Abstract
Sphingolipids are well established sources of important signaling molecules. For example, ceramide (Cer) has been described as a potent inhibitor of cell growth and inducer of apoptosis. In contrast, ceramide 1-phosphate (C1P) has been reported to have mitogenic properties and to inhibit apoptosis. Our understanding of the distinct biological roles of C1P in the regulation of DNA synthesis, inflammation, membrane fusion, and intracellular Ca2+ increase has rapidly expanded. C1P is a bioactive sphingolipid formed by the phosphorylation of ceramide catalyzed by ceramide kinase (CERK). This chapter specifically focuses on the role of C1P in phagocytosis and Ca2+ homeostasis. Studies of the metabolism of C1P during phagocytosis, may lead to a better understanding of its role in signaling. Potentially, the inhibition of CERK and C1P formation may be a therapeutic target for inflammation.
Keywords: Ceramide-1-phosphate, ceramide kinase, phagocytosis, calcium, transient potential channel, fusion
Ceramide-1-phosphate in Phagocytosis
The clearance of pathogens by the phagocytosis of opsonized, infectious agents is a vital biological process that is part of the innate immune system [1]. Phagocytosis is usually triggered by the interaction of target-bound opsonins with specific receptors on the surface of phagocytes. These receptors include the Fc receptors (FcRs), which bind to the Fc portion of immunoglobins [2], and the complement receptors [3], which bind to the complement deposited on targets. FcRs recognize the Fc portion of immunoglobins, and are expressed differentially on many cell types of the immune system [1]. Receptors for IgG (FcγR), IgE (FcεR) and IgA (FγA) have been characterized [1]. There are three classes of FcγRs: FcγRI, FcγRII, and FcγRIII. Each class consists of several receptor isoforms that are the product of different genes and splicing variants [2]. The interaction of FcRs with their immunoglobulin ligands triggers a series of leukocyte responses that include phagocytosis, the respiratory burst, antibody-dependent cell mediated cytotoxicity, the release of pro-inflammatory mediators, and the production of cytokines [1, 4]. The activation of these receptors leads also to a reorganization of the plasma membrane that profoundly affects the function of phagocytes. The plasma membrane forms pseudopods that extend around an extracellular particle followed by fusion to form a membrane-bounded intracellular vesicle, termed the phagosome. As the process of phagocytosis proceeds, cytoplasmic granules fuse with the phagosome membrane to deliver hydrolytic and anti-bacterial enzymes to the phagosome [5]. Phagolysosome formation requires an increase in intracellular Ca2+ and in addition to the recruitment of a complex containing docking and fusion proteins [6, 7]. In contrast to apoptotic cells, Fc receptor-mediated phagocytosis of microorganisms is often associated with a robust inflammatory response. Recently it has been shown that in immune cells, sphingolipid metabolism triggered by phagocytosis results in the formation of several lipid second messengers, including ceramide (Cer), sphingosine, C1P, and sphingosine-1-phosphate (S1P) [8]. It has been observed that the sphingolipid Cer is generated coincident with the termination of the respiratory burst and phagocytosis. Furthermore, the addition of cell-permeable ceramide blocks oxidant release and Fc-mediated phagocytosis [9]. In related work, ceramide kinase (CERK) has been identified as a central enzyme that regulates the levels of Cer via its phosphorylation to the bioactive sphingolipid metabolite, C1P [10].
Ceramide kinase is a highly conserved lipid kinase, present in animals and plants [11, 12], brain synaptic vesicles [13], human leukemia (HL-60) cells [14], and primary neutrophils [10]. The cDNA sequence for CERK was cloned by Sugiura and colleagues in 2002 [11]. hCERK encodes a protein of 537 amino acids that has a catalytic region with a high degree of similarity to the glycerol kinase catalytic domain. hCERK also has a putative N-myristoylation site on its NH2 terminus followed by a pleckstrin homology domain (PH). The PH domain in its N-terminus is known to bind the β/γ subunit of heterotrimeric G-proteins [15], phosphoinositol-4,5-bisphosphate [16], and phosphorylated tyrosine residues [17, 18]. Several studies have demonstrated that the PH domain may be an important regulatory site for CERK and is required for the proper localization of the enzyme in cells. CERK also contains a Ca2+/calmodulin (Ca2+/CaM) binding motif [19]. Recently, Igarashi and colleagues demonstrated that the activation of CERK and the formation of its product, C1P, in response to an increased intracellular concentration of Ca2+ were dependent on CaM [20] . Using calcium chelator BAPTA Boath demonstrated the dependence of CERK on Ca2+ ions [21].
The cloning of CERK also afforded the opportunity to examine, in addition to the structure, the function of the enzyme and its product [11]. C1P has been reported to have mitogenic effects [22] and to mediate arachidonic acid release [23]. In addition to cell growth, C1P has been found to mediate various inflammatory responses, such as the translocation of cytosolic phospholipase A2α (cPLA2α) to the Golgi apparatus, and directly interacts with cPLA2α in vitro [24]. Other studies have further documented a role of C1P as a mediator of Ca2+-dependent degranulation in mast cells [25] and its important role in phagolysosome formation in polymorphonuclear leukocytes (PMN) and Ca2+ signaling [10, 26].
The signaling pathways involved in phagocytosis are determined by the activation state of the PMN, as well as by the type of agonist used for activation. Membrane fusion plays an important role in the degranulation process by creating a pathway by which granule contents have access to phagosomes or to the extracellular milieu. Using a cell-free fusion assay, Ca2+ alone cannot promote fusion between neutrophil granules and plasma membrane fractions [27]. Therefore, other components must be required to bring about phagolysosomal formation, factors such as annexin, VAMP-2, and lipids [28]. The existence of CERK activity in PMNs has been previously established [10]. Calcium-dependent CERK is localized to both the PMN plasma membrane and secretory vesicles based on colocalization with the plasma membrane marker HLA and with the secretory vesicle marker latent alkaline phosphatase [10].
