Abstract
A recurrent hepatitis C virus (HCV) infection after liver transplantation (LT) can lead to accelerated allograft injury and fibrosis. The aim of this article is to report the first ever use of daclatasvir (DCV; also known as BMS-790052), a potent orally administered nonstructural 5A replication complex inhibitor, in combination with peginterferon α (PEG-IFNα) and ribavirin in an LT recipient. A 49-year-old female developed a severe recurrent HCV genotype 1b infection 4 months after transplantation with severe cholestasis on biopsy, an HCV RNA level of 10,000,000 IU/mL, an alkaline phosphatase level of 1525 IU/mL, and a total bilirubin level of 8.4 mg/dL. Despite partial virological suppression with PEG-IFNα and ribavirin, progressive allograft failure ensued and culminated in retransplantation at 9 months. Three months after the second transplant, DCV (20 mg/day), PEG-IFNα2a (180 μg/week), and ribavirin (800 mg/day) were prescribed for early recurrent cholestatic HCV. Serum HCV RNA became undetectable at week 3 of treatment and remained undetectable during 24 weeks of triple therapy and during the posttreatment follow-up. DCV was well tolerated, and the trough drug levels were within the targeted range throughout the treatment. The cyclosporine trough levels were also stable during and after therapy. In conclusion, the lack of anticipated drug-drug interactions between DCV and calcineurin inhibitors and the potent antiviral efficacy of DCV make this agent (in combination with PEG-IFN and ribavirin) an attractive antiviral regimen worthy of further study in LT recipients with recurrent HCV.
Liver failure and hepatocellular carcinoma due to chronic hepatitis C virus (HCV) infections are the leading indications for liver transplantation (LT) in the United States.1 However, the recurrence of HCV infections after LT is nearly universal and is characterized by high serum HCV RNA levels and allograft hepatitis of variable severity.2,3 The rate of fibrosis progression is greatly accelerated in these patients versus nontransplant HCV patients, with 10% to 30% developing cirrhosis within 5 years of transplantation.4,5 The accelerated course of recurrent HCV infections is attributable in part to the use of potent immunosuppressants, the treatment of acute rejection, and other donor and recipient factors. Not surprisingly, LT recipients with chronic HCV have a significantly lower 5-year survival rate in comparison with other recipients because of the higher rate of graft failure due to recurrent disease.6,7
Peginterferon (PEG-IFN)/ribavirin combination therapy is frequently used for select LT recipients with recurrent HCV infections.8–10 In addition, several studies have shown that LT recipients who achieve a sustained virological response (SVR) have significantly improved survival in comparison with partial responders as well as untreated patients.11–13 However, many LT recipients are incapable of starting interferon therapy because of pancytopenia, renal insufficiency, and/or other medical and psychiatric comorbidities. In addition, antiviral response rates are lower in LT recipients versus nontransplant patients (SVR rates of 30% and 45% for patients with genotype 1), in part because of the use of immunosuppressive agents and the need for more frequent antiviral medication dose reductions and/or early discontinuation.8–10 Therefore, better tolerated and more effective treatments for LT recipients with recurrent HCV are urgently needed. The direct-acting antiviral agents telaprevir and boceprevir, in combination with PEG-IFN and ribavirin, were recently shown to significantly improve SVR rates in both treatment-naive patients and previously treated patients with genotype 1.14–17 However, both of these agents are contraindicated in LT recipients because of potentially severe and life-threatening drug-drug interactions with cyclosporine and tacrolimus as well as the lack of efficacy and safety data in this patient population.18,19 Daclatasvir (DCV), which is also known as BMS-790052, is an investigational oral nonstructural 5A replication complex inhibitor in development with demonstrated antiviral efficacy when it is combined with PEG-IFN and ribavirin in treatment-naive patients and previously treated patients with HCV genotypes 1 and 4.20–25 In phase 2 clinical trials, DCV has generally been well tolerated in combination therapy, with no unique adverse events identified to date. Furthermore, in vitro and in vivo testing suggests that DCV should not lead to any clinically significant drug-drug interactions when it is coadministered with other drugs that are metabolized by cytochrome P450 3A4 (eg, cyclosporine, tacrolimus, and sirolimus).24 The aim of this article is to report the first successful use of a direct-acting antiviral agent (DCV) in combination with PEG-IFN and ribavirin in an LT recipient with a severe, interferon-refractory cholestatic HCV infection.
