Abstract
Emotional reactivity has been theorized to play a central role in borderline personality (BP) pathology. Although growing research provides evidence for subjective emotional reactivity in BP pathology, research on physiological or biological reactivity among people with BP pathology is less conclusive. With regard to biological reactivity in particular, research on cortisol reactivity (a neurobiological marker of emotional reactivity) in response to stressors among individuals with BP pathology has produced contradictory results and highlighted the potential moderating role of PTSD-related pathology. Thus, this study sought to examine the moderating role of PTSD symptoms in the relation between BP pathology and both subjective (self-report) and biological (cortisol) emotional reactivity to a laboratory stressor. Participants were 171 patients in a residential substance use disorder treatment center. Consistent with hypotheses, results revealed a significant main effect of BP pathology on subjective emotional reactivity to the laboratory stressor. Furthermore, results revealed a significant interaction between BP pathology and PTSD symptoms in the prediction of cortisol reactivity, such that BP pathology was associated with heightened cortisol reactivity only among participants with low levels of PTSD symptoms. Similar findings were obtained when examining the interaction between BP pathology and the reexperiencing and avoidance/numbing symptoms of PTSD specifically. Results highlight the moderating role of PTSD symptoms in the BP-reactivity relation.
1. Introduction
Borderline personality disorder (BPD) is a serious mental illness associated with substantial economic, societal, and personal costs [1]. Characterized by pervasive dysfunction across multiple domains, the symptoms of this disorder include marked emotional reactivity, difficulties controlling anger, unstable relationships and fears of abandonment, identity disturbance, and suicidal, self-harm, and other self-destructive behaviors [2]. Although the prevalence of BPD in the general population ranges from 2% [3] to 6% [4], individuals with BPD are over-represented in psychiatric outpatient settings [5] and are major consumers of health care resources [6,7].
Although numerous factors have been implicated in the development of BPD (including impulsivity, interpersonal sensitivity, and a fragile or disorganized self-structure; see [8,9,10,11]), most theories highlight the centrality of emotional reactivity to the pathogenesis of this disorder. Indeed, emotional reactivity, defined as the degree of emotional response to internal or external stimuli across subjective, physiological, or expressive domains [12], is a key component of the biologically-based emotional vulnerability described by Linehan [11] as central to BPD, and is considered to be a “core” personality trait underlying this disorder [13,5].
Some consistent patterns have emerged in studies of subjective emotional reactivity in BPD (see [14]). Generally, studies suggest a positive association between borderline personality (BP) pathology and self-reported emotional reactivity at a trait level (e.g., [15,16]), with some studies finding higher self-reported trait-level emotional reactivity among participants with BPD, compared to those without any disorder [17] or without other personality disorders [18]. Using ecological momentary assessment, participants with BPD have also been found to have more frequent shifts in negative affect, compared with healthy controls [19,20], as well as to report greater negative emotional reactivity in response to daily negative interactions [21,22]. Finally, laboratory-based studies provide further support for heightened subjective emotional reactivity in BPD (for an exception see [23]), finding greater self-reported emotional reactivity in response to laboratory stressors among patients with (vs. without) BPD [24] and individuals high (vs. low) in BP pathology [25].
Research on physiological or biological reactivity among people with BP pathology is less conclusive, with studies providing evidence for both hyper- and hypo-reactivity within BPD. With regard to physiological reactivity, one study found evidence for lower sympathetic nervous system reactivity (as evidenced by skin conductance responses) among individuals with BPD, compared to healthy controls [26]. However, other studies have found evidence for heightened sympathetic reactivity in BPD when controlling for the effects of dissociation [27]. For example, Ebner-Priemer and colleagues [27] found that women with BPD and low levels of state dissociation exhibited enhanced startle responses, in comparison with reduced startle evident among women with BPD and high levels of dissociation. Still other studies have found no differences in physiological reactivity between BPD and control groups [17,28,29].
As for research on biological reactivity in BPD, increasing attention is being paid to cortisol responses, a well-established neurobiological marker of emotional reactivity [30,31]. Specifically, abnormalities in cortisol levels, both in general and in response to laboratory stressors, are considered to reflect hypothalamic-pituitary-adrenal (HPA) axis dysfunction [31] – an area of dysfunction proposed to be central to BPD (e.g., [32,33,34]). Mirroring the inconclusive evidence for autonomic reactivity in BPD, however, the small body of literature on HPA axis dysfunction in BPD reveals mixed results, with some studies finding hyper-suppression of cortisol in response to the dexamethasone suppression test (DST; [35]), others finding within-BPD group differences in cortisol levels (with one subset of BPD patients evidencing hypo-suppression of cortisol and another evidencing hyper-suppression of cortisol [36]), and still others finding that cortisol levels do not differentiate between BPD and other psychiatric disorders [37,38].
Research on cortisol reactivity in response to stressors among participants with BPD has likewise produced contradictory results. For example, one preliminary study found no differences in cortisol reactivity among women with BPD (compared to healthy controls) in response to a conflictual discussion with their mothers [39]. Another study examining cortisol reactivity in response to a standardized psychosocial stressor [40] found that BPD participants (vs. healthy controls) demonstrated cortisol hypo-reactivity. Finally, another study examining plasma cortisol reactivity to a standardized psychosocial stressor among participants with BPD (compared to healthy controls; [41]) found that although the BPD group as a whole did not exhibit differential cortisol reactivity compared to the healthy controls, the BPD participants with high levels of dissociative features demonstrated heightened cortisol reactivity. Given the inconclusive nature of extant research on cortisol reactivity to stressors among individuals with BP pathology, it is important to search for moderators that might clarify the relationship between BP features and cortisol reactivity.
One factor that may account for some of the discrepant findings in this line of research is the presence of PTSD symptoms among some of the participants with BPD. Given the considerable overlap and high rates of co-occurrence between BPD and PTSD [42,43,44], many of the existing studies of cortisol responding in BPD likely included participants with co-occurring PTSD symptoms. For instance, in the aforementioned study that found cortisol hypo-reactivity among participants with BPD, one-third of the BPD participants had co-occurring PTSD [40]. Given mounting evidence that dissociation and other symptoms of PTSD may attenuate autonomic reactivity among participants with BPD (see [27,45,46]), the co-occurrence of PTSD symptoms among some participants with BPD could explain the discrepancies in the literature on cortisol reactivity within BPD. In support of this premise, one recent study found evidence for heightened startle potentiation in response to standardized emotional cues among BPD participants only after controlling for state dissociation [27], a common correlate of both BPD and PTSD (e.g., [47,43]). Similarly, whereas BPD participants without co-occurring PTSD exhibited startle potentiation in response to idiographic rejection and abandonment scripts (compared with non-psychiatric controls), BPD participants with co-occurring PTSD demonstrated attenuated startle responses [45]. Furthermore, a recent study of overnight cortisol release in BPD found a negative association between cortisol release and PTSD symptoms [46]. Taken together, these findings suggest that PTSD symptoms may moderate the relationship between BP pathology and cortisol reactivity.