The presence of CERK activity in brain synaptic vesicles and the plasma membrane of PMNs led to the hypothesis that C1P may attenuate membrane charge, regulate vesicle transport, or play a role in regulating the secretion of neurotransmitters by promoting the fusion of vesicle membranes [13]. Phospholipid composition is known to play a significant role in membrane fusogenicity. Because phosphorylation of ceramide would produce an acidic phospholipid similar to phosphatidic acid (PA), a lipid shown to be highly fusogenic [29], the role of C1P in phospholipid-dependent vesicle fusion was examined in several studies. The addition of exogenous C1P was shown to promote liposome fusion in a cell-free system [10]. In mast cells, C1P formation is associated with Ca2+-dependent degranulation, and that C1P formation is enhanced during activation induced by IgE-antigen complex or by the Ca2+-ionophore A23187 [25]. Exogenous introduction of CERK into permeabilized RBL-2H3 cells is also sufficient to cause degranulation [25]. Additionally a newly developed synthetic inhibitor of CERK efficiently inhibits mast cell degranulation [30].
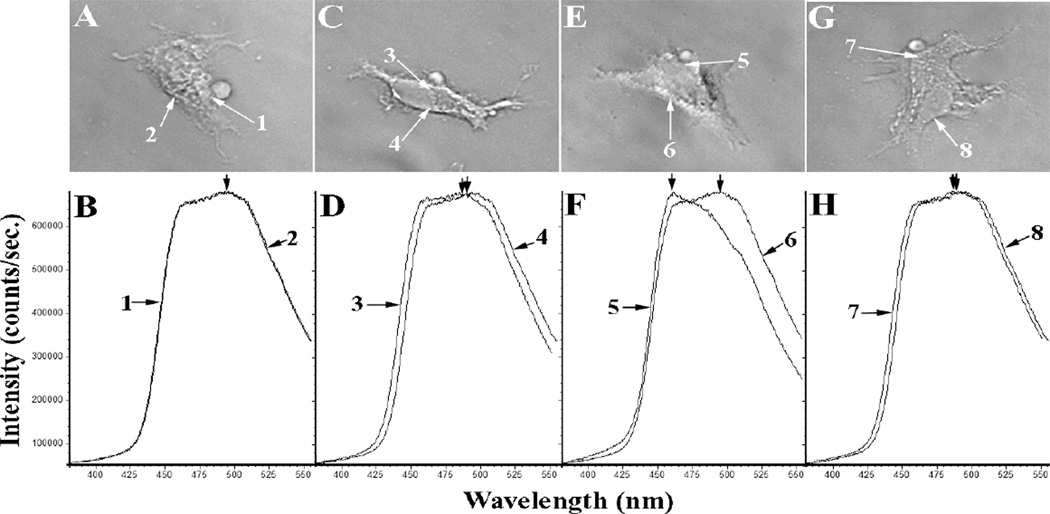
Both phagocytosis and degranulation require a membrane fusion step. The fusion of opposed membranes requires the destabilization of the membranes to render them susceptible to fusion. This destabilization may result from the Ca2+-induced phase separation of rigid (more ordered Lo) crystalline domains of acidic phospholipids (e.g. phosphatidic acid) within mixed lipid membranes [31]. Fusion can be initiated between closely opposed membranes at the boundaries between crystalline and the surrounding non-crystalline domains. Such boundaries represent structurally unstable points and thus offer focal points for the mixing of molecules from opposed membranes. To establish whether C1P might contribute to such destabilization, COS-1 cells expressing FcγRIIA were employed. The overexpression of CERK in these cells enhanced C1P generation and phagocytosis during activation with EIgG [32]. Labeling these cells with Laurdan demonstrated a dramatic shift in emission during phagocytosis, corresponding to a distinct change in lipid-ordered structure (Fig.1). The change in Laurdan emission provides strong evidence of a lipid raft-like Lo domain in COS-1 cells at the site of phagosome formation1.
Figure 1. Lipid-ordered membrane domains in association with sites of phagocytosis in COS-1 cells expressing FcγRIIA, FcγRIIA/vector, FcγRIIA/hCERK, and FcγRIIA/G198DhCERK.
COS-1 cells were labeled with Laurdan. Regions 1, 3, 5, and 7 (panels A,C,E, and G) correspond to membrane areas participating in phagocytosis. The Laurdan emission spectra for those regions are denoted with the same numbers as shown below each micrograph. FcγRIIA cells demonstrated a 0-nm shift (panel B), and the cells treated with FcγRIIA/vector demonstrated a ~3-nm shift (panel D). The FcγRIIA/hCERK transfectants exhibited a shift in emission maximum of ~37 nm (panel F), and FcγRIIA/G198DhCERK-transfected cells (panel G) exhibited a shift in the emission of 2.2+0.6-nm shift (panel H). Quantitatively, the calculated general polarization of membrane regions 1, 2, 3, 6, and 7 were (-0.030), region 8 was (-0.024), region 4 was (-0.016), and membrane regions 5 was (+0.088). Fifteen separate spectra were averaged to generate the spectra shown in panels B, D, F, and H. The difference in both emission maximum and general polarization was highly significant.
