Abstract
Objective
Factors responsible for the variability in outcomes after lower extremity vein bypass grafting (LEVBG) are poorly understood. Recent evidence has suggested that a single nucleotide polymorphism (SNP) in the promoter region of the p27Kip1 gene, a cell-cycle regulator, is associated with coronary in-stent restenosis. We hypothesized an association with vein graft patency.
Methods
This was a retrospective genetic association study nested within a prospective cohort of 204 patients from three referral centers undergoing LEVBG for claudication or critical ischemia. The main outcome measure was primary vein graft patency.
Results
All patients were followed up for a minimum of 1 year with duplex graft surveillance (median follow-up, 893 days; interquartile range, 539-1315). Genomic DNA was isolated and SNP analysis for the p27Kip1-838C>A variants was performed. Allele frequencies were correlated with graft outcome using survival analysis and Cox proportional hazards modeling. The p27Kip1-838C>A allele frequencies observed were CA, 53%; CC, 30%; and AA, 17%, satisfying Hardy-Weinberg equilibrium. Race (P = .025) and history of coronary artery disease (P = .027) were different across the genotypes; all other baseline variables were similar. Primary graft patency was greater among patients with the −838AA genotype (75% AA vs 55% CA/CC at 3 years; P = .029). In a Cox proportional hazards model including age, sex, race, diabetes, critical limb ischemia, redo (vs primary) bypass, vein type, and baseline C-reactive protein level, the p27Kip1-838AA genotype was significantly associated with higher graft patency (hazard ratio for failure, 0.4; 95% confidence interval, 0.17-0.93). Genotype was also associated with early (0-1 month) changes in graft lumen diameter by ultrasound imaging.
Conclusions
These data suggest that the p27Kip1-838C>A SNP is associated with LEVBG patency and, together with previous reports, underscore a central role for p27Kip1 in the generic response to vascular injury.
First described more than 60 years ago,1 autogenous vein bypass grafting remains a key therapeutic option for patients with extensive peripheral artery disease as well as coronary artery disease. In the United States Medicare population, more than 100,000 lower extremity and 200,000 coronary bypass graft procedures are performed each year for relief of ischemia.2,3 Although vein grafts in the lower extremity are durable in many cases, the development of de novo stenosis within the graft occurs in 30% to 50% of patients within the first several years, often necessitating repeat intervention.4-8 Despite attention to vein harvesting trauma, improved surgical techniques, modification of conventional atherosclerosis risk factors (eg, smoking cessation, lipid-lowering drugs), and antithrombotic therapies, the incidence of vein graft disease has not changed perceptibly for 3 decades. Furthermore, there is limited understanding, beyond technical factors, of the variable nature of vein graft remodeling and clinical outcomes among individual patients.9
The prototypic response of blood vessels to mechanical trauma, namely, the development of neointimal thickening, may become clinically manifest as lumenal renarrowing after angioplasty, stent placement, and bypass grafting. The acute injury triggers a proliferative responsive in resident vascular smooth muscle cells (VSMCs) and adventitial cells via cell-cycle activation. Normally quiescent in the uninjured vessel, VSMCs rapidly respond to local cytokine and growth factor signals and are released from growth inhibition by coordinated activity of cell cycle proteins.10 The cyclin-dependent kinase (CDK) inhibitor p27Kip1 is a critical gatekeeper of the G1-S checkpoint, blocking cell-cycle entry by inhibiting CDK-cyclin interactions, specifically that between cyclin E-CDK2 and cyclin D-CDK4.11 Numerous lines of evidence suggest that p27Kip1 plays an important role in the response to vascular injury and in atherosclerosis.12-14
Recent studies have demonstrated the potential role of genetic variability as a determinant of clinical outcomes in patients with cardiovascular disease and after clinical interventions. Of interest, a single nucleotide polymorphism (SNP) in the p27Kip1 gene (−838C>A; rs36228499) was recently identified as a potential risk factor for myocardial infarction.15 In a retrospective association study in two Dutch cohorts of patients who had undergone percutaneous placement of bare-metal stents (BMS) in coronary arteries, this single nucleotide polymorphism (SNP) was identified as a strong predictor of in-stent restenosis.16 We hypothesized that genetic factors related to neointimal disease in venous bypass grafts would be similar to those in injured arteries and that variability in the p27Kip1 gene would be associated with vein graft disease. Our findings support a potentially central role for p27Kip1 as a global determinant of cardiovascular intervention outcomes.
METHODS
Study design and cohorts
This was a retrospective study designed to test the specific hypothesis that the p27Kip1-838C>A SNP (rs36228499) is associated with primary patency of lower extremity vein bypass grafts (LEVBGs).
The primary cohort of 204 patients was derived from a prospective study examining the relationship between systemic inflammation and clinical outcomes after LEVBG at three Boston hospitals (Brigham and Women’s Hospital, Beth Israel Deaconess Medical Center, Boston VA Medical Center). This study was sponsored by the National Heart, Lung and Blood Institute (HL 75771). The inclusion and exclusion criteria of this cohort have been described elsewhere.17,18 Briefly, patients were eligible for enrollment if they were undergoing primary or redo lower extremity bypass surgery with autogenous vein for lifestyle-limiting claudication or critical limb ischemia. Importantly, patients were excluded if they had a recent pre-existing condition likely to influence systemic inflammation, including myocardial infarction, stroke, major illness, or major operation ≤30 days of the bypass surgery, evidence of foot infection, or current use of immunosuppressant medications. Patients were also excluded if any portion of the bypass was constructed with nonautogenous material.