PATIENTS AND METHODS
Antiviral Treatment Regimen
An investigator-initiated, emergency investigational new drug application (#109,999) was approved by the US Food and Drug Administration to provide antiviral treatment for this patient with a severe recurrent cholestatic HCV infection after LT. The treatment protocol included DCV (Bristol-Myers Squibb, Princeton, NJ; 20 mg/day), PEG-IFNα2a (Genentech, Nutley, NJ; 180 μg/week), and ribavirin (400 mg twice daily) for 24 weeks. A reduced dose of DCV (20 mg) was selected because of the potential for a theoretically mild drug-drug interaction with cyclosporine. DCV and cyclosporine are substrates and inhibitors of cytochrome P450 3A4 and P-glycoprotein, respectively; therefore, it was anticipated that the exposure to DCV might be increased when it was concomitantly administered with cyclosporine. The patient was seen at weeks 0, 1, 2, 3, 4, 8, 12, 16, 20, and 24 and at follow-up weeks 12 and 24 at the University of Michigan Institute for Clinical and Health Research (Ann Arbor, MI). HCV RNA levels were tested with the Roche Cobas TaqMan test (lower limit of detection = 43 IU/mL) at weeks 0, 1, 2, 3, 4, 8, 12, and 24 and at follow-up weeks 12 and 24. Written informed consent was obtained from the patient after a review and approval by the local institutional review board.
DCV Pharmacokinetics
A morning blood sample was obtained at weeks 2, 4, 6, 8, and 12 so that the DCV trough levels could be assessed with a validated liquid chromatography/tandem mass spectrometry method.21 In addition, semi-intensive 6-hour pharmacokinetic sampling was performed at treatment week 2 to estimate the overall exposure and to determine the maximum concentration and the time to the maximum concentration in the steady state.
RESULTS
Initial Pretransplant and Posttransplant Course
A 49-year-old female health care provider acquired an HCV genotype 1b infection after an inadvertent needle stick in 1990. She was initially treated with interferon monotherapy without clearance and was retreated with full-dose PEG-IFN and ribavirin for 48 weeks in 2002; HCV RNA was suppressed to undetectable levels, but this was followed by a posttreatment relapse. The patient also had a history of diabetes mellitus, hypertension, and a body mass index of 35 kg/m2. Her interleukin-28B genotype at rs12979860 was CT. The patient progressed to decompensated cirrhosis with ascites, gastrointestinal bleeding, and encephalopathy and was listed for transplantation with a Model for End-Stage Liver Disease (MELD) score of 11. After the diagnosis of a 2-cm hepatocellular carcinoma, she received a MELD upgrade to 22 points. While she was hospitalized with a MELD score of 32 and was on hemodialysis, she received a cadaveric liver graft from a donor who was less than 40 years old with a cold ischemia time of 9 hours. The induction immunosuppression consisted of basiliximab (Simulect, Novartis, East Hanover, NJ) on postoperative days 1 and 4 as well as intraoperative steroids. She was discharged home on postoperative day 22 with stable liver biochemistries and was receiving cyclosporine, mycophenolate mofetil, and prednisone.
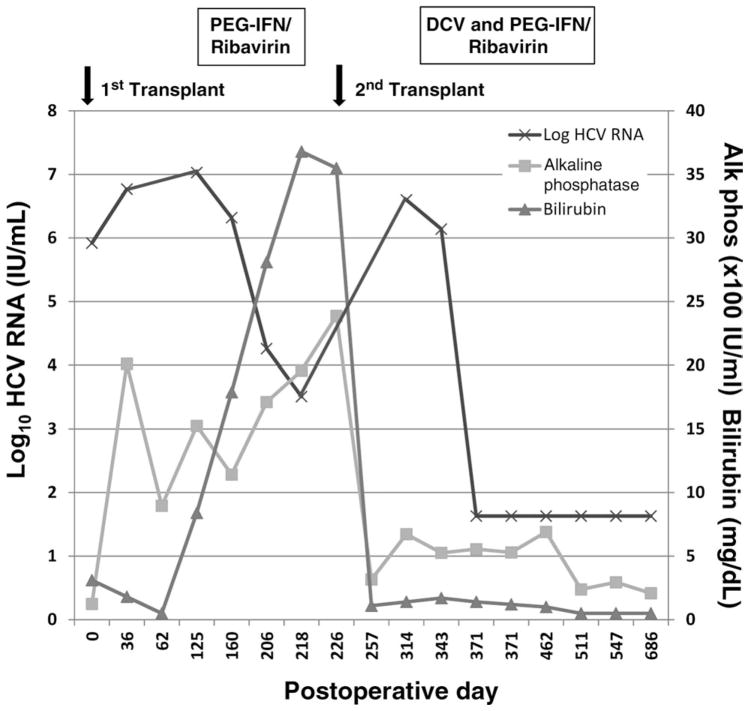
Four months after the first transplant, her serum alkaline phosphatase level was 1525 IU/mL, her bilirubin level was 8.4 mg/dL, and her HCV RNA level was 10.8 × 106 IU/mL. The evaluation for biliary strictures was negative, and a liver biopsy sample showed an intense pericentral cholestatic reaction with lymphocytic inflammation indicative of recurrent HCV (Fig. 1A). As a result, PEG-IFNα2a (180 μg/week) and ribavirin (1200 mg/day) were initiated. At week 4 of the antiviral therapy, her bilirubin level increased to 17.9 mg/dL, and repeat liver biopsy demonstrated severe cholestatic HCV with perisinusoidal fibrosis. Although her HCV RNA level had declined to 3.2 × 103 IU/mL at treatment week 12, her cholestasis worsened with an alkaline phosphatase level of 1959 IU/mL and a bilirubin level of 36.8 mg/dL; the antiviral therapy was discontinued because of her worsening clinical status (Fig. 2).
