Abstract
Cirrhosis is a common chronic condition with high rates of morbidity and mortality. Optimal medical management involves a multidisciplinary approach, but coordination between medical specialties needs to be improved. This clinical perspective discusses care coordination interventions that have been successful in other disease states and how they could be applied to the management of cirrhosis.
Keywords: Communication, Multidisciplinary Care, Chronic Liver Disease
A histologic entity describing bands of fibrous tissue encircling nodules of hepatocytes, cirrhosis is also a clinical syndrome reflecting the final common pathway for most chronic liver diseases such as viral hepatitis, alcoholic hepatitis, and nonalcoholic fatty liver disease.1 Although true prevalence data are unknown, new diagnoses of cirrhosis are estimated to occur at a rate of 30,000/y in the United States.2 The serious complications of later stages of cirrhosis include ascites, encephalopathy, variceal bleeding, and hepatocellular carcinoma, which cause more than 150,000 hospitalizations, costing nearly $4 billion each year in the United States.3 Earlier stages of cirrhosis also can cause significant morbidity from sequelae such as fatigue, diabetes, minimal hepatic encephalopathy, insomnia, and other factors that impair quality of life and place a heavy burden on patients and their caregivers.4–9 In fact, because the majority of patients live more than a decade after their diagnosis, cirrhosis should be viewed as a chronic condition similar to diabetes and cardiovascular disease.10
There is every reason to suspect that the burden of cirrhosis will only increase. The prevalence of cirrhosis in hepatitis C virus (HCV)-infected individuals has increased dramatically from 9% in 1996 to 18% in 2006, largely owing to an aging cohort with years of chronic infection. Alcoholic liver disease may become more common owing to an increase in alcohol abuse among women.11,12 Finally, patients with nonalcoholic fatty liver disease constitute a large and growing population, with a prevalence estimated at 25% to 30% overall and increasing to 70% to 90% in diabetic patients.13 Approximately one third of patients with nonalcoholic fatty liver disease will have some progression to fibrosis and one third of these patients will have progression to severe fibrosis and cirrhosis; these proportions may increase with increasing prevalence of the metabolic syndrome.14 Thus, it is not surprising that ambulatory care visits for patients with liver disease have been increasing steadily between 1992 and 1993 and 2003 and 2005, when compared with the 1970s to 1980s.15 Clearly, cirrhosis represents a substantial and growing health care burden for both patients and society at large.
Management of Cirrhosis: How Are We Doing?
Medical management of cirrhosis is an area that fortunately benefits from a large body of high-quality evidence. Many randomized trials have been performed over the years, and practice guidelines exist that outline standards of care for cirrhosis.16–22 Table 1 summarizes the class 1A recommendations, which are based on multiple, high-quality, randomized, controlled trials or meta-analyses. For example, a meta-analysis of prophylactic antibiotics for patients with cirrhosis and gastrointestinal bleeding showed reduced all-cause mortality, infection-related mortality, rebleeding, and hospitalization length.23 Another meta-analysis of 8 randomized trials showed that nonselective β-blockers reduced the risk of bleeding among patients with cirrhosis and moderate to large esophageal varices.24 Randomized trial data also exist to guide diuretic therapy, variceal ligation, and many other clinical scenarios.16
Table 1.
| Cirrhosis care category | Recommendation |
|---|---|
| TIPS | In patients with good liver function, either a TIPS or a surgical shunt is an appropriate choice for the prevention of rebleeding in patients who have failed medical therapy |
| TIPS will decrease the need for repeated large-volume paracentesis in patients with refractory cirrhotic ascites | |
| Prophylactic use of nonabsorbable disaccharides or antibiotics does not appear to lower the risk of encephalopathy after TIPS creation | |
| ePTFE-covered stents are preferred to bare stents to lower the risk of shunt dysfunction | |
| Varices | In patients with medium/large varices who have not bled but have a high risk of hemorrhage, nonselective β-blockers or EVL may be recommended for prevention of first variceal hemorrhage |
| In patients with medium/large varices who have not bled and are not at highest risk for hemorrhage, nonselective β-blockers are preferred and EVL should be considered in patients with contraindications, intolerance, or noncompliance to β-blockers | |
| Short-term (maximum, 7 d) antibiotic prophylaxis should be instituted within 24 hours in any patient with cirrhosis and gastrointestinal hemorrhage: oral norfloxacin or intravenous ciprofloxacin are the recommended antibiotics | |