All patients provided written informed consent, and the study protocol was approved by the respective Institutional Review Boards (IRBs) at the participating sites. Patients were enrolled between 2004 and 2007 and were followed up for a minimum of 1 year (median follow-up, 32 months). A preoperative blood sample was drawn on all study participants, and aliquots of anticoagulated whole blood were frozen at −80° C. Of the 225 individuals enrolled in this study, DNA samples were available for genotyping from 204, which are the subject of the analysis.
A second cohort of 51 patients examined in this study was derived from two Seattle hospitals (University of Washington Medical Center and the VA Puget Sound Health Care System). These individuals were enrolled in a series of prospective, observational pilot studies examining the associations between graft stenosis, platelet/monocyte activity, and growth patterns of cells obtained from vein grafts (Supplementary Table I).19,20 The studies were approved by the IRBs at both institutions, and all patients gave informed consent. Patients were excluded if they were unable to give informed consent or to return for follow-up examinations. Participants in the Seattle cohort were recruited between 2004 and 2009. Patients were followed up for a minimum of 12 months. Anticoagulated whole blood was obtained at baseline and frozen at −80°C.
Clinical assessments and end point definitions
All patients in the Boston cohort were followed up by their vascular surgeons for clinical and graft-related events at 1, 3, 6, 9, and 12 months, and every 6 months thereafter until termination from the study. Study personnel recorded clinical or graft-related events during the postoperative visits, including rehospitalizations, major adverse cardiovascular events, amputations, graft revisions, or graft occlusions. Under the study protocol, patients underwent duplex ultrasound surveillance of their bypass grafts at each visit at 1, 3, 6, 9, and 12 months, and thereafter at 6-month intervals. For the Seattle cohort, the follow-up assessment schedule included clinical and duplex ultrasound graft examinations at 6 weeks and at 3, 6, and 12 months after surgery. Primary and secondary graft patency were defined in accordance with accepted guidelines for reporting of lower extremity revascularization.21
Genomic DNA and SNP analysis
Genomic DNA was isolated from whole blood using a purification kit and the manufacturer’s suggested protocol (Wizard; Promega Corp, Madison, Wisc). The p27Kip1-838C>A SNP was genotyped by polymerase chain reaction using the following primers: forward: TCCAGGTCCCGGCTTCCCGGt, reverse: CCTGCTCTGGCTGGCCTCGGAG. A mismatch creating a Taq1 site when −838C is present is shown in lower case. Reactions were performed using a programmable thermocycler (MJ Research, St. Bruno, Quebec, Canada), and the reaction product was digested with Taq1 (10 hours at 65°C) and resolved on a 3.5% agarose gel with ethidium bromide staining.
Ultrasound imaging substudy
We prospectively enrolled patients from one of the study sites (Brigham and Women’s Hospital in Boston) in an IRB-approved imaging substudy designed to examine remodeling patterns in vein bypass conduits using high-resolution ultrasound imaging. The methods for this substudy have been reported previously.18 In brief, after informed consent, participants underwent serial ultrasound assessment of a defined, registered 5-cm region of their bypass graft using B-mode, M-mode, and Doppler modalities. The designation of the region of interest of the conduit (index segment) was made in the operating room and was specifically selected as a straight, valveless vein segment ≥5 cm away from the proximal anastomosis and in a superficial location. Surgical clips were placed as a reference, and the distance from the proximal anastomosis was recorded for subsequent identification. The initial set of images was acquired in the operating room after completion of the bypass graft and before wound closure. Five high-resolution M-mode cross-sectional images of the vein were recorded at each 1-cm interval along the index segment using an ATL HDI 3000 ultrasound machine (Advanced Technology Laboratories, Bothell, Wash) with a 10-MHz transducer and cardiac gating. Lumen diameter was calculated as the mean of these 25 measurements. At postoperative visits, in addition to standard Duplex graft surveillance, these patients had detailed imaging acquisition of the index segment of the conduit using the same protocol.
Statistical methods
Hardy-Weinberg equilibrium was first evaluated among the full Boston cohort and then in the subset who self-reported as white. There were no significant deviations from Hardy-Weinberg equilibrium (P > .20). The primary analyses were performed using the larger Boston cohort, with the Seattle cohort analyzed separately as a confirmatory population. Graft patency rates were estimated by life-table analysis. Univariate associations between genotype and graft outcomes were performed by log-rank test. A Cox proportional hazards model was used incorporating demographic (age, race, sex) variables and other variables relevant to LEVBG outcomes, including diabetes, critical limb ischemia as the indication, redo bypass, and baseline high-sensitivity C-reactive protein level, in addition to the p27 genotype. A value of P < .05 was considered statistically significant for all tests.