Figure 1.
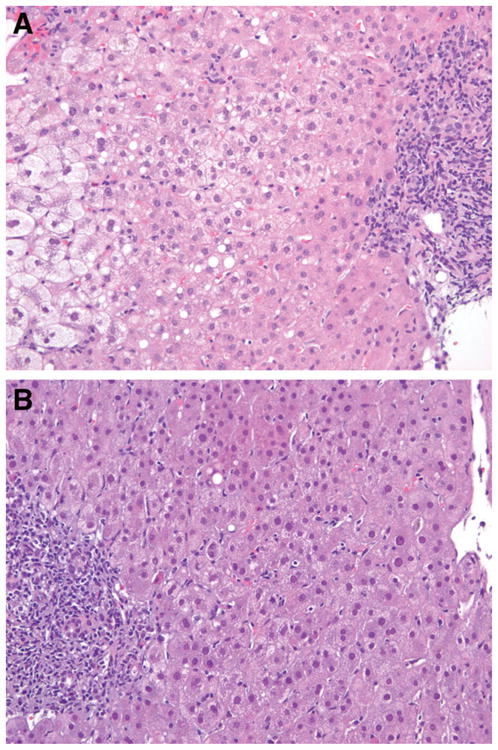
(A) Severe cholestatic HCV infection 4 months after the first transplant. The left side of the slide shows intense pericentral hepatocyte balloon degeneration. On the right, a portal tract can be seen with focal periportal cholestatic ductular proliferation and lymphocytic inflammation indicative of recurrent cholestatic DCV (hematoxylin and eosin stain, magnification ×200). (B) Recurrent HCV infection 3 months after the second transplant. On the right side, a central vein can be seen with normal pericentral hepatocytes and without ballooning. On the left, a portal tract can be seen with moderate lymphocytic inflammation that is characteristic of an early recurrent HCV infection. In the lobular parenchyma between the portal tract and central vein, there is mild inflammation with scattered sinusoidal lymphocytes and a single necrotic hepatocyte near the portal tract; these are typical features of recurrent HCV infections.
Figure 2.
Liver biochemistries and HCV RNA levels during antiviral therapy. Five months after the first transplant, the patient was started on PEG-IFNα2a (180 μg/week) and ribavirin (1200 mg/day). Despite the partial suppression of HCV RNA, the serum alkaline phosphatase and bilirubin levels continued to rise, and antiviral therapy was discontinued 12 weeks later. After retransplantation, the serum alkaline phosphatase and total bilirubin levels nearly normalized, but they began to rise again with the reemergence of HCV RNA. Four months after the second transplant, the patient was started on DCV (20 mg/day), PEG-IFNα2a (180 μg/week), and ribavirin (800 mg/day). Within 3 weeks of the initiation of therapy, HCV RNA became undetectable, and there were concomitant improvements in the serum alkaline phosphatase and bilirubin levels. After 24 weeks of triple therapy, the patient received another 4 weeks of PEG-IFN/ribavirin consolidation therapy. The patient remained well with HCV RNA undetectable at her last follow-up (32 weeks after the completion of treatment).
