Abstract
Background
To determine outcomes in breast cancer patients treated with neoadjuvant chemotherapy.
Methods
72 consecutive patients receiving neoadjuvant chemotherapy for breast cancer were enrolled.
Results
Mastectomy was avoided in 46% of patients and 42% converted to negative nodes after neoadjuvant chemotherapy. Thirteen patients (18%) achieved a pathologic complete response (pCR), which was associated with the ER−/Her2− subtype (58%), and was significantly less likely to occur in the ER+/Her2− subtype (2%), p<0.01. Patients with ER+/Her2+ subtype were most likely to have no response or progression (NR/P) during chemotherapy compared to ER−/Her2− subtype (50% vs 0%, p=0.01). The five-year survival for patients achieving a pCR was 100% compared to 74% in the partial response group and 48% in the NR/P group (p=0.01).
Conclusions
Neoadjuvant chemotherapy for patients with advanced breast cancer provided prognostic information, allowed evaluation of response to chemotherapy, decreased mastectomy rate and potentially reduced the need for axillary node dissection.
Keywords: Breast Cancer, Neoadjuvant Chemotherapy, Surgery, Mastectomy, Triple Negative, Pathologic Complete Response
INTRODUCTION
Breast cancer remains the most common cancer that affects women, with an estimated 230,480 new diagnoses in the United States in 2011. Despite the evolution of treatment, breast cancer persists as the second most common cause of cancer death in women 1. Treatment modalities for breast cancer have evolved significantly over the past 25 years. Historically, neoadjuvant chemotherapy was utilized only in patients with inoperable disease. However, since 1995, studies have shown that neoadjuvant chemotherapy is also beneficial in those with operable breast cancer. 2–5 There is the potential for neoadjuvant chemotherapy to downstage disease allowing for breast conserving treatment (BCT) in patients who otherwise might require a mastectomy. 6–8 Meta-analysis of trials looking at the accuracy of sentinel lymph node biopsy (SLNB) in clinically node negative patients after neoadjuvant chemotherapy has found that SLNB can be performed in this patient population and predicts axillary disease with similar accuracy to patients with early stage breast cancer who did not receive neoadjuvant chemotherapy9. Therefore, patients with clinically node positive disease who have a good clinical response to neoadjuvant therapy and convert to clinically node negative disease, with a negative SLNB may be able to avoid axillary node dissection.
Receptor status [estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor (HER2)] has led to the classification of subtypes of breast cancer. Each subtype represents a different tumor biology with distinct behavior, response to treatment and recurrence and survival patterns. 10–12 Her2 overexpressing tumors have been shown to have a high rate of pathologic complete response (pCR) when treated with neoadjuvant chemotherapy including trastuzumab13. Despite lower rates of pCR, patients with ER expressing breast cancers have been shown in a large series to have an improved five-year survival over other subtypes14.
Neoadjuvant chemotherapy is currently regarded as equivalent to adjuvant chemotherapy for advanced breast cancer in terms of overall and disease free survival. Theoretical advantages of neoadjuvant chemotherapy include an identifiable tumor to measure effectiveness of a therapeutic regimen and decreasing disease burden to allow less extensive surgery. Previous studies have investigated the response of advanced breast cancers to neoadjuvant chemotherapy. Improved survival has been noted in patients achieving pathologic complete response (pCR) and increased rates of locoregional recurrence have been observed in patients with triple negative breast cancer 11,15. However, less is understood about how each subtype responds distinctly to neoadjuvant chemotherapy, and for which, if any, subtype there is a differential benefit to neoadjuvant chemotherapy. The goal of this study was to determine the outcomes for patients with advanced breast cancer treated with neoadjuvant chemotherapy at a single institution. We analyzed, by stage and subtype, rates of conversion to BCT, nodal clearance, pCR, and recurrence and death to better understand the role of neoadjuvant chemotherapy for each tumor subtype.