RESULTS
Characterization of the study population by p27 genotype
Characteristics of subjects in the Boston cohort by p27Kip1-838C>A genotype are summarized in Table I (see Supplementary Table II, online only, for the Seattle cohort). Mean age was 70 years, 82% were white, and 45% were women. Race (P = .025) and history of coronary artery disease (P = .027) were different across the genotypes; all other baseline variables including use of anti-platelet and statin medications were similar between the groups. There were no failures of genotyping for this SNP.
Table I.
Characteristics of Boston lower extremity vein bypass (LEVB) graft cohort by the p27Kip1-838 genotype
| Variable a | −838AA | −838CA | −838CC | Pa |
|---|---|---|---|---|
| Patients | 35 (17.2) | 108 (52.9) | 61 (29.9) | |
| Age, years | 67.3 ± 12.1 | 66.8 ± 67.5 | 67.8 ± 9.95 | .891 |
| Male sex | 26 (74.3) | 81 (75.0) | 40 (65.6) | .402 |
| Race | ||||
| Caucasian | 31 (88.6) | 99 (91.7) | 47 (77.1) | .025 |
| African | 0 | 8 (7.4) | 8 (13.1) | .069 |
| American Hispanic |
3 (8.6) | 1 (0.93) | 6 (9.8) | .020 |
| hsCRP >5 mg/L | 14 (40.0) | 36 (33.3) | 24 (39.3) | .650 |
| CLI | 20 (57.1) | 62 (57.4) | 37 (60.7) | .908 |
| Diabetes mellitus | 19 (54.3) | 53 (49.1) | 35 (57.4) | .567 |
| CAD | 14 (40.0) | 56 (51.9) | 41 (67.2) | .027 |
| Current tobacco use |
10 (28.6) | 46 (42.6) | 22 (36.1) | .305 |
| BMI, kg/m2 | 27.1 ± 5.03 | 29.1 ± 7.4 | 28.7 ± 7.36 | .325 |
| Tissue loss | 9 (25.7) | 33 (30.6) | 21 (34.4) | .670 |
| Redo LEVB | 3 (8.6) | 11 (10.2) | 6 (9.8) | .962 |
| SSGSV conduit | 32 (91.4) | 91 (84.3) | 46 (75.4) | .114 |
| Nonreversed SSGSV |
23 (65.7) | 66 (61.1) | 35 (57.4) | .719 |
| Infrapopliteal target |
17 (48.6) | 47(43.5) | 36 (59.0) | .153 |
| Statin use | 30 (85.7) | 89 (82.4) | 47 (77.1) | .532 |
| Antiplatelet Rx | 29 (82.9) | 86 (79.6) | 49 (80.3) | .916 |
BMI, Body mass index; CAD, coronary artery disease; CLI, critical limb ischemia; hsCRP, high-sensitivity C-reactive protein; SSGSV, single-segment great saphenous vein.
Continuous data re shown as mean ± standard deviation and categoric data as number (%).
χ2 test.
The p27Kip1-838C>A allele frequencies observed were CA, 53%; CC, 30%; and AA, 17% in the Boston cohort and CA, 65%, CC 23%, and AA 12% in the Seattle cohort. Age, race, and sex distributions for the Seattle cohort were similar to that of the Boston cohort.
Clinical outcomes
For the Boston cohort, 78 patients lost primary patency during follow-up. By life-table analysis, the overall primary patency rate was 69% ± 3% at 1 year and 60% ± 4% at 3 years. Secondary patency was 85% ± 3% and 82% ± 3% at 1 and 3 years respectively.
Early (30-day) event rates were not different by genotype (Table II). Primary graft patency tended to be associated with p27Kip1-838 genotype, with the patients having the AA genotype demonstrating improved patency (P = .066 by log-rank test; Table II; Fig 1). Because the observed pattern was consistent with a recessive model, we combined the CA and CC groups for subsequent analysis. In this analysis, AA genotype was significantly associated with primary graft patency (P = .029 by log-rank test; Fig 2). In the Seattle cohort, a similar trend was observed in primary patency by p27Kip1-838C>A genotype (83% AA vs 60% CA/CC at 1 year; P = .27 by χ2; Supplementary Table III, online only).
Table II.
Summary of clinical outcomes in Boston cohort by p27Kip1-838 genotype
| Variablea | AA No. (%) |
CA No. (%) |
CC No. (%) |
Pb |
|---|---|---|---|---|
| 30-day | ||||
| Graft failure | 3 (8.6) | 6 (5.6) | 0 (0) | .921 |
| MACE | 3 (8.6) | 7 (6.5) | 2 (3.3) | .908 |
| % (SEM) | % (SEM) | % (SEM) | ||
| Primary patency | ||||
| 1 year | 81.0 (7.0) | 67.5 (4.7) | 62.1 (6.4) | .066 |
| 3 years | 76.5 (7.9) | 58.2 (5.4) | 51.2 (7.4) | .066 |
| Secondary patency | ||||
| 1 year | 90.6 (5.1) | 80.4 (4.3) | 86.1 (8.6) | .163 |
| 3 years | 90.6 (5.1) | 76.8 (4.6) | 83.8 (5.0) | .163 |
| 3-year outcome | ||||
| Limb loss | 91.0 (4.9) | 95.0 (2.1) | 96.5 (2.4) | .721 |
| Survival | 74.9 (8.5) | 74.9 (8.5) | 83.5 (5.1) | .903 |
MACE, Major adverse cardiovascular event, including death, myocardial infarction, stroke; SEM, standard error of the mean.