Thus, the primary aim of the current study was to examine the association between BP pathology and both subjective (i.e., self-report) and biological (i.e., cortisol) emotional reactivity, as well as to explore the moderating role of PTSD symptoms in these relations. To this end, we examined the interactive effects of BPD and PTSD symptoms on emotional reactivity to a standardized laboratory stressor among an at-risk sample of substance use disorder (SUD) patients – a population with elevated rates of both BPD and PTSD pathology compared to the general population [48,49]. Building on past research, we hypothesized that BPD symptoms would be associated with greater cortisol and self-report indices of emotional reactivity. Further, we hypothesized that PTSD symptoms would attenuate the relationship between BPD symptoms and cortisol reactivity in particular.
2. Method
2.1. Participants
Participants in this study were 171 (68.4% male) SUD patients, consecutively admitted to a residential SUD treatment facility in central Mississippi. Participants were ethnically diverse (54% White; 37% African-American), and ranged in age from 18 to 61 (M = 35.25; SD = 9.97). Additional demographic and clinical characteristics are available in Table 1. This study was approved by a human subjects review committee, and all participants provided informed consent.
Table 1.
Demographic and clinical characteristics of the sample.
| Variables | M (SD) or N (%) |
|---|---|
| Age | 35.25 (9.97) |
| Female | 54 (31.6%) |
| Race | |
| White | 92 (53.8%) |
| Black/African American | 63 (36.8%) |
| Native American | 8 (4.7%) |
| Other | 8 (4.7%) |
| Income | |
| <$9,999 | 77 (45%) |
| $10,000–19,999 | 26 (15.2%) |
| $20,000–29,999 | 26 (15.2%) |
| $30,000–49,999 | 21 (12.3%) |
| >$50,000 | 18 (10.7%) |
| Education | |
| 1st–12th grade | 50 (29.2%) |
| High school graduate or GED | 56 (32.8%) |
| Some college or Technical school | 53 (31%) |
| College graduate | 7 (4.1%) |
| Graduate degree | 5 (2.9%) |
| Marital status | |
| Married or cohabitating | 50 (29.2%) |
| Single | 121 (70.8%) |
2.2. Measures
2.2.1. BPD symptom severity
To assess BPD symptom severity, we used the Borderline Evaluation of Severity Over Time (BEST; [50]), a 15-item, self-report measure of BPD-specific symptom severity, or the degree of impairment from each of the nine BPD criteria over the past month. Research indicates that the BEST has adequate test-retest reliability, as well as good convergent and discriminant validity [51,52]. Specifically, scores on the BEST have been found to be associated with other self-report measures of BPD symptoms, as well as to distinguish patients with BPD from control participants [52]. Furthermore, the use of a dimensional measure of overall BPD symptom severity was considered a strength, given increasing evidence that BPD is best conceptualized as a dimensional (vs. categorical) construct (e.g., [53,54]). In the present sample, the BEST demonstrated good internal consistency (α = . 83).
2.2.2. PTSD symptom severity
To assess PTSD symptom severity, participants were administered the Life Events Checklist (LEC; [55,56]) and PTSD Checklist (PCL; [57]). The LEC is a 17-item, self-report measure designed to screen for potentially traumatic events (PTEs) in a respondent’s lifetime. The LEC assesses exposure to 16 PTEs and includes one item assessing any other extraordinarily stressful event not captured in the first 16 items. To determine whether or not participants met Criterion A traumatic exposure for PTSD [2], and consistent with past research [58,59], respondents who reported direct (i.e., the event happened to them personally) or indirect (i.e., they witnessed or learned of the event) exposure to at least one PTE were also asked to indicate which of the events was most traumatic and whether or not they experienced fear, helplessness, and/or horror as a result. The LEC has demonstrated convergent validity with measures assessing varying levels of exposure to PTEs and psychopathology known to relate to traumatic exposure [56].
The PCL is a widely used, 17-item, self-report measure of the severity of reexperiencing, avoidance/emotional numbing, and hyperarousal symptoms experienced in response to a PTE. In completing the PCL, and consistent with past research [58,59], participants were instructed to refer to the event they indicated as being most traumatic on the LEC. Using a five-point Likert-type scale (1 = not at all, 5 = extremely), participants rate the extent to which each symptom has bothered them in the past month. The PCL has demonstrated strong test-retest reliability (r = .96) and good construct validity among military, civilian, and substance-using populations [60,61,57]. Further, the subscales of the PCL demonstrate high levels of agreement with the Clinician-Administered PTSD Scale [55] – a well-established and empirically-supported interview-based measure of PTSD (e.g., [62]). Given evidence that PTSD is best represented as a dimensional construct [63,64,65], participants’ responses to each item on the PCL were summed to provide a total score representing overall PTSD symptom severity and cluster scores representing the severity of reexperiencing, avoidance/numbing, and hyperarousal symptoms. Internal consistency in the present sample was excellent for the total scale (α = .95) and good for all cluster subscales (αs ≥ .85).
2.2.3. Laboratory stressor
To elicit emotional distress (and facilitate the assessment of emotional reactivity), this study used a modified version of the Paced Auditory Serial Addition Task – Computerized (PASAT-C), an empirically-supported laboratory stressor shown to induce emotional distress in the form of anxiety, frustration, and irritability [66,67,68]. During this task, numbers are sequentially flashed on a computer screen, and participants are instructed to sum the most recent number with the previous number (using the computer mouse to click on the correct answer). After providing each sum, the participant must ignore the sum and add the following number to the most recently presented number. When a correct answer is provided, a point is obtained. If an incorrect answer is provided, or if the participant fails to provide an answer before the next number is presented, an “explosion” sound is played and the score does not change.
The version of the PASAT-C used in this study consisted of 3 levels with increasingly shorter latencies between number presentations (Level 1 = 3 seconds; Level 2 = 2 seconds; Level 3 = 1 second). Because the correct answer must be provided before the presentation of the next number to obtain a point, difficulty increases as latencies decrease. The first level lasted 3 minutes, the second level lasted 5 minutes, and the third level lasted 7 minutes and included an option to terminate the task at any time. In support of its construct validity, this task has been shown to induce emotional distress in the form of anxiety, anger, frustration, and irritability among both nonclinical and clinical samples [24,68], including SUD patients [69].
