Abstract
There are many human extra-renal tissues and cells that biosynthesize 1α,25-dihydroxyvitamin D (1α,25(OH)2D) by the action of CYP27B1/1α-hydroxylase. Human marrow stromal cells (hMSCs), also known as mesenchymal stem cells, were isolated from marrow discarded from well-characterized, consented subjects during common orthopedic procedures. Human MSCs can give rise to osteoblasts, chondrocytes, adipocytes, and other lineages. Their in vitro differentiation to osteoblasts is stimulated by 1α,25(OH)2D, and recent evidence indicates that they have the capacity to metabolize vitamin D in a regulated manner. Human MSCs express the vitamin D receptor, 25-hydroxylases, 1α-hydroxylase, and 24-hydroxylase; stimulation of in vitro osteoblastogenesis by 25(OH)D depends on the activity of CYP27B1/1α-hydroxylase. Finding that hMSCs are a both a producer and target of 1α,25(OH)2D suggests a potential autocrine/paracrine role of vitamin D metabolism in osteoblast differentiation. Expression and enzyme activity of CYP27B1/1α-hydroxylase are upregulated by substrate 25(OH)D and Parathyroid Hormone (PTH) and are downregulated by 1α,25(OH)2D. With subject age, there is a decrease in basal osteoblast potential and in stimulation of osteoblastogenesis by 1α,25(OH)2D, 25(OH)D, and PTH. In vitro treatment with a combination of 25(OH)D and PTH rejuvenated osteoblastogenesis with hMSCs from elders; this was attributable to increases in CYP27B1/1α-hydroxylase and in receptor for each hormone by the reciprocal factor. Other clinical variables beside age, i.e. low serum 25(OH)D or low estimated glomerular filtration rate, are correlated with reduced osteoblastogenesis. These studies suggest that osteoblastogenesis may not be optimal unless there is sufficient serum 25(OH)D substrate for hMSCs to synthesize and respond to local 1α,25(OH)2D.
Keywords: Osteoblast differentiation, CYP27B1, Aging, 25-hydroxyvitamin D
1. Introduction
Human bone marrow stromal cells (hMSCs) are also known as human mesenchymal stem cells or marrow-derived skeletal stem cells. Human MSCs are multipotential progenitor cells capable of differentiation into osteoblasts, chondrocytes, adipocytes, other connective tissue cells [1–3], and possibly other cell types such as neuronal cells [4] or hepatocytes [5]. The active metabolite of vitamin D, 1α,25-dihydroxyvitamin D (1α,25(OH)2D3) or calcitriol, is an important regulator of mineral and bone metabolism. Calcitriol regulates proliferation, differentiation, and function of many cell types, both normal and malignant [6]. Human MSCs are a target of calcitriol action to promote their differentiation to osteoblasts [7]. Osteoblastogenesis is also stimulated by 25-hydroxyvitamin D3 (25(OH)D3) [8], an effect that requires conversion to 1α,25(OH)2D3 by 1α-hydroxylase (CYP27B1) [9]. This review summarizes the latest information concerning the significance of vitamin D metabolism in hMSCs.
2. Impact of method to isolate hMSCs
Stem cells or progenitors are defined by the ability to proliferate and to differentiate; for hematopoietic cells, those properties are monitored by colony assays in semi-solid media. For MSCs, proliferative capacity is recognized by the development of monolayer colonies of plastic-adherent cells from a single cell; these are termed Colony-Forming Unit-Fibroblasts (CFU-F). MSCs are defined functionally by adherence to plastic surfaces and by the potential to give rise to osteoblasts, chondrocytes, and adipocytes [1–3]. In vitro differentiation is demonstrated by specific lineage markers that arise when the cells are cultured in lineage-specific media and supplements for osteoblasts [8–13], adipocytes [14–16], or chondrocytes [16]. Because there are changes in cell behaviors associated with prolonged culture, such as culture stress or in vitro senescence, it is important to use hMSCs from different patients at the same passage for each experiment [17]. In addition, many in vitro behaviors and baseline characteristics of hMSCs depend on clinical features of the subjects from whom the cells were isolated, including age [10–13,17], gender [17], vitamin D status [8,18], and kidney status [18]. Human MSCs can be obtained from marrow aspirates from volunteers, from cadaveric bones, or from tissues discarded during orthopedic surgery, for example, for joint replacement due to debilitating hip or shoulder osteoarthrosis. It is also important to be aware of the method by which the hMSCs were prepared because of potential impact of accompanying cell types and their products that are present within the preparation.
Recent studies demonstrate that various tissues and organs are sources of multipotential progenitors, including marrow, adipose tissue, and dental tissues, but different MSC populations can exhibit significant differences in their proliferation, differentiation, and molecular phenotype [19]. The major methods for isolation hMSCs from bone marrow are outlined here to highlight differences in the composition of the sample. Bone marrow contains hematopoietic stem cells and the adherent fraction that gives rise to hMSCs. When whole marrow is transferred to tissue culture dishes, the hematopoietic fraction does not adhere to plastic, whereas the skeletal progenitor cells are adherent. The adherent layer also includes mature cells like macrophages, macrophage colonies, endothelial cells, and epithelioid cells. When the hematopoietic cells are left in the dish and an adherent stromal layer is allowed to develop, the former will adhere to the attached cells and will persist, depending on the factors produced by the stroma. The interactions between those fractions of cells depend on cell-to-cell contacts and are referred to as “juxtacrine” stimulation between cell surface ligands on one cell type and cell-surface receptors on the other; the interactions can be reciprocal [20]. When whole marrow is cultured at high initial seeding density, the adherent layer forms rapidly and produces factors that support continuous hematopoiesis in vitro [21]. If the non-adherent hematopoietic cells are thoroughly removed a day or two after seeding, the adherent cells expand and gives rise to proliferative MSCs, thus diluting the non-adherent and non-proliferating cells. The early principles for identification and characterization of MSCs were established by Friedenstein [22] and others [23] for animal species that have small amounts of available marrow. The general procedure for studying mouse MSCs includes mature and progenitor cells and small aggregates of cells. Many studies with human cells follow a similar protocol. It is important to appreciate the methods used in order to properly interpret results and to compare studies.
2.1 Human MSCs from whole marrow cultures
There are several ways to establish whole marrow cultures for hMSC studies. In D’Ippolito’s study, for example, bone marrow was obtained from trabecular bone chips by gentle rocking with medium [24]. Adherent cells from this preparation include fibroblastic spindle-shape cells, monocytes, macrophages, endothelial cells, and multinucleated osteoclasts.
