Abstract
As a part of the UIC-based ICBG project in Laos, plants were collected based on ethnomedical interviews and evaluated for antimalarial activity. A CHCl3 extract from the vine of Gongronema napalense (Wall.) Decne. (Asclepiadaceae) showed promising anti-malarial activity while exhibiting low levels of cytotoxicity and was thus followed up with further fractionation and biological evaluation. Bioassay-guided fractionation led to the isolation of a new steroidal glycoside, gongroneside A, which showed antimalarial activity in vitro with an IC50 value of 1.60 and 1.39 μM against the Plasmodium falciparum D6 and W2 clones, respectively.
Keywords: Gongronema napalense, asclepiadaceae, steroid glycoside, gongroneside A, Plasmodium falciparum, antimalarial activity
Introduction
As part of our continuing effort in the search for biologically active molecules from plants of Laos under the ICBG (International Cooperative Biodiversity Group) program[1–3], ethnomedical interviews, designed to uncover information on plants used in traditional therapy against diseases of interest to this ICBG (AIDS, cancer, malaria, tuberculosis), were performed throughout Laos. In this effort, investigators of the Traditional Medicine Research Center of the Ministry of Health of Laos collaborated with investigators from the University of Illinois at Chicago.
Malaria has always been considered one of the main public health problems in Laos, due to its wet, humid climate which supports high levels of mosquito reproduction, and due to the absence of, or difficulty in, acquiring the effective treatments. There are 20,000 to 55,000 cases of, and 200 to 600 deaths, from malaria recorded in Laos each year[4, 5]. These statistics, together with the facts that plants represent the major component of traditional medicinal materials of Laos[6], that they have been previously used in malarial therapy, and that there is increasing resistance to standard antimalarial drugs[7], were the bases for the effort in the ICBG project to search for new antimalarials in medicinal plants.
Biological evaluation of extracts of samples collected through the interviews of local healers in Laos led to the identification of one flowering plant species, Gongronema napalense (Wall.) Decne. (Asclepiadaceae) (synonym: Gymnema napalense Wall.), that showed activity in vitro in an antimalarial assay.
G. napalense is a vine with simple, opposite, thin, cordate leaves and thin, green fruits, longer than a finger length. White latex exudes from the stem when it is cut. Known in Laos as “Kheuang nguan mu”, the plant is used locally in combination with one other species to treat polio. An equal amount of the vine is mixed with the stem wood of “Kok mak kham”; one handful of each is boiled in 2 L of water until the volume reaches 500 ml. The decoction is then taken orally as needed for 7 to 15 days. Species distribution is confined to east and southeast Asia (including Laos), southern China, India, and Nepal[8, 9], and the plant has been previously reported for the treatment of leucorrhea, blennorrhea, and traumatic injury[10].
The present paper reports the isolation of a new antimalarial pregnane glycoside from this plant species, which is used in Champasak in southern Laos in traditional medical practices.
Material and methods
General
Optical rotations were measured with a Perkin-Elmer 241 polarimeter (Perkin-Elmer; Wellesley, MA, USA). UV spectra were measured on a Beckman DU-7 spectrometer (Beckman Coulter, Inc., Fullerton, CA, USA). IR spectra were run on a Jasco FT/IR-410 spectrometer spectrometer (JASCO Co., Tokyo, Japan), equipped with a Specac Silver Gate ATR system by applying a film on a Germanium plate. NMR spectra were recorded on a Bruker DPX-400 NMR spectrometer (Bruker, Karslruhe, Germany). Chemical shifts (δ) were expressed in ppm with reference to the solvent signals. All NMR data were obtained by using standard pulse sequences supplied by the vendor. LREIMS were recorded on a Thermo Finnigan LCQ mass spectrometer (Thermo Finnigan, San. Jose, CA, USA). HRFABMS spectra were recorded on a JEOL GCmate II spectrometer (Jeol Ltd., Tokyo,. Japan). Reverse-phase preparative HPLC was carried out on a Waters 600E Delivery System pump, equipped with a Waters 996 photodiode detector (Waters, Milford, MA, USA), and a Phenomenex LUNA C18 (2) column (120 Å, 10 μm, 50×250 mm) (Phenomenex Inc., Torrance, CA, USA) at 20 ml/min. Silica gel 60 (230–400 mesh, Natland International Corp., Research Triangle Park, USA) and Si gel RP-18 (40–63 μm, EM Science, N.J., USA) were used for column chromatography. Thin-layer chromatography was performed on Whatman glass-backed plates coated with 0.25 mm layers of Si gel 60 (Whatman,. Clifton, NJ, USA) or Merck aluminium-backed plates coated with 0.2 mm layers of Si gel RP-18 F254 (E Merck, Darmstadt, Germany). Fractions were monitored by TLC and spots were visualized by heating Si gel plates sprayed with 10% H2SO4 in MeOH.
