Abstract
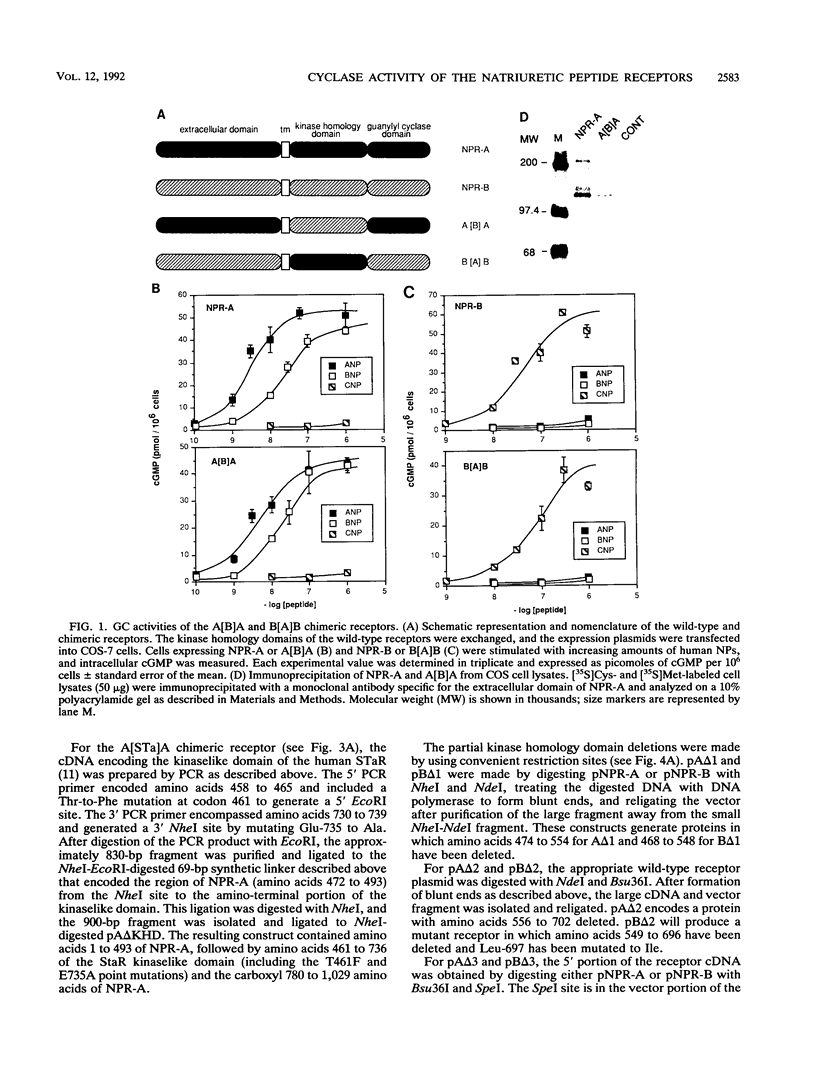
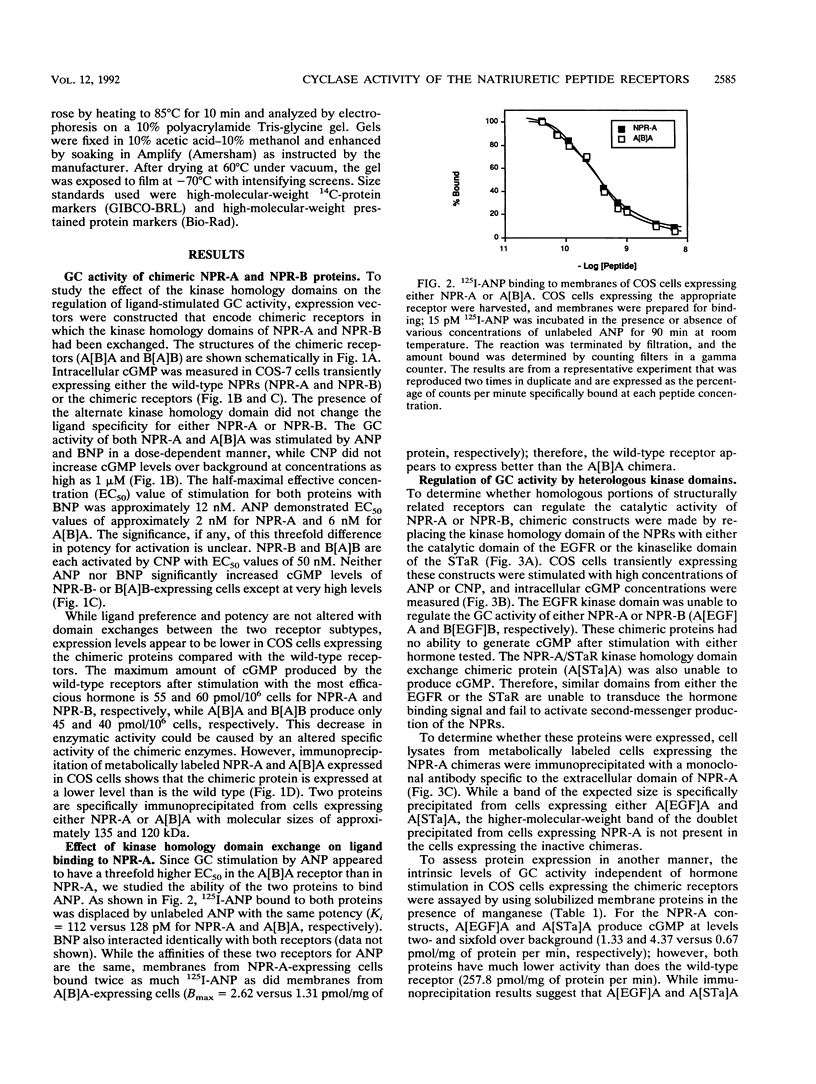
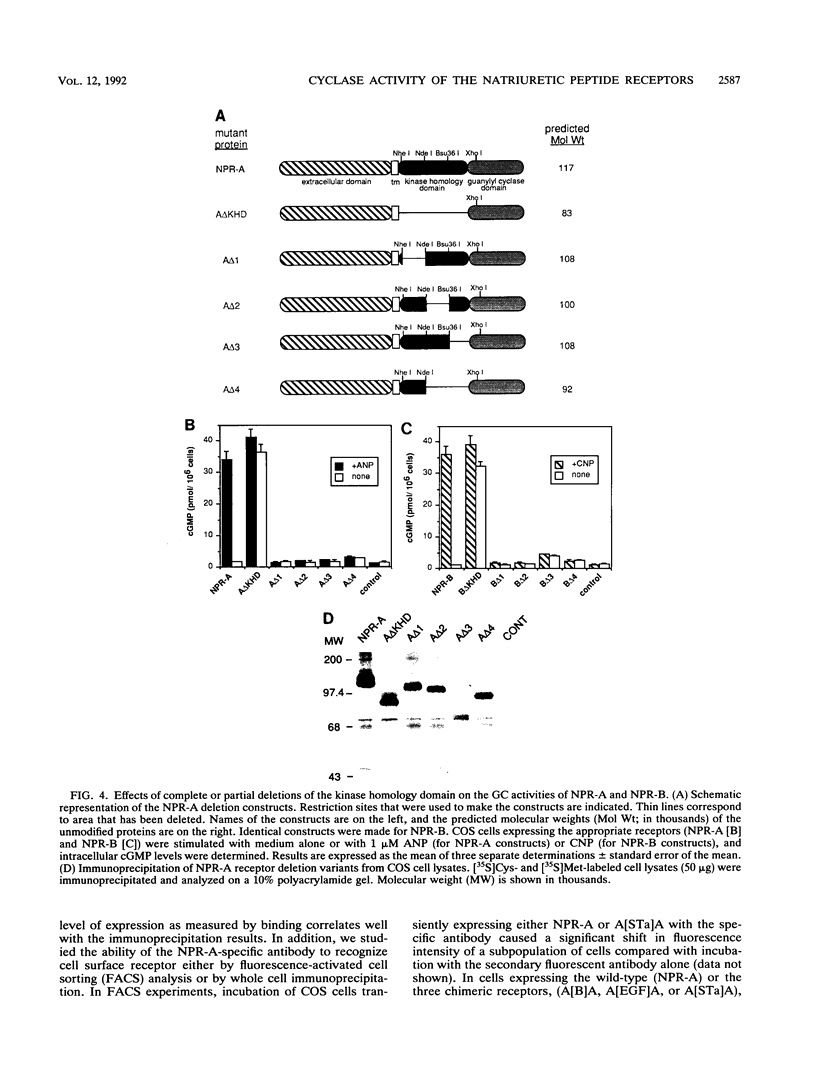
The natriuretic peptide receptors, NPR-A and NPR-B, are two members of the newly described class of receptor guanylyl cyclases. The kinaselike domain of these proteins is an important regulator of the guanylyl cyclase activity. To begin to understand the molecular nature of this type of regulation, we made complete and partial deletions of the kinase domain in NPR-A and NPR-B. We also made chimeric proteins in which the kinase domains of NPR-A and NPR-B were exchanged or replaced with kinase domains from structurally similar proteins. Complete deletion of the kinase homology domain in NPR-A and NPR-B resulted in constitutive activation of the guanylyl cyclase. Various partial deletions of this region produced proteins that had no ability to activate the enzyme with or without hormone stimulation. The kinase homology domain can be exchanged between the two subtypes with no effect on regulation. However, structurally similar kinaselike domains, such as from the epidermal growth factor receptor or from the heat-stable enterotoxin receptor, another member of the receptor guanylyl cyclase family, were not able to regulate the guanylyl cyclase activity correctly. These findings suggest that the kinaselike domain of NPR-A and NPR-B requires strict sequence conservation to maintain proper regulation of their guanylyl cyclase activity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley J. K., Tubb D. J., Garbers D. L. Receptor-mediated activation of spermatozoan guanylate cyclase. J Biol Chem. 1986 Nov 15;261(32):14859–14862. [PubMed] [Google Scholar]
- Bertics P. J., Chen W. S., Hubler L., Lazar C. S., Rosenfeld M. G., Gill G. N. Alteration of epidermal growth factor receptor activity by mutation of its primary carboxyl-terminal site of tyrosine self-phosphorylation. J Biol Chem. 1988 Mar 15;263(8):3610–3617. [PubMed] [Google Scholar]
- Bertics P. J., Gill G. N. Self-phosphorylation enhances the protein-tyrosine kinase activity of the epidermal growth factor receptor. J Biol Chem. 1985 Nov 25;260(27):14642–14647. [PubMed] [Google Scholar]