Ceramide-1-phosphate as a Regulator of Calcium Homeostasis
Calcium is a ubiquitous intracellular messenger, controlling a diverse range of cellular processes, such as gene transcription, muscle contraction, cell proliferation, and apoptosis [35–37]. Of importance to immunity, Ca2+ waves have been observed in migrating polymorphonuclear leukocytes (PMN), fibroblasts, and tumor cells [38, 39]. The level of intracellular Ca2+ is determined by a balance between the “on” reactions that introduce Ca2+ into the cytoplasm, and the “off” reactions through which this signal is removed by the combined action of buffers, pumps and exchangers. Each cell type expresses a unique set of components for Ca2+ signaling which create Ca2+-signaling systems with different spatial and temporal properties [39]. Calcium influx is mediated through both Ca2+ release from intracellular stores and Ca2+ entry from the extracellular environment. In the case of the latter, there are many different plasma membrane channels that control Ca2+ entry from the external medium in response to stimuli that include membrane depolarization, stretch, noxious stimuli, extracellular agonists, intracellular messengers and the depletion of intracellular stores [39]. The plasma membrane Ca2+ channels can be divided into several different types. Voltage operated channels (VOC) are employed largely by excitable cell types such as muscle and neuronal cells where they are activated by plasma membrane depolarization. Receptor operated channels are structurally and functionally diverse channels found on secretory cells and nerve terminals. Small molecule operated channels are activated by a number of small messenger molecules, such as diacylglycerol [40] and arachidonic acid [41]. In addition to these more clearly defined channel-opening mechanisms, store operated channels are sensitive to a diverse array of stimuli. Many of these channels belong to the large transient receptor protein (TRP) ion-channel family, which are encoded by up to 29 different genes [42]. The mammalian TRP superfamily of ion channels consists of voltage-independent, non-selective cation channels that are expressed in excitable and non-excitable cells. The biologic roles of TRP channels are diverse and include vascular tone, thermo sensation, irritant stimuli sensing and flow sensing in the kidney. Growing evidence supports the notion that most cells possess different TRP channels (TRPCs) that are located in association with Ca2+ stores where they are capable of functioning as Ca2+-release channels [40, 43, 44].
It been established that members of a subgroup of closely related TRP channels (TRPC3/6/7) can be activated by diacyglycerol, a product of PLC activation [40, 45, 46]. However another subgroup of TRP channels (TRPC1/4/5), although dependent on receptor-induced PLC activation, are completely unresponsive to DAG [47], suggesting that different TRPC proteins may have different mechanisms of activation. Notably, recent data have shown that TRPC1 and TRPC5 can be activated by S1P [48]. Sphingolipids, including sphingosine, S1P, and sphingosylphosphorylcholine, have diverse effects on the regulation of intracellular free Ca2+ concentration in nonexcitable and excitable cells [49–51]. C1P has emerged as a putative modulator of cellular functions that are in part regulated by Ca2+ signaling [52]. Studies in the role of C1P in modulating Ca2+ flux have produced somewhat controversial results. In some reports C1P did not modulate [Ca2+]i nor did it affect Ca2+ mobilization in mouse fibroblasts [22, 53–55]; however, others have clearly shown that C1P enhanced store-operated Ca2+ entry into thyroid cells [26, 56]. The precise role of C1P in Ca2+ signaling is therefore not yet well established and is discussed in more detail below.
Gijsbers et al. reported that C1P exogenously added in calf pulmonary artery endothelial cells is more potent than S1P for causing a fast and transient intracellular rise in Ca2+ [57]. Colina et al. showed that C1P increased intracellular Ca2+ in Jurkat T-cells. In this study C1P elevated the concentration of InsP3, inducing the liberation of Ca2+ from the endoplasmic reticulum, which in turn provoked the opening of a store operated Ca2+ channel at the plasma membrane [58]. Hogback et al. [59] reported that C1P evoked a concentration-dependent increase in [Ca]i, both in calcium-containing and calcium-free buffer in FRTL-5 cells. In this report, the effect of C1P was mediated, at least in part, by a pertussis toxin–sensitive G protein. The phospholipase C inhibitor U73122 attenuated the effect of C1P. C1P invoked a small, but significant increase in inositol InsP3. However, the effect of C1P on Ca2+ was not inhibited by Xestospongin C, 2-aminoethoxydiphenylborate, or neomycin indicating independent activation of IP3R. The effect of C1P on Ca2+ was potently attenuated by dihydrosphingosine and dimethylsphingosine, two inhibitors of sphingosine kinase. This attenuation may be the result of the C1P evoked increase in the production of intracellular S1P [59]. C1P also induced Ca2+ mobilization in GH4C1 rat pituitary cells, but indirectly, through voltage-operated Ca2+ channels [57].
Most studies to date have examined the mechanism of C1P by its exogenous addition to cells. The cloning of CERK provided a new tool to study the role of C1P in Ca2+ signaling. Using COS-1 cells stably transfected with FcγRIIA and hCERK, our laboratory previously showed that the activation of CERK with the concomitant accumulation of C1P altered Ca2+ signaling near the phagosome and significantly promoted phagocytosis and phagolysosomal formation [26]. EIgG-mediated ligation of FcγRIIA leads to the accumulation of CERK and TRPC-1 within lipid rafts, which are key sites associated with signal transduction. High-speed microscopy was used to study the contribution of CERK to phagosomal Ca2+ signaling. The high-speed imaging indicated that Ca2+ signaling in this model system occurs at “hot spots” that represent brief, but intense, Ca2+ release events. These sites of enhanced Ca2+ signaling are not random but often repeat at the same spatial location. These Ca2+ signaling “hot spots” exist for a period of time (<100ms) that is consistent with channel-gating times. The quantal Ca2+ burst seen in these studies at the pseudopods and phagosome membranes may represent Ca2+ release at local regions of signaling (Fig.2) [26]. It was shown previously in neutrophils that C1P is formed by a calcium-dependent CERK located in the plasma membrane during IgG-dependent phagocytosis [10]. Although CERK may participate in Ca2+ signaling, the kinase does not directly mediate Ca2+ movement across cellular membranes. These studies suggest that TRPC-1 is a leading candidate for a potential signaling partner of CERK/C1P. The store operated Ca2+ channel blockers CdCl2, CAI, and SKF93365 significantly decreased the ability of COS-1 cells transfected with hCERK to undergo Fc-mediated phagocytosis and phagolysosomal fusion [26]. These findings are consistent with the idea that store operated calcium channels, possibly mediated by TRP channels, are participants in phagocyte function. These findings are also consistent with the previous findings that exogenously added C1P enhances store operated Ca2+ entry [56] and that store operated calcium channels participate in phagocyte function in vivo and in vitro [25, 60]. To date the mechanism of CERK/C1P modulation of Ca2+ signaling has not been determined.
