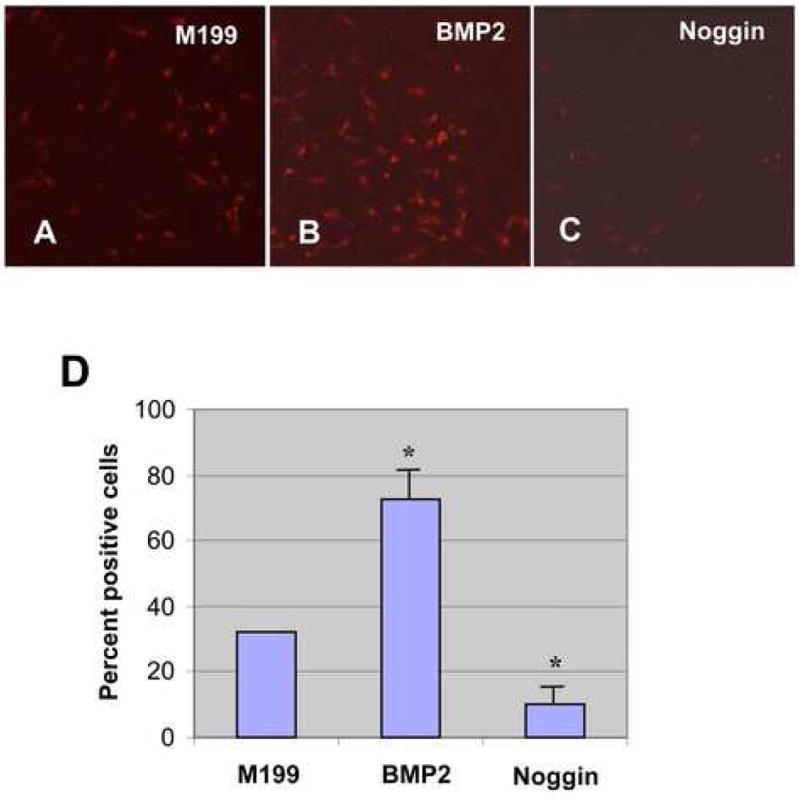
Periostin expression (mRNA and protein) was assessed in AV cushion mesenchymal cell aggregates cultured on collagen gels. Periostin protein expression was analyzed by immunostaining (Fig. 5A–F) and quantitatively evaluated by immunointensity (Fig. 5G). Periostin expression in AV cushion mesenchymal cell aggregates was elevated when treated with BMP-2 (Fig. 5B, C and D). Significant promotion of periostin protein expression was detected when AV cushion mesenchymal cell aggregates were treated with as little as 20ng/ml BMP-2 (Fig. 5G). Noggin treatments inhibited BMP-2-supported induction of periostin expression (Fig. 5E and G). Treatments with noggin alone with Medium 199 also significantly repressed endogenous periostin expression by cultured AV cushion mesenchymal cell aggregates as compared to the control treatment (Fig. 5F and G).
Fig. 5.
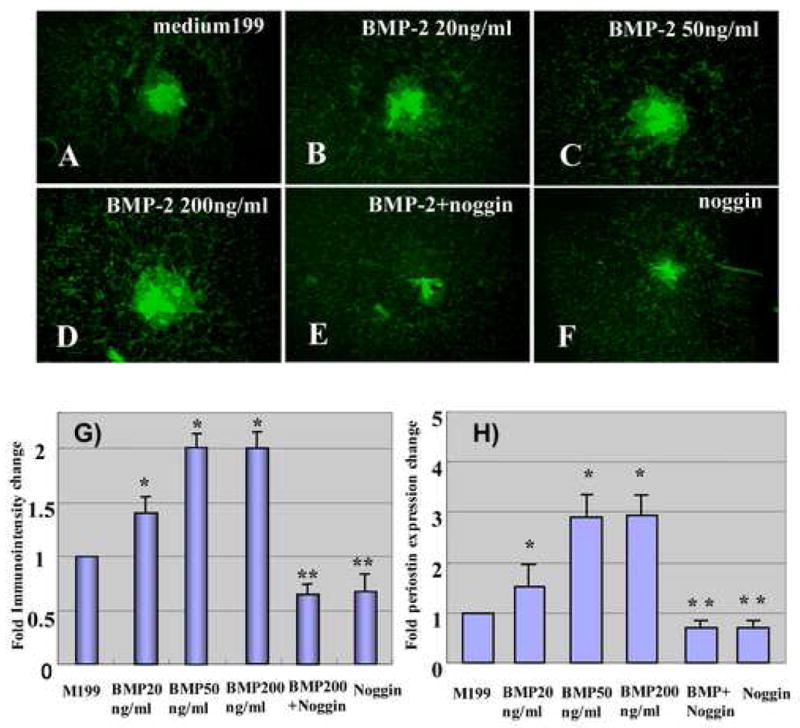
A–F. Periostin immunostaining in AV cushion mesenchymal cell aggregate culture. The mesenchymal cells were cultured with control medium 199 (A), BMP-2, 20ng/ml (B), BMP-2, 50ng/ml (C), BMP-2, 200 ng/ml (D), BMP-2 200 ng/ml plus noggin, 500 ng/ml (E) or noggin, 500 ng/ml (F). as low as 20 ng/ml of BMP-2 promoted periostin immunostaining by cultured cushion mesenchymal cell aggregates. G, Quantitative evaluation of periostin immunostaining in AV cushion mesenchymal cell aggregate culture. Immunointensity of the mesenchymal cell aggregates treated with as little as 20 ng/ml of BMP-2 was significantly higher than the control (*p<0.01 ). Conversely, immunointensity in mesenchymal cell aggregates cultured with noggin was significantly lower than the control (**p<0.05). H, Quantitative evaluation of periostin mRNA expression in AV cushions mesenchymal cell aggregate culture. Real-time PCR data revealed an approximately 3-fold increase of periostin expression in AV cushion mesenchymal cell aggregate culture treated with BMP-2 over the culture treated with control Medium199 (*p<0.01). BMP-2-supported elevation of periostin expression was abolished when noggin was added to the culture (**p<0.05).