Other studies use hMSCs prepared from single-cell-colonies and from pooled colonies [2]. Marrow aspirates and surgical fragments of trabecular bone and marrow were scraped gently and repeatedly to release a suspension of marrow cells. The hMSCs from single-colonies varied widely in differentiation capacities; some strains developed extensive bone and hematopoietic tissue in vitro, some strains formed little bone, and others formed only fibrous tissue. Clonal stromal cell lines immortalized by transfection with SV40 for studies of regulation of stromal support of hematopoiesis [25] have been used for comparison of properties with normal hMSCs [16,26]. Although immortalized MSCs and cell lines can be useful because of the availability of large numbers of cells, they may not represent the biology of normal cells.
2.2 Human MSCs from density centrifugation protocols
This method is designed to enrich for progenitor cells by centrifugation with a viscous medium that separates cells based upon their density. We developed a standardized protocol that isolates hMSCs from bone and marrow discarded during orthopedic surgery for hip and shoulder osteoarthrosis (Figure 1), with a yield of low-density mononuclear cells between 40 to 800 million per subject [27]. This fraction contains progenitors for both hematopoietic and mesenchymal cells, but the non-adherent hematopoietic progenitor cells can be removed to allow the adherent hMSCs to proliferate. Chang et al. compared the ability of two products, Ficoll and Percoll, to isolate MSCs from human bone marrow [28]. They demonstrated that the Ficoll methodology was superior in isolating cells with osteoblast lineage potential. A Ficoll-containing device was commercialized and shown to increase yield of hMSCs from marrow aspirate, compared with a manual procedure [29].
Figure 1. Ficoll isolation method for hMSCs.
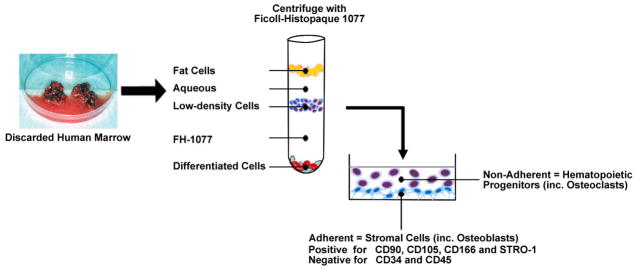
Discarded human bone and marrow tissues are minced in PBS with EDTA to release cells. After passage through a sieve to remove bone particles, cells are collected by centrifugation and resuspension in PBS. Ficoll-Histopaque 1077 (FH-1077, a medium with density of 1.077 g/mL) is carefully added beneath the aqueous cell suspension. Centrifugation separates marrow cells into three major fractions. The fat cells float at the top, and the mature, differentiated erythrocytes and leukocytes sediment at the bottom. Low-density undifferentiated cells accumulate at the interface between the aqueous and FH-1077 layers. This fraction contains progenitors for both hematopoietic and mesenchymal cells. They are collected, washed, and seeded into tissue culture dishes. After 2–3 days incubation, the non-adherent hematopoietic lineage cells, which includes osteoclast progenitors, are removed, allowing the adherent stromal cells to expand. The enriched adherent cells are called hMSCs, which are positive for CD90, CD105, CD166, and STRO-1, and negative for CD34 and CD45.
Currently, there are several commercial sources of hMSCs. The companies state that they use Ficoll or Histopaque protocols for their products.
2.3 Human MSCs from sorting with various antibodies
Fluorescence Activated Cell-Sorting (FACS) methods can be used to isolate a population of cells enriched for a phenotype on the basis of cell-surface epitopes. The sorting machine physically separates a mixture of cells into different containers, one cell at a time, based upon cell size and amount of attached fluorescent antibody. STRO-1 antibody is one of the first antibodies shown to enrich hMSCs, when it was found to bind with high affinity to an uncharacterized cell surface epitope expressed by hMSCs and erythrocytic cells [30]. In a direct comparison of protocols, we found similar biological properties in cell populations isolated with STRO-1 sorting and with the simpler Ficoll method [13]. Enrichment of hMSCs by FACS can be achieved by combining antibodies [31–33]. Alternatively, immunomagnetic isolation methods can be used for smaller samples [34].
Although sorting techniques are helpful in separating MSCs from hematopoietic cells, the methods are approximations dependent on the specificity and binding affinity of antibodies and on compromises between threshold settings and recovery yields. Wagner et al. have warned that cell surface markers were not useful to discriminate between human MSCs from different sources and fibroblasts that have no differentiation potential [35]. A panel of 22 surface markers did not discern any differences in hMSCs from marrow, adipose tissue, and cord blood, but neither could it distinguish those hMSCs from dermal fibroblasts.
3. Vitamin D metabolism
Cholecalciferol (vitamin D3) is synthesized from 7-dehydrocholesterol in the skin by exposure to ultraviolet light from the sun. Alternatively, vitamin D, in the form of ergocalciferol (vitamin D2) from plants or vitamin D3 (from animals), can be obtained from supplements or dietary sources. Biological activation of vitamin D, a two-step process, starts with carbon-25-hydroxylation to calcidiol (25-hydroxyvitamin D, 25(OH)D) primarily by the cytochrome enzymes CYP2R1 and CYP27A1, and subsequent carbon-1α-hydroxylation by CYP27B1/1α-hydroxylase [36]. The CYP24A1/24-hydroxylase regulates and inactivates 1α,25(OH)2D or 25(OH)D in kidney, skin, and bone cells [37–39].
Cells that contain functional CYP27B1/1α-hydroxylase, such as kidney cells, can convert calcidiol (25(OH)D) to 1α,25(OH)2D. Emerging data suggest that besides kidney cells, many other cells including bone cells have the ability to generate 1α,25(OH)2D (Table 1). There are differences that have been reported for extra-renal biosynthesis of 1α,25(OH)2D. For example, the regulation of 1α,25(OH)2D3 production in keratinocytes is more sensitive to inhibition by exogenous 1,25(OH)2D than is the renal production of 1α,25(OH)2D [40,41]. Thus, at normal circulating levels of free 1α,25(OH)2D, production of that metabolite by epidermal cells may be more inhibited than is its production by renal tubules. Biosynthesis of 1α,25(OH)2D3 also occurs by cells in the immune system [42], such as human monocyte-derived dendritic cells [43], myelomonocytic cell line [44], cultured alveolar macrophages [45], and in human prostate and other cancer cells [46].
Table 1.