METHODS
Patients
Following institutional review board approval, retrospective chart review was performed on all patients who received neoadjuvant chemotherapy followed by surgical resection for primary breast cancer at the University of Iowa Holden Comprehensive Cancer Center from June 2005 to March 2011. Patients were categorized according to the sixth edition of the AJCC staging manual and patients with stage II and stage III disease were enrolled. Patients with stage IV disease and inflammatory cancer were excluded because they could not be evaluated for conversion to breast conserving treatment and recurrence. Chemotherapy regimen was determined by the treating medical oncologist in collaboration with the institutional tumor board and was based on clinical trials and national standards at the time of treatment. The majority of patients were initially started on combination therapy with adriamycin, cyclophosphamide, and paclitaxel. Patients received adjuvant endocrine therapy and trastuzumab according to institutional standards and receptor status.
Subtype was stratified according to receptor status on immunohistochemistry with relation to estrogen receptor (ER) and Her2 overexpression, which was confirmed with a fluorescent in situ hybridization (FISH) score of greater than or equal to 2+. Staining for progesterone receptor (PR) was also performed on all patients but not used for stratification in this study. Conversion to breast conserving therapy was defined as patients that were not eligible for breast conserving treatment at their initial visit and who were clinically determined to require a mastectomy, who following neoadjuvant chemotherapy did undergo a breast conserving operation. Nodal clearance was defined as patients with clinically node positive disease (90% proven on cytology), who were subsequently found to have no nodal disease on surgical resection. Patient responsiveness to chemotherapy was categorized as no response or progression (NR/P), partial response, or pathologic complete response (pCR). NR/P was defined by lack of clinical response or progression during the primary chemotherapy treatment resulting in a change in planned chemotherapy regimen at the discretion of the treating medical oncologist. A pCR was defined as no evidence of malignant disease, including DCIS, in the primary tumor site or lymph nodes at surgical excision. All patients not having NR/P or pCR as above were defined as having a partial response. RECIST criteria were not used to monitor response in this study because our patients are not routinely imaged before and after neoadjuvant chemotherapy. Recurrence was defined by evidence of local, regional, or distant disease not present in the immediate post-operative period, while death was defined as patient death over the study period.
Statistical Analysis
Univariate analysis was performed with Student T test with continuous variables and frequency association with categorical variables. Chi square test and Fisher’s exact test were performed for all frequency association analysis. P values from Chi square test were used when the results were consistent and there was no concern over continuity correction. Otherwise p values from Fisher’s exact test were used. We used Cox regression models consistently to evaluate survival when the proportional hazard model assumptions were met. Stata 11.2 (StataCorp College Station, Texas) was used for all analyses. Significance was defined as p < 0.05.
RESULTS
Seventy-two patients were treated with neoadjuvant chemotherapy followed by surgical resection for stage II or III breast cancer at the University of Iowa Holden Comprehensive Cancer Center from June 2005 to March 2011. The mean age was 49 years (range 28 – 72). The mean length of follow up was 33 months. Patients enrolled by subtype were ER+/Her2− 39 (54%), ER+/Her2+ 4 (6%), ER−/Her2+ 10 (14%), ER−/Her2− 19 (26%), table 1. Clinical stage at diagnosis was stage II, 33 patients (46%) and stage III, 39 patients (54%).
Table 1.
