Abstract
Amyotrophic Lateral Sclerosis (ALS) is a fatal neurodegenerative disease characterized the progressive loss of brain and spinal cord motor neurons. Epidemiological studies have shown a higher incidence of ALS in men than women. Interestingly, there are clear gender differences in disease onset and progression in both mouse and rat models of familial ALS over-expressing mutated human superoxide dismutase-1 (SOD1G93A). To determine a possible involvement of sex steroids and neurosteroids in ALS, we first asked whether the reduction of serum steroid levels by gonadectomy can modulate disease progression in SOD1G93A rats. Although sexual dimorphism was observed in intact and gonadectomized SOD1G93A rats, there is no significant effect of gonadectomy on disease onset and progression. We next tested whether hormonal treatment using a neurosteroid dehydroepiandrosterone (DHEA) can ameliorate motor neuron loss in both sexes of SOD1G93A rats. DHEA treatment did not alter disease progression or survival in SOD1G93A rats. Our results indicate that gonadal steroids and neurosteroids are not one of the possible modulators for the occurrence or disease progression in a rat model of ALS. Further analysis will be necessary to understand how sexual dimorphism is involved in ALS disease progression.
Keywords: Amyotrophic lateral sclerosis (ALS), SOD1G93A rats, sexual dimorphism, gonadectomy, dehydroepiandrosterone (DHEA)
Introduction
The exact etiology of Amyotrophic lateral sclerosis (ALS) is still unclear, but most epidemiological studies have shown a higher incidence of ALS in men than women (1). Sexual dimorphism in disease onset and progression is also observed in mouse (2;3) and rat (4) models for familial ALS using mutant human superoxide dismutase 1 gene (SOD1G93A). Furthermore, it has been demonstrated that ovariectomy led to a significant acceleration of disease progression in SOD1G93A female mice (3;5). These observations suggest an involvement of sex steroids on the occurrence or disease progression of ALS.
Steroids have pronounced neurotrophic effects on normal neuronal activity during development and in adulthood. Particularly, neurosteroids such as dehydroepiandrosterone (DHEA) are the most abundant steroids in the blood of young adult humans and have neuroprotective properties in vitro (6;7) and in vivo (8;9). Significant interest has arisen from the hypothesis that declining DHEA concentrations in adults may serve as an indicator of a number of conditions (10).
In the present study we asked whether the alternations of serum steroid levels by gonadectomy or chronic DHEA can modulate disease progression in SOD1G93A rats.
Materials and methods
We prepared a cohort of SOD1G93A rats as described previously (4) in accordance with the guidelines for UW-Madison and NIH standards of animal care. Male (n=8) and female SOD1G93A rats (n=9) were gonadectomized at 60 days of age. Chronic DHEA treatments were performed by using medical grade silastic tubing implants or hormone pellets as described previously (11). SOD1G93A rats (85 days old) received subcutaneous implants into the cervical region with a silicone-tube containing crystalline DHEA (Sigma-Aldrich) or cholesterol (control). At endpoint, trunk blood samples were collected and measured serum steroid concentration by radioimmunoassay.
We performed the routine analyses of locomotor testing using the Basso-Beattie-Bresnahan (BBB) score until endpoint as previously described (4;12). Disease onset was estimated by using the BBB rating score of 16 or lower (4). Statistical analysis of disease onset and survival was performed using the Kaplan-Meier method with log rank test. In Table 1, survival periods of the animals were expressed as means ± SEM and analyzed by two-tailed t-test. Differences in BBB score were calculated using two-way ANOVA with Bonferonni post hoc test. Differences were considered significant when P<0.05.
Table 1.
Disease onset and survival period in gonadectomized SOD1G93A rats.
| Group | Sex | Disease onset, (days) |
Survival (days) |
|---|---|---|---|
| Control SOD1G93A | Male | 97 ± 5.1 | 141 ± 6.3 |
| Female | 104 ± 9.6 | 161 ± 12.1* | |
| Gonadectomized SOD1G93A | Male | 100 ± 8.3 | 142 ± 8.0 |
| Female | 101 ± 6.5 | 162 ± 13.2** |
P<0.05 vs. control male;
P<0.05 vs. gonadectomized male.