2.2.4. Subjective emotional reactivity
In line with the methods used in previous studies assessing reactivity to the PASAT-C, participants were asked to report on their levels of anxiety, irritation, and frustration before the task (pre-task) and following exposure to several minutes of the task (post-task). Each item was rated from 0 to 100, with the average rating across all three forms of negative affect combined to create a composite subjective emotional distress variable. The internal consistency of this emotional distress scale in this sample was adequate (αs = 0.85 at pre-task and 0.75 at post-task).
2.2.5. Biological (cortisol) reactivity
For a subset of participants (see below), saliva samples were obtained at two time points during the study: (a) immediately before the PASAT-C, and (b) 20 min following the PASAT-C (given evidence that cortisol levels do not peak for approximately 20 min following presentation of an emotionally-evocative cue; see [31,70]). Saliva samples were collected by having participants place a swab under their tongue for at least 1 min. Once the swab was saturated with saliva, participants were asked to place the swab into a plastic vial that was then sealed and stored in a freezer. All samples were assayed in duplicate for salivary cortisol off-site by Salimetrics, LLC using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test used 25 μL of saliva per determination, has a lower limit of sensitivity of 0.003 μg/dL, standard curve range from 0.012 μg/dL to 3.0 μg/dL, an average intra-assay coefficient of variation (CV) of 3.8%, and an average inter-assay CV of 5.1%.
Salivary cortisol data were available for only the subset of participants (n = 127) who: (a) completed the study after 1:00 pm (to limit the influence of diurnal fluctuations in cortisol levels), and (b) did not eat, drink caffeine, or smoke in the 60 minutes prior to the study session (given the influence of caffeine, nicotine, and food intake on cortisol levels; see [70] for guidelines in assessing cortisol response to acute stressors).
2.2.6. Clinical covariates
Consistent with past studies of inpatient substance users [71,72], past year severity of alcohol and drug use was assessed through a self-report measure of the frequency of use of a variety of substances (e.g., alcohol, cannabis, cocaine, stimulants, opiates) in the past year. Modeled after other well-established, empirically-supported measures (e.g., the Alcohol Use Disorders Identification Test; [73]), this measure characterizes frequency of use in a manner consistent with the SUD module of the Structured Clinical Interview for DSM-IV (SCID-IV; [74]). Responses are summed to create an overall score representing past year substance use. In support of the measure’s construct validity, scores on this measure have been found to be associated with a number of constructs theoretically- and empirically-linked to SUD, including impulsivity [72], emotion dysregulation [71], and PTSD symptoms. Further, scores on this measure demonstrate convergence with SCID-IV SUD diagnoses in associations with relevant outcomes [72]. This variable was examined as a potential covariate in subsequent analyses. Internal consistency in the current study was good (α = 0.90).
We also assessed other factors that may affect cortisol reactivity. Extant research suggests that demographic characteristics (age, sex, and ethnicity) may be linked with differential basal cortisol levels and cortisol reactivity [70]. Furthermore, both nicotine and medications have also been shown to affect cortisol, and we therefore collected data on nicotine use (coded as yes or no) and psychiatric medication status (coded as yes or no). These variables were examined as potential covariates in subsequent analyses.
2.3. Procedures
All procedures were reviewed and approved by the University of Mississippi Medical Center’s Institutional Review Board. Data were collected as part of a larger ongoing study examining predictors of residential SUD treatment dropout. To be eligible for inclusion in the study, participants were required to have a Mini-Mental Status Exam [75] score of ≥ 24 and no psychotic symptoms. Eligible participants were recruited for this study no sooner than 72 hrs after entry in the facility (to limit the possible interference of withdrawal symptoms on study engagement). Those who met inclusion criteria were provided with information about study procedures and associated risks, following which written informed consent was obtained.
The larger study from which these data were drawn involved two sessions conducted on separate days (to limit participant burden). All procedures for the current study occurred during the second assessment session. Specifically, following completion of a battery of questionnaires (including those noted above), participants were provided instructions for the PASAT-C. Saliva samples were then collected, following which participants were asked to rate their current levels of anxiety, irritation, and frustration (providing a baseline assessment of subjective emotional responses). After this baseline assessment period, participants completed the PASAT-C. Following completion of the PASAT-C, participants were once again instructed to rate their current levels of anxiety, irritation, and frustration (providing an assessment of subjective reactivity to the PASAT-C). Finally, a second saliva sample was obtained approximately 20 min after the PASAT-C. Participants received $15 for completing this session.
2.4. Planned Analyses
Prior to data analysis, all variables of interest were inspected for non-normality (skew > 3.0, kurtosis > 10.0; [76,77]). Extremely skewed or kurtotic variables were square root transformed, consistent with recommended guidelines [78]. In addition, residual plots and normal quantile plots were examined to assess for multivariate normality. All continuous predictor variables were grand-mean centered to allow for clearer interpretation of interactions and to reduce multicollinearity.
Next, we examined the associations of the dependent variables (subjective and biological emotional reactivity indices) with potential covariates, including age, gender, ethnicity, smoker status, psychiatric medication status, substance use severity, and baseline emotional distress. Any variables found to be associated with the dependent variables were included as covariates in the relevant analyses.
The utility of the PASAT-C as a laboratory stressor was examined using a repeated measures analysis of covariance (controlling for relevant covariates) examining changes in subjective emotional distress from pre- to post-task.
To examine the primary question of whether BPD symptoms, PTSD symptoms, and/or their interaction predict emotional reactivity to the laboratory stressor, we conducted two hierarchical multiple regression analyses with post-PASAT-C measurements of self-reported emotional distress and salivary cortisol levels serving as the dependent variables. As stated previously, given the critical effect of diurnal fluctuations and food, caffeine, and nicotine intake on cortisol reactivity (see [70]), we restricted our analyses of cortisol reactivity to those individuals (N = 127) for whom cortisol was collected after 1:00 pm and who did not eat, smoke, or drink caffeine within 60 minutes of saliva sample collection. In each of these models, baseline measurements of self-reported emotions or salivary cortisol, as well as other identified covariates, were entered in the first step, BPD and PTSD symptoms were entered in the second step, and the interaction of BPD symptoms and PTSD symptoms was entered in the final step. Any significant interactions were explored following the methods described by Aiken and West [79]. First, regressions lines were plotted one standard deviation above and below mean levels of BPD symptoms and PTSD symptoms, following which follow-up tests were conducted to test whether the slopes of the regression lines differed significantly from zero.
