Summary
Translational control provides numerous advantages in regulation of gene expression including rapid responsiveness, intracellular localization, non-destruction of template mRNA, and coordinated regulation of transcript ensembles. Transcript-selective, translational control is driven by the specific interaction of factor(s) with the 5′ or 3′ untranslated region (UTR), thereby influencing initiation, elongation, or termination of mRNA translation. The mean length of human 3′UTRs is greater than that of 5′UTR, indicating the expanded potential for motifs, structural elements, and binding sites for trans-acting factors that exert transcript-selective translation control. New and unexpected mechanisms of 3′UTR-mediated translational control and their contributions to disease have received increasing attention during the last decade. Here, we briefly review a few recent and representative discoveries of 3′UTR-mediated translational control, emphasizing the novel aspects of these regulatory mechanisms and their potential pathophysiological significance.
The GAIT system as archetype for 3′UTR-mediated translational control and its modulation
Recognition and binding of proteins or protein complexes to defined sequence or structural RNA elements in the mRNA 3′UTR is a common translational control mechanism (Figure 1). This mechanism is generally directed by stimulus-dependent post-translational modifications that regulate protein-protein interactions and complex assembly, as well as the RNA-binding properties of these assemblages. Recent attention has focused on an additional layer of complexity in which the processes involved in translational control can themselves be modulated by environmental inputs. In this section, we review new paradigms for physiological and pathological modulation of translational control. We focus attention on the GAIT (interferon [IFN]-γ-activated inhibitor of translation) system, a mechanism of translational control in human myeloid cells.
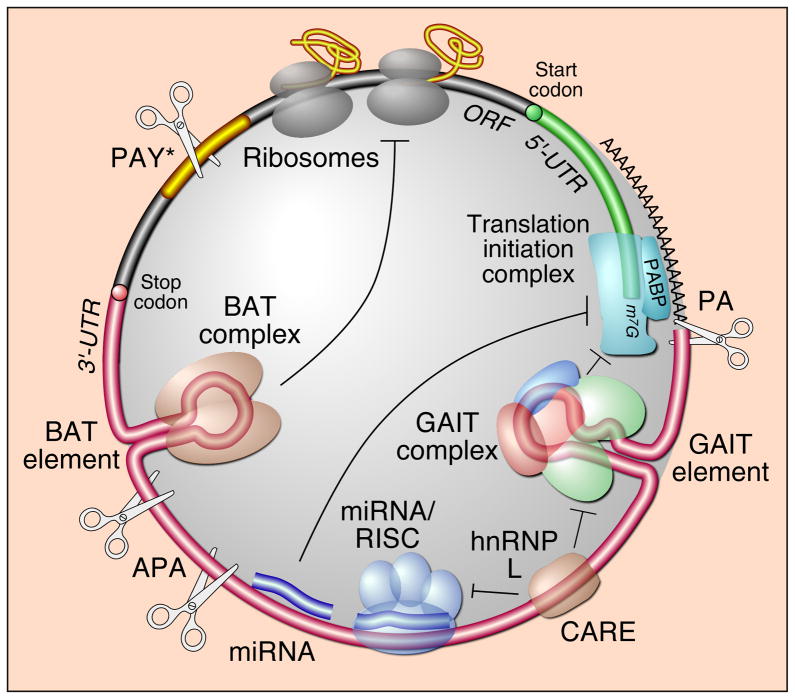
Figure 1.
Multi-modal control of mRNA translation by the 3′UTR. Shown is 3′UTR-mediated translational control by cis-acting structural RNA elements, i.e., GAIT and BAT elements and their cognate trans-acting factors, i.e., heterotetrameric GAIT complex and BAT complex; miRNA-mediated translational control; 3′UTR shortening by alternative polyadenylation (APA); generation of truncated, 3′UTR-less open reading frame (ORF) by polyadenylation-mediated Tyr-to-stop codon conversion (PAY*); and translational control by competitive binding of a trans-acting factor to a sequence-specific element, i.e., hnRNPL-binding to CARE, for secondary regulation of protein- and miRNA-mediated translational control. Some of these regulatory processes might be facilitated by end-to-end mRNA closure mediated by interaction of polyA-binding protein (PABP) with the polyA tail and with the translation initiation complex.
