Abstract
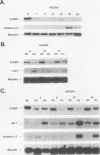
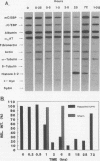
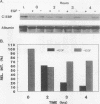
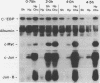
As an approach to understanding physiological mechanisms that control the proliferation of highly differentiated cells, we are addressing whether certain hepatic transcription factors participate in mechanisms that control the growth of hepatocytes. We have focused on CCAAT enhancer-binding protein (C/EBP alpha), a transcription factor which is highly abundant in normal liver and is considered to regulate expression of many genes, including some involved in energy metabolism (S. L. McKnight, M. D. Lane, and S. Gluecksohn-Walsh. Genes Dev. 3:2021-2024, 1989). Using Northern (RNA) blot analysis, we have examined the expression of C/EBP alpha mRNA during liver regeneration and in primary cultures of hepatocytes. C/EBP alpha mRNA levels decrease 60 to 80% within 1 to 3 h after partial hepatectomy as the cells move from G0 to G1 and decrease further when cells progress into S phase. Run-on transcription analysis is in agreement with the Northern blot data, thus suggesting that C/EBP alpha is transcriptionally regulated in regenerating liver. C/EBP alpha mRNA expression also decreases dramatically during the growth of freshly isolated normal hepatocytes cultured under conventional conditions (on dried rat tail collagen; stimulated to proliferate by epidermal growth factor [EGF] and insulin). Cultures of hepatocytes on rat tail collagen in the presence or absence of EGF clearly show that within 3 h, EGF depresses C/EBP alpha mRNA expression and that this effect is substantially greater by 4 h. Inhibition of protein synthesis in the liver by cycloheximide or in cultured hepatocytes by puromycin or cycloheximide effectively blocks the down-regulation of C/EBP alpha gene expression, apparently by stabilizing the normal rapid turnover of the C/EBP alpha mRNA (half-life of <2 h). This drop in C/EBP alpha gene expression in response to activation of hepatocyte growth is consistent with the proposal that C/EBP alpha has an antiproliferative role to play in highly differentiated cells (R. M. Umek, A. D. Friedman, and S. L. McKnight, Science 251: 288-292, 1991).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990 Jun;9(6):1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellas R. E., Bendori R., Farmer S. R. Epidermal growth factor activation of vinculin and beta 1-integrin gene transcription in quiescent Swiss 3T3 cells. Regulation through a protein kinase C-independent pathway. J Biol Chem. 1991 Jun 25;266(18):12008–12014. [PubMed] [Google Scholar]
- Ben-Ze'ev A., Robinson G. S., Bucher N. L., Farmer S. R. Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2161–2165. doi: 10.1073/pnas.85.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Bond J. F., Farmer S. R. Regulation of tubulin and actin mRNA production in rat brain: expression of a new beta-tubulin mRNA with development. Mol Cell Biol. 1983 Aug;3(8):1333–1342. doi: 10.1128/mcb.3.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. F., Robinson G. S., Farmer S. R. Differential expression of two neural cell-specific beta-tubulin mRNAs during rat brain development. Mol Cell Biol. 1984 Jul;4(7):1313–1319. doi: 10.1128/mcb.4.7.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher N. L., Patel U., Cohen S. Hormonal factors concerned with liver regeneration. Ciba Found Symp. 1977;(55):95–107. doi: 10.1002/9780470720363.ch5. [DOI] [PubMed] [Google Scholar]
- Bucher N. L., Robinson G. S., Farmer S. R. Effects of extracellular matrix on hepatocyte growth and gene expression: implications for hepatic regeneration and the repair of liver injury. Semin Liver Dis. 1990 Feb;10(1):11–19. doi: 10.1055/s-2008-1040453. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Chang C. J., Chen T. T., Lei H. Y., Chen D. S., Lee S. C. Molecular cloning of a transcription factor, AGP/EBP, that belongs to members of the C/EBP family. Mol Cell Biol. 1990 Dec;10(12):6642–6653. doi: 10.1128/mcb.10.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Kaestner K. H., Geiman D. E., Lane M. D. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescenzi M., Fleming T. P., Lassar A. B., Weintraub H., Aaronson S. A. MyoD induces growth arrest independent of differentiation in normal and transformed cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8442–8446. doi: 10.1073/pnas.87.21.8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Kent R. B., Sonenshein G. E. Transcriptional activation of immunoglobulin alpha heavy-chain genes by translocation of the c-myc oncogene. 1983 Sep 29-Oct 5Nature. 305(5933):443–446. doi: 10.1038/305443a0. [DOI] [PubMed] [Google Scholar]