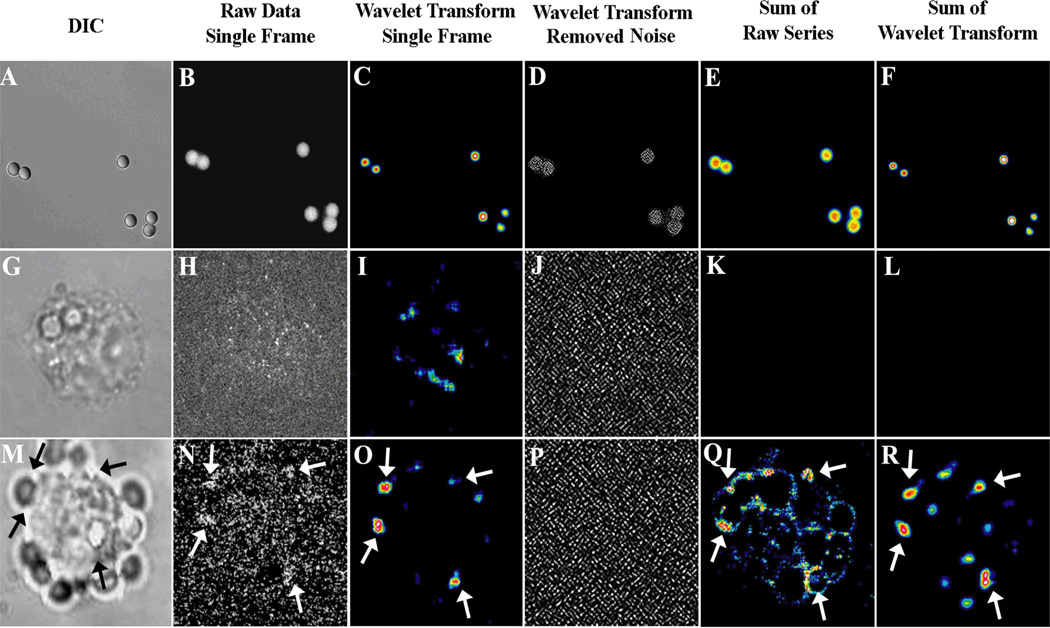
Figure 2. Analyses of high speed images.
Differential interference contrast (DIC; column 1,A, G, M) and fluorescence images (columns 2 through 6) are shown. A. Panels A-F denote a series of experiments using blue fluorescent beads. A single exposure of the flash-lamp yields a high quality image of the beads (B). A-F shows data which were obtained using the same excitation/emission filters and excitation flash as those used for the cells. When the beads were exposed to a single 6 µsec. flash, the fluorescence image of Fig. 2B was obtained. This image, suggests that the illumination field provided by the flash lamp was acceptable. Wavelet software is an important emerging technology and has many advantages including the ability to remove uncorrelated noise [64, 65]. Because the cell data utilize wavelet filtration for noise removal, the image of fluorescence beads was processed using this software for comparison. Wavelet transformation of the single frame in Fig. 2B returned a very similar but somewhat sharper image (Fig. 2C). As the image was very clean, very little noise was removed (Fig. 2D). If image stacks of 100 frames are summed of the raw or wavelet filtered images, essentially the same images are again returned (Fig. 2E and F, respectively).
B. Panels G–L and M–R are comparable images for two different transfectants (G-L for FcγRIIA transfected cells and M-R for FcγRIIA/hCERK transfected cells). The result using a wavelet coefficient of 1 is shown in panel D. When 100 frames of a high speed series are summed, the raw data and the wavelet filtered data yield matching results. Transfectants expressing only FcγRIIA did not yield a definitive fluorescence signal (G-L). However, transfectants expressing FcγRIIA/hCERK displayed a signal near pseudopods and phagosomes. As indicated in the raw data of Fig. 2N, two peri-phagosomal calcium signals were noted in this frame. After wavelet filtration of the data was performed to minimize the contribution of noise to this image, these same two regions were quite apparent in the filtered image (Fig. 2O). A substantial component of the uncorrelated noise removed from the original raw image is shown in Fig. 2P. To confirm that the wavelet-filtered single frame of Fig. 2O was contained in the summed (long time scale) image, the 500 raw images were collapsed into a single frame (Fig. 2Q) then compared with the filtered single frame (Fig. 2O). When the 500 wavelet-filtered images are collapsed onto a single frame, all of the significant signaling areas represented in the summed raw data (Fig. 2Q) are represented in the summed wavelet-filtered image (Fig. 2R). Regions with noted repetitions throughout the sequence of 500 images are indicated with arrows. Wavelet filtration indicated two particularly strong signals near the same target (wavelet coefficient=16). Much of the noise removed from the image is shown in panel 2P, which employed a wavelet coefficient of 2. These data indicate that CERK transfection leads to punctate Ca2+ signaling events regions of signaling near targets at phagosomes or phagocytic cups.