The human GAIT complex is heterotetrameric, consisting of glutamyl-prolyl tRNA synthetase (EPRS), ribosomal protein L13a (RPL13a), NS1-associated protein 1 (NSAP1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [1]. The GAIT complex binds a split stem-loop element in the 3′UTR of target mRNAs, and inhibits recruitment of the small ribosomal subunit-containing pre-initiation complex, thereby preventing translation initiation. Phosphorylation of EPRS and RPL13a is essential for GAIT complex assembly and for translation-inhibition of a post-transcriptional regulon of inflammation-related genes in IFN-γ-activated monocytic cells [2]. EPRS is phosphorylated at two sites in its linker domain connecting the catalytic subunits. EPRS is initially phosphorylated by cyclin-dependent kinase (Cdk)5 at Ser886, and later by an unidentified Cdk5-dependent AGC kinase at Ser999 [3,4]. Still later, RPL13a is phosphorylated at Ser77 by activation of a cascade of death-associated protein kinases, namely, DAPK and ZIPK [2]. Phosphorylation of EPRS and RPL13a coordinates multiple events including release of both proteins from parental complexes, assembly of the GAIT complex, binding of the complex to 3′UTR GAIT elements, and finally, translation-inhibition of target mRNAs including vascular endothelial growth factor (VEGF)-A and ceruloplasmin [4,5]. The GAIT system might act as a gate that restricts expression of inflammation-related proteins that can be injurious in excess.
Recently, a C-terminus truncated form of EPRS, EPRSN1, has been observed which contains the GAIT element binding domain but lacks the two phosphorylation sites required for GAIT complex formation [6••]. Interestingly, EPRSN1, is generated by an unprecedented mechanism in which a “UA”-bearing Tyr codon in the coding sequence of EPRS mRNA is targeted by alternative polyadenylation to generate a “UAA” stop codon (PAY*). The PAY* mechanism generates a very stable transcript entirely lacking any 3′UTR, and is thus not susceptible to post-transcriptional control mechanisms. EPRSN1 acts as a dominant-negative inhibitor, constitutively binding with high affinity to GAIT-element bearing mRNAs, thereby shielding them from the inhibitory GAIT complex. In this context, the GAIT complex act as a gate that blocks excessive expression of potentially injurious proteins, whereas, EPRSN1 can be considered as a “door-stop” that prevents complete gate closure, permitting a “translational trickle” of target expression. Application of the PAY* mechanism to the GAIT target VEGF-A may be particularly significant in the context of disease. VEGF-A is a potent macrophage-derived angiogenic factor that facilitates tumor growth and metastasis [7]. Anti-VEGF therapy limits growth of certain carcinomas [8]; however, adverse effects suggest VEGF-A is essential for maintenance, as well as for development, of healthy vessels. Moreover, deletion of macrophage-derived VEGF-A accelerates tumor growth [9]. Thus, a major function of EPRSN1 might be to maintain VEGF-A, and other GAIT targets, at basal levels required for tissue and organism well-being.
The GAIT regulatory system is itself subject to regulation by environmental signals. When myeloid cells are simultaneously subjected to IFN-γ and hypoxia, the stimulatory activity of hypoxia prevails over the inhibitory activity of the GAIT complex, and high-level VEGF-A expression continues unabated [10•]. Immediately upstream of the GAIT element, the VEGF-A 3′UTR contains a CA-rich element (CARE) that is a binding site for heterogenous nuclear ribonucleoprotein (hnRNP) L. In response to hypoxia, hnRNP L binding to the CARE induces a conformational change in the 3′UTR that prevents GAIT complex binding and translational silencing. Analogous to metabolite-dependent bacterial riboswitches, the protein-dependent VEGF-A RNA switch exemplifies a new class of regulatory systems in which combinatorial utilization of nearby RNA elements can optimally integrate disparate input signals to produce the appropriate output.