| Therapy with somatostatin or its analogues, octreotide and vapreotide, or terlipressin should be initiated as soon as variceal hemorrhage is suspected and continued for 3–5 days after diagnosis is confirmed | |
| Esophagogastroduodenoscopy, performed within 12 hours, should be used to make the diagnosis and to treat variceal hemorrhage, either with EVL or sclerotherapy | |
| If patients with cirrhosis are found to have bleeding esophageal varices, they should receive EVL or sclerotherapy at time of index endoscopy | |
| Patients with cirrhosis who survive an episode of active variceal hemorrhage should receive therapy to prevent recurrence of variceal hemorrhage | |
| Combination of nonselective β-blockers plus EVL is the best option for secondary prophylaxis of variceal hemorrhage | |
| Ascites | If patients have clinically apparent moderate to severe ascites, they should be managed with a combination of sodium-restricted diet and diuretics (including a combination of both spironolactone and loop diuretics) |
| If hospitalized patients with ascites have ascitic fluid PMN count ≥250 cells/mm3, they should receive empiric antibiotics within 6 hours of their test result | |
| If ambulatory patients with ascites have an ascites fluid PMN count ≥250 cells/mm3, they should receive empiric antibiotics within 24 hours of their test result | |
| If patients have ascites fluid total protein <1.1 g/dL and serum bilirubin >2.5 mg/dL, they should receive prophylactic antibiotics | |
| Patients who have survived an episode of spontaneous bacterial peritonitis should receive long-term outpatient prophylaxis with daily norfloxacin (or trimethoprim/sulfamethoxazole) | |
| Hepatic encephalopathy | Patients with cirrhosis who have persistent hepatic encephalopathy should receive oral disaccharides or rifaximin |
| Hepatocellular carcinoma | If patients have cirrhosis, they should receive surveillance for HCC by using imaging with or without α-fetoprotein every 6–12 mo |
Despite the presence of such evidence-based guidelines, mounting evidence suggests that many patients fail to receive proven treatments. In various studies, between 7% and 62% of patients with cirrhosis received vaccination against hepatitis A or B,25,26 and as few as 6% to 22% of patients with known grades II to III varices received primary prophylaxis with β-blockers.27,28 Rates of screening for hepatocellular carcinoma were dismally low, at 16% to 28%.29 Only 33% of patients with ascites received all recommended treatments.30,31 Furthermore, among patients hospitalized for complications of cirrhosis, more than one third are readmitted within 1 month after discharge.32 Clearly, the current management of cirrhosis leaves room for improvement.
Improving Cirrhosis Care: The Need for Coordination
Why do patients with cirrhosis fail to receive treatments that are supported by evidence-based guidelines? Most would assume that much of the deficit lies in physician education. However, which physicians? The expanding body of medical knowledge makes it difficult for all physicians to follow up the literature on all subjects. As a result of this difficulty, a number of studies have shown differences in specialty vs generalist care.33 In their study of quality care for cirrhotic patients with ascites, Kanwal et al31 found that being seen either by a sub-specialist or at an academic tertiary care center was associated with statistically significantly greater adherence to quality indicators. Similarly, in patients with hepatitis C, subspecialty care as part of pretreatment processes of care produced improved outcomes, including improved sustained virologic response rates.34 Bini et al35 found that gastroenterology consultation was associated with improved outcomes for patients hospitalized with decompensated cirrhosis. Differences in generalist vs specialist care also have been noted in inflammatory bowel disease, where a recent study found that the presence of early postoperative gastroenterology care in Crohn’s patients led to improved outcomes.36
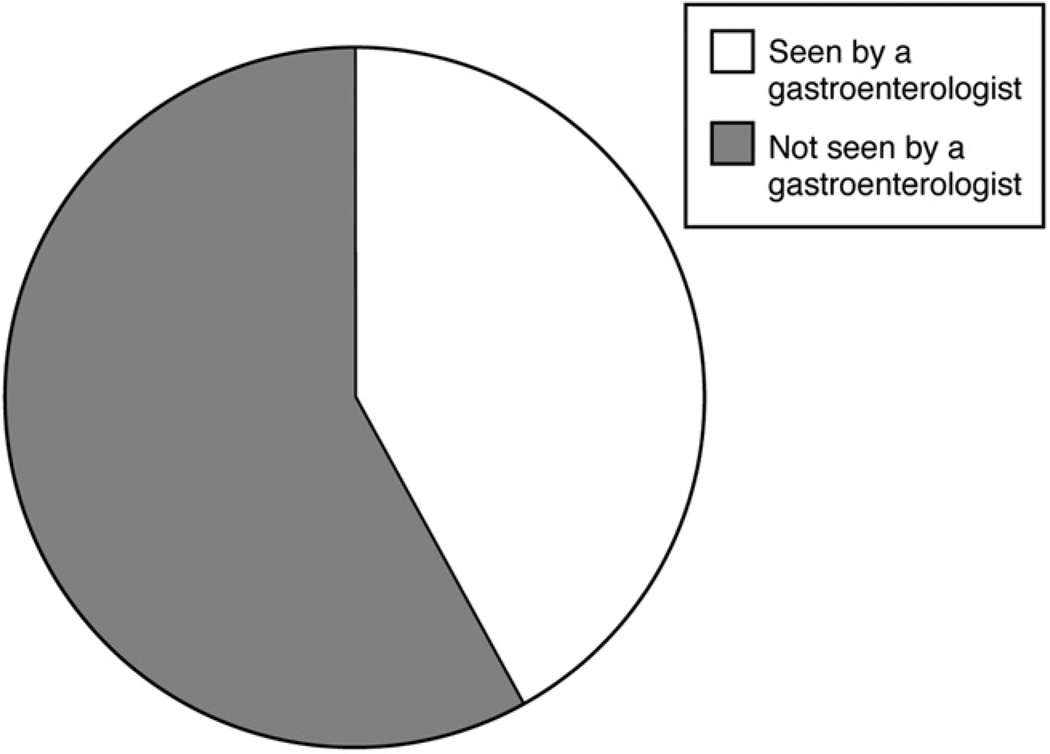
In light of these findings, what proportion of patients with cirrhosis receives subspecialty care? We analyzed Medicare billing data on 276 patients in the Health and Retirement Study who were hospitalized with an International Classification of Diseases, 9th revision, code for cirrhosis (57.2, 571.5) in 1998 to 2006, and who had continuous enrollment in Medicare parts A and B. As shown in Figure 1, only 42% had an Evaluation and Management code submitted by a gastroenterologist during their hospital stay. Among those who were discharged and survived the ensuing 365 days, only 45% subsequently had an Evaluation and Management code submitted by a gastroenterologist (unpublished data).