The patient was subsequently hospitalized with severe encephalopathy requiring intubation and coagulopathy with an international normalized ratio of 1.9. She underwent retransplantation with a MELD score of 36 while she was in the intensive care unit. In this instance, a graft from a 19-year-old cadaveric donor was used with a cold ischemia time of only 7 hours. The initial immunosuppression consisted of cyclosporine, mycophenolate mofetil, and steroids. She was discharged home 4 weeks later on cyclosporine and prednisone and fully recovered. Twelve weeks after the second transplant, her alkaline phosphatase level had risen to 673 IU/mL with an alanine amino-transferase (ALT) level of 88 IU/mL and a total bilirubin level of 1.4 mg/dL. A cholangiogram revealed no evidence of obstruction, and her serum HCV RNA level was 4.0 × 106 IU/mL. Because of the diagnostic uncertainty, a liver biopsy sample was obtained, and it showed mild periportal and lobular hepatitis with a lymphocytic infiltrate (Fig. 1B).
DCV in Combination With PEG-IFN and Ribavirin Treatment
The patient was started on DCV (20 mg/day), PEG-IFNα2a (180 μg/week), and ribavirin (800 mg/day) 12 weeks after the second transplant because of the suspicion of an early recurrent HCV infection with cholestatic features. Concomitant medications included cyclosporine (75 mg twice daily), prednisone (4 mg/day), pantoprazole (40 mg/day), nifedipine (60 mg/day), lasix (20 mg/day), ursodeoxycholic acid (600 mg/day), erythropoietin (20,000 U/week), and atenolol (25 mg/day) along with insulin and calcium with vitamin D. Serum HCV RNA became undetectable at week 3 of antiviral therapy, and there were associated improvements in serum ALT and alkaline phosphatase levels (Fig. 2). At week 16 of antiviral therapy, the patient experienced an episode of herpes zoster reactivation that was successfully treated with valacyclovir for 4 weeks.
At treatment week 19, her serum ALT and alkaline phosphatase levels increased to 99 and 517 IU/mL, respectively. A liver biopsy sample showed immune-mediated allograft hepatitis with extensive plasma cell infiltration and hepatic necrosis. At that time, her anti-nuclear antibody and anti–smooth muscle antibody findings were negative, her quantitative immunoglobulin levels were normal, and her cyclosporine trough level was within the therapeutic range at 100 ng/mL. Because this was felt to be immune-mediated allograft dysfunction due to interferon, the PEG-IFN dose was reduced to 135 μg/week, and the prednisone dose was increased from 2 to 20 mg/day for the remainder of the treatment course.
After 24 weeks of triple antiviral therapy, PEG-IFN/ribavirin consolidation therapy was administered for another 4 weeks. After the cessation of the antiviral medications at week 28, her serum ALT level was 82 IU/mL, her alkaline phosphatase level was 294 IU/mL, and her total bilirubin level was normal. At post-treatment weeks 12 and 24, HCV RNA remained undetectable, and she had a serum ALT level of 50 IU/mL, an alkaline phosphatase level of 220 IU/mL, and a total bilirubin level of 0.6 mg/dL. At week 32 after treatment, the patient was feeling well with normal liver biochemistries and was receiving cyclosporine (75 mg twice daily), prednisone (5 mg/day), and azathioprine (100 mg/day); HCV RNA remained undetectable.
Pharmacokinetic Assessment
The plasma concentrations of DCV at treatment week 2 were 137, 102, 112, 218, 451, and 376 ng/mL before dosing and 0.5, 1.0, 2.0, 4.0, and 6.0 hours after dosing, respectively. The trough values at weeks 4, 6, 8, 12, and 24 were 129, 160, 270, 161, and 87 ng/mL, respectively.
DISCUSSION
A recurrent HCV infection after LT can lead to accelerated allograft injury and fibrosis because of the high levels of HCV replication and the ongoing suppression of the host immune response to viral antigens.4,5 Patients who receive monoclonal antibody induction therapy or require treatment for acute rejection with pulse steroids are at particular risk for severe recurrent HCV infections, and so are recipients of older donor allografts.7,26–28 Our patient developed a severe recurrent cholestatic HCV infection within 4 months of her initial transplant in the absence of monoclonal antibody induction therapy and despite the receipt of a younger donor liver with a short cold ischemia time. Despite the minimization of her immunosuppression and the early introduction of PEG-IFN/ribavirin combination therapy, the patient developed progressive and life-threatening cholestatic graft failure. Liver biopsy samples at months 4 and 5 showed classic features of a severe cholestatic HCV infection (Fig. 1A).28,29 This particular recurrent HCV infection variant tends to occur within the first year of LT and is characterized by the development of inflammatory infiltrates, cholestasis, and hepatocyte ballooning, which can frequently lead to early graft failure and premature death. Although there have been case reports of salvage antiviral therapy, most patients die of infection or progressive allograft failure.27
Although previous data have shown poor outcomes with retransplantation in patients with severe recurrent HCV infections, a second transplant was offered to this patient because of her young age, excellent functional status, and good general health.30–32 Fortunately, she had an adequate physiological reserve to recover and was discharged home within 4 weeks of retransplantation. However, when the patient began to manifest early recurrent cholestasis with associated histological changes 3 months after her second transplant (Fig. 1B), a potent direct-acting antiviral–based antiviral therapy (DCV) in combination with PEG-IFN and ribavirin was initiated.