- Bovy P. R. Structure activity in the atrial natriuretic peptide (ANP) family. Med Res Rev. 1990 Jan-Mar;10(1):115–142. doi: 10.1002/med.2610100105. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Ballermann B. J., Gunning M. E., Zeidel M. L. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990 Jul;70(3):665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Kohse K. P., Chang B., Hirata M., Jiang B., Douglas J. E., Murad F. Characterization of ATP-stimulated guanylate cyclase activation in rat lung membranes. Biochim Biophys Acta. 1990 Apr 9;1052(1):159–165. doi: 10.1016/0167-4889(90)90071-k. [DOI] [PubMed] [Google Scholar]
- Chang M. S., Lowe D. G., Lewis M., Hellmiss R., Chen E., Goeddel D. V. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature. 1989 Sep 7;341(6237):68–72. doi: 10.1038/341068a0. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L., Chang M. S., Lowe D. G., Chin H. M., Goeddel D. V., Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989 Mar 2;338(6210):78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L. The protein kinase domain of the ANP receptor is required for signaling. Science. 1989 Sep 22;245(4924):1392–1394. doi: 10.1126/science.2571188. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Singh S., Garbers D. L. Adenine nucleotides are required for activation of rat atrial natriuretic peptide receptor/guanylyl cyclase expressed in a baculovirus system. J Biol Chem. 1991 Mar 5;266(7):4088–4093. [PubMed] [Google Scholar]
- Fuller F., Porter J. G., Arfsten A. E., Miller J., Schilling J. W., Scarborough R. M., Lewicki J. A., Schenk D. B. Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones. J Biol Chem. 1988 Jul 5;263(19):9395–9401. [PubMed] [Google Scholar]
- Gibbs C. S., Zoller M. J. Rational scanning mutagenesis of a protein kinase identifies functional regions involved in catalysis and substrate interactions. J Biol Chem. 1991 May 15;266(14):8923–8931. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Kambayashi Y., Nakao K., Mukoyama M., Saito Y., Ogawa Y., Shiono S., Inouye K., Yoshida N., Imura H. Isolation and sequence determination of human brain natriuretic peptide in human atrium. FEBS Lett. 1990 Jan 1;259(2):341–345. doi: 10.1016/0014-5793(90)80043-i. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N. H., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Kojima M., Minamino N., Kangawa K., Matsuo H. Cloning and sequence analysis of a cDNA encoding a precursor for rat C-type natriuretic peptide (CNP). FEBS Lett. 1990 Dec 10;276(1-2):209–213. doi: 10.1016/0014-5793(90)80544-s. [DOI] [PubMed] [Google Scholar]
- Koller K. J., Lowe D. G., Bennett G. L., Minamino N., Kangawa K., Matsuo H., Goeddel D. V. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science. 1991 Apr 5;252(5002):120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- Kurose H., Inagami T., Ui M. Participation of adenosine 5'-triphosphate in the activation of membrane-bound guanylate cyclase by the atrial natriuretic factor. FEBS Lett. 1987 Jul 27;219(2):375–379. doi: 10.1016/0014-5793(87)80256-9. [DOI] [PubMed] [Google Scholar]
- Lowe D. G., Camerato T. R., Goeddel D. V. cDNA sequence of the human atrial natriuretic peptide clearance receptor. Nucleic Acids Res. 1990 Jun 11;18(11):3412–3412. doi: 10.1093/nar/18.11.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. G., Chang M. S., Hellmiss R., Chen E., Singh S., Garbers D. L., Goeddel D. V. Human atrial natriuretic peptide receptor defines a new paradigm for second messenger signal transduction. EMBO J. 1989 May;8(5):1377–1384. doi: 10.1002/j.1460-2075.1989.tb03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maack T., Suzuki M., Almeida F. A., Nussenzveig D., Scarborough R. M., McEnroe G. A., Lewicki J. A. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987 Oct 30;238(4827):675–678. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- McGregor A., Richards M., Espiner E., Yandle T., Ikram H. Brain natriuretic peptide administered to man: actions and metabolism. J Clin Endocrinol Metab. 1990 Apr;70(4):1103–1107. doi: 10.1210/jcem-70-4-1103. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Pohl G., Källström M., Bergsdorf N., Wallén P., Jörnvall H. Tissue plasminogen activator: peptide analyses confirm an indirectly derived amino acid sequence, identify the active site serine residue, establish glycosylation sites, and localize variant differences. Biochemistry. 1984 Jul 31;23(16):3701–3707. doi: 10.1021/bi00311a020. [DOI] [PubMed] [Google Scholar]