Figure 1.
Proportion of Medicare patients hospitalized with cirrhosis who are seen by a gastroenterologist during their stay.
Why is it that many patients with cirrhosis never see a gastroenterologist? One possibility is access. Not all rural communities have even the first-line expert, namely a gastroenterologist. Furthermore, the practice of hepatology within the subspecialty of gastroenterology means that there exists a large spectrum of interest and experience in caring for liver patients among general gastroenterologists. Thus, even those communities with gastroenterologists may not have a subspecialist willing or able to manage cirrhosis. Hepatologists, who focus entirely on treating liver disease, tend to be located in transplant centers. Most of these centers are in urban areas, and consequently many patients have to travel long distances to be seen. Even in areas where patients would not have to travel large distances for care, such as major metropolitan centers, safety-net organizations who serve the uninsured in these cities experience barriers to subspecialty access.37
Our experience suggests that even patients who manage to see a subspecialist receive care that is fragmented and poorly coordinated. Fragmentation occurs between both inpatient and outpatient settings as well as between primary care providers (PCPs) and subspecialists. The coordination of care between these providers is poor, particularly in the United States, which ranked next to last in a group of 7 nations with respect to coordinated care.38 For example, Australian primary care physicians reported receiving relevant information back from specialists 96% of the time, as compared with only 75% of the time for US PCPs.38 Other studies have shown large discrepancies between receipt of referral information and consult recommendations that resulted in providers feeling less able to provide high-quality care.39 Not surprisingly, patients with a higher number of chronic illnesses and more subspecialists involved in their care report worse care coordination.40
When performed correctly, co-management can lead to better patient care than that by a single specialty. In other diseases, co-managed care has been shown to improve things such as mortality after myocardial infarction, providing support for the importance of coordinated care as opposed to the either/or dichotomy of generalist vs subspecialist-only care.41,42 Outpatient multidisciplinary management of congestive heart failure has been found to reduce all-cause mortality and hospital admissions, both for heart failure–related reasons as well as other reasons.43,44 Despite these robust outcomes, few studies have evaluated such interventions in cirrhosis. Given the evidence of care deficiencies, and the barriers to subspecialty access, we call for efforts to improve multidisciplinary care coordination and to apply these concepts to cirrhosis care.
Coordinated Care for Cirrhosis: Directions for Improvement
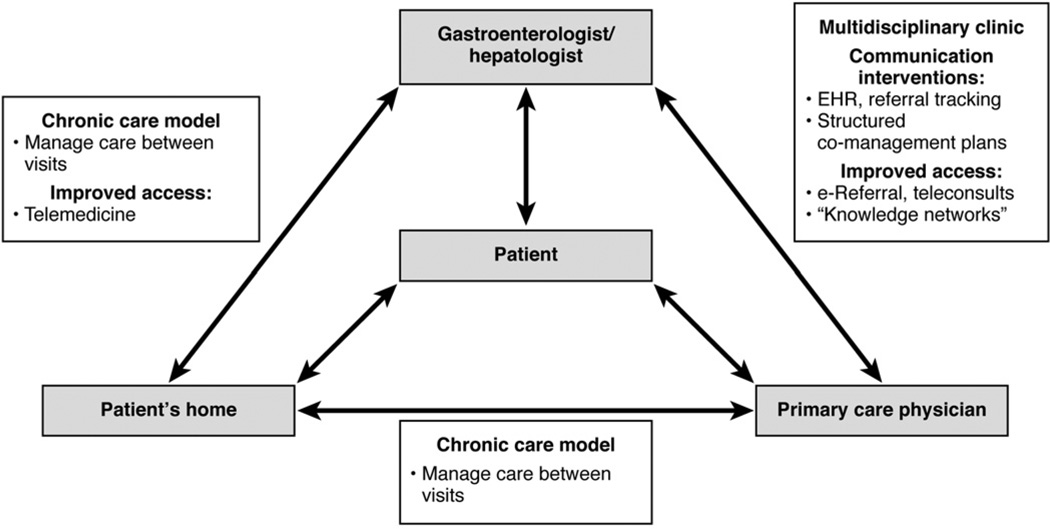
Potential directions for improving cirrhosis care can be grouped into 4 categories: (1) chronic care models, in which active disease management occurs between clinic visits, (2) multidisciplinary clinic models, in which co-location of specialists helps integrate care, (3) communication interventions, which attempt to improve information transfer, and (4) improving access to specialty care. As shown in Figure 2, a combination of one or more of these interventions may be applicable, depending on geographic distances and the types of providers involved.