Expression of CYP27B1 in hMSCs, Kidney, and Other Tissues/Cells
| Tissue | Cell type | RNA | Protein | Activity | References |
|---|---|---|---|---|---|
| Bone Marrow | hMSCs | yes | WB | 1 | [8,9,12] |
| Kidney | Human kidney | yes | yes | 2 | [76] |
| Bone | Bone biopsy | yes | N.D. | N.D. | [39] |
| Human primary ostoblast | yes | N.D. | 1 | [48] | |
| Osteoclast RAW 264.7 cells | yes | IF | 1 | [39,50,51] | |
| Human osteoblast-like cells | MG-63 | yes | N.D. | 1 | [39] |
| SAOS-2 | yes | N.D. | 1 | [47] | |
| G-292 | yes | N.D. | 1 | [47] | |
| HOS | yes | IF | 1 | [49] | |
| SV-HFO | yes | WB | 1 | [39] | |
| Human keratinocytes | Primary human keratinocytes HPK1A cell line | yes | ICC | 3 | [41,77] |
| Prostate | Primary normal prostatic epithelial cells | yes | WB | 2 | [46] |
| Cancer-derived prostatic epithelial cells | yes | WB | 2 | [46] | |
| Benign prostatic hyperplasia | yes | N.D. | 2 | [46] | |
| Human prostate cancer cell lines | LNcaP | yes | WB | 2 | [46] |
| PC-3 | yes | WB | 2 | [46] | |
| Colon | Human normal and malignant Colon mucosa and colon tumors | yes | WB IF IHC |
N.D. | [42] |
| Immune system Blood cells | Peripheral blood Mononuclear cells Dendritic cells Macrophages | yes | ICC | 2 | [42–45] |
| Human colon cell line | Caco-2 | yes | N.D. | 3 | [78] |
| BBe | yes | N.D. | 3 | [78] | |
| Adipocyte | 3T3-L1 | yes | N.D. | 2 | [52] |
| Others | human umbilical vein endothelial cells | yes | WB IHC |
2 | [53] |
| Brain (cerebellum/purkinje cells) | yes | IHC | N.D. | [42] | |
| Epidermis (statum basalis) | yes | IHC | N.D. | [42] | |
| Parathyroid glands | yes | IHC | N.D. | [79] | |
| Pancreatic islets | yes | IHC WB ICC |
N.D. | [42] | |
| Mammary, breast (cancer or normal) | yes | IHC | N.D. | [80] | |
| Ovary | yes | IHC | 2 | [81] | |
| Human trophoblast cells | yes | N.D. | N.D. | [82] | |
| Human Vascular Smooth Muscle Cells | yes | N.D. | 4 | [83] | |
| Lymph node Spleen cells | yes | IHC | 2 | [42,84] | |
| Human small cell lung cancer cell line NCI H82 | N.D. | N.D. | 3 | [85] |
RNA: yes means by in situ hybridization, RT-PCR, or real-time RT-PCR;
Protein: detected by Western immonblot (WB), Immunohistochemistry (IHC) or Immuno Fluorescence (IF);
Activity: 1. by radioimmunoassay or enzyme-linked immunosorbent assay kits from IDS; 2. by thin-layer chromatography; 3. by high-performance liquid chromatography; 4. by radioimmunoassay kit from DiaSorin.
N.D.: Not detected.
4. Effects of vitamin D on osteoblasts and hMSCs
Cells with the vitamin D receptor (VDR) can be targets of vitamin D action, depending on the receptor’s affinity for the metabolites. 1,25(OH)2D3, is the most active metabolite, with high affinity for VDR. In vivo, 1,25(OH)2D3 acts to maintain normocalcemia by regulating intestinal calcium absorption and PTH activity. In addition to its role in calcium homeostasis, 1α,25(OH)2D3 affects cell proliferation, differentiation, and function [6]. The differentiation of hMSCs to osteoblasts is enhanced by 1α,25(OH)2D3 [7]. Our finding that both 25(OH)D3 and 1α,25(OH)2D3 stimulated osteoblastogenesis in hMSCs and, in some cases, to equal extents [8,9] suggests a potential autocrine/paracrine role of vitamin D metabolism in osteoblast differentiation. Similar ideas have been proposed for 25(OH)D3 metabolism in regulating bone matrix formation by differentiated human osteoblasts [47].
The presence of CYP27B1 in extra-renal tissues especially in bone raises several important questions: (1) What are the effects of vitamin D on bone cells? (2) What is the amount of local 1,25(OH)2D synthesis in bone? (3) Do hMSCs have all the vitamin D enzymes? (4) Is extra-renal CYP27B1 in hMSCs regulated in a manner like kidney cells? (5) Does age affect the relative expression of vitamin D enzymes? (6) What is the significance of marrow synthesis of 1α,25(OH)2D?
5. Vitamin D metabolism in bone and in marrow
Human bone tissue includes osteoblasts, osteoclasts, and osteocytes; marrow contains hMSCs, adipocytes, and hematopoietic lineage cells. In 1981, Howard et al. reported that human osteoblasts activate and inactivate 25(OH)D3 [48], subsequently confirmed by others as being dependent on 1α-hydroxylase/CYP27B1 [39,48,49]. In vitro, 1α,25(OH)2D stimulates bone formation and matrix mineralization but also stimulates bone resorption under different circumstances. Because cells of the monocyte/macrophage lineage are known to express CYP27B1 and convert 25(OH)D3 into 1α,25(OH)2D [44], it was of interest whether differentiated osteoclasts would also do so. Kogawa et al. determined that osteoclasts that were derived in vitro from human peripheral blood monocytes (PBMCs) produced 1α,25(OH)2D from added 25(OH)D3 [50,51]. In addition, 25(OH)D3 significantly reduced bone resorption in other osteoclast models [50].
In addition to bone cells per se, Li et al. found that 1α-hydroxylase was expressed in adipose tissue, with measurable 1α-hydroxylase enzymatic activity in adipocytes (1.16 ± 0.07 pmol/mg protein/h) [52] comparable to that in other cell lines, including prostate (0.07 – 3.08) [46], vascular endothelial cells (0.32) [53], human bone marrow stromal cells (1.50 – 4.41) [12], and in human renal tissue (0.60) [54]. Thus, the combined presence of CYP27B1 and VDR in various skeletal cells and their progenitors indicate possible autocrine/paracrine roles for 25(OH)D3 to regulate bone cell growth, differentiation, and skeletal homeostasis.