Plant material
The initial collection of the entire vine sample (SL7530, 500 g) of Gongronema napalense was made on August 4, 2003 at Pontong District of Champasak Province (southern Laos), and was documented by a voucher specimen AL 148 (LAOS 541). A larger amount of the vine sample (SLA7530, 8.0 kg) for the current isolation work was subsequently re-collected in the same location by the staff of the Traditional Medicine Research Center in June 2005. Duplicate voucher specimens of both the initial collection and the recollected samples have been deposited at the herbaria of the Traditional Medicine Research Center, Vientiane, Laos, and at the John G. Searle Herbarium of the Field Museum of Natural History (Chicago, IL, U.S.A.).
Extraction and isolation
Eight kilograms dry weight of the recollected sample was extracted with ethanol at the TMRC facilities. The ethanol extract was then sent to UIC for further work. The extract was re-dissolved in a MeOH-H2O/9:1 solution and defatted with n-hexane. The ratio of MeOH/H2O was adjusted to 1:1. and the de-fatted extract was partitioned with CHCl3 (×3) to yield a CHCl3 extract (19.2 g).
The CHCl3 extract was loaded on to a 45 cm × 3.5 cm Si gel column (192 g) for initial separation (S1). The stationary phase was packed wet in 100% CHCl3. Elution of the CHCl3 fraction was accomplished under gravity with a solvent gradient beginning at 100% CHCl3 (F1–F3) and running through CHCl3/MeOH solvent ratios of 99:1 (F4–F9), 98:2 (F10–F15), 96:4 (F16–F21), 92:8 (F22–F30), 88:12 (F31–F39), 8:2 (F40–F49), 1:1 (F50–F53), and finally 100% MeOH (F54–F58). An eluant (250 ml) was collected for each fraction. The solvent systems were adjusted based on the TLC results of the fractions and activity assays which were performed throughout the fractionation process.
The resulting fractions were tested in the antimalaria bioassay. The results demonstrated that the fractions F25–F29 from S1 showed significant inhibition against P. falciparum parasites. A portion (500 mg) of these fractions (F25–F29) was subjected to a second separation (S2) on a reverse phase C-18 Si gel column (45 cm × 1.5 cm, 117 g). Fractionation was accomplished under air pressure with solvent gradients of MeOH/H2O 7:3 (F59–F98). 8:2 (F99–F113), and finally 100% MeOH (F114–F120). An eluant (100 ml) was collected for each fraction. Fractions from S2 were combined based on the TLC analyses. S2 fractions F100–F102 that had the most pronounced spots on TLC were selected for further fractionation.
The combined S2 fractions F100–F102 (84.5 mg) were chromatographed over a reverse phase C-18 Si gel column (45 cm × 1.5 cm. 117 g), using CH3CN-H2O in a gradients ranging from 2:8 (F121–F126), 3:7 (F127–F133), 4:6 (F134–F148), to 1:1 (F149–F169). An eluant (100) was collected from each fraction. The resulted fractions F14–F28 were pooled (29.2 mg) and subjected to preparative HPLC separation (solvent system: CH3CN/ H2O 55:45) to afford gongroneside A (1, 5.2 mg).