Figure 2.
A conceptual model of coordination and communication interventions for improving the care of patients with cirrhosis.
Chronic Care Models
Chronic care models target primarily the patient rather than the providers and attempt to improve care by active management (by telephone or other means) between clinic visits. The disease state most similar to cirrhosis in which chronic care models have been well studied is congestive heart failure. In their meta-analysis of heart failure outcomes, Holland et al43 grouped studies by 4 specific interventions: home visits, videophone or other remote home-monitoring systems, scheduled home telephone calls and mailings, or clinic or hospital visits only without any home contact. Overall, risk of all-cause admission, mortality, and heart failure admission decreased by 12%, 20%, and 30%, respectively.43 The home-based interventions produced the greatest benefit, with clinic or hospital visit– only interventions showing minimal benefit.43 Because of this robust evidence, applications of the chronic care model are now included as a class 1 recommendation in the heart failure practice guidelines.45
Multidisciplinary Care Models
Optimal cirrhosis management involves an experienced gastroenterologist/hepatologist, primary care physician, and often by a radiologist, endocrinologist, psychiatrist, and infectious disease specialists. Thus, one way to improve care would be co-location in a single clinic. This approach in other chronic diseases, such as diabetes, coronary artery disease, and chronic obstructive pulmonary disease, has shown improvements in quality of care, preventive care, and guideline adherence.46 Within gastroenterology, multidisciplinary care also has been shown to improve outcomes. Multidisciplinary hepatocellular carcinoma care produces increased quality of life, decreased disease progression, earlier stage diagnosis, and improved survival, likely owing to the highly individualized care patients receive in such clinics.47–49 This success in other areas suggests that multidisciplinary clinics for patients with cirrhosis may be one method to improve outcomes.
Communication Interventions
Many patients receive subspecialty care at a distance from their home, which makes multidisciplinary clinics impractical. How can providers improve communication and coordination across the miles? With respect to the generalist–subspecialist interface, referrals remain a major way in which subspecialist expertise is elicited, although approximately half of all new visits to a specialist originate from direct patient request, even in settings with gatekeeper policies, such as health maintenance organizations.50 Mehrotra et al51 performed a narrative review of the literature concerning communication between specialists and generalists. They identified 4 key steps in the process of referral and communication51: (1) referral tracking, (2) information transfer from generalist to specialist (ie, reason for referral and prior work-up), (3) information transfer from specialist to generalist (ie, recommendations after a visit to a subspecialist), and (4) care integration between generalist and subspecialist.
Studies on reasons for referral and appropriateness of referrals have shown both under-referral and over-referral, although such studies are hampered by differences in study design.51 However, both specialist and PCPs overwhelmingly find the communication process to be unsatisfactory.52 Between 50% and 68% of the time there was no communication from referring physician to specialist and between 4% and 45% of the time there was no communication from the specialist to the referring physician.52–54 Furthermore, specialists and generalists often do not agree on communication, with 87% of specialists in one study reporting sending postreferral communication within 7 days whereas only 36% of generalists in the same study reported receiving communication within 7 days.52 This lack of communication often is noted by patients and can contribute to poorer outcomes including decreased ability to provide adequate care and delayed diagnosis and treatment.54,55
Some of this confusion may be owing to a lack of clarity regarding the expected role of the subspecialist consultant. Several roles for the consultant have been described in the literature: cognitive consultant (providing diagnostic or therapeutic advice), procedural consultant (providing technical expertise and performing procedure), co-manager with shared care (sharing long-term management with PCP), co-manager with principal care (assuming total responsibility for long-term management), and primary care (providing the medical home for a group of patients).30 Other than when only a procedure is requested, such as for open-access colonoscopy, the subspecialist rarely is informed of the requested level of involvement, generating confusion. Within the field of gastroenterology, cirrhosis care likely falls into the categories of either co-manager with shared care or co-manager with principal care. This relationship becomes even more complicated when a PCP, local gastroenterologist, and transplant hepatologist all are involved.
