Abstract
Background:
A wide range of responses of patients with CPCML to IM has been reported. Several factors were proposed to predict response including molecular response at 3 and 6 months. Purpose: To study the impact of pretreatment BCR-ABL transcript level on molecular response to IM, and to assess the value of the milestone ; ≤10% transcript at 3 months on PFS and OS.
Patients and Methods:
Fifty five adult CP-CML patients receiving daily dose of 400 mg IM were subjected to molecular and cytogenetic analysis at diagnosis and at regular time intervals. Median follow up period was 36 months (15-48). Hematologic, cytogenetic, and molecular responses were rated according to ELN.
Results:
Two Patient groups were distinguished regarding response to IM therapy. A group of 22/55 patients (40%) having pretreatment BCR-ABLIS level ≤200% and a second patient group 33/55 (60%) having transcript level >200%. The ≤10% milestone was achieved by 15/22 patients (68%) versus 7/33 patients (21%), p=0.04 in favor of the first group. Optimal responders in first group were 14/22 (64%) compared to 13/33 (39%) in second group, p=0.02. Achievement of 10% transcript level significantly correlated with longer PFS. The median BCR-ABLIS transcripts levels in optimal responders at 3, 6 and 18 months was 10%, 2% and 0.1%, respectively compared to 100%, 65% and 10%, in suboptimal/resistant patients p=0.001. Resistance in 11 patients was correlated with identifiable ABL Kinase mutations.
Conclusions:
The Pretreatment 200% cutoff and the 3 month BCR-ABLIS ≤10% transcript levels proved strong predictors of response to IM and significantly correlated with probability of CCyR, MMR and PFS.
Keywords: CPCML, BCRABL transcript, cytogenetic, molecular responses
INTRODUCTION
The introduction of Imatinib Mesylate (IM) in 1998 has revolutionized the management of Chronic Myeloid Leukemia (CML). This selective tyrosine kinase inhibitor offers a durable response in a high percentage of patients with favorable long term safety profile and decreasing rate of relapse (1). Impressive response rates and good tolerability have made IM the standard front line therapy for CML. Despite its remarkable efficacy for the treatment of chronic phase (CP) CML, a growing number of patients either fail IM therapy due to emergence of ABL kinase domain (AKD) mutations or develop intolerance to the drug (2). All treatment trials with IM insisted on the close sequential molecular monitoring of patients under IM therapy to assess the kinetics of BCR ABL transcripts level regression and its impact on the remote cytogenetic response and progression free survival (PFS) (3-5). We have previously reported that patients achieving two log reduction of BCR-ABL transcript level after 6 months of IM therapy could benefit of a sustained cytogenetic and molecular responses (6). Several reports have focused on the correlation between earlier molecular response at 3 months and the probability of longer disease free survival (DFS) and higher rate of overall survival (OS) (7, 8). A BCR-ABLIS transcript level of ≤10% after 3 months or <1% after 6 months of IM therapy has been found to be associated with significant increase of the probability of obtaining a complete cytogenetic (CCyR) and molecular (CMR) responses (9). In addition, a high BCR-ABLIS transcript level at diagnosis was found to be related to a non-optimal response to IM therapy (7).
In the present study we aimed at
Exploring the relation between pretreatment BCR-ABLIS transcript level and molecular and cytogenetic responses to IM.
Assessing the impact of achieving ≤10% BCR-ABLIS transcripts level at 3 months of IM therapy on progression free survival (PFS) and overall survival (OS) in a cohort of Egyptian patients.
PATIENTS AND METHODS
Study group
Fifty five adult Egyptian patients with CP-CML were evaluable for molecular analysis at diagnosis and at 3 months interval of IM therapy. The patients presented to the outpatient clinics of the Medical Oncology Unit, National Cancer Institute (NCI) and hematology Unit of the Medical school, Cairo University from May 2007 to May 2011. The study was carried out according to declaration of Helsinki and approved by the institutions review boards. Informed consent was provided by all patients.