Periostin mRNA expression was assessed with quantitative (Q) RT-PCR with AV cushion mesenchymal cell aggregates cultured on collagen gels (Fig. 5H). QRT-PCR data revealed an approximately 3-fold increase of periostin expression by AV cushion mesenchymal cell aggregates treated with BMP-2 over the control treatments with Medium199 (Fig. 5H). As low as 20 ng/ml of BMP-2 induced periostin expression (Fig. 5H). BMP-2-supported elevation of periostin mRNA expression was abolished when noggin was added to the culture. Noggin alone with Medium 199 also significantly repressed endogenous periostin mRNA expression by cultured AV cushion mesenchymal cell aggregates.
BMP-2 treatment increases Id1 and Twist expression by AV cushion mesenchymal cells
In our present work, RT-PCR analysis revealed endogenous expression of chick Id1 and Twist in HH stage-24 chick AV canal. Id1 and Twist were detected in the AV cushion mesenchyme but not clearly detected in the myocardium (Fig. 6A). Chick Twist has been localized in HH stage 22–30 chick AV cushion mesenchyme by in situ hybridization (Dr. R. Heimark, University of Arizona; personal communication). To test whether BMP signaling regulates expression of these transcription factors, chick Twist and Id1 mRNA expression was assessed with QRT-PCR with AV cushion mesenchymal cell aggregates cultured on collagen gels (Figs.6B and C). QRT-PCR data revealed an approximately 8-fold increase of both Twist and Id1 expression by AV cushion mesenchymal cell aggregates treated with BMP-2 over the control treatments with Medium199. BMP-2-supported elevation of Twist and Id1 mRNA expression was abolished when noggin was added to the culture. Noggin alone with Medium 199 also significantly repressed endogenous Id1 and Twist mRNA expression by cultured AV cushion mesenchymal cell aggregates.
Fig. 6.
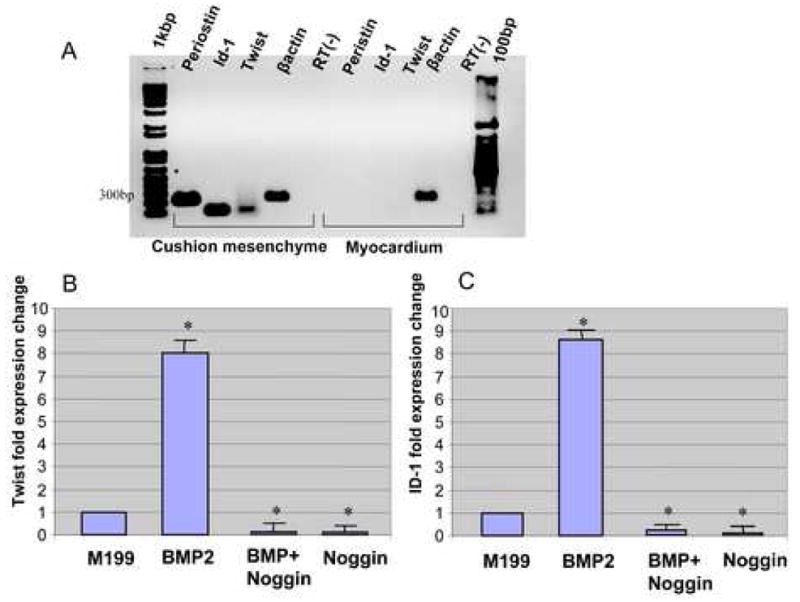
A. RT-PCR analysis revealed mRNA expression of chick Id1 and Twist in HH stage-24 chick AV canal. Id1 and Twist were detected in AV cushion mesenchyme but not detected in myocardium. B and C. Quantitative evaluation of chick Id1 and Twist mRNA expression in AV cushions mesenchymal cell aggregate culture. Real-time PCR data revealed an approximately 8-fold increase in expression of both Twist (A) and Id1 (B) in AV cushion mesenchymal cell aggregate culture treated with BMP-2 over the culture treated with control Medium199 (*p<0.01). BMP-2-supported elevation of Twist and Id1 expression was abolished when noggin was added to the culture (*p<0.01).
Modulation of BMP signaling by dn- or caBMPR virus treatments is associated with alteration of cell migration and periostin expression by cultured AV cushion mesenchymal cells
To examine whether BMP signaling through BMP receptors which are expressed by the AV cushion mesenchymal cells regulates mesenchymal cell migration and periostin expression, dn-and caBMPR-1B -RCAS-virus were applied to AV cushion mesenchymal cell aggregates cultured on collagen gels. First, we tested whether dn- or caBMPR-1B virus infection altered intracellular BMP signaling in AV cushion mesenchymal cells. AV cushion mesenchymal cell aggregates treated with dn-and caBMPR-1B virus were examined for activation of Smad 1/5/8 phosphorylation, a down stream effector of BMP signaling, by immunohistochemical detection of phospho-Smad 1/5/8 expression and a viral marker, AMV3c2 staining. caBMPR-1B-infected, AMV3c2-positive, cushion mesenchymal cells showed elevated phospho-Smad 1/5/8-expression (arrowheads in Fig. 7D and H). By contrast, dnBMPR-1B-infected, AMV3c2-positive, cushion mesenchymal cells repressed endogenous nuclear staining of phospho-Smad 1/5/8 (arrowhead in Fig. 7B and F) as compared to the control (Fig. 7A and E). When BMP-2 was added to dnBMPR-1B infected cushion mesenchymal cells, a significant reduction of Smad 1/5/8 expression was also observed (Fig. 7C and G). These data indicate that infection of dnBMPR-1B or caBMPR-1B inhibits or enhances cytoplasmic BMP signaling respectively, in AV cushion mesenchymal cells.
Fig. 7.