3. Results
3.1. Preliminary Analyses
All variables of interest fell within the acceptable range of normality, with the exception of cortisol levels. The post-PASAT-C cortisol levels exhibited high leptokurtosis (kurtosis = 19.88, SE = .37); thus, we applied a square root transformation to all cortisol variables, consistent with other research [78,80].
To ensure that the subset of participants with cortisol data were similar in terms of demographic characteristics to the larger sample, we conducted a series of ANOVAs and χ2 analyses. As expected, these analyses revealed no significant differences between the samples in age, F(1,169) = 1.73, p = .19, proportion of males, χ2(1) = 1.37, p = .24, proportion of White race/ethnicity, χ2(1) = 2.31, p = .13, education level, χ2(1) = .67, p = .41, income, χ2(1) = .59, p = .44, or employment status, χ2(1) = .02, p = .89. Furthermore, the samples were comparable in terms of psychiatric medication status, χ2(1) = .12, p = .73, severity of BPD symptoms, F(1,169) = .08, p = .78, severity of PTSD symptoms, F(1,169) = .08, p = .78, and self-reported emotional distress, F(1,169) = .04, p = .85.
Correlation analyses examining zero-order associations between the dependent variables and potential covariates revealed a significant association. In regard to subjective emotional reactivity, self-reported emotional distress in response to the PASAT-C was significantly associated with substance use severity, r = .24, p <.01. In regard to cortisol reactivity, post-PASAT-C cortisol levels were significantly associated with baseline self-reported emotional distress, r = .19, p =.04. No other associations were significant.
3.2. Manipulation check
Providing support for the use of the PASAT-C as a laboratory stressor, results of the repeated measures analysis of covariance (controlling for substance use severity) demonstrated a significant effect of time, F (1, 168) = 116.10, p < .01, η2 = .41, with participants reporting a significant increase in negative emotions from pre-task (M = 23.53, SE = 1.86) to post-task (M = 48.47, SE = 2.29).
3.3. Self-reported emotional reactivity
The hierarchical multiple regression analysis examining self-reported emotional distress in response to the PASAT-C was significant, R2 = .25, f 2 = .33, p < .01. BPD symptoms emerged as a significant predictor of self-reported emotional reactivity, β = .19, p = .02, whereas PTSD symptoms did not, β = .08, p = .26. The addition of the BPD x PTSD interaction to the model in the third step did not significantly improve the model, ΔR2 < .01, Δf 2 < .01, p = .63 (see Table 2).
Table 2.
Hierarchical regression analysis exploring the roles of borderline personality symptoms, posttraumatic stress disorder symptoms, and their interaction in self-reported emotional reactivity to a laboratory stressor
| Predictors | β | R2 (Adj. R2) | ΔR2 | F | f2 | Δf2 |
|---|---|---|---|---|---|---|
| Step 1 | .20 (.19) | .20*** | 20.90*** | .25 | ||
| Baseline self-report | .38*** | |||||
| Substance use severity | .23** | |||||
| Step 2 | .25 (.23) | .05* | 13.62*** | .33 | .07 | |
| PTSD symptoms | .08 | |||||
| BPD symptoms | .19* | |||||
| Step 3 | .25 (.23) | .001 | 10.89*** | .33 | .00 | |
| BPD x PTSD interaction | .03 |
Notes: BPD = borderline personality disorder; PTSD = posttraumatic stress disorder.
p < .05.
p < .01.
p < .001.
3.4. Cortisol reactivity
The hierarchical multiple regression analysis examining salivary cortisol in response to the PASAT-C was also significant, R2 = .67, f 2 = 2.03, p < .01. The inclusion of BPD and PTSD symptoms in the second step significantly improved the model, ΔR2= .02, Δf 2 = .06, p = .02, with BPD symptoms emerging as a significant predictor of cortisol reactivity, β = .17, p = .01; PTSD symptoms did not emerge as a significant predictor, β = −.08, p = .19. Furthermore, the addition of the BPD x PTSD interaction in the third step significantly improved the model, R2= .01, f 2 = .03, p = .047 (see Table 3). Tests of the slopes of the regression lines (see Figure 1) revealed that the relationship between BPD symptoms and cortisol reactivity increased in magnitude as PTSD symptoms moved from high (b = −.05, t = −.48, p = .63) to low (b = .35, t = 3.34, p < .01).
Table 3.
Hierarchical regression analysis exploring the roles of borderline personality symptoms, posttraumatic stress disorder symptoms, and their interaction in cortisol reactivity to a laboratory stressor
| Predictors | β | R2 (Adj. R2) | ΔR2 | F | f2 | Δf2 |
|---|---|---|---|---|---|---|
| Step 1 | .64 (.63) | .64*** | 109.14*** | 1.78 | ||
| Baseline cortisol | .78*** | |||||
| Baseline emotional distress | .15** | |||||
| Step 2 | .66 (.65) | .02* | 59.25*** | 1.94 | .06 | |
| PTSD symptoms | −.08 | |||||
| BPD symptoms | .17** | |||||
| Step 3 | .67 (.66) | .01* | 49.38*** | 2.03 | .03 | |
| BPD x PTSD interaction | −.11* |
Notes: BPD = borderline personality disorder; PTSD = posttraumatic stress disorder.
p < .05
Figure 1.