(3β, 12β, 14β, 17α, 20S*)-3-[(O-β-D-glucopyranosyl-(l→4)-O-2, 6-dideoxy-3-O-methyl-β-D-ribo-hexopyranosyl-(1→4)-O-6-deoxy-3-O-methyl-β-D-allopyranosyl (1→4)-O-2, 6-dideoxy-3-O-methyl-β-D-arabino-hexopyranosyl-(1→4)-0-2, 6-dideoxy-3-O-methyl-β-D-ribo-hexopyranosyl-(1→4)-2, 6-dideoxy-3-0-methyl-β-D-ribo-hexopyranosyl)oxy]-12-acetoxy-20-[2-(methylamino)benzoxy]-pregn-5-ene-3, 8, 12, 14, 17, 20-pentol (Gongroneside A, 1)
Colorless gum; : + 0.45 (MeOH; c 1.0); UV nm (MeOH) λmax (log ε): 358 (2.51), 255 (1.87), 241 (2.69), 215 (2.08), 201 (1.52); IR cm−1 (dried film) νmax: 3853, 3750, 3675, 3649, 2359, 1684, 1653, 1540, 1080; 1H and 13C NMR: (see Table 1); positive HRFABMS m/z: 1478.7243 [M+Na]+ (calcd. for C72H113NO29Na, 1478.7296).
Table 1.
NMR spectral data of gongroneside A (1) (400 MHz for 1H NMR and 100 MHz for 13C NMR. pyridine-d5, J in Hz)
| δ C | δ H | |
|---|---|---|
|
| ||
| C | Steroid | |
| 1 | 38.8 t | 1.01 m, 1.69 m |
| 2 | 29.8 t | 1.76 m, 2.01 m |
| 3 | 77.6 d | 3.80 m |
| 4 | 39.2 t | 2.44 m, 2.52 m |
| 5 | 139.2 s | |
| 6 | 119.4 d | 5.29 m |
| 7 | 34.9 t | 2.42 m, 2.42 m |
| 8 | 74.3 s | |
| 9 | 44.0 d | 1.68 m |
| 10 | 37.2 s | |
| 11 | 25.6 t | 1.91 m, 2.25 m |
| 12 | 74.5 d | 5.13 dd (10.6, 5.0) |
| 13 | 56.9 s | |
| 14 | 88.9 s | |
| 15 | 33.7 t | 2.04 m, 2.08 m |
| 16 | 33.9 t | 1.96 m, 1.99 m |
| 17 | 87.6 s | |
| 18 | 11.3 q | 2.00 s |
| 19 | 18.0 q | 1.28 s |
| 20 | 74.9 d | 5.17 q (6.0) |
| 21 | 15.6 q | 1.52 d (6.1) |
|
| ||
| 12-O-Acetyl moiety | ||
|
| ||
| C=O | 171.3 s | |
| Me | 22.0 q | 2.10 s |
|
| ||
| 20-O-N-Methylanthraniloyl moiety | ||
|
| ||
| 1' | 168.2 s | |
| 2' | 111.0 s | |
| 3' | 152.6 s | |
| 4' | 111.5 d | 6.70 d (8.7) |
| 5' | 135.1 d | 7.40 ddd (10.9, 8.4, 2.8) |
| 6' | 114.7 d | 6.55 t (7.6) |
| 7' | 132.6 d | 8.32 dd, 8.2, 2.4) |
| NH | 8.09 q (5.1) | |
| Me | 29.5 | 2.75 d (5.1) |
| δ C | δ H | δ C | δ H | |
|---|---|---|---|---|
|
| ||||
| C | Sugar A | Sugar D | ||
| 1 | 96.4 d | 5.25 d (9.7, 1.5) | 102.0 d | 5.24 d (8.6) |
| 2 | 37.0 t | 1.90 m, 2.31 m | 72.4 d | 3.83 m |
| 3 | 78.0 d | 4.08 q (3.5) | 83.0 d | 4.35 t (3.3) |
| 4 | 83.3 d | 3.50 m | 83.3 d | 3.48 dd (8.0, 3.3) |
| 5 | 69.0 d | 4.20 dq (9.0, 6.5) | 69.4 d | 4.18 dq (9.0, 6.3) |
| 6 | 18.5 q | 1.37 d (6.1) | 18.3 q | 1.34 d (6.0) |
| OMe | 58.9 q | 3.60 s | 61.7 q | 3.86 s |
| C | Sugar B | Sugar E | ||
|---|---|---|---|---|
| 1 | 100.5 d | 5.09 brd (8.4) | 100.5 d | 5.10 brd (9.1) |
| 2 | 36.8 t | 1.79 m, 2.26 m | 36.8 t | 1.74 m, 2.24 m |
| 3 | 77.9 d | 3.98 q (3.5) | 78.2 d | 4.10 q (3.4) |
| 4 | 83.1 d | 3.41 dd (9.5, 3.4) | 82.9 d | 3.65 dd (9.7. 3.4) |
| 5 | 69.1 d | 4.14 dq (8.4, 6.3) | 69.0 d | 4.22 dq (8.9, 6.2) |
| 6 | 18.3 q | 1.35 d (6.1) | 18.6 q | 1.56 d (6.1) |
| OMe | 58.7 q | 3.53 s | 58.9 q | 3.52 s |
| C | Sugar C | Sugar F | ||
|---|---|---|---|---|
| 1 | 101.9 d | 4.64 dd (9.6, 3.3) | 106.6 d | 4.92 d (7.6) |
| 2 | 37.2 t | 1.73 m, 2.46 m | 75.5 d | 4.02 m |