In contrast to her initial post-LT treatment course, a rapid suppression of HCV RNA at week 3 was observed with associated improvements in serum ALT and alkaline phosphatase levels while she was receiving DCV in combination with PEG-IFN and ribavirin (Fig. 2). In addition, HCV RNA remained undetectable throughout the 24-week course of triple antiviral therapy even though she had been previously treated 3 times without success. Furthermore, viral replication remained suppressed despite an intensification of her immunosuppressive regimen due to allograft dysfunction at week 19 and an intentional reduction in the dose of PEG-IFN. We considered discontinuing all 3 antiviral agents at this point, but we wanted her to complete the full course of treatment (24 weeks) because of her previous lack of clearance with PEG-IFN and ribavirin. A reduced dose of DCV (20 mg) was used throughout her treatment because of the potential for cyclosporine to increase the plasma levels of DCV. The plasma trough levels of DCV at weeks 2, 4, 8, 12, and 24 ranged from 87 to 270 ng/mL and were similar to the levels reported for nonimmunosuppressed HCV patients receiving DCV at 60 mg/day.21 In addition, the observed maximum concentration of DCV (451 ng/mL) was similar to the values observed in nontransplant patients receiving a once daily dose of 30 mg.21 The time to the maximum concentration for the patient was 4 hours, which was longer than the mean time to the maximum concentration previously reported for nontransplant patients under fasting conditions but was within the range observed when patients received DCV under fed conditions. Overall, the exposure observed for this subject was within the therapeutic range of DCV for which safety and efficacy have been observed; this suggests that cyclosporine did not have a clinically significant effect on her DCV exposure.
Antiviral therapy was well tolerated in this LT recipient, who was receiving concomitant cyclosporine, prednisone, and several other medications. The episode of herpes zoster at antiviral treatment week 19 was not felt to be related to the DCV study medication or the PEG-IFN/ribavirin treatment because solid organ transplant recipients are known to be at increased risk for zoster reactivation.33–35 In addition, the zoster infection improved despite continued antiviral therapy. The other adverse event experienced by this patient, immune-mediated allograft hepatitis, is being increasingly reported in LT recipients receiving interferon.8,36,37 Many HCV patients with immune-mediated hepatitis have detectable serum autoanti-bodies and develop a plasma cell–rich infiltrate in the allograft that responds to increased immunosuppression. Some investigators have speculated that immune-mediated hepatitis may be due to the rapid suppression of HCV RNA and may reflect an immune reconstitution syndrome like that reported for human immunodeficiency virus patients treated with antiretroviral therapy.37 In support of this, interferon therapy can promote HCV-specific CD4 and CD8 responses in the liver, but additional prospective studies of viral kinetics and intrahepatic immune responses are needed.38 HCV patients with cholestasis and plasma cell hepatitis on their baseline biopsy samples or with elevated alkaline phosphatase levels before antiviral therapy may be at particular risk for this complication.36,37 Other studies have suggested that virological responders to interferon therapy who experience improved liver function and metabolism of calcineurin inhibitors may be at increased risk of developing rejection during antiviral therapy.39,40 Although our understanding of this clinicopathological entity is evolving, many patients improve with a reduction or discontinuation of interferon therapy or with an increase in their immunosuppression, but cases of progressive hepatitis with graft failure and death have been reported.36 In the current patient, we were reluctant to stop the antiviral therapy because of her progressive liver failure with her first transplant and her rapid response to the 3-drug antiviral treatment. Therefore, the dose of PEG-IFN was reduced, and the corticosteroid dose was increased to treat the immune-mediated allograft hepatitis. Fortunately, this patient was successfully supported through a full 24-week course of triple antiviral therapy, and her liver biochemistries further improved during follow-up. In addition to being highly effective, DCV was not associated with any clinically significant drug-drug interactions in our patient. In particular, the trough levels of cyclosporine remained within the target range of 75 to 125 ng/mL during and after treatment.