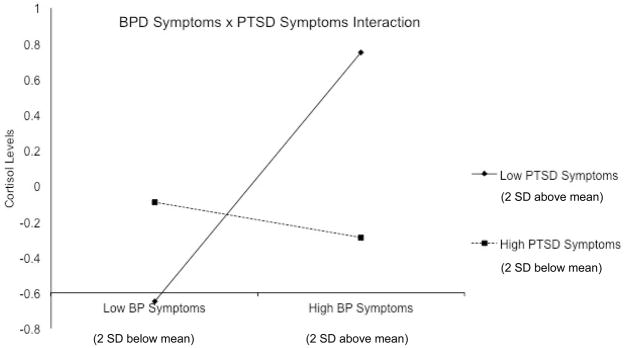
Interactive effect of borderline personality (BP) symptoms and posttraumatic stress disorder (PTSD) symptoms on cortisol levels following a laboratory stresso
3.5. Exploratory analyses
To examine whether the moderating role of PTSD symptoms in the relation between BPD symptoms and cortisol reactivity to the PASAT-C was driven by any particular PTSD symptom cluster, we ran separate hierarchical multiple regression analyses similar to those above (see section 3.4) for each PTSD symptom cluster (i.e., reexperiencing, avoidance/numbing, and hyperarousal). The overall model including PTSD reexperiencing symptoms was significant (R2 = .66, f 2 = 1.94, p < .001), and the BPD x PTSD reexperiencing symptom cluster interaction emerged as a significant predictor of cortisol reactivity in the final step of the model, ΔR2= .01, Δf 2 = .03, β = −.12, p = .03. Likewise, the model including PTSD avoidance/numbing symptoms was significant (R2 = .66, f 2 = 1.94, p < .001), and the BPD x PTSD avoidance/numbing symptom cluster interaction emerged as a significant predictor of cortisol reactivity in the final step of the model, ΔR2= .01, Δf 2 = .06, β = −.12, p = .02. In contrast, the BPD x PTSD hyperarousal symptom cluster interaction was not a significant predictor of cortisol reactivity, ΔR2=.01, Δf 2 = .01, β = −.07, p = .21 (although the overall model including this symptom cluster was significant; R2 = .65, f 2 = 1.98, p < .001). Tests of the slopes of the regression lines exploring the interactions between BPD symptoms and both the reexperiencing and avoidance/numbing symptom clusters revealed a similar pattern to that found with overall PTSD symptom severity, with a significant relation between BPD symptoms and cortisol reactivity only among participants with low levels of these particular PTSD symptoms (bs > .37, ts > 3.02, ps <.003).
4. Discussion
The primary aim of this study was to extend research on the relationship between emotional reactivity and BP pathology by examining both subjective and biological (cortisol) reactivity to a laboratory stressor and exploring the moderating role of PTSD symptoms in these relationships. In line with theoretical literature emphasizing the centrality of emotional reactivity in BPD [13,11], we hypothesized that BPD symptoms would be associated with heightened subjective emotional reactivity to a laboratory stressor. The findings from the present investigation supported this hypothesis, in line with past findings of subjective emotional reactivity among participants with BPD or BP pathology [25,24]. Results of this study also provide preliminary evidence for the role of PTSD symptoms as a moderator of the relationship between BP pathology and cortisol reactivity. Consistent with our hypotheses, BPD symptoms predicted heightened cortisol reactivity to a laboratory stressor only among participants with low levels of PTSD symptoms. Among participants with elevated levels of PTSD symptoms, there was no evidence of an association between BPD symptoms and cortisol reactivity. Notably, the moderating role of PTSD symptoms in the relation between BP pathology and cortisol reactivity seems to be driven by the reexperiencing and avoidance/numbing symptoms of PTSD in particular, as only these symptom clusters emerged as significant moderators of this relation. Together, these findings help to clarify some of the contradictory findings in the literature with regard to the relationship between BP pathology and cortisol reactivity, and are consistent with past findings suggesting the attenuating effect of PTSD on autonomic arousal and cortisol levels (e.g., [27,45,46]).
The present study represents an important step forward in research on emotional reactivity in BP pathology. Given the inconclusive and contradictory nature of extant research on physiological and biological indices of emotional reactivity in BP pathology (e.g., [27,26,17]), the identification of potential moderators of the BPD-reactivity association is crucial. The present findings also suggest that increasing levels of PTSD symptoms result in a greater discrepancy between self-reported and physiological reactivity among participants with BP symptoms. Thus, PTSD symptoms may be one factor contributing to the discordant patterns of subjective and physiological patterns of arousal evident among participants with BPD pathology (e.g., [14]).
Several limitations of the present study warrant consideration. First, although the use of a mixed-gender sample was a strength of this study (given the typical reliance on female samples within the BPD literature; see [81,82]), it also complicates the interpretation of cortisol data. In particular, the present study did not control for menstrual cycle, which may influence cortisol findings [83]. However, it is worth noting that gender was not found to be associated with cortisol reactivity in our sample. Second, our use of a standardized stressor may have led to greater heterogeneity of responses to the emotion induction. Given evidence that individual differences in cognitive appraisals lead to differential HPA axis response (e.g., [31]), a personalized stressor may provide a more potent and ecologically-valid means of examining emotional reactivity. Third, our measure of self-reported emotional reactivity was limited to only a few emotions. Although this confers the advantage of quick administration with minimal interference with emotion inductions (and included several key emotions considered to be particularly relevant to BPD and the emotion induction used here, including anxiety, irritability, and frustration; see [24]), it did not provide a comprehensive measure of current emotional state. In particular, despite examining reactions across anxiety and anger-spectrum emotions, this measure did not assess for other emotions theorized to be central to BPD, including shame [24]. Future studies should examine self-reported reactivity to this and other emotion inductions across a wider range of negative emotions.
Fourth, given that we did not directly assess state dissociation in this study, the role of dissociation in the relations examined here remains unclear. Indeed, dissociation is an associated feature of both BPD and PTSD [47,43], and has been found to attenuate autonomic reactivity in BPD [27]. However, in the absence of a direct measure of dissociation, its impact on the interrelations observed here cannot be determined. Nonetheless, findings that both the avoidance/numbing and reexperiencing symptom clusters of PTSD moderated the relation between BP pathology and cortisol reactivity suggest that it may be PTSD symptoms more broadly, rather than dissociation or numbing in particular, that are responsible for the attenuation of cortisol reactivity in BPD. Fifth, although the focus on BPD and PTSD symptoms within a substance-using population is clinically-relevant (as SUD patients have been found to evidence high rates of BPD and PTSD pathology, as well as heightened levels of emotion-related difficulties; see [48,49]), the results from this study may not generalize to non-SUD samples. Future research may benefit from examining the impact of PTSD symptoms on the relationship between BP pathology and emotional reactivity across diverse samples. Finally, it is important to note that our assessment of PTSD and BPD was limited to the use of self-report measures, and confined to PTSD and BPD symptoms, rather than PTSD or BPD diagnoses per se. Therefore, the extent to which these findings generalize to populations with BPD and PTSD diagnoses warrants further examination.
Despite these limitations, the results of this study underscore the importance of emotional reactivity to BP pathology. The pattern of findings suggests that individuals suffering from BP pathology may experience not only greater subjective emotional reactivity, but abnormal cortisol reactivity as well. Furthermore, these results suggest that PTSD symptoms may obscure emotional reactivity among individuals suffering from BP pathology. Given the inconclusive nature of many of the studies of biological and psychophysiological indices of emotional reactivity in BPD [14], future research should examine the role of PTSD symptoms in moderating the relationship between BP features and emotional reactivity on other biological (e.g., psychophysiological) markers of emotional arousal.