To address these deficiencies, the electronic health record (EHR) may show promise for facilitating communication and coordination of care across the specialist–generalist interface. The Institute of Medicine advocates for the use of an EHR by all providers, specifically citing gains in coordination and communication between providers as a goal of policy change. The Affordable Care Act of 2009 as well as the Health Information Technology for Economic and Clinical Health (HITECH) Act both established financial incentives to encourage EHR adoption based on evidence showing that the EHR would improve medical care across multiple domains, including quality of care, care coordination, efficiency, and communication.56,57 Use of an electronic medical record has been shown to be particularly important to improving coordination and quality of care for patients with chronic illnesses, and as a result of the growing body of literature as well as policy incentives, EHR use is growing.58 As of 2011, 55% of physicians had adopted an EHR system and approximately 75% reported that they currently met federal meaningful use criteria.59 With the increased incentives for EHR adoption, this percentage likely will increase.
With increasing use of EHR, streamlined communication between generalists and specialists may become easier, with improvements in quality of care and patient outcomes as an expected result. A large review of multiple meta-analyses of health information technology use and outcomes concluded that use of an EHR led to improvements in quality of care, particularly with respect to preventive care measures and medication management.60 A separate qualitative study of EHR implementation in a series of small primary care practices showed that EHR implementation improved efficiency across several domains, including coordination of care and communication with specialists.61 Specifically, PCPs reported greater ease of tracking specialist care and improved communication between providers and staff.61 It is important to note that these studies have been performed almost exclusively in large integrated health systems or medical groups and would not be applicable to practice settings where multiple providers have different EHRs.62
Despite the promise of health information technology, there are still deficits. In a study of referrals performed within the Veterans Affairs system, which uses a uniform EHR for both inpatient and outpatient care, communication deficits in the referral process were still noted.63 Inadequate justification for follow-up evaluation of unresolved referrals after 30 days was noted in 77% of such incomplete referrals.63 In more than 15% of discontinued referrals, there was no subspecialist follow-up evaluation and no justification given for discontinuation.63 Furthermore, provider perceptions of care coordination at the generalist–subspecialist interface revealed that the existence of an EHR, although necessary, was not sufficient to ensure adequate communication, particularly when providers were operating outside an integrated medical system.62 Thus, widespread use of an EHR likely will improve many aspects of communication and shows great promise in improving the generalist–specialist interface; however, it likely will not be enough on its own to substantially improve care coordination for patients with cirrhosis.
Improving Access to Specialty Care
According to one study, gastroenterology was one of the most highly requested but difficult-to-access referrals, largely owing to poor specialist distribution and low overall supply of specialists.37 Setting aside the obvious solution of increasing the workforce, how could access to gastroenterologists/ hepatologists be improved for patients with cirrhosis, particularly in underserved areas? Closely tied to and dependent on the establishment of EHR, electronic referral systems, or e-referrals, have been shown to increase efficiency of the referral process, as well as increasing access to subspecialty care. PCPs who used e-referral noted improvements in multiple aspects of communication and coordination of care, including in referral tracking, access, wait time, answering of clinical questions, and overall care improvement.64 In one study evaluating the implementation of e-referral in a public safety-net system, an e-referral system was established between PCPs and outpatient subspecialists.65 The program started with gastroenterology and liver clinics and subsequently has expanded to include multiple other subspecialty clinics. In this system, consult requests are placed electronically and then reviewed by specialty-specific clinician reviewers to evaluate urgency, appropriateness of specialty, and whether or not any work-up should be completed before the appointment. In many cases, the clinician reviewer can guide the PCP through the e-referral interface, effectively providing an electronic curbside consultation. Through this e-referral system, providers have noted improvements in wait times, clinical and administrative efficiency, and, importantly, in communication and coordination.65 The iterative discussion, which can occur via the referral portal, also provided an educational benefit to providers, giving them valued information about the condition being referred as well as appropriate work-up. The success of e-referral, particularly for underserved populations, has led to its implementation in the Veterans Administration system at Kaiser Permanente in California, as well as county health systems in some areas of California.65,66
In addition to e-referral, subspecialist expertise has been extended to underserved areas via telemedicine. Alternately referred to in the literature as telemedicine, teleconsultation, or telemanagement, telemedicine initiatives seek to find ways of transforming the traditional doctor–patient visit, using various technologies including telephone, video, audio, and Internet-based networking platforms to connect providers to each other and to patients. An example of a successful telemedicine program is the University of New Mexico’s Project Extension for Community Healthcare Outcomes (ECHO), originally developed for hepatitis C treatment. Noting that New Mexico had the highest rates of deaths from chronic liver disease and cirrhosis as well as having a large rural poor population, access to HCV treatment before Project ECHO was scarce.67 Project ECHO uses videoconferencing in addition to specially designed HCV education software to create a knowledge network, linking remote clinicians to the University of New Mexico specialists. In weekly telemedicine clinics, remote PCPs present their HCV patients to a panel of academic health center physicians, which can include gastroenterologists, infectious disease doctors, addiction specialists, pharmacists, and also other PCPs connected in the “learning loops.”67 Although other telemedicine initiatives have focused on treatment only, Project ECHO adds an educational component as well. Results of the initiative showed that an increased number of patients were receiving treatment and that there was no difference between sustained virologic response rates between the ECHO cohort and the University Clinic cohort.68 It is unclear whether a similar level of success would be achieved in telemedicine for cirrhosis, which requires a broader knowledge base and access to advanced therapies such as transjugular intrahepatic portosystemic shunt. Furthermore, logistical barriers remain in most practice settings, particularly in regard to reimbursement for these activities.