- Ramarao C. S., Garbers D. L. Purification and properties of the phosphorylated form of guanylate cyclase. J Biol Chem. 1988 Jan 25;263(3):1524–1529. [PubMed] [Google Scholar]
- Rosenzweig A., Seidman C. E. Atrial natriuretic factor and related peptide hormones. Annu Rev Biochem. 1991;60:229–255. doi: 10.1146/annurev.bi.60.070191.001305. [DOI] [PubMed] [Google Scholar]
- Schulz S., Green C. K., Yuen P. S., Garbers D. L. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990 Nov 30;63(5):941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- Schulz S., Singh S., Bellet R. A., Singh G., Tubb D. J., Chin H., Garbers D. L. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989 Sep 22;58(6):1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- Song D. L., Kohse K. P., Murad F. Brain natriuretic factor. Augmentation of cellular cyclic GMP, activation of particulate guanylate cyclase and receptor binding. FEBS Lett. 1988 May 9;232(1):125–129. doi: 10.1016/0014-5793(88)80400-9. [DOI] [PubMed] [Google Scholar]
- Spellman M. W., Basa L. J., Leonard C. K., Chakel J. A., O'Connor J. V., Wilson S., van Halbeek H. Carbohydrate structures of human tissue plasminogen activator expressed in Chinese hamster ovary cells. J Biol Chem. 1989 Aug 25;264(24):14100–14111. [PubMed] [Google Scholar]
- Sudoh T., Kangawa K., Minamino N., Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988 Mar 3;332(6159):78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- Sudoh T., Minamino N., Kangawa K., Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990 Apr 30;168(2):863–870. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- Thorpe D. S., Morkin E. The carboxyl region contains the catalytic domain of the membrane form of guanylate cyclase. J Biol Chem. 1990 Sep 5;265(25):14717–14720. [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]