All patients had morphologic and cytogenetic evidence of Philadelphia positive CP-CML (defined as less than 12 months from diagnosis); age 18 years or older; having normal blood chemistry and normal cardiac function. Women at childbearing age were required to have a negative pregnancy test before starting IM and to use contraception during therapy. Excluded from the study were patients receiving CML therapies other than IM (busulfan, IFN-α, or Ara-C). Exceptions included anagrelide and hydroxyurea for the treatment of elevated platelet (>700 × 109/L) and WBC count (>50 × 109/L), respectively; for more than 4 weeks.
Treatment
Patients were treated within an international Novartis-sponsored protocol with Imatinib Mesylate (IM) at a daily oral dose of 400 mg. The dose was reduced to 300 mg for any ≥ Grade 3 drug-related hematologic toxicity. No dose adjustments were made for grade 1 or 2 hematologic toxicities.
Methods
BCR-ABLIS transcript level according to International Scale (IS) was determined usingReal Time quantitative polymerase chain reaction (RQ-PCR) detection system (Ecco Illumina, USA). The ABL control gene and the BCR-ABL target gene transcript levels were measured using Universal PCR Master Mix (Nanogen advanced diagnostics, SrL, Italy) and specific primers and hydrolysis probe (Philadelphia p210 and Abl QPCR alert Amplimix and QPCR alert Ampliprobe).A set of reference RNA was usedto convert results into IS. Quantitations were made against specific Abl and p210 standards (Philadelphia p210 QPCR standards, Nanogen) (10). Cytogenetic analysis was performed by G banding technique according to standard methods (11). At least twenty metaphases were analyzed for each sample. Fluorescent insitu hybridization (FISH) analysis for BCR and ABL genes using probes from Vysis Inc. (Downers Groove, IL, USA). A minimum of 100 interphase nuclei were evaluated for each patient.
Mutation Screening
Genomic DNA was extracted from peripheral blood using Gentra Puregene blood kit (QIAGEN, Hilden, Germany). ASO-PCR assay was established for the detection of 16 known mutations which were selected according to their frequency in IM-resistant CML patients. Mutation panel selected in this study included M244V, L248V, G250E, Q252H (a), Q252H (b), Y253H, Y253F, E255K, E255V, V299L (a), V299L (b), F311L, T315I, F317L, M343T, M351T, E355G and F359V. Mutated or wild-type sequences were specifically amplified in a PCR reaction to analyze the most frequently identified mutations in the AKD (amino acids 220 to 498). The amplified products were detected by electrophoresis on 2% ethidium bromide-stained agarose gel (13, 14).
Definition of treatment responses
Hematologic, molecular and cytogenetic responses were defined according to European Leukemia Net criteria. MMR was defined as a 3-log reduction of BCR-ABL transcripts level, corresponding to ≤0.1% on IS (12). Progression free survival (PFS) defined as loss of hematologic or cytogenetic response, death, or development of advanced CML. Overall (OS) defined by the absence of death from any reason (15).
Statistical analysis
All analyses were performed using the statistical package for the social sciences (SPSS software 17; SPSS Inc., Chicago, USA). We used a receiver operating characteristic curve (ROC) to identify the cutoffs in pretreatment transcript level that would best predict patient outcome (16).
RESULTS
Fifty five Egyptian patients; 33 males and 22 females were enrolled in this study. Median age of the whole cohort at diagnosis was 40.5 years (19-60). Median total leukocytic count was 150 × 109/L (27-583). Median hemoglobin and platelet count was 11.4 gm/dl (8.2-16.9) and 340 × 109/L (115-1000), respectively. Median follow up period was 36 months (15-48). Complete hematologic response (CHR) at 3 months of IM therapy was achieved by 54/55 patients (98.2%). Complete Cytogenetic Response at 12 months and MMR at 18 months of IM therapy were achieved by 28/55 patients (51%).