Results
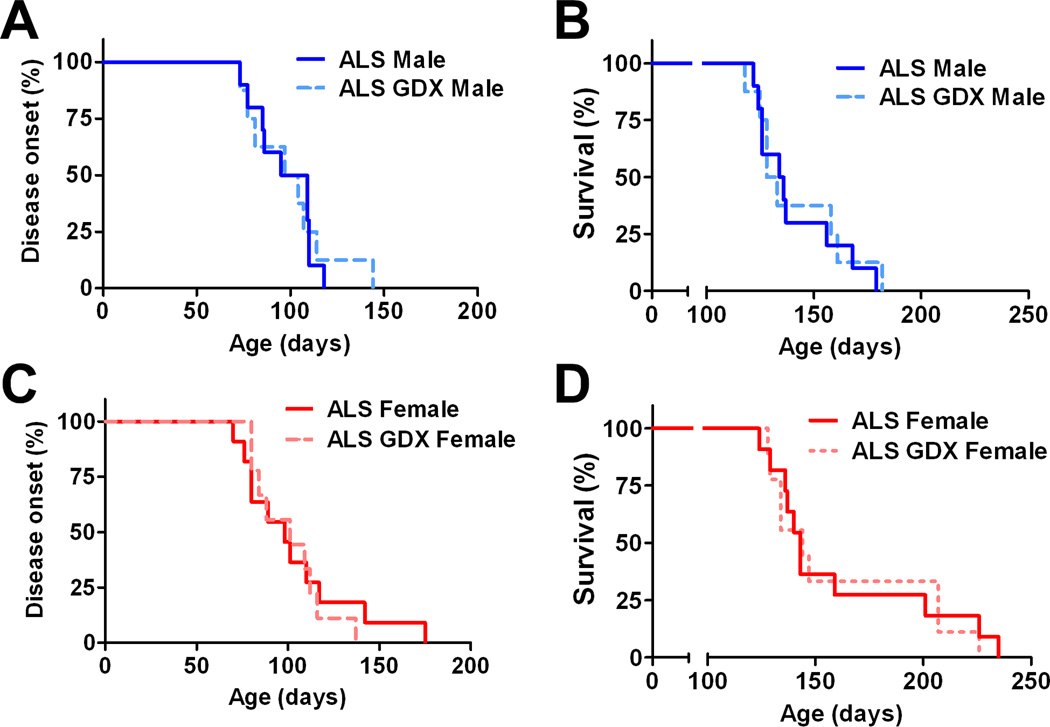
As we have shown previously (4), survival periods for intact females were significantly longer when compared to males (Fig. 1; P<0.05). Gonadectomy did not affect disease onset in either males (Fig. 1A) or females (Fig. 1C) when compared to the non-surgery control SOD1G93A rats. Furthermore, there was no significant difference in survival between gonadectomized SOD1G93A rats and their same-sexual counterparts from onset until death (Figs. 1B and D). The mean time to reach endpoint was 141 days in control males (n=10), 161 days in control females (n=11), 142 days in gonadectomized males (n=8), and 162 days in gonadectomized females (n=10) (Table 1). We next analyzed whether gonadectomy affects to motor function in SOD1G93A males and females. Regardless of the treatment, SODG93A females showed higher BBB scores when compared to SOD1G93A males (P<0.05). However, gonadectomy did not alter progression of motor dysfunction in both SODG93A males and females (Fig. 2).
Figure 1. Effects of gonadectomy on disease onset and survival in SOD1G93A rats.
Kaplan-Meier curves generated from gonadectomized and intact SOD1G93A rats depicting disease onset and survival. Castration did not affect to disease onset (A) and survival period (B) in SOD1G93A males. Ovariectomized SOD1G93A females showed similar trends in disease onset (C) and survival (D) compared to intact females.