A recent paper by Beech provides a brief and focused review of their latest findings that show that TRPC5 is a sensor of important signaling phospholipids including S1P [61]. When Cav1.2 channels are lost, there is no concomitant loss of Ca2+ entry. Ca2+ entry is instead enabled by other ion channels (TRPC), which are often resistant to therapeutic concentrations of Ca2+ antagonists and permeable to Na+ and K+ as well as Ca2+. In a screen of potential lipid regulators of TRPCs, Xu et al identified S1P as an activator of TRPC5. Ion permeation involving TRPC5 is crucial because S1P-evoked motility is also suppressed by the channel blocker 2-aminoethoxydiphenyl borate or a TRPC5 ion-pore mutant [48]. Although TRP channels are structurally related to voltage-gated ion channels, they do not require depolarization in order to be active; instead they are activated by several different endogenous chemical substances.
As regulation of entry of Ca2+ and other cations through store operated Ca2+ channels occurs in a number of cells, identifying TRP channels and their function has broad significance in a variety of states of cell activation. The regulation of TRPC1/TRPC5 may depend on different lipids. S1P, which is suggested to have pivotal roles in mural cell recruitment during both vascular development [62] and atherosclerosis [63] is a novel bipolar activator of the TRPC1/5 heteromultimeric channel. Blocking these channels could potentially be used for drug targeting.
At present, the manner in which C1P regulates Ca2+ signaling is not firmly established. One hypothesis is that the calmodulin binding motif could be responsible for the increase in Ca2+ signaling during activation of CERK. However, it has been shown that there is residual Ca2+ signaling after the deletion of this motif [20].
Conclusion
Sphingolipid-metabolizing enzymes control the dynamic balance of the cellular levels of the bioactive lipid ceramide. C1P is a bioactive lipid which is extensively studied in inflammation. Collectective, sudies addressing the role of C1P in Ca2+ signaling are limited and often conflict. Recent findings from studies of the role of C1P in Ca2+ signaling emphasize the importance of discerning the mechanism of Ca2+ signaling under different physiological and pathological conditions. Data obtained from our model have provided the basis of our hypothesis of phagocytosis-triggered cellular signaling, wherein EIgG-mediated ligation of FcγRIIA leads to the activation of CERK and the subsequent accumulation of C1P. This leads to punctuate distribution of Ca2+ release at pseudopods and the periphagosomal vicinity. The higher Ca2+ signal observed in hCERK transfected cells as well as the fact that CERK co-localized with EIgG during phagocytosis support our hypothesis that Ca2+ signaling is an important factor for increasing phagocytosis and is regulated by CERK in a manner that likely involves TRPCs (Fig 3).
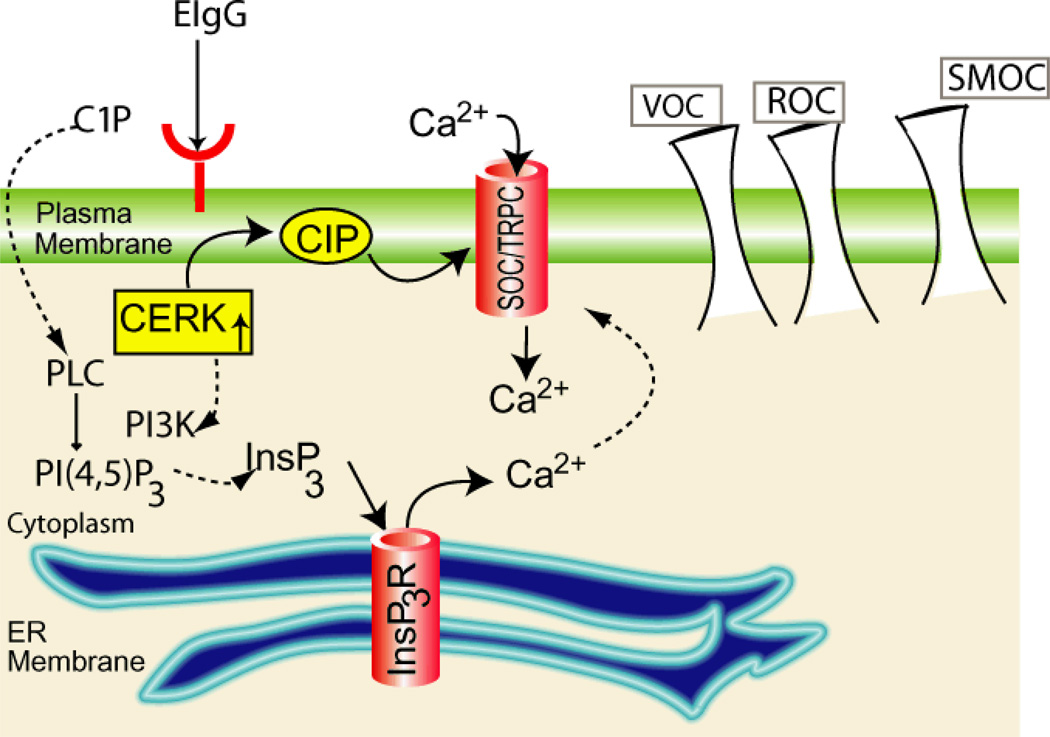
Fig. 3. Proposed mechanisms of Ca2+ entry in hCERK transfected COS-1 Cells.