| 3 | 79.2 d | 3.53 m | 78.2 d | 4.21 t (9.1) |
| 4 | 82.9 d | 3.54 m | 71.8 d | 4.19 t (9.8) |
| 5 | 71.9 d | 3.53 m | 78.5 d | 4.00 m |
| 6 | 18.6 q | 1.60 d (6.3) | 63.1 t | 4.39 dd (11.5, 5.5) |
| OMe | 57.2 q | 3.51 s | 4.59 dd (11.7, 1.5) |
Antimalarial and cytotoxicity assays
Antimalarial and cytotoxicity assays were performed using cultured Plasmodium falciparum clones (W2, D6) and the oral epidermoid cancinoma (KB) cell line, respectively, as previously described[14]. Chloroquine was used as the positive control for antimalarial assay. DMSO was used as negative control for both antimalarial and cytotoxicity assays. The IC50 values of antimalarial assays and ED50 values of cytotoxicity assays were determined using nonlinear regression analysis.
Results and discussion
A sample of G. napalense, was collected from the Pontong District of Champasak Province (southern Laos). After promising antimalarial activity was observed during the initial testing of the crude CHCl3 extract with low levels of cytotoxicity, 8.0 kg of the entire vine (SLA7530) was recollected and an EtOH extract obtained. This extract was re-dissolved in MeOH, followed by de-fatting and partitioning with CHCl3. Bioassay-directed fractionation of the CHCl3 fraction by repeated flash column chromatography on Si gel, followed by a subsequent preparative HPLC separation afforded a new steroid glycoside, gongroneside A (1).
Compound 1 was isolated as a colorless gum with a molecular formula of C72H113NO29 based on HRFABMS (m/z 1455.7398 [M+Na]+ 1478.7243 calcd. 1478.7296) and NMR studies (Table 1).
In the 1H and l3C NMR spectra, anomeric signals attributed to six sugar units were observed at δH 5.25 (dd, J = 9.7, 1.5 Hz), 5.24 (d, J = 8.7 Hz), 5.10 (brd, J = 9.1 Hz), 5.09 (brd, J = 8.4 Hz), 4.92 (d, J = 7.6 Hz), and 4.64 (dd, J = 9.6, 3.3 Hz) and δC 96.4, 100.5 (2C), 101.9, 102.0, and 106.6. The signals of an acety] and an N-methyl anthraniloyl groups were also observed in the 1D NMR spectra (Table 1). An examination of the literature with respect to compounds previously isolated from various species of Asclepiadaceae and the NMR data that were reported for them together with a comparison of the preliminary survey of the 1D NMR data for 1, indicated that the compound possessed a similar steroidal glycosidic structure to stephanosides A–E which had been isolated from Stephanotis lutchuensis Koidz. var. japonica (Makino) Hatusima[11], a species of the same family. The aglycone of 1 was determined to be a pregnane steroid by comparison of its NMR data with those of stephanosides A–E and was identified as sarcostin due to its NMR data being identical to those reported in the literature[11]. The stereochemistry of the sarcostin aglycone was further confirmed by ROESY experiment. ROE interactions were observed between 19-Me (δH 1.28)/H-11β (δH 2.25), 19-Me/H-7β (δH 2.44), 18-Me (δH 2.00)/H-11β, H-4α (δH 2.52)/H-6 (δH 5.29), H-1α (δH 1.01)/H-9 (δH 1.68), and H-2α (δH 2.01)/ H-4α.