The GAIT system is also subject to pathological dysregulation. Treatment of myeloid cells with low density lipoprotein (LDL) oxidatively modified (LDLox) by exposure to a physiological oxidant system, completely suppresses GAIT pathway activity, and enhances expression of VEGF-A and other GAIT targets [11]. LDLox selectively induces S-nitrosylation at Cys247 of GAPDH, a GAIT complex constituent, and blocks its interaction with RPL13a. In the absence of GAPDH binding, phosphorylated RPL13a released from the large ribosomal subunit is rapidly degraded by the polyubiquitin and proteasome pathway. Thus, LDLox in hyperlipidemic disorders such as atherosclerosis might contribute to chronic inflammation by impairing GAIT-mediated translational control. Moreover, the results suggest a more general mechanism in which extraribosomal activities of the ribosomal protein might require protective chaperones. Pathological disruption of either protein, or their interaction, presents an alternative mechanism of diseases due to ribosomal protein defects, and new targets for therapeutic intervention.
Translational control by 3′UTR-targeting miRNA
microRNAs (miRNAs) are 20- to 23-nt, non-coding RNAs that can target mRNA [12], DNA [13], and even protein [14], to regulate gene expression at both transcriptional and post-transcriptional levels. miRNAs bind complementary sequences, most often in the 3′UTR of target mRNAs, to alter expression by regulating either mRNA decay or translation. A ribosome profiling study pointed to mRNA decay, rather than translational silencing, as the dominant mechanism for miRNA-mediated inhibition of gene expression in human and mouse cells [15]. However, kinetic analyses in flies and zebrafish have suggested a unifying mechanism in which translational repression at the initiation step is coupled with mRNA deadenylation and decay [12,16]. These conclusions were derived from short time-course studies, and they suggest that similar high-resolution kinetic analyses might be required to determine whether translational silencing is a priming event for mRNA decay in mammalian systems, and to elucidate mechanisms of dysfunctional miRNAs underlying human pathology.
The stoichiometry of miRNAs and their target mRNAs is a critical parameter affecting regulatory efficiency. miRNAs are generally present at low levels and contribute to fine-tuning of translation. Recent studies implicate circumstances in which the stoichiometry is dysregulated and accompanied by potent modulation of target mRNA translation with pathologic consequences. Consistent with this concept, two classes of natural human miRNA “sponges” inside and outside of cells have been reported. In one case, the PTEN pseudogene, PTENP1, exhibits tumor suppressive activity by sponging PTEN 3′UTR-binding oncogenic miRNAs, and stimulating PTEN translation [17•]. Moreover, genomic analysis of sporadic colon cancer revealed PTENP1 genetic loss and consequent low-level PTEN expression in cancer patients. In addition, RNA deep-sequencing revealed that multiple competitive endogenous RNAs (ceRNAs) overexpressed in cancer tissues, e.g., ATP8A2-ψ (pseudogene of ATP8A2), could inactivate tumor suppressor miRNAs [18]. In another example, HSUR1 and HSUR2 are Herpesvirus saimiri-derived, U-rich, non-coding RNAs that reduce miR-27 expression in host T-cells, thereby increasing the amount of miR-27-targeted FoxO1 protein [19]. In summary, an excess of miRNA-binding RNA can act as a potent miRNA decoy.
The availability of miRNAs and the accessibility of target mRNAs can be stringently regulated both in cis and trans, and contribute importantly to human pathology. As one example, alternative polyadenylation can generate a truncated 3′UTR lacking certain cis-acting, miRNA targeting seed sequences [6,20]. Removal of these elements generally results in enhanced transcript stability and translatability. Alternative poly-adenylation decreases the mean 3′UTR length in cancer cells and tissues, and is correlated with elevated oncogene expression [21••]. On the other hand, mRNAs containing long, intact 3′UTRs have greater potential for combinatorial regulation by multiple trans-acting factors, both positive and negative, binding to spatially distinct cis-acting elements. Secondary regulation of miRNA activity or accessibility by RNA-binding proteins has gained recent attention. These proteins can directly bind and sequester miRNAs, or alternatively, bind the target mRNA at or near the miRNA binding site and block its interaction with miRNA:RISC (RNA-induced silencing complex). In an example of the former mechanism, plant AGO10 functions as a decoy for miR166/165 to maintain the shoot apical meristem in Arabidopsis, preventing their incorporation into AGO1 complexes, and consequent repression of target gene expression [22]; this type of mechanism has not yet been reported in any animal system. In an example of the latter case, the CARE-binding protein heterogeneous ribonucleoprotein (hnRNP) L prevents miR-297 recognition of the CARE in the human VEGF-A mRNA 3′UTR, resulting in increased VEGF-A translation and possibly enhanced angiogenesis [23].