The roles of ceramide kinase (CERK) and ceramide-1-phosphate (C1P) in modulating Ca2+ flux are controversial. C1P may act through a store operated (SOC). Alternatively, C1P may mediate voltage operated channels (VOC) or exert its effects through an increase in inositol-1,4,5-trisphosphate (InsP3) levels. In the latter case InsP3 in concert with phosphatidylinositol-3-kinase (PI3K) activation and a subsequent activation of InsP3 receptor (InsP3R)-operated Ca2+ channels from the endoplasmic reticulum (ER). Overexpression of hCERK results in the elevation of C1P during activation by opsonized erythrocytes (EIgG) and stimulates Ca2+ entry through SOC/TRPCs. PI(4,5)P2 Phosphatidylinositol-4,5-bisphosphate, PLC phospholipase C, TRPC, transient potential channels.
Future studies will be required to focus on understanding the activation of different channels involved in these processes with the hope of elucidating the role of TRPC as lipid responsive ionotropic receptors.
Acknowledgements
We thank Robin G. Kunkel Department of Pathology for helping with the figures in the chapter.
Footnotes
Lo phase lipids have been observed in reconstructed lipid rafts using Laurdan [33] C. Dietrich, Bagatolli L, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E., Lipid rafts reconstituted in model membranes, Biophys J. 80 (2001) 1417–1428‥ The demonstration of Lo phase lipids in non-transformed cells has been limited to neutrophils at the site of the lamellipodium [34] A. Kindzelski, Sitrin R, Petty H., Cutting edge:Optical microspectrophotometry supports the existence of gel phase lipid rafts at the lamellipodium of neutrophils: apparent role in calcium signalling., J. Immunol. 172 (2004) 4681–4685‥
References
- 1.Jones S, Lindberg FP, Brown EJ. Phagocytosis. In: Paul WE, editor. Fundamental Immunology. Philadelphia,PA: Lippincott-Raven; 1999. pp. 997–1020. [Google Scholar]
- 2.Ravetch J, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 3.Brown E. Complement receptors,adhesions, and phagocytosis. Infect. Agents Dis. 1992;1:63–70. [PubMed] [Google Scholar]
- 4.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J.Cell Biochem. 2004;92:913. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 5.Beron W, Alvarez-Dominquez C, Mayorga L, Stahl PD. Memebrane traficing along the phagocytic pathway. Trends Cell Biol. 1995;5:100–104. doi: 10.1016/s0962-8924(00)88958-8. [DOI] [PubMed] [Google Scholar]
- 6.Burgoyne R, Geisow MJ. The annexin family of calcium-binding proteins. Cell Calcium. 1989;10:1–10. doi: 10.1016/0143-4160(89)90038-9. [DOI] [PubMed] [Google Scholar]
- 7.Borregard N, Boxer LA. Williams Hematology. In: Lichtman M, Beutler E, Kipps TJ, Seligsohn V, Kaushansky K, Prchal JT, editors. Williams Hematology. 7th ed. New Yourk: McGraw-Hill Publishers; 2005. pp. 921–957. [Google Scholar]
- 8.Hannun Y, Bell RM. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989;243:500–506. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- 9.Hinkovska-Galcheva V, Kjeldsen L, Mansfield J, Boxer A, Shayman JA, Suchard J. Activation of a plasma membrane associated neutral sphingomyelinase and concomitant ceramide accumulation during IgG dependent phagocytosis in human polymorphonuclear leukocytes. Blood. 1998;91:4761–4769. [PubMed] [Google Scholar]
- 10.Hinkovska-Galcheva V, Boxer L, Mansfield P, Harsh D, Blackwood A, Shayman JA. The formation of ceramide-1-phosphate during neutrophil phagocytosis and its role in liposome fusion. J.Biol. Chem. 1998;273:33203–33209. doi: 10.1074/jbc.273.50.33203. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura M, Kono K, Liu H, Shimizugawa T, Minekura H, Spiegel S, Kohama T. Ceramide Kinase, a novel lipid kinase. Molecular cloning and functional characterization. J. Biol. Chem. 2002;277:23294–23300. doi: 10.1074/jbc.M201535200. [DOI] [PubMed] [Google Scholar]
- 12.Liang H, Yao N, Song JT, Luo S, Lu H, Greenberg JT. Ceramides modulate programed cell death in plants. Genes Dev. 2003;17:2636–2641. doi: 10.1101/gad.1140503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajjalieh S, Martin T, Floor E. Synaptic vesicle ceramide kinase. A calcium-stimulated lipid kinase that co-purifies with brain synaptic vesicles. J Biol Chem. 1989;264:14354–14360. [PubMed] [Google Scholar]
- 14.Kolesnick R, Hemer M. Characterization of a ceramide kinase activity from human leukemia (HL-60) cells. Separation from diacylglycerol kinase activity. J. Biol. Chem. 1990;265:18803–18808. [PubMed] [Google Scholar]
- 15.Lemmon M, Ferguson KM, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 16.Harlan J, Hajduc PJ, Yoon HS, Fesik SW. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature. 1994;371:168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- 17.Gibson T, Hyvonen M, Musacchio A, Saraste M, Birney E. PH domain: the first anniversary. Trends. Biochem. Sci. 1994;19:349–353. doi: 10.1016/0968-0004(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 18.Kavanough W, Turck CW, Williams LT. PTB domain binding to signaling proteins through a sequence motif containing phosphotyrosine. Science. 1995;268:1177–1179. doi: 10.1126/science.7539155. [DOI] [PubMed] [Google Scholar]