The observation that both 1α,25(OH)2D3 and 25(OH)D3 stimulated osteoblastogenesis in the majority of hMSCs samples led to the discovery that hMSCs expressed the VDR and vitamin D hydroxylases, CYP27B1, CYP27A1, CYP24A1 [8], as well as CYP2R1 (unpublished). It is notable that the in vitro hydroxylation of 25(OH)D3 to 1α,25(OH)2D and the stimulation of osteoblastogenesis by 25(OH)D3 were blocked by ketoconazole, a cytochrome P450 inhibitor [8], and by knock-down of CYP27B1 with gene silencing technology [9]. These lines of evidence indicate that 1α-hydroxylation of 25(OH)D3 to 1α,25(OH)2D by CYP27B1 is necessary for the biological effects of 25(OH)D3. In the kidney, CYP24A1 converts vitamin D metabolites to water-soluble forms for excretion [37]. In hMSCs, basal expression of CYP24A1 is usually low and is upregulated by 1 nM 1α,25(OH)2D3 and by 1 μM 25(OH)D3 [9]. In vitro studies [8] showed that CYP27B1 in hMSCs is regulated by substrate feed-forward stimulation and product feedback inhibition (Figure 2). There was dose-dependent upregulation of CYP27B1 by 25(OH)D3 and its downregulation by 1α,25(OH)2D3. Further, high doses of 25(OH)D3 and 1α,25(OH)2D3 upregulated CYP24A1 in hMSCs. Another important way that regulation of CYP27B1 in hMSCs is similar to that in renal cells is in upregulation by PTH [12]. Thus, hMSCs can regulate the concentration of 1α,25(OH)2D by both the rates of its production and inactivation [8,9].
Figure 2. Vitamin D metabolism, regulation, and action in hMSCs.
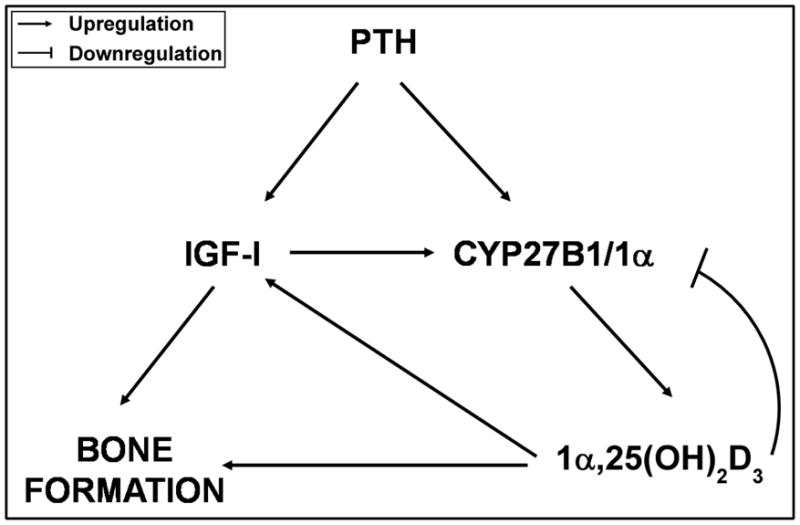
Vitamin D3 (cholecalciferol) is hydroxylated at carbon-25 by CYP27A1 to 25(OH)D3 (calcidiol), which downregulates CYP27A1 and upregulates CYP27B1 and, at higher concentrations, upregulates CYP24A1. In a dose-dependent manner, 25(OH)D3 is hydroxylated at carbon-1α by CYP27B1 to 1α,25(OH)2D3 (calcitriol). Higher concentrations of 1α,25(OH)2D3 downregulate CYP27B1 and upregulate CYP24A1. Both 25(OH)D3 and 1α,25(OH)2D3 upregulate IGF-I which mediates their stimulation of osteoblast differentiation in hMSCs. These findings indicate an autocrine/paracrine role for vitamin D metabolism in human osteoblastogenesis in hMSCs.
6. Effects of age on osteoblastogenesis and on vitamin D metabolism in hMSCs
A decline in the numbers of or differentiation potential of stem cell populations in adult organs can contribute to human aging and age-related disease such as arthrosis, tendinosis, and osteoporosis [55]. There are many properties of hMSCs that are dramatically affected by the age of the subject (Table 2). The basal differentiation of hMSCs to osteoblasts declines with age, as shown by our group [10–13] and by others [24]. In addition, we showed that there are age-related intrinsic changes in hMSCs associated with decreased proliferation and differentiation potential [13]. There is also an age-related decline in stimulation of osteoblastogenesis by 1α,25(OH)2D3 [18].
Table 2.
Effects of Age on Human Marrow Stromal (Mesenchymal Stem) Cells
| Decrease with Age | Increase with Age |
|---|---|
| Proliferation potential Osteoblast differentiation Stimulation of osteoblastogenesis by 1α,25(OH)2D3, 25(OH)D3, and PTH Constitutive expression of CYP27B1 and biosynthesis of 1α,25(OH)2D3 Constitutive expression PTHR1 PTH signaling of CREB and β-catenin Constitutive expression of anti- osteoclastogenic Osteoprotegerin |
Cells positive for Senescence Associated-β-galactosidase Apoptotic cells Constitutive expression of P53, P21, BAX Support of osteoclastogensis by increased constitutive expression of pro-osteoclastogenic RANKL, IL-6, IL- 11 |
Previously, we found that in two-thirds of hMSCs from elders osteoblastogenesis was stimulated by both 25OHD3 and 1α,25(OH)2D3 [8]. Indeed, in hMSCs, there was an age-related decline in expression and activity of CYP27B1, in biosynthesis of 1α,25(OH)2D, and in stimulation of osteoblastogenesis by 25(OH)D3 [12]. Expression of CYP27B1 in MSCs from subjects older than 55 years of age was 56% of that in MSCs from subjects younger than 50 years of age. In studies with rats, Ishida M et al. showed conversion of 25OHD3 to 1α,25(OH)2D in the kidney was decreased with age of the animal [56].
7. Rejuvenation of osteoblastogenesis and vitamin D metabolism in hMSCs from elders
Findings from our lines of research on PTH effects on hMSCs and on vitamin D metabolism in hMSC converged in an endeavor to rejuvenate osteoblast differentiation in hMSCs from elders. PTH is an important stimulus of CYP27B1 transcription and activity in the kidney [57] and likewise dose-dependently upregulated CYP27B1 expression and biosynthesis of 1α,25(OH)2D in hMSCs [57,58]. To date, other than hMSCs, there is no evidence that PTH upregulates CYP27B1 in extra-renal cells or tissue. In contrast to the bone-resorptive effects from chronically elevated PTH, it is clear that intermittent administration of low doses of PTH is osteoanabolic [59]. PTH is also known to prevent osteoblast apoptosis [60]. PTH stimulates osteoblast differentiation of hMSCs, but the responsiveness to low doses declines with the age of the subject [11]. Several model systems show that the actions of PTH to stimulate bone formation are mediated by skeletal insulin-like growth factor-I (IGF-I) [61,62]. In hMSCs, PTH induced IGF-I and IGF-signaling (Figure 3); moreover, experiments with small molecule signaling inhibitors revealed that PTH induction of CYP27B1 was mediated directly through CREB and indirectly by IGF-I signaling [12]. Not only does IGF-I stimulate osteoblast differentiation in hMSCs, it stimulates biosynthesis of 1α,25(OH)2D in synergy with 25OHD3 [9]. Finding that with age there is a decline in PTH/PTHrP receptor (PTHR1) expression and consequent decline in PTH signaling of CREB and β-catenin helps to explain why PTH stimulation of osteoblastogenesis decreases with age of the subject from whom the hMSCs were obtained (Table 2). As proof-of-principle, we showed that dexamethasone upregulated PTHR1 and restored the effects of PTH on hMSCs [11]. More recent evidence that 25(OH)D3 upregulates PTHR1 [63] indicates further beneficial interactions between PTH and vitamin D metabolism in hMSCs.