Thus, technological advances such as EHRs, e-referrals, and telemedicine may help improve deficiencies in referral tracking and information transfer between the PCP and specialists. These programs will not, however, fully address deficiencies in care integration and co-management. For example, who should the patient call if changes to the diuretic regimen are needed? Who is responsible for surveillance of hepatocellular carcinoma? Our experience is that the answers to these questions are often unclear to both the providers and the patient. Not only do providers need to clearly articulate their roles in the co-management relationship, but these roles need to be shared with the patient. We have been told by patients over and over that in many situations they have no idea which provider they are supposed to call. Further research is needed to determine whether structured co-management plans would decrease confusion, duplicate testing, and medical errors.
Conclusions
In summary, a growing body of literature exists that patients with cirrhosis fail to receive treatments that are supported by guidelines and high-quality evidence. Because cirrhosis management is often multidisciplinary, we propose that improving care coordination between providers may be a mechanism to help remedy this failure and improve quality and efficiency of care. Multiple interventions to improve care coordination have been shown to be effective across a variety of other chronic diseases, and these interventions should be studied in patients with cirrhosis. The challenge for gastroenterologists and hepatologists who co-manage cirrhosis patients alongside PCPs then will be to apply these care coordination interventions to our own practices, realizing that each physician will need to choose those interventions that match their practice needs. Such care coordination actually may represent a business opportunity for gastroenterologists. The US health care system is in the midst of massive changes; physician practices and hospital systems are merging, and reimbursement mechanisms are changing to focus on accountable care rather than fee-for-service care.69 Care coordination has become a high policy priority, and new revenue sources are becoming available to subspecialists willing to partner with PCPs to manage patient populations.56 The American Gastroenterology Association and American Association for the Study of Liver Disease recognize these trends and are working to establish performance metrics for cirrhosis care. In addition to hopefully improving patient outcomes, these efforts also may serve to increase cirrhosis care to greater national prominence. However, to extend the expertise of gastroenterologists and hepatologists to the PCPs and patients who need it, methods of communication and delineation of responsibilities will need to be improved.
Acknowledgments
The authors would like to thank Dr Theodore J. Iwashyna for assistance with Medicare data analysis.
Funding
Supported by National Institutes of Health T32DK062708 (J.L.M.) and K23DK085204 (M.L.V.).
Abbreviations used in this paper
- ECHO
Extension for Community Healthcare Outcomes
- EHR
electronic health record
- HCV
hepatitis C virus
- PCP
primary care provider
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell BP, Manos MM, Zaman A, et al. The epidemiology of newly diagnosed chronic liver disease in gastroenterology practices in the United States: results from population-based surveillance. Am J Gastroenterol. 2008;103:2727–2736. doi: 10.1111/j.1572-0241.2008.02071.x. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen GC, Segev DL, Thuluvath PJ. Nationwide increase in hospitalizations and hepatitis C among inpatients with cirrhosis and sequelae of portal hypertension. Clin Gastroenterol Hepatol. 2007;5:1092–1099. doi: 10.1016/j.cgh.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 4.Gralnek IM, Hays RD, Kilbourne A, et al. Development and evaluation of the Liver Disease Quality of Life instrument in persons with advanced, chronic liver disease—the LDQOL. Am J Gastroenterol. 2000;95:3552–3565. doi: 10.1111/j.1572-0241.2000.03375.x. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, et al. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280–288. doi: 10.3748/wjg.15.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalaitzakis E, Josefsson A, Castedal M, et al. Factors related to fatigue in patients with cirrhosis before and after liver transplantation. Clin Gastroenterol Hepatol. 2012;10:174–181. doi: 10.1016/j.cgh.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Rakoski MO, McCammon RJ, Piette JD, et al. Burden of cirrhosis on older Americans and their families: analysis of the health and retirement study. Hepatology. 2012;55:184–191. doi: 10.1002/hep.24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646–1653. doi: 10.1038/ajg.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solà E, Watson H, Graupera I, et al. Factors related to quality of life in patients with cirrhosis and ascites: relevance of serum sodium concentration and leg edema. J Hepatol. 2012;57:1199–1206. doi: 10.1016/j.jhep.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Ginés P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122–128. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 11.Grucza RA, Norberg K, Bucholz KK, et al. Correspondence between secular changes in alcohol dependence and age of drinking onset among women in the United States. Alcohol Clin Exp Res. 2008;32:1493–1501. doi: 10.1111/j.1530-0277.2008.