- 19.Rhoads A, Friedberg F. Sequence motifs for calmudolin recognition. FASEB J. 1997;11:331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- 20.Mitsutake S, Igarashi Y. Calmodulin is involved in the Ca2+ - dependent activation of ceramide kinase as a calcium sensor. J.Biol.Chem. 2005;280:40436–44044. doi: 10.1074/jbc.M501962200. [DOI] [PubMed] [Google Scholar]
- 21.Boath A, Gray C, Lidome E, Ullrich T, Nussbaumer P, Bornancin F. Regulation and traffic of ceramide-1-phosphate produced by ceramide kinase. J.Biol.Chem. 2008;283:8517–8526. doi: 10.1074/jbc.M707107200. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Munoz A, Frago LM, Alvarez L, Varela-Nieto I. Stimulation of DNA synthesis by natural ceramide 1-phosphate. Biochemical. Journal. 1997;325:435–440. doi: 10.1042/bj3250435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pethus B, Bielawski A, Spiegel S, Roddy P, Hannun Y, Chelfant C. Ceramide kinase mediates cytokine-and calcium ionophore- induced arachidonic and release. Biol. Chem. 2003;278:38206–38213. doi: 10.1074/jbc.M304816200. [DOI] [PubMed] [Google Scholar]
- 24.Stahelin R, Subramanian P, Vora M, Cho W, Chalfant CE. Ceramide-1-phosphate binds group IVA cytosolic phospholipase A2 via a Novel Site in the C2 Domain. J.Biol.Chem. 2007:20467–20474. doi: 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- 25.Mitsutake S, Kim T-J, Inagaki Y, Kato M, Yamashita T, Igarashi Y. Ceramide kinase is a mediator of calcium-dependent degranulation in mast cells. J. Biol. Chem. 2004;279:17570–17577. doi: 10.1074/jbc.M312885200. [DOI] [PubMed] [Google Scholar]
- 26.Hinkovska-Galcheva V, Clark A, VanWay S, Huang J-B, Hiraoka M, Abe A, Borofsky M, Kunkel R, Shanley T, Shayman JA, Lanni F, Petty HR, Boxer LA. Ceramide kinase promotes Ca2+ signaling near IgG-opsonized targets and enhances phagolysosomal fusion in COS-1 cells. J. Lipid Res. 2008;49:531–542. doi: 10.1194/jlr.M700442-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Francis J, Smolen W, Balazovich KJ, Sandborg RD, Boxer LA. Calcium-dependent fusion on the plasma membrane fraction from human neutrophils with liposomes. Biochim. Biophys. Acta. 1990;1025:1–9. doi: 10.1016/0005-2736(90)90183-o. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Abe A, Balazovich K, Wu D, Suchard S, Boxer L, Shayman J. Ceramide regulates oxidant release in adherent human neutrophils. J Biol Chem. 1994;269:18384–18389. [PubMed] [Google Scholar]
- 29.Blackwood R, Transue T, Harsh M, Brower C, Zacharek J, Smolen E, Hessler J. PLA2 promotes fusion between PMN-specific granules and complex liposomes. J Leuk Biol. 1996;59:663–667. doi: 10.1002/jlb.59.5.663. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Inagaki Y, Mitsutake S, Maezawa N, Katsumura S, Ryu YW, Park CS, Taniguchi M, Igarashi Y. Suppression of mast cell degranulation by a novel ceramide kinase inhibitor, the F-12509A olefin isomer K1. Biochim Biophys Acta. 2005;1738:82–90. doi: 10.1016/j.bbalip.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Papahadjopoulos D, Vail W, Newton C, Nir S, Jacobson K, Poste G, lazo R. Studies on membrane fusion. III The role of calcium -induced phase changes. Biochim. Biophys. Acta. 1977;465:579–598. doi: 10.1016/0005-2736(77)90275-9. [DOI] [PubMed] [Google Scholar]
- 32.Hinkovska-Galcheva V, Boxer L, Kindzelcki A, Hiraoka M, Abe A, Petty HR, Shayman JA. Ceramide-1-phosphate: A mediator of phagocytosis. J.Biol.Chem. 2005;280:26612–26621. doi: 10.1074/jbc.M501359200. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich C, Bagatolli L, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kindzelski A, Sitrin R, Petty H. Cutting edge:Optical microspectrophotometry supports the existence of gel phase lipid rafts at the lamellipodium of neutrophils: apparent role in calcium signalling. J. Immunol. 2004;172:4681–4685. doi: 10.4049/jimmunol.172.8.4681. [DOI] [PubMed] [Google Scholar]
- 35.Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeel P, MacEwan DJ, Scott RH. TNF-a receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurons. Neuropharmacology. 2002;42:95–106. doi: 10.1016/s0028-3908(01)00163-0. [DOI] [PubMed] [Google Scholar]
- 36.Lennartz M. Phospholipases and phagocytosis: the role of phospholipid-derived second messengers in phagocytosis. Int. J. Biochem. 1999;31:415–430. doi: 10.1016/s1357-2725(98)00108-3. [DOI] [PubMed] [Google Scholar]
- 37.Tas P, Koschel K. Sphingosine-1-phosphate induces a Ca2+ signal in primary astrocytes and a Ca2+ signal and shape change in C6 rat glioma cells. J. Neurosci Res. 1998;52:427–434. doi: 10.1002/(SICI)1097-4547(19980515)52:4<427::AID-JNR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 38.Mandeville J, Ghosh R, Maxfield E. Intracellular calcium levels correlate with speed and persistent forward motion in migrating neutrophils. Biophys. J. 1995;68:1207–1217. doi: 10.1016/S0006-3495(95)80336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berridge M, Bootman MD, Roderick HL. Calcium signalling:Dynamics, homeostasis and remodeling. Nature Reviews/Molecular and Cellular Biology. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann T, Obukhov G, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of TRPC-6 and TRPC-3 chanels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 41.Mignen O, Shuttleworth T. IARC a novel arachidonate-regulated, noncapacitative Ca2+ entry channel. J Biol Chem. 2000;275:9114–9119. doi: 10.1074/jbc.275.13.9114. [DOI] [PubMed] [Google Scholar]