All rates >30 days are by life-table analysis, shown as % (SEM); 30-day event rates are raw proportion.
Univariate P-values by log-rank or logistic regression analysis.
Fig 1.
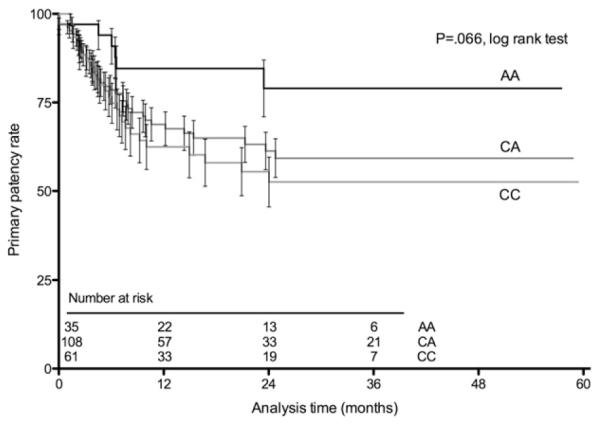
Life-table plot shows primary lower extremity vein bypass graft patency by p27Kip1-838 genotype for the 204 individuals in the Boston cohort. Data are shown with the standard error of the mean brackets.
Fig 2.
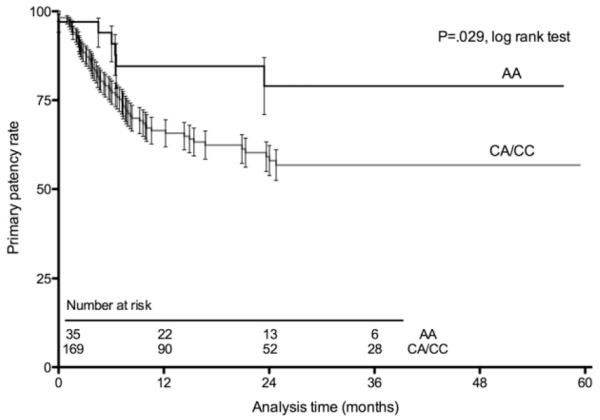
Life-table plot shows primary lower extremity vein bypass graft patency by p27Kip-838 genotype, using a recessive model (AA vs CA + CC). Data are shown with the standard error of the mean.
Multivariable model for primary graft patency
A Cox proportional hazards model using the Boston cohort data revealed that the p27Kip1-838C>A genotype was significantly associated with primary graft patency (hazard ratio for AA, 0.41; 95% confidence interval, 0.18-0.97; P = .039), adjusting for age, race, diabetes, redo bypass, indication (critical ischemia vs claudication), and baseline high-sensitivity C-reactive protein (Table III). Analyses restricted to the individuals who self-reported as white race had very similar point estimates (hazard ratio for AA, 0.39; 95% confidence interval, 0.16-0.99; P = .048) including adjustment for the same set of covariates.
Table III.
Cox proportional hazards model for primary graft patency
| Variable | HR (95% CI) | Pa |
|---|---|---|
| Age | 1.01 (0.99-1.03) | .387 |
| Non-white race | 2.0 (1.12-3.60) | .019 |
| Diabetes | 1.58 (0.95-2.62) | .075 |
| Critical limb ischemia | 1.52 (0.86-2.69) | .151 |
| Re-do bypass | 1.99 (1.03-3.84) | .041 |
| Baseline hs-CRP >5 mg/L | 1.23 (0.77-2.16) | .335 |
| p27Kip1-838AA | 0.41 (0.18-0.97) | .039 |
CI, Confidence interval; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein.
There is no corresponding footnote for “a” in Table III.
Vein remodeling
We analyzed data from the imaging substudy to look for associations between patterns of vein remodeling after arterialization and the p27Kip1-838 genotype. There were 55 patients who participated in the ultrasound substudy, had imaging data available from the intraoperative and 1-month scans, and had been genotyped for the p27 SNP of interest. We found that individuals with the homozygous CC genotype had significantly less early dilation (0-1 month) of the venous conduit (P = .045 by analysis of variance; Fig 3). This association was unchanged when restricted to the 45 white patients. Owing to the modest size of the substudy cohort, we were unable to define significant associations between genotypes and later remodeling changes or to conduct further multivariable analysis across genotypes.
Fig 3.