Figure 3. Summary of the interactions among PTH, IGF-I, and 1,25(OH)2D3 on CYP27B1/1α-hydroxylase expression and bone formation in hMSCs.
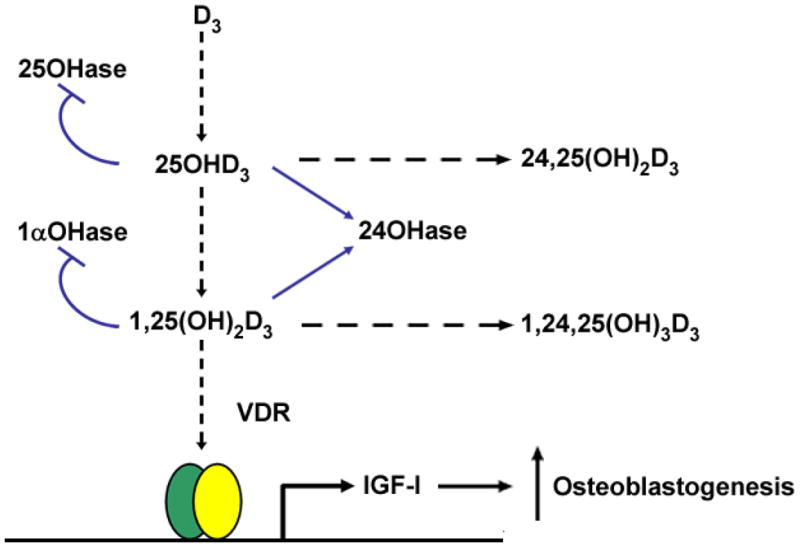
PTH upregulates IGF-I [12] which stimulates bone formation in hMSCs [8]. PTH upregulates CYP27B1 expression and stimulates 1α,25(OH)2D3 production [12], and at higher concentration, 1α,25(OH)2D3 downregulates CYP27B1 [8]. In addition, IGF-I upregulates CYP27B1 expression and stimulates 1α,25(OH)2D3 biosynthesis [8]. 1α,25(OH)2D3 induces IGF-I and bone formation in hMSCs [8].
In sum, hMSCs from elders are resistant to stimulation of osteoblastogenesis by 1α,25(OH)2D3 [18], by 25(OH)D3 [12], and by PTH [11] (Table 2). That PTH upregulated CYP27B1 in a dose-dependent manner even in hMSCs from elders [12] suggested possibile synergy between PTH and 25OHD3. In the first series of studies, 12-hour pre-treatment of hMSCs from elders with PTH(1-34) resulted in biosynthesis of 1α,25(OH)2D equivalent to that in hMSCS from young subjects [12]. The increases in biosynthesis and CYP27B1 expression were mediated through CREB and IGF-I pathways. Accordingly, 12-hour pre-treatment with PTH1-34 provided hMSCs from elders with responsiveness to the pro-osteoblastogenic effects of 25(OH)D3, with increased osteoblast differentiation. In the second series of studies, hMSCs were treated simultaneously and continuously with PTH and 25(OH)D3 [63]. Osteoblast differentiation was significantly stimulated 170% by PTH1-34 (100 nM) and 280% by 25(OH)D3, but by 650% with simultaneous combination of PTH1-34 and 25(OH)D3. Not only was the synergy due to upregulation of CYP27B1, but in addition, PTH upregulated the VDR, and 25OHD3 upregulated PTHR1. Further, the synergistic effects on osteoblast differentiation were blocked in the presence of a small molecule inhibitor of histone deacetylase, Scriptaid(R) [63]. Thus, epigenetic regulation may be central to rejuvenating osteoblastogenesis in hMSCs from elders.
8. Clinical implications
Loss of bone mass associated with human skeletal aging can be explained in part by the age-related decline in in vitro osteoblast differentiation [10–13,24] and by the age-related increase in in vitro osteoclast differentiation [64] with bone cell progenitors from bone marrow. Finding vitamin D-hydroxylases and regulated activity in hMSCs provides support for the hypothesis of an autocrine/paracrine role of vitamin D metabolism in human osteoblast differentiation. Analysis of a cohort of subjects whose hMSCs were used for osteoblast differentiation experiments showed that several clinical attributes were significantly associated with in vitro behavior. Stimulation of osteoblastogenesis by α,25(OH)2D3 was reduced in hMSCs from subjects with advanced age, with low serum 25(OH)D levels, and with low estimated glomerular filtration rate (eGFR) [18]. Those observations suggest that it is clinically important to correct vitamin D-deficiency, especially in elders, in order to enhance bone cell differentiation and bone formation. There is an ongoing controversy about the optimal level of serum 25(OH)D to ensure skeletal health [65–67] and circulating 25(OH)D may be very important to support non-renal production of 1α,25(OH)2D. It is also controversial whether to monitor/correct serum 25(OH)D in patients with chronic kidney disease (CKD) [68]. We recently reported that all three metabolites, D3, 25(OH)D3, and 1α,25(OH)2D3, stimulated in vitro osteoblastogenesis with hMSCs from a subject who had been undergoing hemodialysis for 2+ years as well as an age/gender-matched control subject [69]. We propose that osteoblastic bone formation in CKD patients may not be optimal unless there is sufficient serum 25(OH)D substrate for MSCs to synthesize and respond to local 1α,25(OH)2D.