In summary, we have reported the first successful use of a direct-acting antiviral agent in combination with PEG-IFN and ribavirin in an LT recipient with severe, interferon-refractory cholestatic HCV. Despite 3 previous attempts at antiviral therapy (including a course of PEG-IFN and ribavirin after the first transplant), the patient developed early cholestatic HCV after her second transplant. The rapid suppression of HCV RNA to undetectable levels within 3 weeks of the initiation of treatment with DCV and PEG-IFN/ribavirin along with the improved liver biochemistries suggests that effective antiviral therapy can lead to improved outcomes in these patients. In addition, the continued suppression of HCV RNA in this patient despite the need to reduce the PEG-IFN dose and increase the dose of steroids is encouraging with respect to the potency and efficacy of DCV-based combination antiviral therapy. Furthermore, the fact that only 24 weeks of triple antiviral therapy was required to achieve an SVR in this patient is also promising for other LT recipients with genotype 1, who frequently have difficulty tolerating 48 weeks of treatment. However, the optimal duration of triple antiviral therapy for LT recipients with HCV genotype 1a versus LT recipients with HCV genotype 1b requires further investigation. The favorable safety profile of DCV in combination with PEG-IFN and ribavirin that was observed in this patient and other studies (including the potentially minimal drug-drug interactions with calcineurin inhibitors) also makes it an attractive agent to explore in future studies of LT recipients. Finally, it is hoped that if combination regimens of potent oral antiviral agents such as DCV and other direct-acting antiviral classes such as nonstructural 3 protease inhibitors (asunaprevir and TMC-435) and nonstructural 5B nucleoside polymerase inhibitors (INX-189 and GSI-7977) with or without ribavirin can be safely administered to nontransplant patients, many additional LT recipients with recurrent HCV will be able to be treated. However, additional prospective studies will be needed.41,42
Acknowledgments
Robert J. Fontana has conducted research and consulted for Bristol-Myers Squibb. Eric A. Hughes, Dessislava Dimitrova, and Marc Bifano are employees of Bristol-Myers Squibb. Robert Hindes was formerly employed by Bristol-Myers Squibb and is currently employed by Gilead Sciences.
This work was supported in part by the National Institutes of Health through its support of the Michigan Institute for Clinical and Health Research (grant UL1RR024986). The investigational agent, daclatasvir, was supplied by Bristol-Myers Squibb.
The authors thank the following individuals for their assistance with this study: study coordinators Suzanne Welch and Sonal Trivedi (University of Michigan Health System), Roberta Tankenow (Investigational Drug Service, University of Michigan Health System), and Tracy Michener and Julia Roach (Collaborative Science Center of Excellence, Bristol-Myers Squibb).
Abbreviations
- ALT
alanine aminotransferase
- DCV
daclatasvir
- HCV
hepatitis C virus
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- PEG-IFN
peginterferon
- SVR
sustained virological response
References
- 1.Verna EC, Brown RS., Jr Hepatitis C virus and liver transplantation. Clin Liver Dis. 2006;10:919–940. doi: 10.1016/j.cld.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Gane EJ, Naoumov NV, Qian KP, Mondelli MU, Maertens G, Portmann BC, et al. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996;110:167–177. doi: 10.1053/gast.1996.v110.pm8536853. [DOI] [PubMed] [Google Scholar]
- 3.Prieto M, Berenguer M, Rayón JM, Córdoba J, Argüello L, Carrasco D, et al. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999;29:250–256. doi: 10.1002/hep.510290122. [DOI] [PubMed] [Google Scholar]