The pathophysiological significance of miRNA has been amply demonstrated in vivo in mice, monkeys, and humans. miRNAs have emerged as important post-transcriptional regulators of lipid metabolism and tumorigenesis, and represent a potentially important class of agents and targets for therapeutic intervention. In one representative example, miR-33a and miR-33b (miR-33a/b) repress expression of the cholesterol transporter ABCA1, thereby attenuating cholesterol efflux to apolipoprotein A1, and suppressing high density lipoprotein (HDL) particle maturation [24]. Combined pharmacological inhibition of miR-33a/b in African green monkeys by systemic delivery of anti-miRNA oligonucleotides increased hepatic expression of ABCA1, induced a sustained increase in plasma HDL, and markedly suppressed the plasma level of very low density lipoprotein (VLDL)-associated triglycerides. These results establish the foundation for a promising therapeutic strategy to treat dyslipidemias, and to decrease the risk for cardiovascular disease.
Pro-oncogenic and anti-oncogenic miRNAs can affect cell proliferation, apoptosis, transformation, carcinogenesis, and metastasis, and they also exhibit differential expression in cancer [25]. In some circumstances, despite the extraordinary complexity of the tumor and its environment, tumorigenesis can be limited by repression of a single oncogene, a process known as “oncogene addiction” [26]. miR-21, which is overexpressed in most human tumor types, is the first validated example of “oncomiR addiction” [27•]. Conditional overexpression of miR-21 in mice generates a pre-B lymphoid-like, malignant phenotype, and inactivation of miR-21 overexpression causes complete tumor regression within a few days. These results establish a promising treatment of human cancers through pharmacological inactivation of miRNAs. In contrast, endogenous miR-126 acts as a tumor suppressor and can inhibit metastatic recruitment of endothelial cells, angiogenesis, and colonization through coordinated targeting of multiple pro-angiogenic mRNAs [28]. However, miR-126 is silenced in a variety of common human cancers and thus might contribute to metastases. It is likely that other 3′UTR-targeting miRNAs that alter translation of disease-related genes will be discovered to be effective therapeutic targets or pharmacological agents for treatment of cancer, cardiovascular disease, and other pathological states.
3′UTR-mediated Translational Control in Disease and Therapeutics
Experimental and clinical studies have suggested that 3′UTR-mediated translational control has a critical role in regulating gene expression in cancer and other pathological states (Table 1). Phosphorylation of a 3′UTR-binding protein was recently shown to significantly impact transforming growth factor (TGF)-β-induced epithelial-mesenchymal transition (EMT), a critical early step in tumorigenesis. The newly recognized BAT (TGF-β-activated translation) complex, consisting of hnRNP E1 and eukaryotic elongation factor-1 A1 (eEF1A1), constitutively binds a 33-nt BAT element in the 3′UTRs of disabled-2 and interleukin-like EMT inducer (ILEI) mRNAs, and inhibits their translation [29,30]. hnRNP E1 inhibits the release of eEF1A1 from the ribosomal A-site thereby stalling translation elongation. Stimulation with TGF-β induces phosphorylation of hnRNP E1 at Ser43 by Akt2, resulting in the release of hnRNP E1 from BAT element-bearing mRNAs, thereby relieving the translational repression of target EMT-inducing transcripts. Attenuation of hnRNP E1 expression in noninvasive breast epithelial cell lines induced EMT and formed metastatic lesions, demonstrating the importance of the BAT system in tumorigenesis in vivo. Thus, phosphorylation-mediated translational control represents a critical checkpoint coordinating the expression of EMT transcripts in tumorigenesis and metastatic progression.