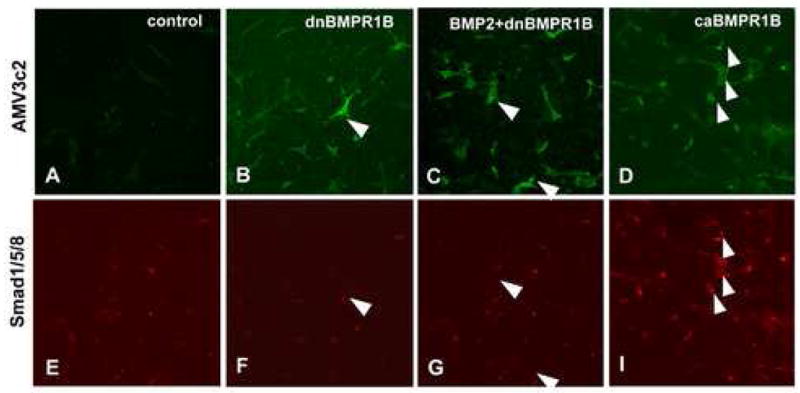
Phospho–Smad 1/5/8 immunostaining. Mesenchymal cells infected with dnBMPR-1B-virus, as seen as AMV3c2 positive cells (B and F) showed less phospho-Smad1/5/8 staining (an arrowhead in F) than the control cells (A and E). Mesenchymal cells infected with dnBMPR-1B-virus and treated with BMP-2 (200 ng/ml) (C and G) also showed less phospho-Smad1/5/8 staining (arrowheads in G) than the control cells (A and E). Mesenchymal cells treated with caBMPR-1B-virus (D and H) showed more intense phopho-Smad1/5/8 staining (arrowheads in H). Panels A–D were stained for, a viral marker, AMV3c2 (green). Panels E–H were stained for phopho-Smad1/5/8 (red).
AV cushion mesenchymal cells treated with dnBMPR-1B or caBMPR-1B virus were evaluated for cell migration. AV cushion mesenchymal cells treated with caBMPR-1B virus migrated deeper into the collagen gels than the mesenchymal cells cultured with the control Medium199 (Fig. 8). Conversely, dnBMPR1B-virus treatments significantly reduced AV cushion mesenchymal cell migration compared to the control culture (Fig. 8). dnBMPR-1B-treated culture had the same level of reduction in cell migration as observed in noggin-treated cushion mesenchymal cell culture (Fig. 3).
Fig. 8.
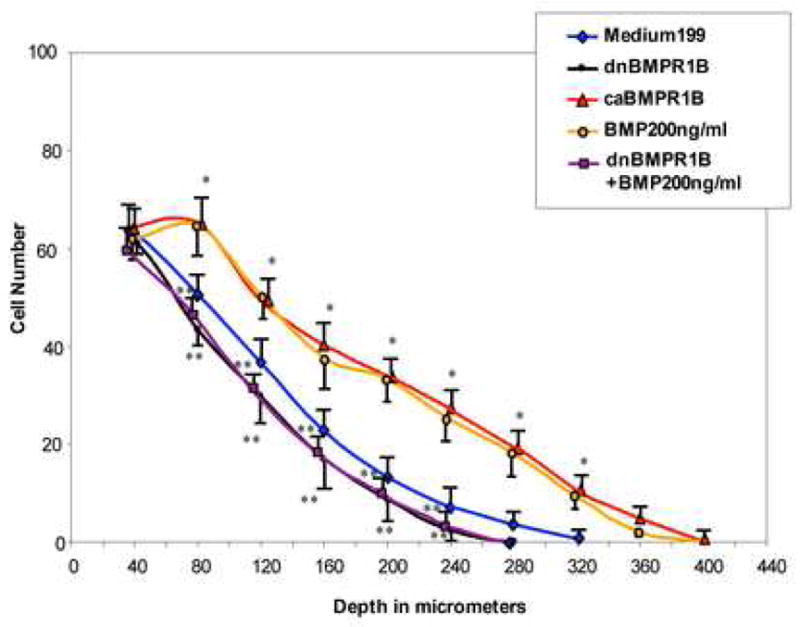
Quantitative evaluation of cell migration by AV cushion mesenchymal cells treated with dnBMPR-1B-virus or caBMPR-1B-virus. Mesenchymal cells treated with caBMPR-1B-virus migrated significantly deeper into the gels than the control (*p<0.01) similar to the level seen in BMP-2-treated mesenchymal cells. Conversely, BMP-2-supported up-regulation of cell migration was significantly reduced by dnBMPR-1B treatments (**p<0.05).
caBMPR1B-virus infection, as detected by a viral marker, AMB3c2 expression, significantly increased periostin protein expression (Fig. 9C and F) whereas dnBMPR-1B-virus infection repressed endogenous periostin protein immunostaining (Fig. 9B and E) in the cushion mesenchymal-cell aggregate culture. A higher magnification view showed that cytoplasmic periostin expression in cushion mesenchymal cells was elevated in caBMPR-1B-infected cells (Fig. 9I and L), whereas periostin expression was reduced in dnBMPR-1B-infected cells (Fig. 9H and L). Quantitative evaluation by analyzing periostin immunointensity showed significant differences among the treatments (Fig. 9M). These data indicate that BMP signaling through the BMP receptors modulate mesenchymal cell migration and periostin expression by AV cushion mesenchymal cell cultures.
Fig. 9.
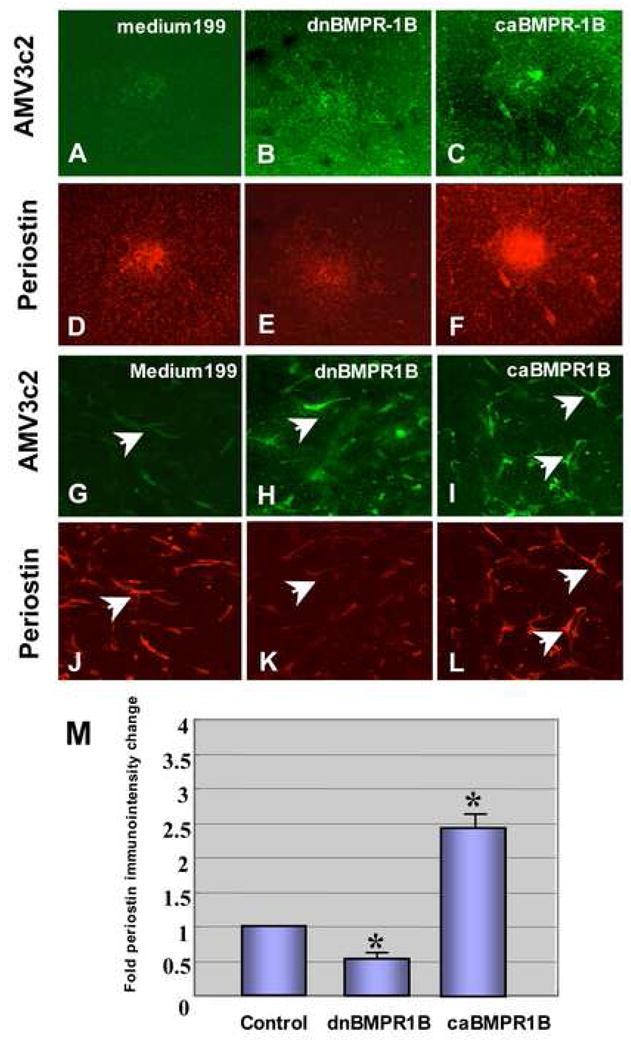
A-F. Periostin Immunostaining in AV cushion mesenchymal cell aggregate culture. Mesenchymal cell aggregates treated with dnBMPR-1B-virus (B and E) resulted in a significant decrease in periostin immunostaining compared to the no-virus control (A and D). Infection with caBMPR-1B-virus significantly increased periostin expression in AV mesenchymal cell culture (C and F). Upper panel (A–C), a viral marker, AMV3c2 immunostaining (green). Lower panel (D–F), periostin immunostaining (red). G–L. Higher magnification view of RCAS-virus-infected AV cushion mesenchymal cells. Cells infected with dnBMPR-1B-virus showed less periostin staining (an arrow in K) than no-virus infected cells (arrow in J). Cells infected with caBMPR-1B showed more intense periostin staining (arrows in L) than no-virus control (an arrow in J). Upper panel (G–I), viral marker AMV3c2 immunostaining (green). Lower panel (J–L), periostin immunostaining (red). M. Quantitative evaluation of periostin immunostaining in AV cushion mesenchymal cell aggregates treated with dn-and caBMPR-1B-virus. Immunointensity of the mesenchymal cell aggregates treated with caBMPR-1B was significantly higher than the control (*p<0.01 ). Conversely, immunointensity in mesenchymal cell aggregates infected with dnBMPR-1B was significantly lower than the control (*p<0.01).