Pretreatment BCR-ABLIS transcript level and response to IM
According to pretreatment BCR-ABLIS transcript level, two groups of patients could be distinguished regarding response to IM therapy. One group of 22 patients (40%) having a pretreatment BCR-ABLIS ≤200% (26-200%, median 75%) and a second group of 33 (60%) patients with a pretreatment BCR-ABLIS transcript level >200% (223-2000%, median 470%) (Table 1). Subgroup analysis of both groups revealed that at 3 months of IM therapy, 15/22 patients (68%) of the first group achieved a BCR-ABLIS transcript level of ≤10% versus 7/33 patients (21%) in the second group, p=0.04. At 3 months of IM therapy, transcript level of 1-5% was achieved by 7/22 patients (32%) of first group versus 1/33 patients (3%) of the second group (p=0.01).
Table 1.
Effect of Pretreatment BCR-ABL transcript level on Molecular Response to IM
| Pretreatment BCR-ABLIS Transcript level | ≤200% | >200% | p-value |
|---|---|---|---|
| No. of patients | 22 | 33 | |
| Median % BCR-ABLIS transcript level (Range) | 75 (26-200%) | 470 (223-2000%) | 0.007 |
| No. of Patients with ≤10% BCR-ABLIS Transcript at 3 Mo (%) | 15 (68%) | 7 (21%) | 0.04 |
| No. of Patients with 1-5% BCR-ABLIS Transcript at 3 Mo (%) | 7 (32%) | 1 (3%) | 0.01 |
| No. of Patients with optimal response; [CCyR at 12 months, MMR at 18 months] (%) | 14 (64%) | 13 (39%) | 0.05 |
The number of optimal responders in patients with pretreatment BCR-ABLIS ≤200% was 14/22 (64%) compared to 13/33 (39%) in the patient group with >200% (p=0.05) (Table 1).
The median BCR-ABLIS transcripts level in optimal responders at 3, 6 and 18 months was 10%, 2% and 0.1%, respectively. On the contrary, patients exhibiting refractoriness and suboptimal response showed a significantly sluggish regression of the median transcript levels, at the corresponding time points (100%, 65% and 10%), (p=0.001) (Table 2).
Table 2.
Correlation between BCR ABL Transcript Regression and Response to IM
| Response Group | Median BCR-ABLIS % at diagnosis (range) | Median BCR-ABLIS % at 3 months (range) | Median BCR-ABLIS % at 6 months (range) | Median BCR-ABLIS % at 18 months (range) |
|---|---|---|---|---|
| Optimal (n:28) | 240.0 (26.0-820) | 10.0 (1.0-100) | 2.0 (0.1-11) | 0.1 (0.003-0.3) |
| Sub-optimal & resistant (n:27) | 380.0 (34.0-2000) | 100.0 (4.0-580) | 65.0 (2.0-3000) | 10.0 (1-287) |
| p-value | 0.09 | <0.001 | <0.001 | <0.001 |
BCR-ABLIS 10% milestone at 3 months and PFS /OS
The rates of PFS and OS of the whole study group at 3 years of IM therapy was 87% and 96.4%, respectively. The estimated PFS at 3 years for patients who achieved the ≤10% at 3 months of IM therapy was 100% versus 80% for patients with >10% (p=0.02), Figure 1. OS for patients according to the 10% BCR-ABLIS transcript level at 3 months of IM therapy is demonstrated in Figure 2.
Figure 1.
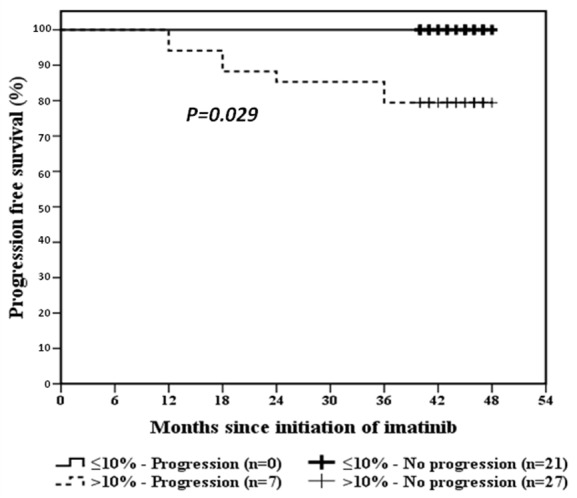
PFS curves of BCR-ABLIS ≤10% and >10% at 3 months of IM therapy
Figure 2.