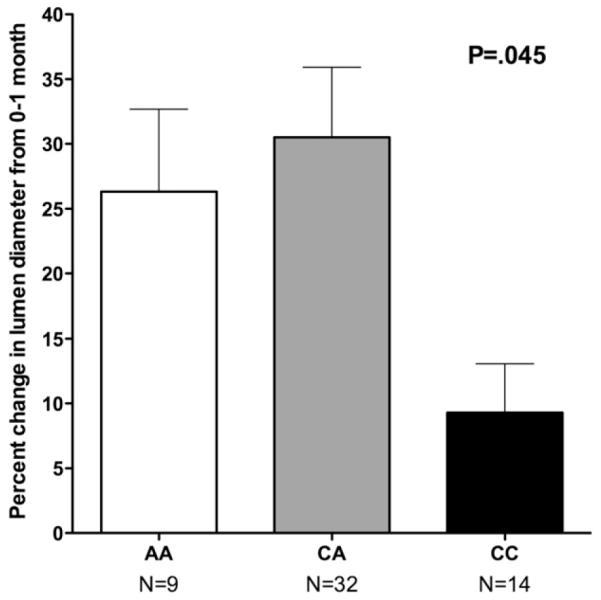
Percentage change (mean ± standard deviation) is shown in vein graft lumen diameter from baseline (intraoperative after implantation) to 1 month, in a subset of 55 patients who took part in a detailed ultrasound imaging substudy,18 by p27Kip1-838 genotype (P = .045 by analysis of variance).
Comparison with other cohorts
Table IV summarizes the genotype frequencies, prevalence of stenosis (artery or graft), and point estimate effect size for the Boston and Seattle vein graft cohorts compared with the coronary BMS outcomes in the Dutch cohort reported by van Tiel et al.16 Reflective of a largely white population in the current study populations, likely enriched for European descent, the genotype frequencies seen are broadly similar across the North American and Dutch cohorts. The protective AA genotype was present in 12% to 21% of these populations. The strikingly similar estimates of a protective association between the AA genotype and target vessel stenosis across the peripheral bypass and coronary BMS studies suggest a fundamental association between p27Kip1-838C>A genotype and the vascular injury response in disparate vessels and circulatory beds.
Table IV.
Relative effects size of the AA genotype in three different cohorts
| Cohort | No. | Overall prevalence of stenosis (%) |
Frequency of −838AA genotype (%) |
Point estimate of effect size |
|---|---|---|---|---|
| Boston | 202 | 34.8 | 17.2% | .41 (HR) |
| Seattle | 51 | 37.3 | 11.8% | .30 (OR) |
| Netherlands16 | 598 | 18 | 21.2% | .29 (HR) |
HR, Hazard ratio, OR, odds ratio.
DISCUSSION
To our knowledge, this report identifies the first potential genetic marker for LEVBG outcomes, a common SNP in the promoter region (position −838) of the gene for the cell-cycle inhibitor, p27Kip1. Patients homozygous for the minor variant A allele, roughly one of six individuals in the study population, experienced a 2.5-fold reduction in subsequent vein graft failure. This association was independent of demographic and clinical risk factors even in a modestsized population, which suggests it is likely robust. Moreover, the magnitude of the effect was strikingly similar across two independent vein graft cohorts, as well as a Dutch coronary BMS population. Although these findings require further prospective validation, they suggest a potentially important global marker of genetic variability in the vascular injury response.
The cell-cycle inhibitor p27Kip1 is known to play a critical role in the regulation of vascular cell proliferation, with complementary evidence from both animal models of disease and human vascular lesions.10,13,22 After arterial injury, increased p27Kip1 expression coincides with decreased cellular proliferation by 5-bromo-2-deoxyuridine staining.12 Genetic studies in mice demonstrate a prominent inhibitory role of p27Kip1 on atherogenesis and injury-induced neointimal hyperplasia.23 In a rabbit vein graft model, local treatment with rapamycin resulted in elevated levels of p27Kip1 that correlated directly with reduced proliferation and less early intimal thickening.24
Current understanding of the adaptive process of vein arterialization and its relationship to subsequent bypass graft disease in humans remains quite incomplete. After implantation in the arterial circulation, veins must undergo structural remodeling in response to acutely elevated shear and tensile forces, leading to some requisite wall thickening. An integrated biomechanical/biochemical approach is needed to explain the observed variability in this response, both along the course of a given conduit and among individual patients.25 Lumen caliber in the vein graft is determined by wall thickness and remodeling. We have previously reported the time course and variability of vein graft remodeling in the lower extremity, highlighting the importance of early outward remodeling on subsequent clinical outcomes.18,26 In our imaging substudy cohort, individuals with the homozygous p27Kip1-838CC genotype had notably inferior remodeling during the first postoperative month. These data, although preliminary, suggest that genetic variability in p27Kip1 may influence the early adaptive dilation response in the arterialized vein. Because ultrasound imaging is limited to lumen dimensions, we are not able to discriminate changes in wall thickness or composition associated with these findings. Graft failure is a complex phenotype consisting of several factors, including remodeling, wall thickness, and thrombosis. We postulate that the apparent discrepancy between a dominant vs a recessive influence of the A allele on early remodeling vs clinical patency may reflect these different components.