The acetyl group was assigned to C-12 due to the presence of the HMBC correlation between the acetyl carbonyl carbon (δC 171.3) and H-12 (δH 5.13). The presence of the long-range heterocorrelation between the H-20 proton (δH 5.17) and the N-methyl anthraniloyl carbonyl carbon (δC 168.2) linked the N-methyl anthraniloyl group to C-20 of the steroid molecule.
Although the aglycone of 1 was found to be the same as those of stephanosides A–E, the finding of 6 anomeric centers indicated that the glycoside portion of the molecule was different than those of stephanoside A–E.
The strategy for elucidation of the structure of the sugar units in 1 followed the evaluation methods described by Duus et al.[12]. Each sugar unit was analyzed one at a time using a combination of gradient-enhanced COSY (gCOSY) data, to determine the coupling network between protons within each sugar residue and gHSQC data to determine one bond heterocorrelations between protons and carbons which would link both the 1H and 13C NMR data for each individual residue. TOCSY data (both 1D selective and 2D TOCSY) was used to determine long-range proton couplings and gHMBC data was used to determine long-range couplings between protons and carbons. ROE interactions from gROESY data were analyzed to assess where linkages were occurring as well as to determine 3D arrangements within each sugar and between sugar residues. For the purposes of this analysis, sugars have been designated as Sugar A through Sugar F, with Sugar A being bonded to the steroid ring at C-3.
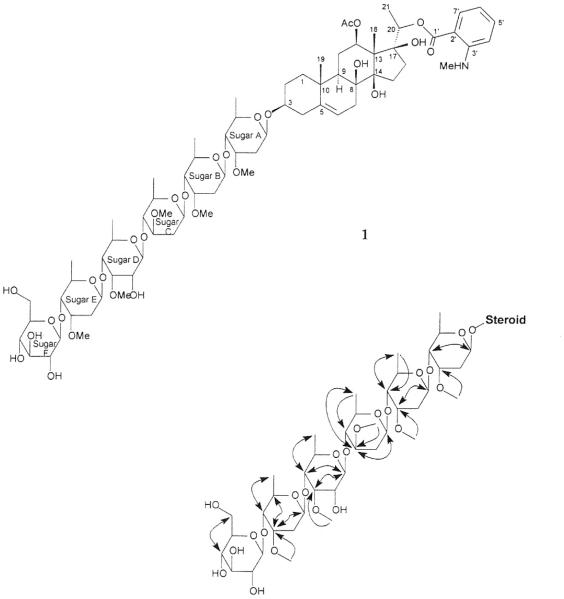
The large values of the coupling constants for the anomeric protons (8 to 10 Hz) indicated that the linkages were in the β-form (equatorial orientation for oxygen) for all of the sugars. Analysis from the gCOSY, gHSQC, gTOCSY, and gHMBC spectral data provided the homonuclear and heteronuclear correlations that occurred within each individual sugar (Table 1, Fig. 1). The attachment of Sugar A to C-3 of the steroid was determined from a ROE interaction between the H-3 proton signal at δH 3.80 and the anomeric proton at δH 5.25 of the sugar. Subsequent ROE interactions were noted between H-4 of Sugar A (δH 3.50) and the anomeric proton of Sugar B (δH 5.09), H-4 of Sugar B (δH 3.41) and the anomeric proton at of Sugar C (δH 4.64), H-4 of Sugar C (δH 3.54) and the anomeric proton of Sugar D (δH 5.24), H-4 of Sugar D (δH 3.48) and the anomeric proton signal at Sugar E (δH 5.10), and H-4 of Sugar E (δH 3.65) and the anomeric proton of Sugar F (δH 4.92).
Fig. 1.