In summary, we provide the evidence that BMP-2 and BMP signaling induce cell migration and expression of a valvulogenic ECM protein, periostin but do not induce cell proliferation by post-EMT AV cushion mesenchymal cells. This evidence was demonstrated by two independent approaches of promoting BMP signaling by gain-of-function experiments: exogenous BMP-2 treatments and activation of BMP receptors by caBMPR-1B-virus infection in post-EMT cushion mesenchymal cells. The effect of BMPs in post-EMT valvulogenesis was further determined by two independent ways of blocking BMP signaling by loss-of-function experiments: exogenous noggin treatment and inhibition of BMP signaling by perturbing the BMP receptors with dnBMPR-1B–virus in post-EMT AV cushion mesenchymal cells. These results are summarized in a diagram, Fig.10.
Fig. 10.
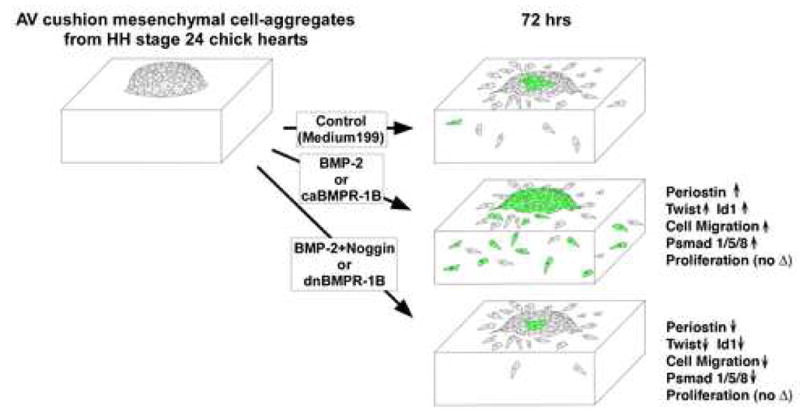
Summary diagram illustrating the results from bioassays to assess the role of BMP-2 and BMP signaling using AV cushion mesenchymal cell aggregates cultured on 3D-collagen gels. Results were evaluated 72hrs after placing the aggregates on the collagen gels. Exogenous BMP-2 or caBMPR-1B treatments induced mesenchymal cell migration and expression of periostin, Twist and Id1, while a BMP antagonist, noggin, or dnBMPR-1B treatment inhibited BMP-2 promoted cell migration and expression of periostin, Twist and Id1. Phospho-Smad 1/5/8 expression was induced by BMP-2 or caBMPR1-B treatments but reduced by noggin or dnBMPR-1B treatments. There was no significant difference in proliferation among treatments.
Discussion
Although accumulated data support the importance of BMPs and BMP signaling in valvuloseptal morphogenesis (Galvin et al., 2000; Delot et al., 2000; Kim et al., 2001; Gaussin et al., 2002; Jiao et al., 2003; Gaussin et al., 2005; Wang et al., 2005), the cell types that are involved and the regulatory mechanisms of BMPs in post-EMT valve formation have not been determined. Recent studies with Nkx2.5-cre/BMP-2 CKO mice showed that myocardially-derived BMP-2 was essential for AV EMT (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006). Moreover, data from Tie1-cre/ALK3 and FLK1-cre/ALK3 CKO mice indicated critical roles of ALK3 in endothelial cells for AV EMT (Park et al, 2006; Song et al., 2007). However, subsequent lethality of the CKO embryos after the EMT stage prevented analysis of the role of BMP signaling in post-EMT valvulogenesis. Although myocardial expression of BMP-2 is down regulated in the AV canal after EMT, BMP-2 is localized in AV cushion mesenchyme during post-EMT valvulogenesis in chicks (Lincoln et al., 2004) and mice (Sugi et al., 2004), suggesting potential autocrine signaling by BMP-2 in cushion mesenchymal cells during post-EMT valvulogenesis. Therefore, in this work we sought to determine the roles of BMP-2 and BMP signaling in post-EMT AV cushion mesenchymal cells by using spatio-temporal viral-gene-transfer technique with chick AV cushion mesenchymal cell cultures in vitro and whole embryo cultures in vivo (ovo). Recent work by Lincoln et al. (2006a) demonstrated that BMP-2 induced aggrecan and sox9—which are characteristics of cartilage cells--in AV cushion mesenchymal cells. This suggests the existence of active BMP signaling in cushion mesenchymal cells. As the first step of our present study, we showed the expression of Type I BMP receptors, BMPR-1A, BMPR-1B and ALK2, as supportive evidence for the presence of BMP signaling in cushion mesenchymal cells and further demonstrated that BMP-2 and BMP signaling through BMP receptors induce mesenchymal cell migration and expression of periostin, both of which were crucial for maturation and differentiation of post-EMT AV cushion mesenchyme. Therefore, our current study supports the idea that the autocrine signaling by BMPs in cushion mesenchymal cells can regulate post-EMT AV cushion mesenchymal cell differentiation.