Response to neoadjuvant chemotherapy as a function of phenotype
| ER+/Her2− | ER+/Her2+ | ER−/Her2+ | ER−/Her2− | P Value | |
|---|---|---|---|---|---|
| Patients | 39 (54%) | 4 (6%) | 10 (14%) | 19 (26%) | |
| Age (Mean±SD) | 50.5±10.6 | 51.8±6.9 | 46.0±11.6 | 48.7±8.4 | 0.597 |
| Convert to BCT# | 18 (53%) | 1 (25%) | 4 (40%) | 8 (42%) | 0.702 |
| Nodal Clearance* | 9 (29%) | 1 (33%) | 5 (71%) | 9 (56%) | 0.094 |
| pCR | 1 (3%) | 0 (0%) | 1 (10%) | 11 (58%) | <0.001 |
| No response or Progression | 5 (13%) | 2 (50%) | 3 (30%) | 0 (0%) | 0.015 |
| Recurrence | 6 (15%) | 2 (50%) | 4 (40%) | 3 (16%) | 0.139 |
| Death | 2 (5%) | 1 (25%) | 3 (30%) | 2 (11%) | 0.064 |
P Value refers to Analysis of Variation (ANOVA) calculation and reports the most significant value in the comparison of all values to all other values in a row.
Excludes patients who were candidates for BCT prior to neoadjuvant chemotherapy
Excludes patients presenting with node negative disease
Conversion to Breast Conserving Treatment
Of the 72 patients enrolled, 5 were eligible for BCT prior to neoadjuvant chemotherapy. All five of these patients underwent BCT and were not included in the conversion to BCT analysis. Thirty-one of the remaining 67 patients (46%) were able to undergo BCT following neoadjuvant chemotherapy. The association between conversion to BCT and subtype is listed in table 1. Conversion to BCT was more common in patients with stage II disease (53%) compared to stage III (41%) without statistical significance. The most likely subtype to convert to BCT was ER+/Her2− (53%) but the association between subtype and conversion to BCT did not approach statistical significance.
Nodal Clearance
Of the 72 enrolled patients, 57 (79%) were staged at the time of presentation as having clinically positive axillary nodes with 52 (91%) of these having cytologic confirmation of nodal metastasis by fine needle aspiration biopsy (FNAB). Of the 57 patients with nodal involvement at presentation, 24 (42%) had negative axillary nodes on surgical resection (9 had a negative SLNB and 15 had ALND with no nodal metastasis identified) after neoadjuvant chemotherapy. There were no instances of axillary recurrence among the patients who converted from a positive to a negative axilla over the study period either in the SLNB or ALND groups. There were 15 patients who presented with clinically negative nodes prior to chemotherapy. All of these 15 patients underwent SLNB after neoadjuvant chemotherapy and no patient had evidence of nodal metastasis and no instance of nodal recurrence over the study period. The most likely subtypes to convert to negative nodal status after neoadjuvant chemotherapy were ER−/Her2+ (71%) and ER−/Her2− (56%), with the ER+/Her2− (29%) and ER+/Her2+ (33%) groups tending to be less likely to have axillary nodal downstaging, p=0.09. There was a trend toward patients with stage II disease to convert to negative nodal status (56%) compared to stage III (31%), p=0.06.
Clinical and Pathologic Response
Response to neoadjuvant chemotherapy was stratified into three categories; no response or progression (NR/P), partial response, and pathologic complete response (pCR). Ten of the 72 patients (14%) enrolled had NR/P to their initial chemotherapy regimen and were switched to a different neoadjuvant treatment at the discretion of the treating medical oncologist. Patients with the ER+/Her2+ subtype were most likely to have NR/P on chemotherapy (50%), while the ER−/Her2− subtype was least likely to have NR/P (0%), p=0.01. There was no difference between stage II (12%) and stage III (15%) in rate of NR/P. Thirteen patients (18%) achieved a pCR following neoadjuvant chemotherapy. A pCR was associated with the ER−/Her2− subtype (58%) and was less likely to occur in patients with the ER+/Her2+ (0%) and ER+/Her2− (3%) subtypes (p<0.001). There was a trend for patients with stage II disease to have a pCR (24%) compared to stage III (13%), but without statistical significance.