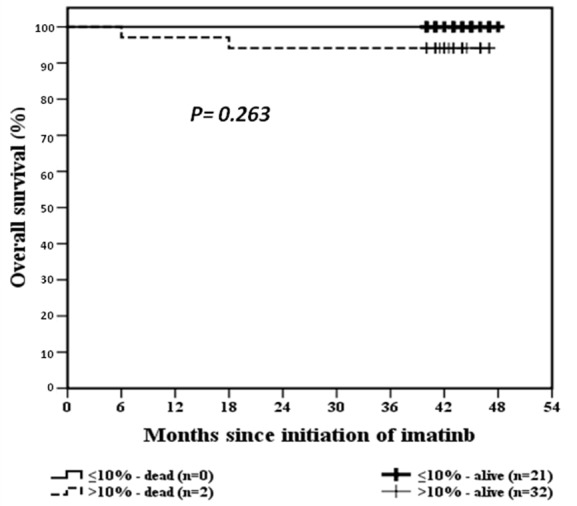
OS curves for BCR-ABLIS ≤10% and >10% at 3 months of IM therapy
Mutational status of resistant patients to IM
Eleven patients (11/55, 20%) have shown resistance to IM and experienced disease progression to acute blastic crisis (ABC) in 3 patients, to accelerated phase (AP) in 2 patients and 2 disease related deaths. None of these patients had demonstrated decrease in transcript level to 10% at 3 months of IM treatment. Mutational analysis revealed P-loop mutations (amino acids 248-255) in 6/11 of patients including Q252Ha and Q252Hb in 3 patients, Y253H in 1 patient and 2 patients having E255K. Non-P loop mutations were found in 4 patients, 3 patients with M351T, one patient with F359V and one patient without any of studied mutations. None of these patients was harboring the gatekeeper mutation T315I.
DISCUSSION
Although CCyR has long been the gold standard of response and survival to IM treated patients with CPCML (17) yet, we and others have stressed the importance of the kinetics of molecular response to IM (3, 6). The application of an international standardization of BCR-ABLIS transcript level enabled a consistent monitoring of patients and more appropriate assessment of the deep molecular responses during the early phase of treatment (4). Most studies have demonstrated that response determined by BCR-ABLIS transcripts level at defined time points has been the more reliable predictive parameter of long term evolution of the disease (7, 8). We have previously reported the predictive value of 2 log reduction of BCR-ABL transcript level after 6 months of IM therapy on PFS and sustained cytogenetic response (6). Recently the kinetics of molecular response at 3 months became a more rising predictive issue (7, 8, 18) where the 3-month BCR-ABLIS ≤10% transcript level was evoked as a strongest predictor milestone of response (9).
In the present study of an Egyptian cohort of 55 CPCML patients (40%) achieved BCR-ABLIS ≤10% transcript level after 3 months of IM therapy. Subgroup analysis of these 22 patients revealed that 15/22 patients (68%) had a pretreatment BCR-ABLIS transcript level ≤200% and 7/33 patients (21%) had a pretreatment level >200% suggesting that the cutoff of 200% could also have a significant impact on the kinetics of molecular response. These data concur with previously reported (7). Complete cytogenetic response and MMR at their specified times were significantly associated with achievement of the 3 months milestone BCR-ABLIS ≤10%, p<0.001. According to some reports, about one-fourth of CML patients were declared high-risk at 3 months (with BCR-ABLIS >10%) and the same proportion of treatment failure was also observed in the IRIS study. This led to a conclusion that failure of achieving ≤10% BCR-ABLIS transcript level after 3 months of IM therapy will lead to disease progression and treatment failure (8). According to failure of achieving this 3 months BCR-ABLIS ≤10% milestone 33/55 patients (60%) in the present study would be considered high risk. However it is importantly to say that among them 7 patients (13%) were optimal responders at 18 months which may raise the issue of distinct biological characteristics of slow responders, such as low hOCT1 or relatively high MDR1 expression (19-21). Both optimal responders and slow responders behaved equally regarding PFS and OS, which is in concordance with our previously reported data on the predictive value of 2 log reduction at 6 months of IM therapy on PFS and OS in another Egyptian series (6). This also stresses on the importance of postponing shift to second generation tyrosine kinase inhibitors (G2TKIs) as changing treatment to second line TKIs after only 3 months would be unusual. Whether an inferior response at 3 months will trigger treatment revision on a considerable proportion of patients should be considered on basis of other biological factors that would affect response.