Table 1.
3′ UTR-regulated translational control of cancer-related genes.
| Target oncogene or tumor suppressor gene | 3′UTR binding factor | Oncogenic role of target gene | References |
|---|---|---|---|
| VEGF-A | GAIT complex, miR-297 | pro-oncogenic | [1–5, 6••,10•, 11, 23, 37] |
| Dab2, ILEI | BAT complex | pro-oncogenic | [29, 30] |
| PTEN, PTENP1 | miR-20a, miR-19b, miR-21, miR-26a, miR-214 | anti-oncogenic | [17•] |
| OCT4, OCT4-pg1, OCT4-pg5 | miR-145, miR-470 (mouse specific) | pro-oncogenic | [17•] |
| NANOG, NANOGP8 | miR-134, miR-296 | pro-oncogenic | [17•] |
| FoxO1 | miR-27a, miR-96, miR-182 | anti-oncogenic | [19, 38] |
| IGFBP2, PITPNC1, MERTK | miR-126 | pro-oncogenic | [28] |
| p53 | CPEB, Gld4, RPL26 | anti-oncogenic | [31–33] |
| ATR | hnRNP A18 | pro-oncogenic | [34] |
Recent reports indicate that expression p53, a critically important tumor suppressor gene, is stringently regulated by translational control mechanisms, primarily acting on the 3′UTR. In one recent study, p53 translation was shown to be regulated by a pair of non-canonical poly(A) polymerases, i.e., germline development (Gld)-2 and -4 [31]. Surprisingly, Gld-2 mono-adenylates miR122, thereby stabilizing its interaction with RISC, and then by targeting its 3′UTR, represses translation of cytoplasmic polyadenyl-ation element-binding protein (CPEB). Because CPEB binding to the 3′UTR of p53 enhances its translation, Gld2 acts as a repressor of p53. In contrast, CPEB recruits Gld4 to the p53 3′UTR, thereby inducing polyadenylation and increased p53 expression. Thus, the Glds function in tandem by acting on the 3′UTRs of two different transcripts, utilizing two different mechanisms, and driving p53 expression in opposing directions.
Radiation stress and DNA damage also regulate translation of p53 and other mRNAs. Ultraviolet irradiation induces binding of ribosomal protein RPL26 binding to a double-stranded RNA motif in p53 mRNA and promotes its translation [32]. The RPL26-binding motif is formed from complementary sequences contained within both the 5′ and 3′UTRs of p53 mRNA suggesting end-to-end transcript closure is important. Interestingly, the same base-pairing motifs in the p53 mRNA termini also bind nucleolin, which competitively blocks RPL26 binding and attenuates p53 translation [33]. In a second example, radiation stress translationally regulates expression of ATR (ataxia telangiectasia mutated and Rad3-related), a kinase that initiates signaling cascades in response to DNA damage [34]. Ultraviolet radiation induces translocation of hnRNP A18 from the nucleus to cytoplasm where it binds the ATR mRNA 3′UTR, enhances its translation, and increases protection against DNA damage.
The translational control apparatus contributes to appropriate stimulus- and condition-dependent responses during development and in maintenance of cell and tissue home-ostasis during health. However, impairment of transcript-selective translational control systems by genetic defects or by environmental stress can greatly influence gene expression patterns, potentially resulting in accumulation of injurious proteins or metabolites, and contribute to pathological outcomes. Given that the mean length of human 3′UTRs is about 600 nt [35] and target sites for miRNA and trans-acting proteins range between approximately 20 and 50 nt, there is a potential for about 10 to 30 non-overlapping RNA elements, and an order of magnitude more elements if overlap is considered. In view of the possible combinatorial effects of pairs (or more) of binding factors, the potential for control is astronomical in scope. Global analysis of disease-oriented translational control networks, including target mRNAs and their binding factors, has derived recent benefit from the rapid development and deployment of next-generation RNA sequencing. As one example of a disease-related, global map of 3′UTR-mediated translation, an extraordinarily complex miRNA regulatory network consisting of 248,000 miRNA-RNA interactions has been described in glioblastoma [36•]. We anticipate that continued explosive growth in this and other new technologies will reveal not just new 3′UTR-mediated translational control elements and factors, but whole new paradigms of physiological and pathological control. It is our hope that some of these next-generation discoveries will also generate candidates for development of next-generation therapeutics.