- 4.Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815–820. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 5.Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, et al. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32:673–684. doi: 10.1016/s0168-8278(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 6.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 7.Lai JC, Verna EC, Brown RS, Jr, O’Leary JG, Trotter JF, Forman LM, et al. for Consortium to Study Health Outcomes in HCV Liver Transplant Recipients (CRUSH-C) Hepatitis C virus-infected women have a higher risk of advanced fibrosis and graft loss after liver transplantation than men. Hepatology. 2011;54:418–424. doi: 10.1002/hep.24390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillouche P, Féray C. Systematic review: anti-viral therapy of recurrent hepatitis C after liver transplantation. Aliment Pharmacol Ther. 2011;33:163–174. doi: 10.1111/j.1365-2036.2010.04505.x. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Marrero JA, Fontana RJ, Greenson JK, Conjeevaram H, Su GL, et al. Sustained virologic response to therapy of recurrent hepatitis C after liver transplantation is related to early virologic response and dose adherence. Liver Transpl. 2007;13:1100–1108. doi: 10.1002/lt.21121. [DOI] [PubMed] [Google Scholar]
- 10.Hanouneh IA, Miller C, Aucejo F, Lopez R, Quinn MK, Zein NN. Recurrent hepatitis C after liver transplantation: on-treatment prediction of response to peginterferon/ribavirin therapy. Liver Transpl. 2008;14:53–58. doi: 10.1002/lt.21312. [DOI] [PubMed] [Google Scholar]
- 11.Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Kremers WK, et al. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant. 2008;8:2426–2433. doi: 10.1111/j.1600-6143.2008.02362.x. [DOI] [PubMed] [Google Scholar]
- 12.Carrión JA, Navasa M, García-Retortillo M, García-Pagan JC, Crespo G, Bruguera M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132:1746–1756. doi: 10.1053/j.gastro.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Picciotto FP, Tritto G, Lanza AG, Addario L, De Luca M, Di Costanzo GG, et al. Sustained virological response to antiviral therapy reduces mortality in HCV reinfection after liver transplantation. J Hepatol. 2007;46:459–465. doi: 10.1016/j.jhep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. for REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. for ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 16.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. for SPRINT-2 Investigators. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. for HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg V, van Heeswijk R, Lee JE, Alves K, Nadkarni P, Luo X. Effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimus. Hepatology. 2011;54:20–27. doi: 10.1002/hep.24443. [DOI] [PubMed] [Google Scholar]
- 19.Lee JE, van Heeswijk R, Alves K, Smith F, Garg V. Effect of the hepatitis C virus protease inhibitor telaprevir on the pharmacokinetics of amlodipine and atorvastatin. Antimicrob Agents Chemother. 2011;55:4569–4574. doi: 10.1128/AAC.00653-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fridell RA, Wang C, Sun JH, O’Boyle DR, II, Nower P, Valera L, et al. Genotypic and phenotypic analysis of variants resistant to hepatitis C virus nonstructural protein 5A replication complex inhibitor BMS-790052 in humans: in vitro and in vivo correlations. Hepatology. 2011;54:1924–1935. doi: 10.1002/hep.24594. [DOI] [PubMed] [Google Scholar]
- 21.Nettles RE, Gao M, Bifano M, Chung E, Persson A, Mar-bury TC, et al. Multiple ascending dose study of BMS-790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology. 2011;54:1956–1965. doi: 10.1002/hep.24609. [DOI] [PubMed] [Google Scholar]
- 22.Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742–748. doi: 10.1002/hep.24724. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki F, Chayama K, Kawakami Y, Toyota J, Karinon Y, Mochida S, et al. Daclatasvir (DCV: BMS-790052), an NS5A replication complex inhibitor, in combination with peginterferon alfa-2b and ribavirin in Japanese treatment naïve and non-responder patients with chronic HCV genotype 1 infection [abstract] Hepatology. 2011;54(suppl 1):LB22. [Google Scholar]
- 24.Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216–224. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 25.Hézode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez-Torres M, Shafran S, et al. Daclatasvir (DCV; BMS-790052), an NS5A replication complex inhibitor, combined with peginterferon-alfa-2a and ribavirin in treatment-naive HCV-genotype 1 or 4 subjects: phase COMMAND-1 study interim week 24 results [abstract] Hepatology. 2011;54(suppl 1):A227. [Google Scholar]
- 26.Bifano M, Sevinsky H, Persson A, Hwang C, Kandoussi H, Jian H, et al. Daclatasvir (DCV; BMS-790052) has no clinically significant effect on the pharmacokinetics of a combined oral contraceptive containing ethinyl estradiol and norgestimate in healthy female subjects [abstract] Hepatology. 2011;54(suppl 1):A1340. [Google Scholar]