Figure 2. Basso-Beattie-Bresnahan (BBB) locomotor rating scales in gonadectomized SOD1G93A rats.
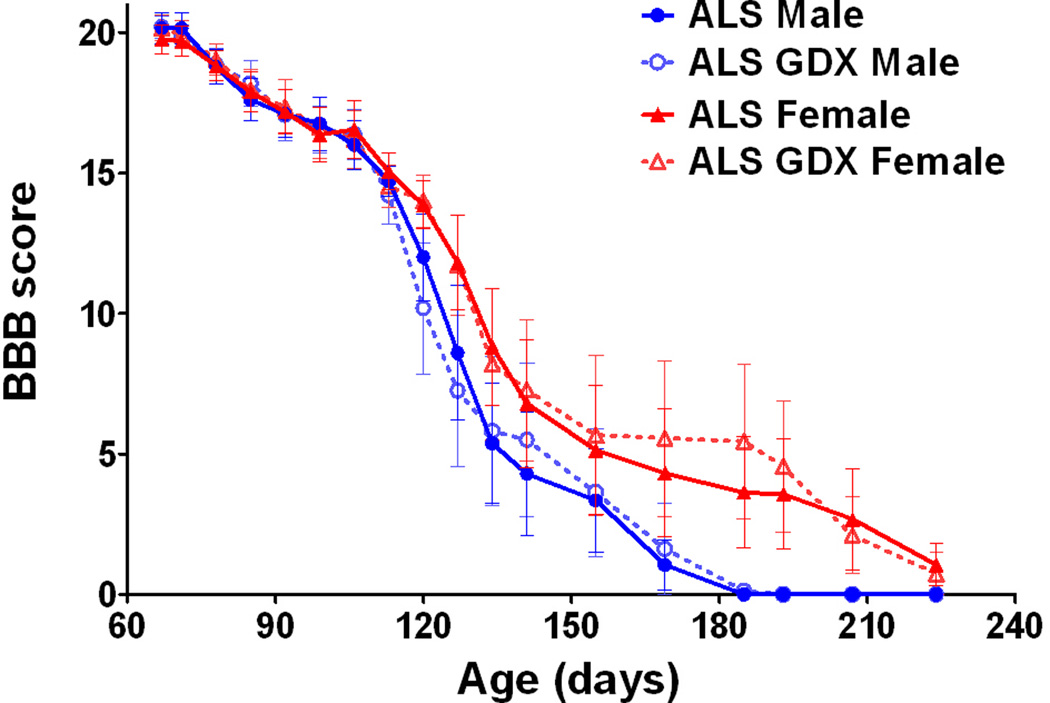
While clear sexual dimorphism was confirmed, gonadectomy did not modulate motor function in both sexes.