Nonetheless, the question of why PTSD symptoms blunt emotional reactivity in the presence of BPD symptoms remains unanswered. Research has identified commonalities between PTSD symptoms and the behaviors observed among animals exposed to uncontrollable stressors, termed “learned helplessness” (see [84]). Consistent with this framework, PTSD symptoms may coincide with a compromised defensive system [45], resulting in blunted sympathetic responses to stressors. Alternatively, it may be that the ways in which individuals with PTSD symptoms respond to emotional stressors leads to attenuated stress hormone reactivity. For instance, PTSD is associated with both dissociation and cognitive avoidance strategies [85]. Although this was not examined in the present study, within other samples, dissociation has been linked with hypo-suppression of cortisol [86]. Future research should examine whether specific strategies associated with PTSD pathology, such as dissociation, may be linked to blunted stress reactivity among individuals with BP pathology. Taken together, the present study adds to the literature suggesting a more nuanced view of emotional reactivity in BPD, and sets the stage for future research aimed at identifying potential contextual and diagnostic moderators of emotional reactivity within this population.
Acknowledgments
Support for this study was provided in part by R21 DA022383 from the National Institute on Drug Abuse of the National Institutes of Health awarded to the last author (MTT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Asselt AD, Dirksen CD, Arntz A, Severens JL. Difficulties in calculating productivity costs: Work disability associated with borderline personality disorder. Value Health. 2007;11:637–44. doi: 10.1111/j.1524-4733.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- 3.Swart M, Blazer D, George L, Winfield I. Estimating the prevalence of borderline personality disorder in the community. J Pers Disord. 1990;4:257–72. [Google Scholar]
- 4.Grant BF, Chou P, Goldstein RB, Huang B, Stinson FS, Saha TD, et al. Prevalence, correlates, disability, and comorbidity of DSM-IIV borderline personality disorder: Results from the wave 2 national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2008;69:533–45. doi: 10.4088/jcp.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis: I. Psychopathology, comorbidity, and personality structure. Biol Psychiatry. 2002;51:936–50. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- 6.Bender DS, Dolan RT, Skodol AE, Sanislow CA, Dyck IR, McGlashan TH, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295–302. doi: 10.1176/appi.ajp.158.2.295. [DOI] [PubMed] [Google Scholar]
- 7.Zanarini MC, Frankenburg FR, Khera GS, Bleichmar J. Treatment histories of borderline inpatients. Compr Psychiatry. 2001;42(2):144–50. doi: 10.1053/comp.2001.19749. [DOI] [PubMed] [Google Scholar]
- 8.Bateman AW, Fonagy P. Psychotherapy for Borderline Personality Disorder: Mentalization Based Treatment. Oxford: Oxford University Press; 2004. [Google Scholar]
- 9.Beauchaine TP, Klein DN, Crowell SE, Gatzke-Kopp LM, Derbidge C. Multifinality in the development of personality disorders: A biology × sex × environment model of antisocial and borderline traits. Dev Psychopathol. 2009;21:735–70. doi: 10.1017/S0954579409000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunderson J, Lyons-Ruth K. BPD’s interpersonal hypersensitivity phenotype: A gene- environment transactional model. J Pers Disord. 2008;22(1):22–41. doi: 10.1521/pedi.2008.22.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford Press; 1993. [Google Scholar]
- 12.Rothbart MK, Derryberry D. Development of individual differences in temperament. In: Lamb ME, Brown AL, editors. Advances in developmental psychology. Vol. 1. Hillsdale (NJ): Erlbaum; 1981. pp. 37–86. [Google Scholar]
- 13.Gunderson JG, Zanarini MC, Kisiel C. Borderline personality disorder. In: Widiger TA, editor. DSM-IV sourcebook. Vol. 2. Washington (DC): American Psychiatric Association Press; 1996. pp. 717–33. [Google Scholar]
- 14.Rosenthal MZ, Gratz KL, Kosson DS, Cheavens JS, Lejuez CW, Lynch TR. Borderline personality disorder and emotional responding: A review of the research literature. Clin Psychol Rev. 2008;28:75–91. doi: 10.1016/j.cpr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Cheavens JS, Rosenthal MZ, Daughters SB, Nowak J, Kosson D, Lynch TR, Lejuez CW. An analogue investigation of the relationships among perceived parental criticism, negative affect, and borderline personality disorder features: The role of thought suppression. Behav Res Ther. 2005;43:257–68. doi: 10.1016/j.brat.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Henry C, Mitropoulou V, New AS, Koenigsberg HW, Silverman J, Siever LJ. Affective instability and impulsivity in borderline personality and bipolar II disorders: Similarities and differences. J Psychiatr Research. 2001;35:307–12. doi: 10.1016/s0022-3956(01)00038-3. [DOI] [PubMed] [Google Scholar]
- 17.Herpertz SC, Schwenger UB, Kunert HJ, Lukas G, Gretzer U, Nutzmann K, et al. Emotional responses in patients with borderline as compared with avoidant personality disorder. J Pers Disord. 2000;14:339–51. doi: 10.1521/pedi.2000.14.4.339. [DOI] [PubMed] [Google Scholar]
- 18.Koenigsberg HW, Harvey PD, Mitropoulou V, Schmeidler J, New AS, Goodman M, et al. Characterizing affective instability in borderline personality disorder. Am J Psychiatry. 2002;159:784–8. doi: 10.1176/appi.ajp.159.5.784. [DOI] [PubMed] [Google Scholar]
- 19.Stein KF. Affect instability in adults with a borderline personality disorder. Arch Psychiatr Nurs. 1996;10:32–40. doi: 10.1016/s0883-9417(96)80084-7. [DOI] [PubMed] [Google Scholar]
- 20.Stiglmayr CE, Grathwol T, Linehan MM, Ihorst G, Fahrenberg J, Bohus M. Aversive tension in patients with borderline personality disorder: A computer-based controlled field study. Acta Psychiatr Scand. 2005;111:372–9. doi: 10.1111/j.1600-0447.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 21.Sadikaj G, Russell JJ, Moskowitz DS, Paris J. Affect dysregulation in individuals with borderline personality disorder: Persistence and interpersonal triggers. J Pers Assess. 2010;92(6):490–500. doi: 10.1080/00223891.2010.513287. [DOI] [PubMed] [Google Scholar]