Recurrence and Death
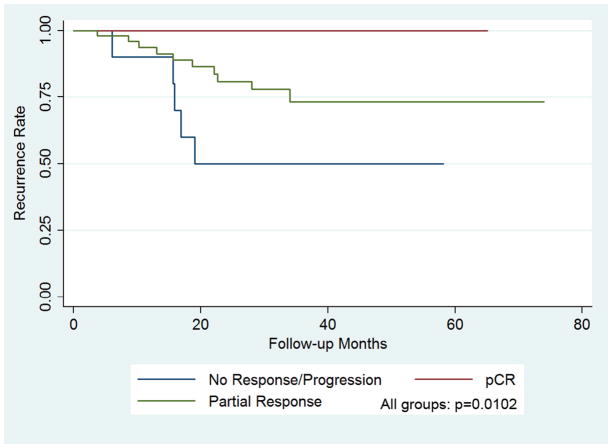
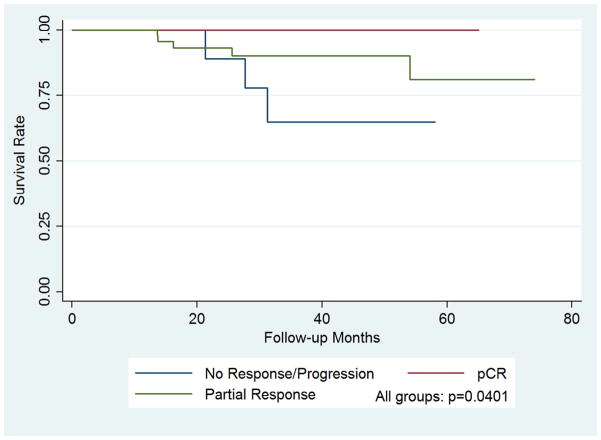
During the study period, 15 of the 72 patients (21%) had a recurrence (two local both following mastectomy, one in the axilla following ALND, and 12 distant). The patient with recurrent axillary disease was staged as positive clinically and pathologically following ALND, and no patient that underwent SLNB without ALND had axillary recurrence over the study period. Patients with the ER+/Her2+ subtype (50%) were most likely to have recurrence but without significance. Additionally, patients with stage II vs. stage III disease at presentation were less likely to have a recurrence (15% vs 26%) without significance. There were 8 deaths (11%) during the study period. There was a trend between death and the ER−/Her2+ subtype compared to the other subtypes (30%, p=0.06). There was a reduction in the incidence of death in patients with stage II disease compared to stage III (3% vs 18%, p=0.06) over the study period. No patient who achieved a pCR had a recurrence compared to patients with a partial response (20%) or NR/P (50%), p=0.01. Additionally, there were no deaths recorded in patients achieving a pCR compared to partial response (10%) and NR/P (30%), p=0.04, table 2. Five-year disease free and overall survival was 100% in the pCR group compared to 74% in the partial response and 48% in the poor response groups, figures 1 and 2.
Table 2.
Recurrence and death as a function of response to neoadjuvant chemotherapy
| pCR | Partial Response | No Response or Progression | P value | |
|---|---|---|---|---|
| Patients | 13 (18%) | 49 (68%) | 10 (14%) | |
| Recurrence | 0 (0%) | 10 (20%) | 5 (50%) | 0.012 |
| Disease Free Survival | 0.010 | |||
| 3-year | 100% | 74% | 48% | |
| 5-year | 100% | 74% | 48% | |
| Death | 0 (0%) | 5 (10%) | 3 (30%) | 0.078 |
| Overall Survival | 0.040 | |||
| 3-year | 100% | 74% | 48% | |
| 5-year | 100% | 74% | 48% | |
Significance calculations were performed with the Cox regression model and represent a comparison between all three groups.
Figure 1. Kaplan-Meier plot demonstrating disease free survival as a function of response to chemotherapy.
Achieving a pCR was predictive of a 100% 5 year disease free survival compared to 74% for patients with a partial response and 48% for patients with no response or progression on neoadjuvant chemotherapy, p=0.01.
Figure 2. Kaplan-Meier plot demonstrating overall survival as a function of response to chemotherapy.