Acknowledgments
P.L.F was supported by NIH grants P01 HL029582, P01 HL076491, and R01 GM086430, A.A. by a National Center Scientist Development Grant 10SDG3930003 from the American Heart Association, and P.Y. by a Fellowship from the American Heart Association, Great Rivers Affiliate. Due to space limitations, combined with remarkable growth in this burgeoning research area, we were not able to include all, or even most, recent discoveries about this topic; we apologize to the authors whose important work was not discussed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sampath P, Mazumder B, Seshadri V, Gerber CA, Chavatte L, Kinter M, Ting SM, Dignam JD, Kim S, Driscoll DM, et al. Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell. 2004;119:195–208. doi: 10.1016/j.cell.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 2.Mukhopadhyay R, Ray PS, Arif A, Brady AK, Kinter M, Fox PL. DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol Cell. 2008;32:371–382. doi: 10.1016/j.molcel.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arif A, Jia J, Moodt RA, DiCorleto PE, Fox PL. Phosphorylation of glutamyl-prolyl tRNA synthetase by cyclin-dependent kinase 5 dictates transcript-selective translational control. Proc Natl Acad Sci U S A. 2011;108:1415–1420. doi: 10.1073/pnas.1011275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arif A, Jia J, Mukhopadhyay R, Willard B, Kinter M, Fox PL. Two-site phosphorylation of EPRS coordinates multimodal regulation of noncanonical translational control activity. Mol Cell. 2009;35:164–180. doi: 10.1016/j.molcel.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukhopadhyay R, Jia J, Arif A, Ray PS, Fox PL. The GAIT system: a gatekeeper of inflammatory gene expression. Trends Biochem Sci. 2009;34:324–331. doi: 10.1016/j.tibs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6••.Yao P, Potdar AA, Arif A, Ray PS, Mukhopadhyay R, Willard B, Xu Y, Yan J, Saidel GM, Fox PL. Coding region polyadenylation generates a truncated tRNA synthetase that counters translation repression. Cell. 2012;149:88–100. doi: 10.1016/j.cell.2012.02.018. This study reveals a “translational trickle” regulatory mechanism that maintains a basal level of gene expression as well as a novel coding region polyadenylation by which a truncated protein is generated via Tyr-to-stop codon conversion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Ray PS, Jia J, Yao P, Majumder M, Hatzoglou M, Fox PL. A stress-responsive RNA switch regulates VEGFA expression. Nature. 2009;457:915–919. doi: 10.1038/nature07598. This study reveals the first protein-dependent RNA switch in human mRNAs. Hypoxia induces a binary conformational change of the VEGF mRNA 3′UTR by altering binding of hnRNP L and GAIT complex to their target RNA elements. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia J, Arif A, Willard B, Smith JD, Stuehr DJ, Hazen SL, Fox PL. Protection of Extraribosomal RPL13a by GAPDH and Dysregulation by S-Nitrosylation. Mol Cell. 2012 doi: 10.1016/j.molcel.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djuranovic S, Nahvi A, Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336:237–240. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khraiwesh B, Arif MA, Seumel GI, Ossowski S, Weigel D, Reski R, Frank W. Transcriptional control of gene expression by microRNAs. Cell. 2010;140:111–122. doi: 10.1016/j.cell.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Eiring AM, Harb JG, Neviani P, Garton C, Oaks JJ, Spizzo R, Liu S, Schwind S, Santhanam R, Hickey CJ, et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. 2010;140:652–665. doi: 10.1016/j.cell.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazzini AA, Lee MT, Giraldez AJ. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science. 2012;336:233–237. doi: 10.1126/science.1215704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. Reports the first example of a pseudogene acting as an miRNA sponge regulating oncomiRNA activity in tumorigenesis. PTEN1, a PETN pseudogene, binds PTEN-specific oncomiRNAs, and sustains PTEN tumor-suppressor activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalyana-Sundaram S, Kumar-Sinha C, Shankar S, Robinson DR, Wu YM, Cao X, Asangani IA, Kothari V, Prensner JR, Lonigro RJ, et al. Expressed pseudogenes in the transcriptional landscape of human cancers. Cell. 2012;149:1622–1634. doi: 10.1016/j.cell.