Most BMP receptors have ligand binding affinity with other TGFβ superfamily proteins, i.e. TGFβ and activin. However, BMPR-1A and BMPR-1B bind specifically with BMP-2 and BMP-4, and bind with BMP-7 at low affinity but do not bind with TGFβs or activin (Dijke et al., 1994; Raftery and Sutherland 1999; de Caestecker, 2004). In this work, we demonstrated that dnBMPR-1B infection as well as treatments with a BMP antagonist, noggin, significantly repressed endogenous phospho-Smad 1/5/8 expression in AV cushion mesenchymal cells, which indicated that intracellular BMP signaling in the cushion mesenchymal cells was effectively inhibited by the treatments with dnBMPR-1B or noggin. Conversely, BMP-2 or caBMPR-1B-virus treatments induced expression of phospho-Smad 1/5/8, indicating that intracellular BMP signaling was effectively enhanced by BMP-2 or caBMPR-1B. Although BMPR-1B does not appear to be the most prominent Type 1 BMP receptor expressed in the AV canal (Fig. 1), our data indicated that overexpression of dnBMPR-B1or caBMPR-1B evidently modulated intracellular BMP signaling in the AV cushion mesenchymal cells. For example, dnBMPR-1B treatments inhibited BMP-2-supported up-regulation of cell migration as well as Smad1/5/8 expression in the cushion mesenchymal cells. The effects of dnBMPR-1B treatments on cushion mesenchymal cell migration and psmad1/5/8 expression are as profound as noggin treatments (Figs. 7 and 8). Because noggin is expected to antagonize BMP signaling through any Type I BMP receptors, the present data strongly suggest that the excess dominant negative forms of BMPR-1B are potentially disrupting BMP signaling by competing with other endogenously expressed Type I BMP receptors, i. e. BMPR-1A and/or Alk2. dnBMPR-1B and caBMPR-1B appear to function as broader reagents that can inhibit or activate BMP signaling through Type I BMP receptors in our AV cushion mesenchymal cell cultures. Therefore, our data do not resolve the specific biological function of BMPR-1B; however, the present study using AV cushion mesenchymal cells provides an example that the dominant-negative and constitutively active approach can be a powerful tool to effectively modulate BMP signaling when multiple Type I BMP receptors may cause functional redundancies.
Cell proliferation is the one of the critical biological processes in elongation and expansion of post-EMT AV cushion tissues in early valvulogenesis (de la Cruz and Markwald, 1998). Recent studies in cardiac valvulogenesis demonstrated that cell proliferation in cushion mesenchyme was high at early stages of valvulogenesis, but significantly decreased after remodeling stages in mice (Hinton et al., 2006) and chicks (Lincoln et al., 2004) and persisted at low levels in juvenile mice (Hinton et al., 2006). Our previous work revealed that fibroblast growth factor 4 (FGF4) induced cell proliferation in HH stage-24 post-EMT AV cushion mesenchyme in vitro and in vivo (ovo) (Sugi et al., 2003). In the present study, we found that BMP-2 and BMP signaling did not enhance mesenchymal cell proliferation in HH stage-24 AV cushion mesenchymal cell culture. BMP-2 is shown to induce Tbx20 that induces cell proliferation but represses expression of an ECM proteoglycan, aggrecan (Shelton and Yutzey, 2007). However, BMP-2 is also reported to induce aggrecan expression from cultured HH stage-25 AV cushion mesenchymal explants (Lincoln et al., 2006a). Tbx20 may exert positive (Cai et al., 2005) or negative feed back (Singh et al., 2005) on BMP-2 expression. Moreover, resent studies on deletion of noggin, which is an antagonist of BMPs and is expressed in both AV myocardium and cushion mesenchyme, showed that proliferation was increased in the myocardium but not in the cushion mesenchyme, suggesting tissue specific responses of BMP signaling (Choi et al., 2007). Therefore, precise signaling mechanisms and molecular interactions of BMP signaling governing cell proliferation in cushion mesenchyme are not fully understood. Taken together, our present study demonstrates that unlike FGF4 or Tbx20, BMP-2 is not responsible for a high proliferation ratio in endocardial cushions during early valvulogenesis but may be a key regulator in other necessary morphological processes in early valvulogenesis, i.e. production of valvulogenic ECM protein such as periostin and induction of cell migration.
Valvulogenesis is thought to be regulated by a complex coordination of growth factor signaling and ECM interactions (Camenisch et al., 2002; Schroeder et al., 2003); however, these processes have not fully elucidated. In the cardiac cushion, periostin expression is observed only after the EMT (Kern et al., 2005) and is closely correlated with the morphogenic processes associated with differentiation of cardiac cushion mesenchyme into dense, fibrous tissues and is proposed to be responsible for delamination of the AV cushion leaflets and the formation of the AV valvular suspensory apparatus (Markwald et al., 2004; Litvin et al., 2005). Periostin is also proposed to potentially regulate differentiation and recruitment of circulating adult bone marrow cells into postnatal hearts (Visconti and Markwald, 2006). Moreover, recent data in post-EMT valvulogenesis using chick primary culture assays indicated that periostin enhanced cell invasion/migration and collagen condensation by cushion mesenchyme which highlighted periostin as a mediator of matrix maturation and remodeling (Butcher et al., 2007). Therefore, periostin is proposed to promote and sustain differentiation of cushion mesenchyme into fibrous connective tissues and is a central player in cushion differentiation and maturation. Although periostin deficient mice do not show early lethality that could be attributed to serious defects in early valvulogenesis (Kii et al., 2006;Rios et al., 2006; Norris et al., 2007), this could be explained by molecular redundancies with a closely related molecule, bigH3, which is in the same gene family and is co-expressed with periostin in developing valvular tissues (Norris et al., 2004). Our present work also demonstrates a striking increase in chick Twist and Id1 expression in cushion mesenchymal cells cultured with BMP-2. Twist1, a member of the bHLH family protein, can specifically bind to the putative binding site, the Twist-box, on the periostin promoter in vitro in osteoblast MC3T3 cells (Oshima et al., 2002). It has also been determined by using Twist1 over-expressing 293T cells that Twist up-regulates mouse periostin transcription (Oshima et al., 2002). Id1 protein, a Class II HLH protein that lack the basics domain which is required for DNA- binding (Benezra, et al., 1990), preferentially dimerizes with E proteins and, hence, enhances Twist homodimer formation (Connerney et al., 2006). Critical evidence generated using in C3H10T1/2 mesenchymal cells is that overexpressing Twist1 homodimers enhances periostin expression, whereas expression of Twist 1 heterodimer inhibits it (Connerney et al., 2006). Therefore, an increase in the transcripts of the chick homologue of Twist1 and Id1 by BMP signaling in our experiments can be associated with activation of the periostin promoter in AV cushion mesenchymal. Collectively, our present work provides initial evidence that BMP-2 and BMP signaling induce periostin expression in post-EMT AV cushion mesenchymal cells.