Chemical struture of compound 1 and selected gTOCSY (double-sided arrowhead) and gHMBC (single-sided arrowhead) correlations of the sugar moiety of 1
Further analysis of the spectral data determined that the six-membered glycoside of 1 contains one molecule each of D-oleandrose (ole) and D-allomethylose (alm), three molecules of D-cymerose (cym), and a terminal D-glucose (glc). Therefore, the sequence was aglycone-cym-cym-ole-alm-cym-glc which was found to be the same as that of the compound marstomentoside I, previously isolated by Abe et al.[13] from another species of the same family, Marsdenia tomentosa Morren & Decne. The structure of compound 1 was thus elucidated to be (3β, 12β, 14β, 17α, 20S*)-3-[(O-β-D-glucopyranosyl-(1→4)-O-2, 6-dideoxy-3-O-methyl-β-D-ribo-hexopyranosyl-(1→4)-O-6-deoxy-3-O-methyl-β-D-allopyranosyl-(1→4)-O-2, 6-dideoxy-3-O-methyl-β-D-arabino-hexopyranosyl-(1→4)-O-2, 6-dideoxy-3-O-methyl-β-D-ribo-hexopyranosyl-(1→4)-2, 6-dideoxy-3-O-methyl-β-D-ribo-hexopyranosyl)oxy]-12-acetoxy-20-[2-(methylamino)benzoxy]-pregn-5-ene-3, 8, 12, 14, 17, 20-pentol and was given the trivial name of gongroneside A.
Gongroneside A (1) was evaluated for antimalarial activity with cultures of Plasmodium falciparum clones D6 (chloroquine-sensitive) and W2 (chloroquine-resistant). The human oral epidermoid carcinoma (KB) cell line was used to evaluate cytotoxicity in order to compute Selective Index values [SI = ED50(KB)/IC50 (malaria clone)]. Chloroquine was employed as the positive control compound in the antimalarial assays. Results of the biological assays are presented in Table 2.
Table 2.
Antimalana activity of G. napalense fractions and gongroneside A (1)
| Compound | D6 | W2 | KB | ||
|---|---|---|---|---|---|
|
|
|||||
| IC50 (μM) | SIa | IC50 (μM) | SI | ED50 (μM) | |
| 1 | 1.600 ± 0.006 | >8.5 | 1.390 ± 0.005 | >9.8 | >13.7 |
| Chloroquine | 0.032 ± 0.007 | 0.390 ± 0.078 | |||
SI = selectivity index = ED50 KB/IC50 P. falciparum.
Compound 1 demonstrated antimalarial activity against the D6 clone with an IC50 value of 1.60 μM and against W2 clones with an IC50 value of 1.39 μM. It showed no apparent toxicities to KB cells at a concentration of 20 μg/ml, and therefore, appears worthy of further evaluation. To the best of our knowledge, this is first report showing a pregnane glycoside to be active against malarial parasites.
Acknowledgements
All work involving plant sample collection, taxonomic identification, bioassay-guided chemical isolation, and structure elucidation described in this paper were carried out under NIH grants 1 UO1-TW01015-01 and 2 UO1-TW-01015-06 administered by the Fogarty International Center through funds from NIH, NSF, and the USDA Foreign Agricultural Service, as part of an International Cooperative Biodiversity Groups (ICBG) program. The plant collection permit was issued by the Ministry of Agriculture and Forestry of Laos. Field interviews were performed under the University of Illinois Institutional Review Board clearance, Protocol No. H-97-1056. The authors are grateful to the NMR Lab of the Department of Medicinal Chemistry and Pharmacognosy, and the Research Resources Center, University of Illinois at Chicago, for access to the Bruker DPX 400 MHz instrument, as well as the acquisition of MS data. Grateful acknowledgements go to Drs. Aleksej Krunic and David Lankin for their assistance with the NMR experiments. The authors would like to acknowledge the assistance of the Traditional Medicine Research Center of the Ministry of Health of Lao P.D.R. (Lao People's Democratic Republic), Vientiane, kindness of the healer who provided the ethnomedical information of the subject plant and who gave permission for the publication of the information, and the assistance of all participating individuals from the Traditional Medicine Station in Champasak Province.