- 27.Sheiner PA, Schwartz ME, Mor E, Schluger LK, Theise N, Kishikawa K, et al. Severe or multiple rejection episodes are associated with recurrence of hepatitis C after orthotopic liver transplantation. Hepatology. 1995;21:30–34. [PubMed] [Google Scholar]
- 28.Machicao VI, Bonatti H, Krishna M, Aqel BA, Lukens FJ, Nguyen JH, et al. Donor age affects fibrosis progression and graft survival after liver transplantation for hepatitis C. Transplantation. 2004;77:84–92. doi: 10.1097/01.TP.0000095896.07048.BB. [DOI] [PubMed] [Google Scholar]
- 29.Narang TK, Ahrens W, Russo MW. Post-liver transplant cholestatic hepatitis C: a systematic review of clinical and pathological findings and application of consensus criteria. Liver Transpl. 2010;16:1228–1235. doi: 10.1002/lt.22175. [DOI] [PubMed] [Google Scholar]
- 30.Schluger LK, Sheiner PA, Thung SN, Lau JY, Min A, Wolf DC, et al. Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology. 1996;23:971–976. doi: 10.1002/hep.510230505. [DOI] [PubMed] [Google Scholar]
- 31.Berenguer M, Prieto M, Palau A, Rayón JM, Carrasco D, Juan FS, et al. Severe recurrent hepatitis C after liver retransplantation for hepatitis C virus-related graft cirrhosis. Liver Transpl. 2003;9:228–235. doi: 10.1053/jlts.2003.50029. [DOI] [PubMed] [Google Scholar]
- 32.Pelletier SJ, Schaubel DE, Punch JD, Wolfe RA, Port FK, Merion RM. Hepatitis C is a risk factor for death after liver retransplantation. Liver Transpl. 2005;11:434–440. doi: 10.1002/lt.20342. [DOI] [PubMed] [Google Scholar]
- 33.Alcaide ML, Abbo L, Pano JR, Gaynor JJ, Tryphonopoulos P, Weppler D, et al. Herpes zoster infection after liver transplantation in patients receiving induction therapy with alemtuzumab. Clin Transplant. 2008;22:502–507. doi: 10.1111/j.1399-0012.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- 34.Levitsky J, Kalil A, Meza JL, Hurst GE, Freifeld A. Herpes zoster infection after liver transplantation: a case-control study. Liver Transpl. 2005;11:320–325. doi: 10.1002/lt.20356. [DOI] [PubMed] [Google Scholar]
- 35.Naoum C, Perissios A, Varnavas V, Lagos D. Treatment of herpes zoster with interferon alpha-2A. Int J Dermatol. 1996;35:749–750. doi: 10.1111/j.1365-4362.1996.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 36.Selzner N, Guindi M, Renner EL, Berenguer M. Immune-mediated complications of the graft in interferon-treated hepatitis C positive liver transplant recipients. J Hepatol. 2011;55:207–217. doi: 10.1016/j.jhep.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Levitsky J, Fiel MI, Norvell JP, Wang E, Watt KD, Curry MP, et al. Risk for immune-mediated graft dysfunction in liver transplant recipients with recurrent HCV infection treated with pegylated interferon. Gastroenterology. 2012;142:1132–1139. doi: 10.1053/j.gastro.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 38.Weston SJ, Leistikow RL, Reddy KR, Torres M, Wertheimer AM, Lewinsohn DM, et al. Reconstitution of hepatitis C virus-specific T-cell mediated immunity after liver transplantation. Hepatology. 2005;41:72–81. doi: 10.1002/hep.20507. [DOI] [PubMed] [Google Scholar]
- 39.Sharma P, Jafri M, Appelman H, McKenna B, Sullivan P, Fontana RJ, Lok ASF. Immunological dysfunction on antiviral therapy for hepatitis C recurrence after liver transplantation is associated with poor graft survival [abstract] Am J Transplant. 2011;11:A828. [Google Scholar]
- 40.Kugelmas M, Osgood MJ, Trotter JF, Bak T, Wachs M, Forman L, et al. Hepatitis C virus therapy, hepatocyte drug metabolism, and risk for acute cellular rejection. Liver Transpl. 2003;9:1159–1165. doi: 10.1053/jlts.2003.50233. [DOI] [PubMed] [Google Scholar]
- 41.Sulkowski MJ, Gardiner D, Lawitz E, Hinestrosa F, Nelson D, Thuluvath P, et al. Potent viral suppression with all-oral combination of daclatasvir (NS5A inhibitor) and GS-7977 (NS5B inhibitor), +/− ribavirin, in treatment naïve patients with chronic HCV genotype 1, 2, and 3 [abstract] J Hepatol. 2012:56. [Google Scholar]
- 42.Suzuki F, Ikeda K, Toyota J, Karino Y, Ohmura T, Chayama K, et al. Dual oral therapy with the NS5A inhibitor daclatasvir (BMS-790052) and NS3 protease inhibitor asunaprevir (BMS-650032) in HCV genotype 1 infected null responders or patients ineligible/intolerant to peginterferon/ribavirin [abstract] J Hepatol. 2012:56. [Google Scholar]