- 22.Stepp SD, Hallquist MN, Morse JQ, Pilkonis PA. Multimethod investigation of interpersonal functioning in borderline personality disorder. Pers Disord: Theory Res Treat. 2011;2(3):175–92. doi: 10.1037/a0020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacob GA, Hellstern K, Ower N, Pillmann M, Scheel CN, Rusch N, et al. Emotional reactions to standardized stimuli in women with borderline personality disorder. J Nerv Men Dis. 2009;197:808–15. doi: 10.1097/NMD.0b013e3181bea44d. [DOI] [PubMed] [Google Scholar]
- 24.Gratz KL, Rosenthal MZ, Tull MT, Lejuez CW, Gunderson JG. An experimental investigation of emotional recovery in borderline personality disorder: The role of shame. Compr Psychiatry. 2010;51:275–85. doi: 10.1016/j.comppsych.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Dixon-Gordon KL, Chapman AL, Lovasz N, Walters KN. Too upset to think: The interplay of borderline personality features, negative emotions, and social problem solving in the laboratory. Personal Disord: Theory Res Treat. 2011;2(4):243–60. doi: 10.1037/a0021799. [DOI] [PubMed] [Google Scholar]
- 26.Herpertz SC, Kunert HJ, Schwenger UB, Sass H. Affective responsiveness in borderline personality disorder: a psychophysiological approach. Am J Psychiatry. 1999;15:1550–6. doi: 10.1176/ajp.156.10.1550. [DOI] [PubMed] [Google Scholar]
- 27.Ebner-Priemer UW, Badeck S, Beckmann C, Wagner A, Feige B, Weiss I, et al. Affective dysregulation and dissociative experience in female patients with borderline personality disorder: A startle response study. J Psychiatr Res. 2005;39:85–92. doi: 10.1016/j.jpsychires.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Herpertz SC, Werth U, Lucas G, Qunaibi M, Schuerkens A, Kunert H, et al. Emotion in criminal offenders with psychopathy and borderline personality disorders. Arch Gen Psychiatry. 2001;58:737–45. doi: 10.1001/archpsyc.58.8.737. [DOI] [PubMed] [Google Scholar]
- 29.Herpertz SC, Koetting K. Startle response in inpatients with borderline personality disorder vs. healthy controls. J Neural Transm. 2005;112:1435–63. doi: 10.1007/s00702-004-0249-1. [DOI] [PubMed] [Google Scholar]
- 30.Denson TF, Spanovic M, Miller N. Cognitive appraisals and emotions predict cortisol and immune responses: A meta-analysis of acute laboratory social stressors and emotion inductions. Psychol Bull. 2008;135(6):823–53. doi: 10.1037/a0016909. [DOI] [PubMed] [Google Scholar]
- 31.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 32.Kontaxakis V, Markianos M, Vaslamatzis G, Markidis M, Kanellos P, Stefanis C. Multiple neuroendocrinological responses in borderline personality disorder patients. Acta Psychiatr Scand. 1998;76:593–7. doi: 10.1111/j.1600-0447.1987.tb02924.x. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan KR, Davidson JR, Rayasam K, Shope F. The dexamethasone suppression test in borderline personality disorder. Biol Psychiatry. 1984;19:1149–53. [PubMed] [Google Scholar]
- 34.Soloff PH, George A, Nathan RS. The dexamethasone suppression test in patients with borderline personality disorders. Am J Psychiatry. 1982;139:1621–3. doi: 10.1176/ajp.139.12.1621. [DOI] [PubMed] [Google Scholar]
- 35.Carrasco JL, Diaz-Marsa M, Pastrana JI, Molina R, Brotons L, Lopez-Ibor MI. Hypothalamic-pituitary-adrenal axis response in borderline personality disorder without post-traumatic features. Br J Psychiatry. 2007;190:357–8. doi: 10.1192/bjp.bp.106.022590. [DOI] [PubMed] [Google Scholar]
- 36.Rinne T, de Kloet ER, Wouters L, Goekoop JG, DeRijk RH, van den Brink W. Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biol Psychiatry. 2002;52:1102–12. doi: 10.1016/s0006-3223(02)01395-1. [DOI] [PubMed] [Google Scholar]
- 37.Beeber AR, Kline MDD, Pie RW, Manning JM. Dexamethasone suppression test in hospitalized depressed patients with borderline personality disorder. J Nerv Ment Dis. 1984;172:301–303. doi: 10.1097/00005053-198405000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Korzekwa M, Steiner M, Links P, Eppel A. The dexamethasone suppression test in borderlines: is it useful? Can J Psychiatry. 1991;36:26–8. doi: 10.1177/070674379103600106. [DOI] [PubMed] [Google Scholar]
- 39.Walter M, Bureau JF, Holmes BM, Bertha EA, Hollander M, Wheelis J, et al. Cortisol response to interpersonal stress in young adults with borderline personality disorder: a pilot study. Eur Psychiatry. 2008;23:201–4. doi: 10.1016/j.curpsy.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nater UM, Bohus M, Abbruzzese E, Ditzen B, Gaab J, Kleindienst N, et al. Increased psychological and attenuated cortisol and alpha-amylase responses to acute psychosocial stress in female patients with borderline personality disorder. Psychoneuroendocrinology. 2010;35:1565–72. doi: 10.1016/j.psyneuen.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Simeon D, Knutelska M, Smith L, Baker BR, Hollander EA. A preliminary study of cortisol and norepinephrine reactivity to psychosocial stress in borderline personality disorder with high and low dissociation. Psychiatry Res. 2007;149:177–84. doi: 10.1016/j.psychres.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Gunderson JG, Sabo AN. The phenomenological and conceptual interface between borderline personality disorder and PTSD. Am J Psychiatry. 1993;150:19–27. doi: 10.1176/ajp.150.1.19. [DOI] [PubMed] [Google Scholar]
- 43.Sar V, Akyuz G, Kugu N, Ozturk E, Ertem-Vehid H. Axis I dissociative disorder comorbidity in borderline personality disorder and reports of childhood trauma. J Clin Psychiatry. 2006;67:1583–90. doi: 10.4088/jcp.v67n1014. [DOI] [PubMed] [Google Scholar]
- 44.Zanarini MC, Frankenburg FR, Dubo ED, Sickel AE, Trikha A, Levin A, Reynolds V. Axis I comorbidity of borderline personality disorder. Am J Psychiatry. 1998;155:1733–9. doi: 10.1176/ajp.155.12.1733. [DOI] [PubMed] [Google Scholar]