- 42.Clapham D, Runnels LW, Stubing C. The TRP ion channel family. Nat.Rev. Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann T, Schaeffer M, Schultz G, Gudermann T. Subunit composition of mammalian receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner H, Fleig A, Stokes A, Kinet JP, Penner R. Discrimination of intracellular calcium store subcompartments using TRPV1 (transient receptor potential channel, vanilloid subfamily member 1) release activity. Biochem. J. 2003;371:341–350. doi: 10.1042/BJ20021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okada T, Inoue R, Yamazaki K, Maeda M, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and Functional Characterization of a Novel Mouse Transient Receptor Potential Protein Homologue TRP7. Ca2+- permeable cation channel that is constitutively activated and enhanced by stimulation of G-protein-coupled receptor. J. Biol. Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto T, Schlegel A, Scherer P, Lisanti M. Caveolins, a family of scaffolding proteins for organizing "Preassembled signaling complexes" at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 47.Schaefer M, Plant TD, Obukhow AG, Hofman T, Gudermann T, Schultz G. Receptor-mediated Regulation of the Nonselective Cation Channels TRPC4 and TRPC5. J.Biol.Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- 48.Xu S-Z, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman A, Flemming F, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter K, Beech DJ. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circulation Res. 2006;98:1381. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spiegel S, Milstein S. Sphingosine-1-phosphate, a key signaling molecule. J.Biol.Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- 50.Mayer zu Heringdort D, van Koppen CJ, Jacobs KH. Molecular diversity of sphingolipid signaling. FEBS Lett. 1997;410:34–38. doi: 10.1016/s0014-5793(97)00320-7. [DOI] [PubMed] [Google Scholar]
- 51.Hinkovska-Galcheva V, VanWay SM, Shanley T, Kunkel RG. The role of sphingosine-1-phosphate and ceramide-1-phosphate in calcium homeostasis. Curent Opinion in Investigational Drugs. 2008;9:1192–1205. [PubMed] [Google Scholar]
- 52.Gomez-Munoz A. Ceramide-1phosphate: a novel regulator of cell activation. FEBS Letters. 2004;562:5–10. doi: 10.1016/s0014-5793(04)00211-x. [DOI] [PubMed] [Google Scholar]
- 53.Pettus B, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, Evans JH, Freiberg J, Roddy P, Hannun YA, Chalfant CE. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J. Biol. Chem. 2004;279:11320–11326. doi: 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- 54.Gomez-Munoz A, Duffy PA, Martin A, O’Brien L, Byun HS, Bittman R, Brindley DN. Short-chain ceramide-1-phosphates are novel stimulators of DNA synthesis and cell division: antagonism by cellpermeable ceramides. Molecular Pharmacology. 1995;47:833–899. [PubMed] [Google Scholar]
- 55.Rile G, Yatomi Y, Takafuta T, Ozaki Y. Ceramide 1-phosphate formation in neutrophils. Acta Haematologica. 2003;109:76–83. doi: 10.1159/000068491. [DOI] [PubMed] [Google Scholar]
- 56.Törnquist K, Ramström C, Rudnäs B, Klika K, Dugué B, Adams J, Zipkin R, Pihlaja K, Pasternack M. Ceramide 1-(2-cyanoethyl) phosphate enhances store-operated Ca2+ entry in thyroid FRTL-5 cells. Eur. J. Biochem. 2002;253:1–11. doi: 10.1016/s0014-2999(02)02362-2. [DOI] [PubMed] [Google Scholar]
- 57.Gijsbers S, Mannaerts GP, Himpens B, Van Veldhoven PP. N-acetyl-sphingenine-1-phosphate is a potent calcium mobilizing agent. FEBS Letters. 1999;453:269–272. doi: 10.1016/s0014-5793(99)00735-8. [DOI] [PubMed] [Google Scholar]
- 58.Colina C, Flores A, Castillo C, delRosario Garrido M, Israel A, DiPolo R, Benaim G. Ceramide-1-phosphate induces Ca2+ mobiliztion in Jurkat T cell by elevation of Ins (1,4,5) P3 and activation of a store-operated channel. Biochem. Biophys. Res. Commun. 2005;336:54–60. doi: 10.1016/j.bbrc.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 59.Hogback S, Leppimaki P, Rudnas B, Bjorklund S, Slotte P, Tornquist K. Ceramide-1-phosphate increses intracellular free calcium in thyroid FRTL-5 cells: evidence for an effect mediated by inositol 1,4,5-trisphosphate and intracellular sphingosine-1-phosphate. Biochem J. 2003;370:111–119. doi: 10.1042/BJ20020970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Itagaki K, Kannan KB, Livingston DH, Deitch EA, Fekete Z, Hauser CJ. Store-operated calcium entry in human neutrophils reflects multiple contributions from independently regulated pathways. J.Immunol. 2002;168:4063–4069. doi: 10.4049/jimmunol.168.8.4063. [DOI] [PubMed] [Google Scholar]
- 61.Beech D. Ion channel switching and activation in smoot-muscle cells of occlusive vascular diseases. Biochemical Society Transactions. 2007;5:890–894. doi: 10.1042/BST0350890. [DOI] [PubMed] [Google Scholar]
- 62.Jain R. Molecular regulation of vessel maturation. Nat.Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 63.Siess W. Athero-and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim.Biophys. Acta. 2002;1582:204–215. doi: 10.1016/s1388-1981(02)00173-7. [DOI] [PubMed] [Google Scholar]
- 64.Carafoli E. Calcium Signaling: a tale for all seasons. Proc. Natl.Acad.Sci.USA. 2002;99:115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Unser M, Albroubi A. A review of wavelets in biochemical applications. Proc. IEEE. 1996;84:626–638. [Google Scholar]