Achieving a pCR was predictive of a 100% 5 year survival compared to 74% for patients with a partial response and 48% for patients with no response or progression on neoadjuvant chemotherapy, p=0.04.
DISCUSSION
This study was designed to assess the response of patients with breast cancer that were treated with neoadjuvant chemotherapy at a single institution. Over the study period neoadjuvant chemotherapy increased eligibility for BCT in 46% of patients, down-staged the axilla in 42%, and provided important prognostic information about recurrence and death risk based on the observed clinical and pathologic response.
As has been previously reported, patients in this study that had a clinical or pathologic response to neoadjuvant chemotherapy had lower rates of recurrence and death over our study period 11,15,16. Our results from a smaller volume institution corroborate that patients with a pCR after neoadjuvant chemotherapy defined patients with improved five year disease free and overall survival. In addition, the ER−/Her2− or triple negative subtype was significantly associated with pCR, demonstrating a more pronounced response to neoadjuvant chemotherapy compared to other subtypes. These findings are in accordance with a large study that also demonstrated improved locoregional recurrence for triple negative breast cancers following neoadjuvant chemotherapy 11. Although five-year survival remains worse in patients with triple negative breast cancer, the data suggest that when given neoadjuvant chemotherapy, a subgroup of patients with this biologic subtype may be more likely to avoid aggressive surgical treatment than those of other subtypes. Furthermore, pCR achieved with neoadjuvant chemotherapy may help define a subtype of triple negative breast cancer patients with an improved predicted outcome.
The current study offers several new findings that relate to conversion to BCT and the possibility to downstage axillary disease with the potential of avoiding ALND. Conversion of patients from mastectomy to BCT is an important outcome as it decreases operative extent, reduces the morbidity associated with mastectomy, and reduces the need for extensive breast reconstruction. Mastectomy has a complication rate ranging from 14%–64% which can be significantly lowered with lumpectomy.17,18 In this cohort, patients that were identified for treatment at stage II were the most likely to become eligible for BCT. A recent study has shown improved clinical outcomes for patients with triple negative breast cancer who undergo lumpectomy instead of mastectomy 16. In our series we did not see a statistical difference for conversion to BCT based on subtype.
Nodal status is an important marker for treatment planning and patient counseling. Recent studies in stage I/II breast cancer patients treated with BCT that included sentinel lymph node biopsy (SLNB) have indicated that there is no survival benefit in clinically node negative patients to performing an axillary node dissection (ALND) after SLNB, even in the presence of positive axillary nodes 19. Previous studies have found no difference in the false negative rates of SLNB between patients treated with neoadjuvant compared to patients not receiving chemotherapy20–23. Further study is still needed in this population to demonstrate the effect on regional recurrence and survival of not performing ALND in this population. We found that 24 of the 57 (42%) patients that were staged with positive nodes prior to chemotherapy had negative nodal pathology on resection. In our study, the mean follow up of 33 months should have been a sufficient time interval for regional recurrence to present. One patient did present with axillary recurrence, but this patient underwent ALND at her initial operation. The data from this study suggest that patients whose axillary status is clinically down-staged by neoadjuvant chemotherapy may be able to avoid ALND and thus be managed in a fashion that is similar to patient who present with clinically node-negative disease. Conversion from positive to negative nodal status after neoadjuvant chemotherapy indicates the effectiveness of neoadjuvant chemotherapy in a high percentage of patients by reducing disease burden regionally. In some patients, metastases have distinct expression profiles and may demonstrate different sensitivity to chemotherapy compared to the primary tumor. However, effectiveness against local disease still provides some indication of distant efficacy. In patients with a clinically positive axilla who convert to a clinically negative axilla after neoadjuvant chemotherapy, further study could suggest that mandatory ALND may not be necessary in these patients. Avoiding an ALND and its additional cost as well as potential adverse sequelae in patients who present with a clinically positive axilla may be an additional benefit of neoadjuvant chemotherapy.