- Descombes P., Chojkier M., Lichtsteiner S., Falvey E., Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990 Sep;4(9):1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- Friedman A. D., Landschulz W. H., McKnight S. L. CCAAT/enhancer binding protein activates the promoter of the serum albumin gene in cultured hepatoma cells. Genes Dev. 1989 Sep;3(9):1314–1322. doi: 10.1101/gad.3.9.1314. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Chung E. Y., Darnell J. E., Jr Gene expression during liver regeneration. J Mol Biol. 1984 Oct 15;179(1):37–53. doi: 10.1016/0022-2836(84)90305-x. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Herbst R. S., Friedman N., Darnell J. E., Jr, Babiss L. E. Positive and negative regulatory elements in the mouse albumin enhancer. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1553–1557. doi: 10.1073/pnas.86.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki H., Akira S., Sugita T., Nishio Y., Hashimoto S., Pawlowski T., Suematsu S., Kishimoto T. Reciprocal expression of NF-IL6 and C/EBP in hepatocytes: possible involvement of NF-IL6 in acute phase protein gene expression. New Biol. 1991 Jan;3(1):63–70. [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Koga M., Ogasawara H. Induction of hepatocyte mitosis in intact adult rat by interleukin-1 alpha and interleukin-6. Life Sci. 1991;49(17):1263–1270. doi: 10.1016/0024-3205(91)90139-3. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Skelly H., Botteri F., van der Putten H., Barber J. R., Verma I. M., Leffert H. L. Proto-oncogene expression in regenerating liver is simulated in cultures of primary adult rat hepatocytes. J Biol Chem. 1986 Jun 15;261(17):7929–7933. [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Lane M. D., Gluecksohn-Waelsch S. Is CCAAT/enhancer-binding protein a central regulator of energy metabolism? Genes Dev. 1989 Dec;3(12B):2021–2024. doi: 10.1101/gad.3.12b.2021. [DOI] [PubMed] [Google Scholar]
- Mead J. E., Braun L., Martin D. A., Fausto N. Induction of replicative competence ("priming") in normal liver. Cancer Res. 1990 Nov 1;50(21):7023–7030. [PubMed] [Google Scholar]
- Mohn K. L., Laz T. M., Melby A. E., Taub R. Immediate-early gene expression differs between regenerating liver, insulin-stimulated H-35 cells, and mitogen-stimulated Balb/c 3T3 cells. Liver-specific induction patterns of gene 33, phosphoenolpyruvate carboxykinase, and the jun, fos, and egr families. J Biol Chem. 1990 Dec 15;265(35):21914–21921. [PubMed] [Google Scholar]
- Mueller C. R., Maire P., Schibler U. DBP, a liver-enriched transcriptional activator, is expressed late in ontogeny and its tissue specificity is determined posttranscriptionally. Cell. 1990 Apr 20;61(2):279–291. doi: 10.1016/0092-8674(90)90808-r. [DOI] [PubMed] [Google Scholar]
- Richman R. A., Claus T. H., Pilkis S. J., Friedman D. L. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3589–3593. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sorrentino V., Pepperkok R., Davis R. L., Ansorge W., Philipson L. Cell proliferation inhibited by MyoD1 independently of myogenic differentiation. Nature. 1990 Jun 28;345(6278):813–815. doi: 10.1038/345813a0. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Williams S. C., Cantwell C. A., Johnson P. F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991 Sep;5(9):1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- Wuarin J., Schibler U. Expression of the liver-enriched transcriptional activator protein DBP follows a stringent circadian rhythm. Cell. 1990 Dec 21;63(6):1257–1266. doi: 10.1016/0092-8674(90)90421-a. [DOI] [PubMed] [Google Scholar]
- Xanthopoulos K. G., Mirkovitch J., Decker T., Kuo C. F., Darnell J. E., Jr Cell-specific transcriptional control of the mouse DNA-binding protein mC/EBP. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4117–4121. doi: 10.1073/pnas.86.11.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]