References
- [1].Soejarto DD, Gyllenhaal C, Regalado JC, Pezzuto JM, Fong HHS, Tan GT, Hiep NT, Xuan LT, Binh DQ, Hung NV, Bich TQ, Thin NN, Loc PK, Vu BM, Southavong BH, Sydara K, Bouamanivong S, O'Neill MJ, Lewis J, Xie X, Dietzman G. Studies on biodiversity of Vietnam and Laos: A UIC-based ICBG program. Pharm Biol. 1999;37:100–13. [Google Scholar]
- [2].Soejarto DD, Pezzuto JM, Fong HHS, Tan GT, Zhang HJ, Tamez P, et al. An international collaborative program to discover new drugs from tropical biodiversity of Vietnam and Laos. Nat Prod Sci. 2002;8:1–15. [Google Scholar]
- [3].Soejarto DD, Zhang HJ, Fong HHS, Tan GT, Ma CY, Gyllenhaal C, Riley MC, Kadushin MR, Franzblau SG, Bich TQ, Miller BE, Thuy TV. Studies on biodiversity of Vietnam and Laos 1998-2005: Examining the impact. J Nat Prod. 2006;69:473–81. doi: 10.1021/np058107t. [DOI] [PubMed] [Google Scholar]
- [4].Petsouvanh R, Inthakone S, Naphayvong R, Phompida S, Sidavong B, Vanisaveth V. The current malaria situation in Lao P.D.R. Mekong Malaria Forum. 2000;1:3–6. [Google Scholar]
- [5].Stohrer JM, Dittrich S, Thongpaseuth V, Vanisaveth V, Phetsouvanh R, Phomida S, Monti F, Christophel EM, Lindegardh N, Annerberg A, Jelinek T. Therapeutic efficacy of artemether-lumefantrine and artesunate-mefloquine for treatment of uncomplicated Plasmodium falciparum malaria in Luang Namtha Province, Lao People's Democratic Republic. Trop Med Int Health. 2004;9:1175–83. doi: 10.1111/j.1365-3156.2004.01320.x. [DOI] [PubMed] [Google Scholar]
- [6].Libman A, Bouamanivong S, Southavong B, Sydara K, Soejarto DD. Medicinal plants: An important asset to health care in a region of Central Laos. J Ethnopharmacol. 2006;106:303–11. doi: 10.1016/j.jep.2005.11.034. [DOI] [PubMed] [Google Scholar]
- [7].Milhous W, Kyle DE. Introduction to the modes of action and mechanisms of resistance to antimalarials. In: Sherman IW, editor. Malaria: Parasite Biology, Pathogenesis, and Protection. Amer. Soc. Microbiol. Press; Washington, D.C.: 1999. pp. 303–6. [Google Scholar]
- [8].Merrill ED. An enumeration of Philippine flowering plants. Manila Bureau of Printing; Manila: 1925. [Google Scholar]
- [9].VAST: Missouri Botanical Garden's Vascular Plants Tropicos Nomenclatural Database. 2005. Available at http://mobot.mobot.org/W3T/Search/vast.html.
- [10].Gongronema napalense. 2005. Flora of China 16: 240. Available at http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=210000672.
- [11].Yoshikawa K, Okada N, Kann Y, Arihara S. Stcroidal glycosides from the fresh stem of Stephanotis lutchuensis var. japonica (Asclepiadaceae). Chemical structures of Stephanosides A-J. Chem Pharm Bull. 1996;44:1790–6. doi: 10.1248/cpb.44.2243. [DOI] [PubMed] [Google Scholar]
- [12].Duus JØ, Gotfredsen CH, Bock K. Carbohydrate structural determination by NMR spectroscopy: Modern methods and limitations. Chem Rev. 2000;100:4589–614. doi: 10.1021/cr990302n. [DOI] [PubMed] [Google Scholar]
- [13].Abe F, Okabe H, Yamauchi T, Honda K, Hayashi N. Pregnane glycosides from Marsdenia tomentosa. Chem Pharm Bull. 1999;47:869–75. doi: 10.1248/cpb.48.154. [DOI] [PubMed] [Google Scholar]
- [14].Zhang HJ, Tamez PA, Vu DH, Ghee TT, Nguyen VH, Le TX, Le MH, Nguyen MC, Do TT, Soejarto DD, Fong HH, Pezzuto JM. Antimalarial compounds from Rhaphidophora decursiva. J Nat Prod. 2001;64:772–7. doi: 10.1021/np010037c. [DOI] [PubMed] [Google Scholar]