Administering chemotherapy when there is an observable tumor allows for evaluation of response to the regimen. This provides an opportunity to change the regimen to a potentially more effective treatment if there is a lack of tumor response or progression. An example in our series illustrates the benefit from this approach: a 48 year old woman presented with a T3, N1, M0 (IIIA) triple negative infiltrating ductal carcinoma. As she desired breast conservation, she was started on neoadjuvant chemotherapy with docetaxel. Following two cycles of treatment she was noted to have clinical progression of the primary tumor and increased fullness in the axilla. Her chemotherapy was changed to adriamycin and cyclophosphamide with bevacizumab, to which she had a clinical response. She underwent BCT with final pathology showing no invasive disease in the breast and only 1 of 17 lymph nodes positive. She is currently 32 months from her initial presentation without evidence of disease. Neoadjuvant chemotherapy provided an opportunity to assess the effectiveness of the initial chemotherapy regimen in this patient, and subsequently change therapy, which had a positive effect on her disease. Had she undergone mastectomy followed by adjuvant chemotherapy there would have been no opportunity to assess for progression, she would not have benefited from the different chemotherapeutic regimen and she would have had a more extensive operation.
In summary, neoadjuvant chemotherapy increases eligibility for BCT by 46% in patients with advanced breast cancer, improving morbidity and cosmesis. Neoadjuvant chemotherapy is also effective in reducing burden of disease in the axilla and could potentially decrease the extent of axillary surgery in a significant percentage of patients who present with clinically node positive disease. These results are in agreement with results from larger centers demonstrating similar effectiveness of neoadjuvant chemotherapy in a lower volume institution. Treating patients with advanced breast cancer with neoadjuvant chemotherapy provides an opportunity to assess responsiveness to a regimen on a patient by patient basis, and provides prognostic information based on clinical and pathologic responsiveness. Neoadjuvant chemotherapy should be considered for patients with stage II and higher breast cancer, and especially the triple negative subtype, to increase eligibility for BCT, decrease need for ALND, decrease tumor burden, and to assess tumor responsiveness to treatment.
SUMMARY.
Neoadjuvant chemotherapy successfully down-staged breast cancer patients thereby reducing the need for mastectomy and potentially eliminating the need for axillary node dissection in selected patients. Response to neoadjuvant chemotherapy predicted disease-free and overall five-year survival. The triple negative subtype demonstrated an increased rate of pathologic and clinical response to neoadjuvant chemotherapy compared to other subtypes and demonstrated the greatest benefit of neoadjuvant treatment.
Acknowledgments
This work was supported by the Kristen Olewine Milke Breast Cancer Research Fund.
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RWE, Brawley O, Jemal A. Cancer Statistics, 2011. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–74. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Powles TJHT, Makris A, et al. Randomized trial of chemoendocrine therapy started before or after surgery for treatment of primary breast cancer. J Clin Oncol. 1995;13:547–52. doi: 10.1200/JCO.1995.13.3.547. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham JDW, SE, Ahmed S, et al. The efficacy of neoadjuvant chemotherapy compared to postoperative therapy in the treatment of locally advanced breast cancer. Cancer Invest. 1998;16:80–6. doi: 10.3109/07357909809039761. [DOI] [PubMed] [Google Scholar]