Interest in blocking cell cycle activation as a means of reducing neointimal thickening in bypass grafts stems from a large body of animal and in vitro studies. Of note, the PRoject of Ex-Vivo vein graft ENgineering via Transfection (PREVENT) clinical trials tested a molecular strategy of cell-cycle inhibition via a transcription factor “decoy” (antagonist of E2F) in two large phase 3 trials (coronary and peripheral), both of which were negative.27,28 The reasons for failure of the test agent in these studies remain unclear; however, variability in clinical outcomes across a range of patient-level factors, including race and sex, was observed in these large multicenter studies. There are few prior reports describing genetic association studies in vein bypass outcomes,29-31 and none involving peripheral grafting. Importantly, our study was not a broad exploratory investigation of genetic associations but rather a hypothesis-based testing of a single suspected genetic marker based on the recent coronary studies.
One of the salient findings from this study is the striking concordance in both prevalence and beneficial association of the p27Kip1-838C>A SNP across our two geographic centers and one in Europe, and two distinct types of vascular injury (stent in the coronary artery and vein bypass in the leg). Although clearly requiring further larger-scale validation studies and expanding to larger numbers of nonwhite individuals, these findings suggest that this marker may be of unique clinical and biologic significance. The initial report from Gonzalez et al16 linking the p27Kip1-838C>A SNP to an increased risk of myocardial infarction is also important to consider because it appears contradictory in clinical terms. We consider the hypothesis proposed by van Tiel et al,16 that is the differential role of VSMC proliferation in maintaining fibrous cap integrity of native atherosclerosis lesions vs promoting restenosis, as a logical explanation that requires further study.
This investigation has a number of important limitations, among which the modest size and limited diversity of the study population is paramount. Nevertheless, this limitation is counterbalanced by the considerable power of long-term imaging surveillance of the bypass grafts in these individuals, allowing for accurate assessment of the timing and progression of vein graft lesions that is not generally obtainable in coronary studies.
Second, we do not have evidence to directly link the p27Kip1-838C>A SNP to expression of p27Kip1 within tissue from the patients. Therefore we cannot discriminate between association and causation in our findings. Of note, van Tiel et al16 examined the potential functional significance of the p27Kip1-838C>A SNP using a recombinant promoter-luciferase construct in human embryonic kidney 293 cells, demonstrating a large increase in promoter activity with the −838A construct compared with the −838C construct. In preliminary studies using these identical constructs (courtesy Carlie J.M. deVries), we have confirmed these relative findings in both human embryonic kidney 293 cells and in primary cultured adventitial fibroblasts from human saphenous vein (Supplementary Fig, online only), However, more direct evidence linking tissue expression to p27Kip1 genotype is needed to support the hypothesis for causation.
CONCLUSIONS
Failure rates of clinical interventions for the treatment of peripheral arterial disease remain high, and the prediction and prevention of such failures is a significant unmet clinical need. Our studies support the need for broader efforts to identify the role of genetic variability in treatment outcomes, which may lead to improvements in patient selection, surveillance, and postinterventional therapies to reduce the burden of restenosis. These findings highlight that such efforts should consider common phenotypic patterns between different types of interventions and vascular beds as well as other related forms of mechanical and surgical trauma. As one example, the p27Kip1-838C>A SNP is a commonly encountered genetic variant that may help to explain some of the unpredictable failure risk associated with therapeutic cardiovascular interventions.
We acknowledge Carlie J.M. deVries (University of Amsterdam) for providing primer sequences, variant p27 promoter constructs, and for scientific advice in the genotyping experiments. Diana Kim assisted with management of the clinical database and blood samples for this project. Genomic DNA extraction was performed by the University of California, San Francisco, DNA Bank. Mark Caldwell provided technical assistance with sample genotyping. We also acknowledge participating surgeons from the Boston and Seattle hospitals who enrolled patients in the respective cohort studies used for this analysis, particularly Drs. Allen D. Hamdan (Beth Israel Deaconess Medical Center, Boston), Frank B. Pomposelli Jr (Beth Israel Deaconess Medical Center, Boston), and Joseph D. Raffetto (Boston VA Medical Center, Boston). Lihua Chen, PhD, assisted with performance of the p27 promoter assays.
Supplementary Material
Supplementary Fig (online only). Effect of the p27kip1-838C>A polymorphism on p27Kip1 promoter activity in human venous fibroblasts. Primary cultured adventitial fibroblasts from human saphenous vein (Kenagy et al J Vasc Surgery 2009; 49:1282-8) were transfected by electroporation with the indicated p27Kip1 promoter-luciferase constructs used by van Tiel et al (Circulation 2009; 120:669-676) containing the −838A or −838C variant. One day after transfection, cells were changed to serum-free medium for 24 hours and firefly luciferase activity measured. Luciferase activity was normalized to Renilla luciferase and expressed as fold of the empty pGL3 shuttle vector. Data shown are mean of four independent experiments (P < .006 by paired t test).
Supplementary Table I (online only). Patient inclusion and exclusion criteria for Seattle cohort
Supplementary Table II (online only). Characteristics of Seattle cohort by p27Kip1-838 genotype
Supplementary Table III (online only). Summary of clinical outcomes in Seattle cohort by p27Kip1-838 genotype
Acknowledgments
Supported by funding from the National Heart, Lung and Blood Institute (HL75771) to M.S.C., C.D.O., and M.A.C.; HL30946 to A.W.C. and R.D.K.; HL098227 to R.C.L.; Department of Veterans Affairs Office of Research Development, Clinical R&D to M.S.; and Vascular Cures Research Foundation to M.S.C. and A.W.C.