The synergistic and reciprocal interactions of 25(OH)D3 and PTH we found in hMSCs may be of relevance to anabolic therapy for osteoporosis. A synthetic form of PTH, teriparatide, has been approved by the US FDA for osteoporosis because of its action to stimulate bone formation [59]. There is some information about the importance of vitamin D status in teriparatide therapy. Samadfam et al. showed that intermittently administered PTH increased bone density in 1α-hydroxylase−/− mice, but that there was a greater effect in mice with an active 1α,25(OH)2D-synthesizing system [70]. They concluded that PTH and endogenous vitamin D may interact to optimize osteoblast differentiation. This concept is also supported by an analysis of factors associated with heterogeneity in skeletal response to full-length PTH therapy for osteoporosis [71]. Of all the variables tested, only an increase in serum 1α,25(OH)2D explained larger gains in bone density in response to PTH. There are data from a PTH trial (Fracture Prevention Trial, PFT) that there was no difference in teriparatide anti-fracture efficacy and bone markers between subjects that were vitamin D- sufficient or insufficient [72], but that trial excluded subjects with serum 25(OH)D <10 ng/mL or with elevated PTH levels that is a secondary response to low 25(OH)D levels. In other words, they compared groups with mean serum 25(OH)D of 24 vs. 38 ng/mL, i.e. “Insufficiency or Sufficiency”. A subsequent analysis of that trial and the Male Osteoporosis Trial, notably all with baseline mean serum 25(OH)D levels of 30–32, i.e. sufficiency, indicated that PTH significantly increased 1α,25(OH)2D levels and lowered serum 25(OH)D [73]. The authors concluded that conversion of 25(OH)D to 1α,25(OH)2D may contribute to the biological effects of teriparatide and that the PTH-induced reduction in serum 25(OH)D may be of clinical importance and should be monitored and corrected, as needed.
There are a number of clinical trials showing efficacy of PTH in elders with osteoporosis, but none directly compared young and old subjects. One analysis [74], for example, compared groups 〈/〉75-years with a mean age of 66.5 vs. 78.3 years. A recent meta-regression analysis of 15 randomized, placebo-controlled trials showed that PTH-induced increments in spine bone density were reduced with increasing age [75]. This suggest that a different clinical regimen of PTH & vitamin D may be needed to optimize their synergy to stimulate bone formation in elders, especially those with osteoporosis or healing disorders.
9. Conclusions
There has been considerable progress in our understanding of vitamin D metabolism and its biological activities. The discoveries of CYP27B1 in a wide variety of extra-renal tissues provide plausible mechanisms for local function of 1α,25(OH)2D, especially in the bone microenvironment. This may explain the clinical consequences of vitamin D-deficiency in the elderly and the marked age-related increase in risk for hip fracture. We summarized features of hMSCs, compared different methods to isolate them, and described the effects of vitamin D on them, and regulation of vitamin D metabolism in them. The striking effects of age on osteoblast differentiation and on responsiveness to 1α,25(OH)2D, 25(OH)D, and PTH suggest potential approaches for rejuvenation. Vitamin D deficiency is common in elders and is associated with impaired calcium absorption and secondary hyperparathyroidism which, in turn, stimulates bone resorption and bone loss. Impaired renal production of 1α,25(OH)2D is seen in CKD, which also increases with age. Discarded orthopedic tissues are precious resources that can provide new information about bone cell differentiation when obtained with consent from well-characterized subjects. Using hMSCs from elders and from subjects with vitamin D-deficiency or with poor renal status allows for new insights into pathophysiological mechanisms of aging and skeletal disorders.
Acknowledgments
This project was supported by NIH grants AG025015 and AG028114 to JG. S.G. was supported by the China Scholarship Council (CSC).
ABBREVIATIONS
- 1α
25(OH)2D, 1α,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- ALP
alkaline phosphatase activity
- CFU-F
colony-forming unit-fibroblasts
- eGFR
estimated glomerular filtration rate
- FACS
fluorescence activated cell sorting
- FBS-HI
heat-inactivated fetal bovine serum
- hMSCs
human marrow stromal cells
- IGF-I
insulin-like growth factor-I
- PBMCs
peripheral blood monocytes
- PTH
parathyroid hormone
- PTHR1
PTH/PTHrP receptor
- VDR
vitamin D receptor
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest associated with this manuscript.
Contributions
SG, SZ, ZB, and JG wrote and revised components of this manuscript. SG prepared Table 1. JG prepared Table 2. JG, SZ, and SG prepared and revised the Figures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 2.Kuznetsov SA, Krebsbach PH, Satomura K, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–47. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Dezawa M, Kanno H, Hoshino M, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–10. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luk JM, Wang PP, Lee CK, et al. Hepatic potential of bone marrow stromal cells: development of in vitro co-culture and intra-portal transplantation models. J Immunol Methods. 2005;305:39–47. doi: 10.1016/j.jim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–8. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu P, Oyajobi BO, Russell RG, et al. Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-beta and 1,25(OH)(2) vitamin D(3) In vitro. Calcif Tissue Int. 1999;65:173–80. doi: 10.1007/s002239900678. [DOI] [PubMed] [Google Scholar]
- 8.Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010;151:14–22. doi: 10.1210/en.2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geng S, Zhou S, Glowacki J. Effects of 25-hydroxyvitamin D(3) on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1alpha-hydroxylase. J Bone Miner Res. 2011;26:1145–53. doi: 10.1002/jbmr.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–90. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 11.Zhou S, Bueno EM, Kim SW, et al. Effects of age on parathyroid hormone signaling in human marrow stromal cells. Aging Cell. 2011;10:780–8. doi: 10.1111/j.1474-9726.2011.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng S, Zhou S, Glowacki J. Age-related decline in osteoblastogenesis and 1alpha-hydroxylase/CYP27B1 in human mesenchymal stem cells: stimulation by parathyroid hormone. Aging Cell. 2011;10:962–71. doi: 10.1111/j.1474-9726.2011.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou S, Greenberger JS, Epperly MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–43. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou S, Lechpammer S, Greenberger JS, et al. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-beta/Smad3 signaling. J Biol Chem. 2005;280:22688–96. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen L, Glowacki J, Zhou S. Inhibition of adipocytogenesis by canonical WNT signaling in human mesenchymal stem cells. Exp Cell Res. 2011;317:1796–803. doi: 10.1016/j.yexcr.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou S, Eid K, Glowacki J. Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19:463–70. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- 17.Shen L, Zhou S, Glowacki J. Effects of age and gender on WNT gene expression in human bone marrow stromal cells. J Cell Biochem. 2009;106:337–43. doi: 10.1002/jcb.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou S, Glowacki J, Kim SW, et al. Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D(3) in human marrow stromal cells. J Bone Miner Res. 2012;27:1992–2000. doi: 10.1002/jbmr.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Nbaheen M, Vishnubalaji R, Ali D, et al. Human Stromal (Mesenchymal) Stem Cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. 2012 Apr 14; doi: 10.1007/s12015-012-9365-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anklesaria P, Teixido J, Laiho M, et al. Cell-cell adhesion mediated by binding of membrane-anchored transforming growth factor alpha to epidermal growth factor receptors promotes cell proliferation. Proc Natl Acad Sci U S A. 1990;87:3289–93. doi: 10.1073/pnas.87.9.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakakeeny MA, Greenberger JS. Granulopoiesis longevity in continuous bone marrow cultures and factor-dependent cell line generation: significant variation among 28 inbred mouse strains and outbred stocks. J Natl Cancer Inst. 1982;68:305–17. [PubMed] [Google Scholar]
- 22.Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47. [PubMed] [Google Scholar]
- 23.Bab I, Ashton BA, Gazit D, et al. Kinetics and differentiation of marrow stromal cells in diffusion chambers in vivo. J Cell Sci. 1986;84:139–51. doi: 10.1242/jcs.84.1.139. [DOI] [PubMed] [Google Scholar]
- 24.D’Ippolito G, Schiller PC, Ricordi C, et al. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–22. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 25.Harigaya K, Handa H. Generation of functional clonal cell lines from human bone marrow stroma. Proc Natl Acad Sci U S A. 1985;82:3477–80. doi: 10.1073/pnas.82.10.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon CM, LeBoff MS, Glowacki J. Adrenal and gonadal steroids inhibit IL-6 secretion by human marrow cells. Cytokine. 2001;16:178–86. doi: 10.1006/cyto.2001.0962. [DOI] [PubMed] [Google Scholar]
- 27.Cheleuitte D, Mizuno S, Glowacki J. In vitro secretion of cytokines by human bone marrow: effects of age and estrogen status. J Clin Endocrinol Metab. 1998;83:2043–51. doi: 10.1210/jcem.83.6.4848. [DOI] [PubMed] [Google Scholar]
- 28.Chang Y, Hsieh PH, Chao CC. The efficiency of Percoll and Ficoll density gradient media in the isolation of marrow derived human mesenchymal stem cells with osteogenic potential. Chang Gung Med J. 2009;32:264–75. [PubMed] [Google Scholar]
- 29.Kasten P, Beyen I, Egermann M, et al. Instant stem cell therapy: characterization and concentration of human mesenchymal stem cells in vitro. Eur Cell Mater. 2008;16:47–55. doi: 10.22203/ecm.v016a06. [DOI] [PubMed] [Google Scholar]
- 30.Gronthos S, Graves SE, Ohta S, et al. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–73. [PubMed] [Google Scholar]
- 31.Gronthos S, Zannettino AC, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 32.Quirici N, Soligo D, Bossolasco P, et al. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–91. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 33.Gang EJ, Bosnakovski D, Figueiredo CA, et al. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–51. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 34.Mrozik K, Gronthos S, Shi S, et al. A method to isolate, purify, and characterize human periodontal ligament stem cells. Methods Mol Biol. 2010;666:269–84. doi: 10.1007/978-1-60761-820-1_17. [DOI] [PubMed] [Google Scholar]
- 35.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–16. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Christakos S, Dhawan P, Liu Y, et al. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- 37.Omdahl JL, May BK. The 25-hydroxyvitamin D 24-hydroxylase. In: Feldman D, Glorieux FH, Pike JW, editors. Vitamin D. Academic Press; San Diego: 1997. pp. 69–85. [Google Scholar]
- 38.Schuessler M, Astecker N, Herzig G, et al. Skin is an autonomous organ in synthesis, two-step activation and degradation of vitamin D(3): CYP27 in epidermis completes the set of essential vitamin D(3)-hydroxylases. Steroids. 2001;66:399–408. doi: 10.1016/s0039-128x(00)00229-4. [DOI] [PubMed] [Google Scholar]
- 39.van Driel M, Koedam M, Buurman CJ, et al. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. Faseb J. 2006;20:2417–9. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 40.Bikle DD, Nemanic MK, Gee E, et al. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest. 1986;78:557–66. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bikle DD, Chang S, Crumrine D, et al. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–92. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- 42.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 43.Hewison M, Freeman L, Hughes SV, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 44.Adams JS, Beeker TG, Hongo T, et al. Constitutive expression of a vitamin D 1-hydroxylase in a myelomonocytic cell line: a model for studying 1,25-dihydroxyvitamin D production in vitro. J Bone Miner Res. 1990;5:1265–9. doi: 10.1002/jbmr.5650051212. [DOI] [PubMed] [Google Scholar]
- 45.Adams JS, Singer FR, Gacad MA, et al. Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J Clin Endocrinol Metab. 1985;60:960–6. doi: 10.1210/jcem-60-5-960. [DOI] [PubMed] [Google Scholar]
- 46.Hsu JY, Feldman D, McNeal JE, et al. Reduced 1alpha-hydroxylase activity in human prostate cancer cells correlates with decreased susceptibility to 25-hydroxyvitamin D3-induced growth inhibition. Cancer Res. 2001;61:2852–6. [PubMed] [Google Scholar]
- 47.Atkins GJ, Anderson PH, Findlay DM, et al. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone. 2007;40:1517–28. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 48.Howard GA, Turner RT, Sherrard DJ, et al. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J Biol Chem. 1981;256:7738–40. [PubMed] [Google Scholar]
- 49.Anderson PH, Atkins GJ, Findlay DM, et al. RNAi-mediated silencing of CYP27B1 abolishes 1,25(OH)2D3 synthesis and reduces osteocalcin and CYP24 mRNA expression in human osteosarcoma (HOS) cells. J Steroid Biochem Mol Biol. 2007;103:601–5. doi: 10.1016/j.jsbmb.2006.12.084. [DOI] [PubMed] [Google Scholar]
- 50.Kogawa M, Findlay DM, Anderson PH, et al. Osteoclastic metabolism of 25(OH)-vitamin D3: a potential mechanism for optimization of bone resorption. Endocrinology. 2010;151:4613–25. doi: 10.1210/en.2010-0334. [DOI] [PubMed] [Google Scholar]