The data in the present study showed that resistance and disease progression occurred in 11/55 (20%) patients who had both higher pretreatment transcript level and in the same time failed to achieve the BCR-ABLIS ≤10% milestone after 3 months of IM . These data point to the pretreatment transcript level as an additional predictive factor of the type of molecular response to IM. However and more importantly the concomitant occurrence of both factors might trigger performing mutational analysis of AKD which may prompt a change of therapeutic strategy , especially if the encountered mutations are Known to be sensitive to second generation Tyrosine Kinase inhibitors (G2TKI) as in the present study.
Although a lower fraction of our patients could achieve the 3 months BCR-ABLIS ≤10% transcript than reported by others (40% versus 64%) (7, 8) yet, optimal response rates at 18 months were not fairly different (51% versus 60%). Our patients seem to be slow responders as shown by median BCR-ABLIS transcripts level in optimal responders at 3, 6 and 18 months (10%, 2% and 0.1%, respectively) compared to others (7, 8). No significant difference in OS at 3 years between patients with 3 month BCR-ABLIS ≤10% and >10% (100% versus 96%, p=0.2). However, we and others, demonstrated a lower rate of 3 year PFS in the >10% group (100% and 80%, p=0.02 (8).
In conclusion, our data point to the pretreatment 200% cutoff transcript level as an additional predictive factor of the type of molecular response to IM and add further proof to the 3 month BCR-ABLIS ≤10% transcript level as a strong predictor of response and correlates significantly with probability of achieving CCyR and MMR at their specified time points with higher estimates of PFS.
CONFLICT OF INTEREST
The authors declare that no conflicting interests exist.
REFERENCES
- 1.Hochhaus A, Druker BJ, Larson RA, O’Brien SG, et al. IRIS 6-Year Follow up: Sustained survival and declining Annual rate of transformation in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [abstract 25] Blood. 2007;110(11) [Google Scholar]
- 2.Deininger M, O’Brien SG, Guilhot F, Goldman JM, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow-up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood, ASH Annual Meeting Abstracts. 2009;114:1126. [Google Scholar]
- 3.Hughes TP, Deininger MWN, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;(108):28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branford S, Fletcher L, Cross NC, Muller MC, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112:3330–3338. doi: 10.1182/blood-2008-04-150680. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoud HK, ElNahass Y, AbdelMoaty M, Abdel Fattah R, et al. Sequential molecular monitoring of patients with chronic phase myelogenous leukemia during Imatinib mesylate treatment: Clinical significance and predictive value. Haematologica. 2008;93(s1):222. abstract 0548. [Google Scholar]
- 6.Mahmoud HK, El Nahass Y, Abdel Moaty M, Abdel Fattah R, et al. Kinetics of BCR-ABL transcripts in Imatinib Mesylate treated Chronic Phase CML (CPCML), A predictor of response and progression free survival. Int. J. Biomed. Sci. 2009;5(3):100–105. [PMC free article] [PubMed] [Google Scholar]
- 7.Vigneri P, Stagno F, Stella S, Cupri A, et al. BCR-ABL (IS) Expression at Diagnosis and After 3 or 6 months of Treatment predicts CML Response to IMATINIB Therapy. Blood ASH Annu. Meet. Abstr. 2011;116:3426. [Google Scholar]