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grucza RA, Bucholz KK, Rice JP, et al. Secular trends in the lifetime prevalence of alcohol dependence in the United States: a re-evaluation. Alcohol Clin Exp Res. 2008;32:763–770. doi: 10.1111/j.1530-0277.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol. 2010;53:713–718. doi: 10.1016/j.jhep.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 15.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Kanwal F, Kramer J, Asch SM, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709–717. doi: 10.1016/j.cgh.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Runyon BA AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 18.Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology. 2009;51:306–306. doi: 10.1002/hep.23383. [DOI] [PubMed] [Google Scholar]
- 19.Division of Viral Hepatitis, National, Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 20.Garcia-Tsao G, Lim JK, Lim J, et al. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol. 2009;104:1802–1829. doi: 10.1038/ajg.2009.191. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 22.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Soares-Weiser K, Brezis M, Tur-Kaspa R, et al. Antibiotic prophylaxis for cirrhotic patients with gastrointestinal bleeding. Cochrane Database Syst Rev. 2002;2 doi: 10.1002/14651858.CD002907. CD002907. [DOI] [PubMed] [Google Scholar]
- 24.D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475–505. doi: 10.1055/s-2007-1007133. [DOI] [PubMed] [Google Scholar]
- 25.Hachem CY, Kramer JR, Kanwal F, et al. Hepatitis vaccination in patients with hepatitis C: practice and validation of codes at a large Veterans Administration Medical Centre. Aliment Pharmacol Ther. 2008;28:1078–1087. doi: 10.1111/j.1365-2036.2008.03827.x. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez B, Hasson NK, Cheung R. Hepatitis C performance measure on hepatitis A and B vaccination: missed opportunities? Am J Gastroenterol. 2009;104:1961–1967. doi: 10.1038/ajg.2009.252. [DOI] [PubMed] [Google Scholar]
- 27.Wilbur K, Sidhu K. Beta blocker prophylaxis for patients with variceal hemorrhage. J Clin Gastroenterol. 2005;39:435–440. doi: 10.1097/01.mcg.0000159222.16032.98. [DOI] [PubMed] [Google Scholar]
- 28.Sorbi D, Gostout CJ, Peura D, et al. An assessment of the management of acute bleeding varices: a multicenter prospective member-based study. Am J Gastroenterol. 2003;98:2424–2434. doi: 10.1111/j.1572-0241.2003.t01-1-07705.x. [DOI] [PubMed] [Google Scholar]
- 29.Davila JA, Weston A, Smalley W, et al. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2007;41:777–782. doi: 10.1097/MCG.0b013e3180381560. [DOI] [PubMed] [Google Scholar]
- 30.Forrest CB. A typology of specialists’ clinical roles. Arch Intern Med. 2009;169:1062–1068. doi: 10.1001/archinternmed.2009.114. [DOI] [PubMed] [Google Scholar]
- 31.Kanwal F, Kramer JR, Buchanan P, et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143:70–77. doi: 10.1053/j.gastro.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 32.Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247–252. doi: 10.1038/ajg.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrold LR, Field TS, Gurwitz JH. Knowledge, patterns of care, and outcomes of care for generalists and specialists. J Gen Intern Med. 1999;14:499–511. doi: 10.1046/j.1525-1497.1999.08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanwal F, Hoang T, Chrusciel T, et al. Process of care for hepatitis C infection is linked to treatment outcome and virologic response. Clin Gastroenterol Hepatol. 2012;10:1270.e3–1277.e3. doi: 10.1016/j.cgh.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089–1095. doi: 10.1053/jhep.2001.29204. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen GC, Saibil F, Steinhart AH, et al. Postoperative healthcare utilization in Crohn’s disease: the impact of specialist care. Am J Gastroenterol. 2012;107:1522–1529. doi: 10.1038/ajg.2012.235. [DOI] [PubMed] [Google Scholar]
- 37.Pourat N, Davis AC, Salce E, et al. In ten California counties, notable progress in system integration within the safety net, although challenges remain. Health Aff (Millwood) 2012;31:1717–1727. doi: 10.1377/hlthaff.2012.0545. [DOI] [PubMed] [Google Scholar]
- 38.Davis K, Schoen C, Stremikis K. Mirror, mirror on the wall: how the performance of the U.S. health care system compares internationally, 2010 update. New York: The Commonwealth Fund; 2010. Jun, [Google Scholar]
- 39.O’Malley AS, Reschovsky JD. Referral and consultation communication between primary care and specialist physicians: finding common ground. Arch Intern Med. 2011;171:56–65. doi: 10.1001/archinternmed.2010.480. [DOI] [PubMed] [Google Scholar]
- 40.Liss DT, Chubak J, Anderson ML, et al. Patient-reported care coordination: associations with primary care continuity and specialty care use. Ann Fam Med. 2011;9:323–329. doi: 10.1370/afm.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willison DJ, Soumerai SB, McLaughlin TJ, et al. Consultation between cardiologists and generalists in the management of acute myocardial infarction: implications for quality of care. Arch Intern Med. 1998;158:1778–1783. doi: 10.1001/archinte.158.16.1778. [DOI] [PubMed] [Google Scholar]
- 42.Ayanian JZ, Landrum MB, Guadagnoli E, et al. Specialty of ambulatory care physicians and mortality among elderly patients after myocardial infarction. N Engl J Med. 2002;347:1678–1686. doi: 10.1056/NEJMsa020080. [DOI] [PubMed] [Google Scholar]