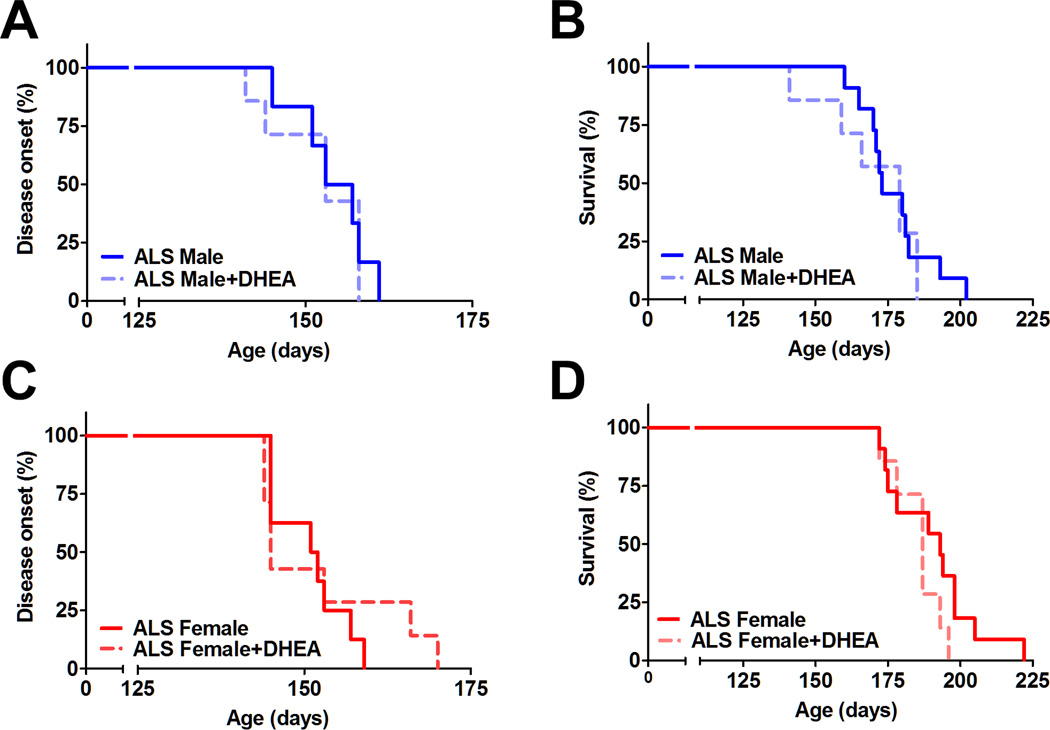
Serum DHEA concentrations were significantly higher in DHEA treated animals (6.6 ± 3.1 ng/ml in males, 10.6 ± 4.9 ng/ml in females), compared to the levels in control males (0.4 ± 0.3 ng/ml) and females (1.2 ± 0.5 ng/ml). However, disease onset progressed in a similar fashion between the two groups for each gender (Fig. 3A and 3B). The survival percentage of the rats demonstrates this trend as well (Fig. 3C and 3D). In this cohort, the means ± SEM to reach endpoint were 177 ± 16.7 days in control males (n=11), 190 ± 13.0 days in control females (n=11), 171 ± 16.3 days in DHEA-treated males (n=7), and 186 ± 8.3 days in DHEA-treated females (n=7). The BBB scores of the ALS males and females and those of both sexes receiving DHEA also support the idea that DHEA does not alter disease progression (data not shown).
Figure 3. Effects of DHEA treatment in SOD1G93A rats.
Chronic DHEA treatment does not alter disease onset and survival period in SOD1G93A males (A, B) and females (C, D).
Discussion
There is no significant effect of gonadectomy on disease onset or progression in SOD1G93A rats. In SOD1G93A mice, ovariectomy accelerated disease progression in females (3;5). The causes for dissimilarities observed here are uncertain at this point, but the difference of species or the timing of the gonadectomies may be a possible reason. In previous experiments using SOD1G93A female mice, ovariectomy was performed at postnatal day 50 (3;5). While our SOD1G93A rat colony indicates the median to reach disease onset and endpoint is 146 and 184 days (13), SOD1G93A mice in those papers showed 70–80 and 130–140 days as disease onset and endpoint, respectively (3;5). This idea of a specific time window that shows the effects of sex steroids could be further analyzed by doing differently timed gonadectomies in SOD1G93A rats. Furthermore, this is the first study to assess the potential role of castration and testicular androgen in a rodent model of ALS. Free testosterone, which can go through the blood-brain barrier as unbound form and potentially metabolize to estrogen in the nervous system, was significantly decreased in the ALS patients of both sexes (14). Further studies are necessary to elucidate how gonadal steroids are linked to the pathophysiology of ALS using different rodent models of ALS.
DHEA has no effect on ALS disease onset, motor function, or survival in both male and female SOD1G93A rats. In a similar fashion to the gonadectomies, examining the ideal dosage of DHEA along with studying the possibility of a time window to see the effects of this neurosteroid could be necessary. However, these current possibilities would suggest that DHEA, which is currently available as a health supplement in the United States, should be carefully considered as a treatment for ALS.