Other than the inductive roles for periostin promoter activation, Id1 and Twist have been known to regulate various aspects of embryonic development including cell migration as well as tumor metastasis (Israel, 1999; Norton et al., 2000; Ruzinova and Benezra, 2003; Kang and Massague, 2004; Yang et al., 2004; Reinhold et al., 2006; Alexander, et al., 2006; Komaki et al., 2007). Particularly relevant to the present study is the recent observation that Id1 and Twist1 are disrupted in AV endocardium in Nkx2.5-cre/BMP-2 CKO mice (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006) as well as in FLK1-cre/ALK3 CKO mice (Park et al, 2006). These findings indicate that expression of Id1 and Twist1 are regulated by BMP-2 and/or BMP signaling in AV endocardial cells and suggest the roles of Id1 and Twist1 in AV EMT in mice. Furthermore, Id1 is known to play a critical role in mediating BMP-induced endothelial cell migration by mouse embryonic endothelial cells in vitro (Valdimarsdottir et al., 2002). Chick homologues of Ids (Id1–Id4) were cloned and localized in early chick embryos (Martinsen and Bronner-Fraser, 1998; Kee and Bronner-Fraser, 2001a, 2001b and 2001c). Our present work with stage-24 chick cardiac cushion mesenchymal cells revealed that Id1 and Twist expression was induced by BMP-2 in post-EMT cushion mesenchymal cells. Because Id1 regulates embryonic vascular endothelial cell migration (Valdimarsdottir et al., 2002), it will be of interest to learn interactions between BMP signaling and these transcription factors and roles of their signaling pathways in cushion mesenchymal cells during post-EMT valvulogenesis.
In conclusion, our current work provides strong evidence that BMP-2 and BMP signaling regulate cushion mesenchymal cell migration and expression of a valvulogenic ECM protein, periostin, indicating that BMP-2 and BMP signaling play a critical role in post-EMT AV cushion mesenchyme differentiation and maturation. Because many molecules are known to be expressed in the post-EMT cushion mesenchyme and involved in valvulogenesis (Reviews, Armstrong and Bischoff, 2004; Schroeder et al., 2003; Person et al., 2006; Lincoln et al., 2006b), further studies are warranted to better characterize the interaction of BMPs and/or BMP signaling with other valvulogenic regulatory molecules and their molecular cascades in post-EMT valvulogenesis.
Supplementary Material
Supplemental Fig. 1. A– H. BrdU incorporation assay. Exogenously added BMP-2 did not stimulate BrdU incorporation by AV cushion mesenchymal cells. Note that incidence of BrdU-positive (green) nuclei appears to be the same in all cultures treated with control Medium 199 (E ), BMP-2 ( F ), BMP-2 plus noggin ( G ) or noggin (H). Upper panels (A–D) show all nuclei stained with propidium iodide (red). I. Quantitative analysis of exogenous BMP-2 treatments in AV cushion mesenchymal cell. BrdU-positive and–negative nuclei in the cultured mesenchymal cells were counted to determine the percentage of cells in cell-cycle transit. A total 1,000 nuclei in each culture was evaluated in 4 random fields. There was no significant difference among the treatments.
Acknowledgments
The authors thank Ms. Pattie L. Tennille for preparation of chick embryos for in situ hybridization. Sections in situ hybridization protocols were kindly provided by Dr. A. Wessels at MUSC and Dr. M. J. B. van den Hoff at the University of Amsterdam, Netherlands. The authors are grateful to the MUSC Proteogenomics Core (Dr. W.S. Argraves, director, and Dr. Jeremy Barth and Mr. Victor Fresco) for RNA quantification with the Agilent Bioanalyzer 2100 (Agilent Tec). The authors are also grateful to the Molecular Morphology Core in the Department of Cell Biology and Anatomy, MUSC, (Dr. Thomas Trusk, director and Mr. Joshua Spruill) for their support with computer-assisted photography and illustration. The authors thank Dr. L. Niswander (Howard Hughes Medical Institute and University of Colorado. Denver, CO) for kindly providing us with two mutant constructs of chick BMPR-1B cloned into RCAS-(A) viral vector. This work was conducted in a facility constructed with support from the NIH (grant C06 RR018823) from the Extramural Research Facilities Program of the National Center for Research Resources. This work was supported by NIH grant HL33756 (RRM and RAN) and AHA Grant-in-Aid 0755525U (YS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander NR, Tran NR, Rekapally H, Summers CE, Glackin C, Heimark RL. N-cadherin gene expression in prostate carcinoma is modulated by integrindependent nuclear translocation of Twist1. Cancer Rec. 2006;66:3365–3369. doi: 10.1158/0008-5472.CAN-05-3401. [DOI] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R, Davis RL, Lockshon E, Turner DL, Weintraub H. The protein: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI-kinase. Dev Biol. 2007;302:256–266. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Zhou E, Yang L, Bu L, Qyang Y, Zhang X, Li X, Rosenfeld MG, Chen J, Evans S. T-box gene coordinate regional rates of proliferation and regional specification during cardiogensis. Development. 2005;132:2475–2487. doi: 10.1242/dev.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Schroeder J, Bradley J, Klewer S, McDonald J. Heart-valve mesenchyme formation is dependent on hyarulonan-augmented activation of ErbB2–ErbB3 receptors. Nat Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- Choi M, Stottmann RW, Yang Yu-Ping, Meyers EN, Klingensmith J. The bone morphogenetic protein antagonist noggin regulates mammalian cardiac morphogenesis. Circ Res. 2007;100:220–228. doi: 10.1161/01.RES.0000257780.60484.6a. [DOI] [PubMed] [Google Scholar]
- Connerney J, Andreeva V, Leshem Y, Muentener C, Mercado MA, Spicer DB. Twist1 dimer selection regulates cranial suture patterning and fushion. Dev Dyn. 2006;235:1345–1357. doi: 10.1002/dvdy.20717. [DOI] [PubMed] [Google Scholar]
- de Caestecker M. The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Reviews. 2004;15:1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- de La Cruz MV, Markwald RR. Living morphogenesis of the heart. New York: Birkhauser/springer-Verlag; 1998. [Google Scholar]