Footnotes
Author conflict of interest: none.
Presented at the 2011 Vascular Annual Meeting of the Society for Vascular Surgery, Chicago, Ill, June 16-18, 2011.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS Conception and design: MC, AC
Analysis and interpretation: MC, CO, MB, MC, KE, WG, RK, RL, MS, AC
Data collection: MC, CO, RK
Writing the article: MC, CO, KE, WG, RK, RL, AC
Critical revision of the article: MC, CO, MB, MC, KE, WG, RK, RL, MS, AC
Final approval of the article: MC, CO, MB, MC, KE, WG, RK, RL, MS, AC
Statistical analysis: MC, CO, KE, WG, RK
Obtained funding: MC
Overall responsibility: MC
REFERENCES
- 1.Kunlin J. Le traitement de l’artere obliterante par la greffe veineuse [The treatment of arterial obstruction by vein grafting] Arch Mal Coeur Vx. 1949:42. [Google Scholar]
- 2.Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg. 2011;54:1021–1031. e1. doi: 10.1016/j.jvs.2011.03.281. [DOI] [PubMed] [Google Scholar]
- 3.Riley RF, Don CW, Powell W, Maynard C, Dean LS. Trends in coronary revascularization in the United States from 2001 to 2009: recent declines in percutaneous coronary intervention volumes. Circ Cardiovasc Qual Outcomes. 2011;4:193–7. doi: 10.1161/CIRCOUTCOMES.110.958744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor LM, Jr, Porter JM. Clinical and anatomic considerations for surgery in femoropopliteal disease and the results of surgery. Circulation. 1991;83(2 Suppl):I63–9. [PubMed] [Google Scholar]
- 5.Shah DM, Darling RC, 3rd, Chang BB, Fitzgerald KM, Paty PS, Leather RP. Long-term results of in situ saphenous vein bypass. Analysis of 2058 cases. Ann Surg. 1995;222:438–46. doi: 10.1097/00000658-199510000-00003. discussion 446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conte MS, Belkin M, Upchurch GR, Mannick JA, Whittemore AD, Donaldson MC. Impact of increasing comorbidity on infrainguinal reconstruction: a 20-year perspective. Ann Surg. 2001;233:445–52. doi: 10.1097/00000658-200103000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berceli SA, Hevelone ND, Lipsitz SR, Bandyk DF, Clowes AW, Moneta GL, et al. Surgical and endovascular revision of infrainguinal vein bypass grafts: analysis of midterm outcomes from the PREVENT III trial. J Vas Surg. 2007;46:1173–9. doi: 10.1016/j.jvs.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen LL, Conte MS, Menard MT, Gravereaux EC, Chew DK, Donaldson MC, et al. Infrainguinal vein bypass graft revision: factors affecting long-term outcome. J Vasc Surg. 2004;40:916–23. doi: 10.1016/j.jvs.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Owens CD. Adaptive changes in autogenous vein grafts for arterial reconstruction: clinical implications. J Vasc Surg. 2010;51:736–46. doi: 10.1016/j.jvs.2009.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanner FC, Yang ZY, Duckers E, Gordon D, Nabel GJ, Nabel EG. Expression of cyclin-dependent kinase inhibitors in vascular disease. Circ Res. 1998;82:396–403. doi: 10.1161/01.res.82.3.396. [DOI] [PubMed] [Google Scholar]
- 11.Nabel EG. CDKs and CKIs: Molecular targets for tissue remodelling. Nat Rev Drug Discov. 2002;1:587–98. doi: 10.1038/nrd869. [DOI] [PubMed] [Google Scholar]
- 12.Chen D, Krasinski K, Sylvester A, Chen J, Nisen PD, Andres V. Downregulation of cyclin-dependent kinase 2 activity and cyclin A promoter activity in vascular smooth muscle cells by p27(KIP1), an inhibitor of neointima formation in the rat carotid artery. J Clin Invest. 1997;99:2334–41. doi: 10.1172/JCI119414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanner FC, Boehm M, Akyurek LM, San H, Yang ZY, Tashiro J, et al. Differential effects of the cyclin-dependent kinase inhibitors p27(Kip1), p21(Cip1), and p16(Ink4) on vascular smooth muscle cell proliferation. Circulation. 2000;101:2022–5. doi: 10.1161/01.cir.101.17.2022. [DOI] [PubMed] [Google Scholar]
- 14.Sedding DG, Seay U, Fink L, Owens CD, Ridker PM, Belkin M, et al. Mechanosensitive p27Kip1 regulation and cell cycle entry in vascular smooth muscle cells. Circulation. 2003;108:616–22. doi: 10.1161/01.CIR.0000079102.08464.E2. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez P, Diez-Juan A, Coto E, Alvarez V, Reguero JR, Batalla A, Andrés V, et al. A single-nucleotide polymorphism in the human p27kip1 gene (−838C>A) affects basal promoter activity and the risk of myocardial infarction. BMC Biol. 2004;2:5. doi: 10.1186/1741-7007-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Tiel CM, Bonta PI, Rittersma SZ, Beijk MA, Bradley EJ, Klous AM, et al. p27kip1-838C>A single nucleotide polymorphism is associated with restenosis risk after coronary stenting and modulates p27kip1 promoter activity. Circulation. 2009;120:669–76. doi: 10.1161/CIRCULATIONAHA.108.842179. [DOI] [PubMed] [Google Scholar]