In conclusion, our results indicate that steroids are not one of the possible modulators for the occurrence or disease progression in a rat model of ALS. Further analysis will be necessary to understand how sexual dimorphism is involved in ALS disease progression.
Acknowledgments
This work was supported by grants from the ALS Association, NIH/NINDS [PO1NS057778 (to C.N.S.) and R21NS06104 (to M.S.)], the University of Wisconsin Foundation, and the Les Turner ALS foundation. The DHEA analysis was performed by Dr. Dan Wittwer and supported in part by the Wisconsin National Primate Research Center, RR000167, a component of the National Institutes of Health.
References
- 1.McCombe PA, Henderson RD. Effects of gender in amyotrophic lateral sclerosis. Gend Med. 2010;7:557–570. doi: 10.1016/j.genm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Veldink JH, Bar PR, Joosten EA, Otten M, Wokke JH, Van den Berg LH. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord. 2003;13:737–743. doi: 10.1016/s0960-8966(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 3.Choi CI, Lee YD, Gwag BJ, Cho SI, Kim SS, Suh-Kim H. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J Neurol Sci. 2008;268:40–47. doi: 10.1016/j.jns.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki M, Tork C, Shelley B, McHugh J, Wallace K, Klein SM, et al. Sexual dimorphism in disease onset and progression of a rat model of ALS. Amyotroph Lateral Scler. 2007;8:20–25. doi: 10.1080/17482960600982447. [DOI] [PubMed] [Google Scholar]
- 5.Groeneveld GJ, Van Muiswinkel FL, Sturkenboom JM, Wokke JH, Bar PR, Van den Berg LH. Ovariectomy and 17beta-estradiol modulate disease progression of a mouse model of ALS. Brain Res. 2004;1021:128–131. doi: 10.1016/j.brainres.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Bastianetto S, Ramassamy C, Poirier J, Quirion R. Dehydroepiandrosterone (DHEA) protects hippocampal cells from oxidative stress-induced damage. Molecular Brain Research. 1999;66:35–41. doi: 10.1016/s0169-328x(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki M, Wright LS, Marwah P, Lardy HA, Svendsen CN. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci U S A. 2004;101:3202–3207. doi: 10.1073/pnas.0307325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiore C, Inman DM, Hirose S, Noble LJ, Igarashi T, Compagnone NA. Treatment with the neurosteroid dehydroepiandrosterone promotes recovery of motor behavior after moderate contusive spinal cord injury in the mouse. J Neurosci Res. 2004;75:391–400. doi: 10.1002/jnr.10821. [DOI] [PubMed] [Google Scholar]
- 10.Celec P, Starka L. Dehydroepiandrosterone - is the fountain of youth drying out? Physiol Res. 2003;52:397–407. [PubMed] [Google Scholar]
- 11.Suzuki M, Bannai M, Matsumuro M, Furuhata Y, Ikemura R, Kuranaga E, et al. Suppression of copulatory behavior by intracerebroventricular infusion of antisense oligodeoxynucleotide of granulin in neonatal male rats. Physiol Behav. 2000;68:707–713. doi: 10.1016/s0031-9384(99)00241-3. [DOI] [PubMed] [Google Scholar]
- 12.Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki M, Klein S, Wetzel EA, Meyer M, McHugh J, Tork C, et al. Acute glial activation by stab injuries does not lead to overt damage or motor neuron degeneration in the G93A mutant SOD1 rat model of amyotrophic lateral sclerosis. Exp Neurol. 2010;221:346–352. doi: 10.1016/j.expneurol.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Militello A, Vitello G, Lunetta C, Toscano A, Maiorana G, Piccoli T, et al. The serum level of free testosterone is reduced in amyotrophic lateral sclerosis. J Neurol Sci. 2002;195:67–70. doi: 10.1016/s0022-510x(01)00688-8. [DOI] [PubMed] [Google Scholar]