- Delot EC, Bahamonde ME, Zhao M, Lyons KM. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development. 2003;130:209–220. doi: 10.1242/dev.00181. [DOI] [PubMed] [Google Scholar]
- Dijke PT, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- Eisenberg CA, Markwald RR. Mixed culture of avian blastoderm cells and the quail mesoderm cell line QCE-6 provide evidence for he pluripotentiality of early mesoderm. Dev Biol. 1997;191:167–181. doi: 10.1006/dbio.1997.8718. [DOI] [PubMed] [Google Scholar]
- Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MAJ, Falb D, Huszar D. A role of smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- Gaussin VC, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci USA. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaussin V, Morley G, Cox L, Zwijsen A, Vance KM, Emile L, Tian Y, Liu J, Hong C, Myers D, Conway S, Depre C, Mishina Y, Behringer RR, Hanks MC, Schneider MD, Huylebroeck D, Fishmen GI, Burch J, Vatner SF. Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrousus. Cir Res. 2005;97:219–226. doi: 10.1161/01.RES.0000177862.85474.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HE. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hinton RB, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Cir Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- Hoffman JIE, Kaplan S. The incidence of Congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Hoffman JIE, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes & Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periostium and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- Hughes SH, Greenhouse JJ, Petropoulos CJ, Sutrave P. Adapter plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- Israel MA, Hrnandez MC, Florio M, Andres-Barquin PJ, Mantani A, Carter JH, Julin CM. Id gene expression as a key mediator of tumor cell biology. Cancer Res. 1999;59:1726s–1730s. [PubMed] [Google Scholar]
- Ji X, Chen D, Xu C, Harris SE, Mundy GR, Yoneda T. Patterns of gene expression associated with BMP-2-induced osteoblast and adipocyte differentiation of mesenchymal progenitor cell 3T3-F442A. J Bone Miner Metab. 2000;18:132–139. doi: 10.1007/s007740050103. [DOI] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of BMP4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Kee Y, Bronner-Fraser M. Temporally and spatially restricted expression of the helix-loop-helix transcription regulator Id1 during avian embryogenesis. Mech Dev. 2001;109:331–335. doi: 10.1016/s0925-4773(01)00574-3. [DOI] [PubMed] [Google Scholar]
- Kee Y, Bronner-Fraser M. The transcriptional regulator Id3 is expressed in cranial sensory placodes during early avian embryonic development. Mech Dev. 2001;109:337–340. doi: 10.1016/s0925-4773(01)00575-5. [DOI] [PubMed] [Google Scholar]
- Kee Y, Bronner-Fraser M. Id4 expression and its relationship to other Id genes during avian embryonic development. Mech Dev. 2001;109:341–345. doi: 10.1016/s0925-4773(01)00576-7. [DOI] [PubMed] [Google Scholar]
- Kern CB, Hoffman S, Moreno R, Damon BJ, Norris RA, Krug EL, Markwald RR, Mjaatvedt CH. Immunolocalization of chick periostin protein in the developing heart. Anat Rec Part A. 2005;284A:415–423. doi: 10.1002/ar.a.20193. [DOI] [PubMed] [Google Scholar]
- Kii I, Amizuka N, Minqi L, Kitajima S, Saga Y, Kudo A. Periostin is an extracellyular matrix protein required for eruption of incisors in mice. Biochem Biophys Res Commun. 2006;342:766–772. doi: 10.1016/j.bbrc.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developmental mouse heart. Dev Biol. 2001;235:449–466. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- Komaki M, Karakida T, Abe M, Oida S, Mimori K, Iwasaki K, Noguchi K, Oda S, Ishikawa I. Twist negatively regulates osteoblastic differentiation in human periodontal ligament cells. J Cell Biochem. 2007;100:303–314. doi: 10.1002/jcb.21038. [DOI] [PubMed] [Google Scholar]
- Kruzynska-Frejtag A, Machnicki M, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech Dev. 2001;103:183–188. doi: 10.1016/s0925-4773(01)00356-2. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn. 2004;230:239–250. doi: 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Alfieri CM, Yutzey KE. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Biol. 2006a;292:290–302. doi: 10.1016/j.ydbio.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Lange AW, Yutzey KE. Hearts and bones: Shared regulatory mechanisms in heart valve. Cartilage, tendon, and bone development. Dev Biol. 2006b;294:292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Litvin J, Zhu S, Norris RA, Markwald RR. Perisotin family of proteins: Therapeutic targets for heart disease. Anat Rec A Disc Mol Cell Evol Biol. 2005;287:1205–1212. doi: 10.1002/ar.a.20237. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Krug EL, Moreno R, Mjaatvedt C, Ogawa M, Drake C, Visconti R, Kruzynska-Frejtag A, Conway SJ. Valvulogenesis: Role of periostin in cushion tissue differentiation. In: Clark EB, Nakazawa M, Takao A, editors. Etiology and Morphogenesis of Congenital Cardiovascular Disease in the Post-Genomic Era. Futura Publishing Company; 2004. [Google Scholar]
- Martinsen BJ, Bronner-Fraser M. Neural crest specification regulated by the helix-loop-helix repressor Id2. Science. 1998;281:988–991. doi: 10.1126/science.281.5379.988. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)- β and bone morphogenetic protein (BMP) Anat Rec. 2000;258:119–127. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Norris RA, Kern CB, Wessels A, Moralez EI, Markwald RR, Mjaatvedt CH. Identification and detection of the periostin gene in cardiac development. Anat Rec. 2004;281A:1227–1233. doi: 10.1002/ar.a.20135. [DOI] [PubMed] [Google Scholar]
- Norris RA, Kern CB, Wessels A, Wirring EE, Markwald RR, Mjaatvedt CH. Detection of βig-H3, a TGFβ induced gene, during cardiac development and its complementary pattern with periostin. Anat Embryol. 2005;210:13–23. doi: 10.1007/s00429-005-0010-z. [DOI] [PubMed] [Google Scholar]
- Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101:195–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JD. Id helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113:3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- Okagawa H, Markwald RR, Sugi Y. Functional BMP receptor in endocardial cells is required in atrioventricular cushion mesenchymal cell formation in chick. Dev Biol. 2007;306:179–192. doi: 10.1016/j.ydbio.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthoek PE, Wenink AC, Vrolijk BC, Wisse LJ, DeRuiter MC, Poelmann RE, Gittenberger-de-Groot AC. Development of the atrioventricular valve tension apparatus in the human heart. Anat Embryol (Berl) 1998;198:317–329. doi: 10.1007/s004290050187. [DOI] [PubMed] [Google Scholar]
- Oshima A, Tanabe H, Yan T, Lowe GN, Glackin CA, Kudo A. A novel mechanism for the regulation of osteoblast differentiation: transcription of periostin, a member of the fasciclin I Family, is regulated by the bHLH transcription factor, Twist. J Cell Biochem. 2002;86:792–804. doi: 10.1002/jcb.10272. [DOI] [PubMed] [Google Scholar]
- Park C, Lavine K, Mishina Y, Deng CX, Ornitz MO, Choi K. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development. 2006;133:3473–3484. doi: 10.1242/dev.02499. [DOI] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cyto. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Raftery LA, Sutherland DJ. TGF-β family signal transduction in drosophila development: From Mad to Smads. Dev Biol. 1999;210:251–268. doi: 10.1006/dbio.1999.9282. [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Kapadia RM, Liao Z, Naski MC. The Wnt-inducible transcription factor Twist1 inhibits chondrogenesis. J Biol Chem. 2006;281:1381–1388. doi: 10.1074/jbc.M504875200. [DOI] [PubMed] [Google Scholar]
- Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cel Biol. 2005;25:1131–1144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Tabin CJ. Bpm2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol. 2006;295:580–588. doi: 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelen BAJ, Goumans MJ, van Ollijen MA, Mummery CL. Int. J Dev Biol. 1997;42:541–549. [PubMed] [Google Scholar]
- Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. TRENDS Cell Biol. 2003;13:410–418. doi: 10.1016/s0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valve: coordination by extracellular matrix and growth factor signaling. J Mol Med. 2003;81:392–403. doi: 10.1007/s00109-003-0456-5. [DOI] [PubMed] [Google Scholar]
- Shelton EL, Yutzey KE. Tbx20 regulation of endocardial cushion cell proliferation and extracellular matrix gene expression. Dev Biol. 2007;302:376–388. doi: 10.1016/j.ydbio.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Christoffels VM, Dias JM, Trowe MO, Petry M, Schuster-Gossler K, Burger A, Ericson H, Kispert A. Tbx20 is essential for cardiac chamber differentiation and repression of Tbx2. Development. 2005;132:2697–2707. doi: 10.1242/dev.01854. [DOI] [PubMed] [Google Scholar]
- Somi S, Buffing AA, Moorman AF, Van Den Hoff MJ. Dynamic patterns of expression of BMP isoforms 2, 4, 5, 6, and 7 during chicken heart development. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:636–651. doi: 10.1002/ar.a.20031. [DOI] [PubMed] [Google Scholar]
- Song L, Faessler R, Mishina Y, Jiao K, Baldwin HS. Essential function of Alk3 during AV cushion morphogenesis in mouse embryonic hearts. Dev Biol. 2007;301:271–286. doi: 10.1016/j.ydbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sugi Y, Markwald RR. Endodermal growth factors promote endocardial precursor cell formation from precardiac mesoderm. Dev Biol. 2003;263:35–49. doi: 10.1016/s0012-1606(03)00433-0. [DOI] [PubMed] [Google Scholar]
- Sugi Y, Ito N, Szebenyi G, Myers K, Fallon JF, Mikawa T, Markwald RR. Fibroblast growth factor (FGF)-4 can induce proliferation of cardiac cushion mesenchymal cells during early valve leaflet formation. Dev Biol. 2003;258:252–263. doi: 10.1016/s0012-1606(03)00099-x. [DOI] [PubMed] [Google Scholar]
- Sugi Y, Yamamura H, Okagawa H, Markwald RR. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol. 2004;269:505–518. doi: 10.1016/j.ydbio.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Valdimarsdottir G, Goumans MJ, Rosendahl A, Brugman M, Itoh S, Lebrin F, Sideras P, ten Dijke P. Stimulation of Id1 expression by bone morphogentic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation. 2002;106:2263–2270. doi: 10.1161/01.cir.0000033830.36431.46. [DOI] [PubMed] [Google Scholar]
- Visconti RP, Markwald RR. Recruitment of new cells into postnatal heart: potential modification of phenotype by periostin. Ann N Y Sci. 2006;1080:19–33. doi: 10.1196/annals.1380.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Dijke PT, Heldin CH, Miyazono K. Bone morphogenetic protein receptors. Bone. 1996;19:569–574. doi: 10.1016/s8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Zou H, Niswander L. Requirement for BMP signaking in interdigital apoptosis and scale formation. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]
- Zou H, Wieser R, Masssague J, Niswander L. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes & Dev. 1997;11:2192–2203. doi: 10.1101/gad.11.17.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. A– H. BrdU incorporation assay. Exogenously added BMP-2 did not stimulate BrdU incorporation by AV cushion mesenchymal cells. Note that incidence of BrdU-positive (green) nuclei appears to be the same in all cultures treated with control Medium 199 (E ), BMP-2 ( F ), BMP-2 plus noggin ( G ) or noggin (H). Upper panels (A–D) show all nuclei stained with propidium iodide (red). I. Quantitative analysis of exogenous BMP-2 treatments in AV cushion mesenchymal cell. BrdU-positive and–negative nuclei in the cultured mesenchymal cells were counted to determine the percentage of cells in cell-cycle transit. A total 1,000 nuclei in each culture was evaluated in 4 random fields. There was no significant difference among the treatments.