- 17.Owens CD, Ridker PM, Belkin M, Hamdan AD, Pomposelli F, Logerfo F, et al. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007;45:2–9. doi: 10.1016/j.jvs.2006.08.048. discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owens CD, Rybicki FJ, Wake N, Schanzer A, Mitsouras D, Gerhard-Herman MD, et al. Early remodeling of lower extremity vein grafts: inflammation influences biomechanical adaptation. J Vasc Surg. 2008;47:1235–42. doi: 10.1016/j.jvs.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenagy RD, Fukai N, Min SK, Jalikis F, Kohler TR, Clowes AW. Proliferative capacity of vein graft smooth muscle cells and fibroblasts in vitro correlates with graft stenosis. J Vasc Surg. 2009;49:1282–8. doi: 10.1016/j.jvs.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno K, Murray-Wijelath J, Yagi M, Kohler T, Hatsukami T, Clowes A, et al. Circulating inflammatory cells are associated with vein graft stenosis. J Vasc Surg. 2011;54:1124–30. doi: 10.1016/j.jvs.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 22.Diez-Juan A, Castro C, Edo MD, Andres V. Role of the growth suppressor p27Kip1 during vascular remodeling. Curr Vasc Pharmacol. 2003;1:99–106. doi: 10.2174/1570161033386709. [DOI] [PubMed] [Google Scholar]
- 23.Boehm M, Olive M, True AL, Crook MF, San H, Qu X, et al. Bone marrow-derived immune cells regulate vascular disease through a p27(Kip1)-dependent mechanism. J Clin Invest. 2004;114:419–26. doi: 10.1172/JCI20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen HL, Liu K, Meng XY, Wen XD, You QS. Local application of rapamycin inhibits vein graft restenosis in rabbits. Transplant Proc. 2011;43:2017–21. doi: 10.1016/j.transproceed.2010.12.063. [DOI] [PubMed] [Google Scholar]
- 25.Owens CD, Ho KJ, Conte MS. Lower extremity vein graft failure: a translational approach. Vasc Med. 2008;13:63–74. doi: 10.1177/1358863X07083432. [DOI] [PubMed] [Google Scholar]
- 26.Gasper WJ, Owens CD, Kim JM, Hills N, Belkin M, Creager MA, et al. Early (30-day) vein remodeling is predictive of mid-term graft patency following lower extremity bypass. J Vasc Surg. 2012 doi: 10.1016/j.jvs.2012.06.098. in press [doi:10.1016/j.jvs.2012.06.098] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 28.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–51. doi: 10.1016/j.jvs.2005.12.058. discussion 751. [DOI] [PubMed] [Google Scholar]
- 29.Ellis SG, Chen MS, Jia G, Luke M, Cassano J, Lytle B. Relation of polymorphisms in five genes to long-term aortocoronary saphenous vein graft patency. Am J Cardiol. 2007;99:1087–9. doi: 10.1016/j.amjcard.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 30.Pereira AC, Miyakawa AA, Lopes NH, Soares PR, de Oliveira SA, Cesar LA, et al. Dynamic regulation of MTHFR mRNA expression and C677T genotype modulate mortality in coronary artery disease patients after revascularization. Thromb Res. 2007;121:25–32. doi: 10.1016/j.thromres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Emiroglu O, Durdu S, Egin Y, Akar AR, Alakoc YD, Zaim C, et al. Thrombotic gene polymorphisms and postoperative outcome after coronary artery bypass graft surgery. J Cardiothorac Surg. 2011;6:120. doi: 10.1186/1749-8090-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig (online only). Effect of the p27kip1-838C>A polymorphism on p27Kip1 promoter activity in human venous fibroblasts. Primary cultured adventitial fibroblasts from human saphenous vein (Kenagy et al J Vasc Surgery 2009; 49:1282-8) were transfected by electroporation with the indicated p27Kip1 promoter-luciferase constructs used by van Tiel et al (Circulation 2009; 120:669-676) containing the −838A or −838C variant. One day after transfection, cells were changed to serum-free medium for 24 hours and firefly luciferase activity measured. Luciferase activity was normalized to Renilla luciferase and expressed as fold of the empty pGL3 shuttle vector. Data shown are mean of four independent experiments (P < .006 by paired t test).
Supplementary Table I (online only). Patient inclusion and exclusion criteria for Seattle cohort
Supplementary Table II (online only). Characteristics of Seattle cohort by p27Kip1-838 genotype
Supplementary Table III (online only). Summary of clinical outcomes in Seattle cohort by p27Kip1-838 genotype