- 8.Hanfstein B, Muler C, Hehlmann R, Erben P, et al. arly molecular and cytogenetic response is predictie for long-term progression-free and overall survival in chronic myeloid leukemia (CML) Leukemia. 2012:1–7. doi: 10.1038/leu.2012.85. [DOI] [PubMed] [Google Scholar]
- 9.Marin D, Ibrahim AR, Lucas C, Gerrard G, et al. Assessment of BCR-ABL transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J. Clin. Oncol. 2012;30:232–238. doi: 10.1200/JCO.2011.38.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabert J, Beillard E, van der Velden VH, Grimwade D, et al. Standardization and quality control studies of real time quantitative - polymerase chain reaction (RQ-PCR) of fusion gene transcripts for minimal residual disease detection in Leukemias – A Europe against cancer Program. Leukemia. 2003;(17):2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 11.Schoh C, Schnittger S, Gerstner D, Hochhaus A, et al. Comparison of chromosome banding analysis, interphase and hypermetaphase-FISH, qualitative and quantitative PCR for diagnosis and follow up in chronic Myeloid Leukemia: A study on 350 cases. Leukemia. 2002;16:53–59. doi: 10.1038/sj.leu.2402329. [DOI] [PubMed] [Google Scholar]
- 12.Baccarani M, Cortes J, Pane F, Niederwieser D, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European Leukemia Net. J. Clin. Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang HY, Hwang JY, Kim SH, et al. Comparison of allele specific oligonucleotide-polymerase chain reaction and direct sequencing for high throughput screening of ABL kinase domain mutations in chronic myeloid leukemia resistant to imatinib. Haematologica. 2006;91:659–662. [PubMed] [Google Scholar]
- 14.Roche-Lestienne C, Soenen-Cornu V, Grardel- Duflos N, et al. Several types of mutations of the ABL gene can be found in chronic myeloid leukemia patients resistant to STI-571, and they can pre-exist to the onset of treatment. Blood. 2002;100:1014–1018. doi: 10.1182/blood.v100.3.1014. [DOI] [PubMed] [Google Scholar]
- 15.Press RD, Love Z, Tronnes AA, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response (CCyR) predict the duration of CCyR in imatinib mesylate - treated patients with CML. Blood. 2006;(107):4250–4256. doi: 10.1182/blood-2005-11-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norusis M. Upper Saddle-River. N.J.: Prentice Hall, Inc.; 2009. SPSS 17.0 advanced statistical procedures companion. [Google Scholar]
- 17.Kantarjian H, Baccarani M, Jabbour E, Sagilo G, et al. Second-generation tyrosine kinase inhibitors; the future frontline CML therapy. Clin. Cancer Res. 2011;17:1674–1683. doi: 10.1158/1078-0432.CCR-10-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanfstein B, Mueller MC, Erben P, Schnittger S, et al. Molecular response after 3 months of 1st line imatinib therapy is predictive for treatment failure and disease progression in patients with chronic phase chronic myeloid leukemia- a follow-up analysis of the German CML study IV. Blood ASH Annu. Meet. Abstr. 2010;116:360. [Google Scholar]
- 19.Thomas J, Wang L, Clark RE, et al. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;(104):3739–3745. doi: 10.1182/blood-2003-12-4276. [DOI] [PubMed] [Google Scholar]
- 20.Crossman LC, Druker BJ, Deininger MW, et al. h-OCT1 and resistance to imatinib. Blood. 2005;106:1133–1134. doi: 10.1182/blood-2005-02-0694. [DOI] [PubMed] [Google Scholar]
- 21.White DL, Hughes TP. Classification of patients with chronic myeloid leukemia on basis of BCR-ABL transcripts level at 3 month fails to identify patients with low organic cation transporter1 activity destined to have poor imatinib response. JCO. 2012;30:1–2. doi: 10.1200/JCO.2011.41.1090. [DOI] [PubMed] [Google Scholar]