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cazalla D, Yario T, Steitz JA. Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science. 2010;328:1563–1566. doi: 10.1126/science.1187197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg MG, Singh LN, Younis I, Liu Q, Pinto AM, Kaida D, Zhang Z, Cho S, Sherrill-Mix S, Wan L, et al. U1 snRNP Determines mRNA Length and Regulates Isoform Expression. Cell. 2012;150:53–64. doi: 10.1016/j.cell.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Mayr C, Bartel DP. Widespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell. 2009;138:673–684. doi: 10.1016/j.cell.2009.06.016. Alternative cleavage and polyadenylation of oncogene pre-mRNAs during tumorigenesis can generate stable mRNAs with short 3′UTRs by removal of miRNA target sites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu H, Hu F, Wang R, Zhou X, Sze SH, Liou LW, Barefoot A, Dickman M, Zhang X. Arabidopsis Argonaute10 specifically sequesters miR166/165 to regulate shoot apical meristem development. Cell. 2011;145:242–256. doi: 10.1016/j.cell.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jafarifar F, Yao P, Eswarappa SM, Fox PL. Repression of VEGFA by CA-rich element-binding microRNAs is modulated by hnRNP L. EMBO J. 2011;30:1324–1334. doi: 10.1038/emboj.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ventura A, Jacks T. MicroRNAs and cancer: short RNAs go a long way. Cell. 2009;136:586–591. doi: 10.1016/j.cell.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. discussion 3080. [DOI] [PubMed] [Google Scholar]
- 27•.Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. miR21 is the first validated example of “OncomiR addiction” in which a single oncogenic miRNA significantly contributes to tumorigenesis in vivo. [DOI] [PubMed] [Google Scholar]
- 28.Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2012;481:190–194. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol. 2010;12:286–293. doi: 10.1038/ncb2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussey GS, Chaudhury A, Dawson AE, Lindner DJ, Knudsen CR, Wilce MC, Merrick WC, Howe PH. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol Cell. 2011;41:419–431. doi: 10.1016/j.molcel.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burns DM, D’Ambrogio A, Nottrott S, Richter JD. CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature. 2011;473:105–108. doi: 10.1038/nature09908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Kastan MB. 5′-3′-UTR interactions regulate p53 mRNA translation and provide a target for modulating p53 induction after DNA damage. Genes Dev. 2010;24:2146–2156. doi: 10.1101/gad.1968910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Guo K, Kastan MB. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J Biol Chem. 2012;287:16467–16476. doi: 10.1074/jbc.M112.349274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang R, Zhan M, Nalabothula NR, Yang Q, Indig FE, Carrier F. Functional significance for a heterogenous ribonucleoprotein A18 signature RNA motif in the 3′-untranslated region of ataxia telangiectasia mutated and Rad3-related (ATR) transcript. J Biol Chem. 2010;285:8887–8893. doi: 10.1074/jbc.M109.013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazumder B, Seshadri V, Fox PL. Translational control by the 3′-UTR: the ends specify the means. Trends Biochem Sci. 2003;28:91–98. doi: 10.1016/S0968-0004(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 36•.Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J, et al. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell. 2011;147:370–381. doi: 10.1016/j.cell.2011.09.041. This study describes a comprehensive regulatory network in which a family of miRNAs coordinates expression of target genes in related signaling pathways in response to metabolic and environmental stress in tumorigenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray PS, Fox PL. A post-transcriptional pathway represses monocyte VEGF-A expression and angiogenic activity. EMBO J. 2007;26:3360–3372. doi: 10.1038/sj.emboj.7601774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guttilla IK, White BA. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J Biol Chem. 2009;284:23204–23216. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]