- 51.Kogawa M, Anderson PH, Findlay DM, et al. The metabolism of 25-(OH)vitamin D3 by osteoclasts and their precursors regulates the differentiation of osteoclasts. J Steroid Biochem Mol Biol. 2010;121:277–80. doi: 10.1016/j.jsbmb.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Byrne ME, Chang E, et al. 1alpha,25-Dihydroxyvitamin D hydroxylase in adipocytes. J Steroid Biochem Mol Biol. 2008;112:122–6. doi: 10.1016/j.jsbmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zehnder D, Bland R, Chana RS, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–9. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 54.Satomura K, Seino Y, Yamaoka K, et al. Renal 25-hydroxyvitamin D3-1-hydroxylase in patients with renal disease. Kidney Int. 1988;34:712–6. doi: 10.1038/ki.1988.237. [DOI] [PubMed] [Google Scholar]
- 55.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5:213–24. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 56.Ishida M, Bulos B, Takamoto S, et al. Hydroxylation of 25-hydroxyvitamin D3 by renal mitochondria from rats of different ages. Endocrinology. 1987;121:443–8. doi: 10.1210/endo-121-2-443. [DOI] [PubMed] [Google Scholar]
- 57.Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000;381:143–52. doi: 10.1006/abbi.2000.1970. [DOI] [PubMed] [Google Scholar]
- 58.Haussler MR, Baylink DJ, Hughes MR, et al. The assay of 1alpha,25-dihydroxyvitamin D3: physiologic and pathologic modulation of circulating hormone levels. Clin Endocrinol (Oxf) 1976;5 (Suppl):151S–65S. doi: 10.1111/j.1365-2265.1976.tb03823.x. [DOI] [PubMed] [Google Scholar]
- 59.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 60.Jilka RL, Weinstein RS, Bellido T, et al. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–46. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCarthy TL, Centrella M, Canalis E. Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor I in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1989;124:1247–53. doi: 10.1210/endo-124-3-1247. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Nishida S, Boudignon BM, et al. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22:1329–37. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou S, Geng S, Glowacki J. Histone deacetylation mediates the rejuvenation of osteoblastogenesis by the combination of 25(OH)D3 and parathyroid hormone in MSCs from elders. J Steroid Biochem Mol Biol. 2012 doi: 10.1016/j.jsbmb.2012.09.002. in press: J Steroid Biochem Mol Biol 2012 Sep 11 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung P-L, Zhou S, Eslami B, Shen L, LeBoff MS, Glowacki J. Effect of age on regulation of human osteoclast differentiation. J Orthp Res. 2013 doi: 10.1002/jcb.24792. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151–4. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 66.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 67.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 68.Moorthi RN, Kandula P, Moe SM. Optimal vitamin D, calcitriol, and vitamin D analog replacement in chronic kidney disease: to D or not to D: that is the question. Curr Opin Nephrol Hypertens. 2011;20:354–9. doi: 10.1097/MNH.0b013e3283470450. [DOI] [PubMed] [Google Scholar]
- 69.Zhou S, LeBoff MS, Waikar SS, Glowacki J. Vitamin D metabolism and action in human marrow stromal cells: Effects of chronic kidney disease. J Steroid Biochem Mol Biol. 2012 Sep 15; doi: 10.1016/j.jsbmb.2012.09.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samadfam R, Xia Q, Miao D, et al. Exogenous PTH and endogenous 1,25-dihydroxyvitamin D are complementary in inducing an anabolic effect on bone. J Bone Miner Res. 2008;23:1257–66. doi: 10.1359/jbmr.080318. [DOI] [PubMed] [Google Scholar]
- 71.Sellmeyer DE, Black DM, Palermo L, et al. Hetereogeneity in skeletal response to full-length parathyroid hormone in the treatment of osteoporosis. Osteoporos Int. 2007;18:973–9. doi: 10.1007/s00198-007-0336-x. [DOI] [PubMed] [Google Scholar]
- 72.Dawson-Hughes B, Chen P, Krege JH. Response to teriparatide in patients with baseline 25-hydroxyvitamin D insufficiency or sufficiency. J Clin Endocrinol Metab. 2007;92:4630–6. doi: 10.1210/jc.2007-0239. [DOI] [PubMed] [Google Scholar]
- 73.Cosman F, Dawson-Hughes B, Wan X, et al. Changes in vitamin D metabolites during teriparatide treatment. Bone. 2012;50:1368–71. doi: 10.1016/j.bone.2012.02.635. [DOI] [PubMed] [Google Scholar]
- 74.Boonen S, Marin F, Mellstrom D, et al. Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc. 2006;54:782–9. doi: 10.1111/j.1532-5415.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 75.Schwarz P, Jorgensen NR, Mosekilde L, et al. Effects of increasing age, dosage, and duration of PTH treatment on BMD increase--a meta-analysis. Calcif Tissue Int. 2012;90:165–73. doi: 10.1007/s00223-011-9564-3. [DOI] [PubMed] [Google Scholar]
- 76.Zehnder D, Hewison M. The renal function of 25-hydroxyvitamin D3-1alpha-hydroxylase. Mol Cell Endocrinol. 1999;151:213–20. doi: 10.1016/s0303-7207(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 77.Pillai S, Bikle DD, Elias PM. 1,25-Dihydroxyvitamin D production and receptor binding in human keratinocytes varies with differentiation. J Biol Chem. 1988;263:5390–5. [PubMed] [Google Scholar]
- 78.Lagishetty V, Chun RF, Liu NQ, et al. 1alpha-hydroxylase and innate immune responses to 25-hydroxyvitamin D in colonic cell lines. J Steroid Biochem Mol Biol. 2010;121:228–33. doi: 10.1016/j.jsbmb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Segersten U, Correa P, Hewison M, et al. 25-hydroxyvitamin D(3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. J Clin Endocrinol Metab. 2002;87:2967–72. doi: 10.1210/jcem.87.6.8604. [DOI] [PubMed] [Google Scholar]
- 80.Segersten U, Holm PK, Bjorklund P, et al. 25-Hydroxyvitamin D3 1alpha-hydroxylase expression in breast cancer and use of non-1alpha-hydroxylated vitamin D analogue. Breast Cancer Res. 2005;7:R980–6. doi: 10.1186/bcr1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evans KN, Taylor H, Zehnder D, et al. Increased expression of 25-hydroxyvitamin D-1alpha-hydroxylase in dysgerminomas: a novel form of humoral hypercalcemia of malignancy. Am J Pathol. 2004;165:807–13. doi: 10.1016/s0002-9440(10)63343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Avila E, Diaz L, Halhali A, et al. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase, 1,25-dihydroxyvitamin D3 24-hydroxylase and vitamin D receptor gene expression by 8-bromo cyclic AMP in cultured human syncytiotrophoblast cells. J Steroid Biochem Mol Biol. 2004;89–90:115–9. doi: 10.1016/j.jsbmb.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 83.Somjen D, Weisman Y, Kohen F, et al. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–71. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 84.Davies M, Hayes ME, Yin JA, et al. Abnormal synthesis of 1,25-dihydroxyvitamin D in patients with malignant lymphoma. J Clin Endocrinol Metab. 1994;78:1202–7. doi: 10.1210/jcem.78.5.8175979. [DOI] [PubMed] [Google Scholar]
- 85.Mawer EB, Hayes ME, Heys SE, et al. Constitutive synthesis of 1,25-dihydroxyvitamin D3 by a human small cell lung cancer cell line. J Clin Endocrinol Metab. 1994;79:554–60. doi: 10.1210/jcem.79.2.8045976. [DOI] [PubMed] [Google Scholar]