- 43.Holland R, Battersby J, Harvey I, et al. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91:899–906. doi: 10.1136/hrt.2004.048389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAlister FA, Stewart S, Ferrua S, et al. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 45.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to update the 2001 guidelines for the evaluation and management of heart failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 46.Hogg W, Lemelin J, Dahrouge S, et al. Randomized controlled trial of anticipatory and preventive multidisciplinary team care: for complex patients in a community-based primary care setting. Can Fam Physician. 2009;55:e76–e85. [PMC free article] [PubMed] [Google Scholar]
- 47.Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford) 2008;10:405–411. doi: 10.1080/13651820802356572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gish RG, Lencioni R, Di Bisceglie AM, et al. Role of the multidisciplinary team in the diagnosis and treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2012;6:173–185. doi: 10.1586/egh.11.105. [DOI] [PubMed] [Google Scholar]
- 49.Guy J, Kelley RK, Roberts J, et al. Multidisciplinary management of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2012;10:354–362. doi: 10.1016/j.cgh.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Forrest CB, Reid RJ. Passing the baton: HMOs’ influence on referrals to specialty care. Health Aff (Millwood) 1997;16:157–162. doi: 10.1377/hlthaff.16.6.157. [DOI] [PubMed] [Google Scholar]
- 51.Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39–68. doi: 10.1111/j.1468-0009.2011.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gandhi TK, Sittig DF, Franklin M, et al. Communication breakdown in the outpatient referral process. J Gen Intern Med. 2000;15:626–631. doi: 10.1046/j.1525-1497.2000.91119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourguet C, Gilchrist V, McCord G. The consultation and referral process. A report from NEON: Northeastern Ohio Network Research Group. J Fam Pract. 1998;46:47–53. [PubMed] [Google Scholar]
- 54.Stille CJ, McLaughlin TJ, Primack WA, et al. Determinants and impact of generalist-specialist communication about pediatric outpatient referrals. Pediatrics. 2006;118:1341–1349. doi: 10.1542/peds.2005-3010. [DOI] [PubMed] [Google Scholar]
- 55.Gandhi TK, Kachalia A, Thomas EJ, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med. 2006;145:488–496. doi: 10.7326/0003-4819-145-7-200610030-00006. [DOI] [PubMed] [Google Scholar]
- 56.Adams K, Corrigan JM. Priority areas for national action: transforming health care quality. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 57.Tang PC. Key capabilities of an electronic health record system: a letter report. Washington DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 58.Burton LC, Anderson GF, Kues IW. Using electronic health records to help coordinate care. Milbank Q. 2004;82:457–481. doi: 10.1111/j.0887-378X.2004.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jamoom E, Beatty P, Bercovitz A, et al. Physician adoption of electronic health record systems: United States, 2011. National Center for Health Statistics data brief (Hyattsville, MD) 2012;98:1–8. [PubMed] [Google Scholar]
- 60.Lau F, Kuziemsky C, Price M, et al. A review on systematic reviews of health information system studies. J Am Med Inform Assoc. 2010;17:637–645. doi: 10.1136/jamia.2010.004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goetz Goldberg D, Kuzel AJ, Feng LB, et al. EHRs in primary care practices: benefits, challenges, and successful strategies. Am J Manag Care. 2012;18:e48–e54. [PubMed] [Google Scholar]
- 62.O’Malley AS, Grossman JM, Cohen GR, et al. Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2010;25:177–185. doi: 10.1007/s11606-009-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh H, Esquivel A, Sittig DF, et al. Follow-up actions on electronic referral communication in a multispecialty outpatient setting. J Gen Intern Med. 2011;26:64–69. doi: 10.1007/s11606-010-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim Y, Chen AH, Keith E, et al. Not perfect, but better: primary care providers’ experiences with electronic referrals in a safety net health system. J Gen Intern Med. 2009;24:614–619. doi: 10.1007/s11606-009-0955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen AH, Kushel MB, Grumbach K, et al. Practice profile. A safety-net system gains efficiencies through “eReferrals” to specialists. Health Aff (Millwood) 2010;29:969–971. doi: 10.1377/hlthaff.2010.0027. [DOI] [PubMed] [Google Scholar]
- 66.Chen C, Garrido T, Chock D, et al. The Kaiser Permanente electronic health record: transforming and streamlining modalities of care. Health Aff (Millwood) 2009;28:323–333. doi: 10.1377/hlthaff.28.2.323. [DOI] [PubMed] [Google Scholar]
- 67.Arora S, Geppert CMA, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82:154–160. doi: 10.1097/ACM.0b013e31802d8f68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–2207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berwick DM. Making good on ACOs’ promise—the final rule for the Medicare Shared Savings Program. N Engl J Med. 2011;365:1753–1756. doi: 10.1056/NEJMp1111671. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823–832. doi: 10.1056/NEJMra0901512. [DOI] [PubMed] [Google Scholar]