- 5.Fisher BBJ, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 6.Chen AM, et al. Breast Conservation After Neoadjuvant Chemotherapy: the MD Anderson Cancer Center Experience. J Clin Oncol. 2004;22(12):2303–12. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 7.Cance WG, et al. Long Term Outcome of Neoadjuvant Therapy for Locally Advanced Breast Carcinoma: Effective Clinical Downstaging Allows Breast Preservation and Predicts Outstanding Local Control and Survival. Ann Surg. 2002;236(3):295–302. doi: 10.1097/01.SLA.0000027526.67560.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonadonna G, et al. Primary Chemotherapy in Operable Breast Cancer: Eight-Year Experience at the Milan Cancer Institute. J Clin Oncol. 1998;16(1):93–100. doi: 10.1200/JCO.1998.16.1.93. [DOI] [PubMed] [Google Scholar]
- 9.Tan VK, Goh BK, Fook-Chong S, Khin LW, Wong WK, Yong WS. The feasibility and accuracy of sentinel lymph node biopsy in clinically node-negative patients after neoadjuvant chemotherapy for breast cancer--a systematic review and meta-analysis. J Surg Oncol. 2011;104:97–103. doi: 10.1002/jso.21911. [DOI] [PubMed] [Google Scholar]
- 10.Sorlie TPC, Tibshirani R, et al. Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses with Clinical Implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayers MOK-DN, Ollila DW, et al. Impact of Breast Cancer Molecular Subtypes on Locoregional Recurrence in Patients Treated with Neoadjuvant Chemotherapy for Locally Advanced Breast Cancer. Ann Surg Oncol. 2011;18:2851–7. doi: 10.1245/s10434-011-1665-8. [DOI] [PubMed] [Google Scholar]
- 12.Rouzier RPC, Symmans WF, et al. Breast Cancer Molecular Subtypes Respond Differently to Preoperative Chemotherapy. Clin Cancer Res. 2005;11(16):5678–85. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 13.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–44. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 15.Fisher CSMC, Gillanders WE, et al. Neoadjuvant Chemotherapy is Associated with Improved Survival Compared with Adjuvant Chemotherapy in patients with Triple-Negative Breast Cancer Only after Complete Pathologic Response. Ann Surg Oncol. 2012;(1):253–8. doi: 10.1245/s10434-011-1877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdulkarim BSCJ, Hanson J, Deschenes J, Lesniak D, Sabri S. Increased Risk of Locoregional Recurrence for Women with T1-2N0 Triple-Negative Breast Cancer Treated with Modified Radical Mastectomy Without Adjuvant Radiation Therapy Compared with Breast-Conserving Therapy. JCO. 2011;29(21):2852–8. doi: 10.1200/JCO.2010.33.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holzgreve WBF. Surgical complications and follow-up evaluation of 163 patients with subcutaneous mastectomy. Aesthetic Plast Surg. 1987;11:45–8. doi: 10.1007/BF01575482. [DOI] [PubMed] [Google Scholar]
- 18.Greco JA, 3rd, Castaldo ET, Nanney LB, et al. Autologous breast reconstruction: the Vanderbilt experience (1998 to 2005) of independent predictors of displeasing outcomes. Journal of the American College of Surgeons. 2008;207:49–56. doi: 10.1016/j.jamcollsurg.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano AEHK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canavese GDB, Vecchio C, Tomei D, Villa G, Carli F, Del Mastro L, Levaggi A, Rosello C, Spinaci S, Bruzzi P, Catturich A. Accuracy of Sentinel lymph node biopsy after neo-adjuvant chemotherapy in patients with locally advanced breast cancer and clinically positive axillary nodes. EJSO. 2011;37(8):688–94. doi: 10.1016/j.ejso.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Newman EA, Sabel MS, Nees AV, et al. Sentinel lymph node biopsy performed after neoadjuvant chemotherapy is accurate in patients with documented node-positive breast cancer at presentation. Ann Surg Oncol. 2007;14:2946–52. doi: 10.1245/s10434-007-9403-y. [DOI] [PubMed] [Google Scholar]
- 22.Shen J, Gilcrease MZ, Babiera GV, et al. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer. 2007;109:1255–63. doi: 10.1002/cncr.22540. [DOI] [PubMed] [Google Scholar]
- 23.Classe JM, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol. 2009;27:726–32. doi: 10.1200/JCO.2008.18.3228. [DOI] [PubMed] [Google Scholar]