- 45.Limberg A, Barnow S, Freyberger HJ, Hamm AO. Emotional vulnerability in borderline personality disorder is cue specific and modulated by traumatization. Biol Psychiatry. 2011;69:574–82. doi: 10.1016/j.biopsych.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Wingenfeld K, Driessen M, Adam B, Hill A. Overnight urinary cortisol release in women with borderline personality disorder depends on comorbid PTSD and depressive psychopathology. Eur Psychiatry. 2007;22:309–12. doi: 10.1016/j.eurpsy.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of re-experiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20(5):713–25. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- 48.Brady TM, Krebs CP, Laird G. Psychiatric comorbidity and not completing jail-based substance abuse treatment. Am J Addict. 2004;13:83–101. doi: 10.1080/10550490490265398. [DOI] [PubMed] [Google Scholar]
- 49.Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: A review and integration. Clin Psychol Rev. 2000;20:235–53. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 50.Pfohl B, Blum N. Unpublished measure. Iowa City: University of Iowa; 1997. Borderline Evaluation of Severity Over Time. [Google Scholar]
- 51.Blum N, Pfohl B, St John D, Monahan P, Black DW. STEPPS: A cognitive– behavioral systems-based group treatment for outpatients with borderline personality disorder – A preliminary report. Compr Psychiatry. 2002;43:301–10. doi: 10.1053/comp.2002.33497. [DOI] [PubMed] [Google Scholar]
- 52.Pfohl B, Blum N, St John D, McCormick B, Allen J, Black DW. Reliability and validity of the Borderline Evaluation of Severity Over Time (BEST): A self-rated scale to measure severity and change in persons with borderline personality disorder. J Pers Disord. 2009;23:281–93. doi: 10.1521/pedi.2009.23.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arntz A, Bernstein D, Gielen D, Van Nieuwenhuyzen M, Penders K, Haslam N, Ruscio J. Taxometric evidence for the dimensional structure of Cluster-C, paranoid and borderline personality disorders. J Pers Disord. 2009;23:606–28. doi: 10.1521/pedi.2009.23.6.606. [DOI] [PubMed] [Google Scholar]
- 54.Trull TJ, Widiger TA, Guthrie P. Categorical versus dimensional status of borderline personality disorder. J Abnorm Psychol. 1990;99:40–8. doi: 10.1037//0021-843x.99.1.40. [DOI] [PubMed] [Google Scholar]
- 55.Blake DD, Weathers FW, Nagy L, Kaloupek DG, Klauminzer G, Charney DS. The Clinician Administered PTSD Scale. Boston: National Center for PTSD-Behavioral Science Division; 1990. [Google Scholar]
- 56.Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric properties of the life events checklist. Assessment. 2004;11:330–41. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- 57.Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist: Reliability, validity, and diagnostic utility. Paper presented at the Annual Meeting of the International Society for Traumatic Stress Studies; San Antonio, TX. 1993. [Google Scholar]
- 58.Tull MT, Barrett HM, McMillan ES, Roemer L. A preliminary investigation of the relationship between emotion regulation difficulties and posttraumatic stress symptoms. Behav Ther. 2007;38:303–13. doi: 10.1016/j.beth.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Weiss NH, Tull MT, Davis LT, Dehon EE, Fulton JJ, Gratz KL. Examining the association between emotion regulation difficulties and probable posttraumatic stress disorder within a sample of African Americans. Cog Behav Ther. 2011 doi: 10.1080/16506073.2011.621970. in press. forthcoming. [DOI] [PubMed] [Google Scholar]
- 60.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behav Res Ther. 1996;34:669–73. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 61.Harrington T, Newman E. The psychometric utility of two self-report measures of PTSD among women substance users. Addict Behav. 2007;32:2788–98. doi: 10.1016/j.addbeh.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 62.Grubaugh AL, Elhai JD, Cusack KJ, Wells C, Frueh BC. Screening for PTSD in public-sector mental health settings: The diagnostic utility of the PTSD Checklist. Depress Anxiety. 2007;24:124–9. doi: 10.1002/da.20226. [DOI] [PubMed] [Google Scholar]
- 63.Broman-Fulks JJ, Ruggiero KJ, Green BA, Kilpatrick DG, Danielson CK, Resnick HS, Saunders BE. Taxometric investigation of PTSD: Data from two nationally representative samples. Behav Ther. 2006;37:364–80. doi: 10.1016/j.beth.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Forbes D, Haslam N, Williams BJ, Creamer M. Testing the latent structure of posttraumatic stress disorder: A taxometric study of combat veterans. J Trauma Stress. 2005;18:647–56. doi: 10.1002/jts.20073. [DOI] [PubMed] [Google Scholar]
- 65.Ruscio AM, Ruscio J, Keane TM. The latent structure of post-traumatic stress disorder: A taxometric investigation of reactions to extreme stress. J Abnorm Psychol. 2002;111(2):290–301. [PubMed] [Google Scholar]
- 66.Brown RA, Lejuez CW, Kahler CW, Strong D. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;111:180–5. [PubMed] [Google Scholar]
- 67.Daughters SB, Lejuez CW, Kahler C, Strong D, Brown R. Psychological distress tolerance and duration of most recent abstinence attempt among residential treatment seeking substance abusers. Psychol Addict Behav. 2005;19:208–11. doi: 10.1037/0893-164X.19.2.208. [DOI] [PubMed] [Google Scholar]
- 68.Lejuez CW, Kahler CW, Brown RA. A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor: Implications for behavioral assessment. Behav Ther. 2003;26:290–3. [Google Scholar]
- 69.Bornovalova MA, Gratz KL, Daughters SB, Nick B, Delaney-Brumsey A, Lynch TR, et al. A multimodal assessment of the relationship between emotion dysregulation and borderline personality disorder among inner-city substance users in residential treatment. J Psychiatr Res. 2008;42:717–26. doi: 10.1016/j.jpsychires.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 70.Nicolson NA. Measurement of cortisol. In: Luecken LJ, Gallo LC, editors. Handbook of Physiological Research Methods in Health Psychology. Thousand Oaks, CA: Sage Publications; 2007. pp. 37–74. [Google Scholar]
- 71.Bornovalova MA, Cashmna-Rolls A, O’Donnell JM, Ettinger K, Richards JB, deWit H, Lejuez CW. Risk taking differences on a behavioral task as a function of potential reward/loss magnitude and individual differences in impulsivity and sensation seeking. Pharmacol Biochem Behav. 2009;93:258–62. doi: 10.1016/j.pbb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 72.Lejuez CW, Bornovalova MA, Reynolds ER, Daughters SB, Curtin JJ. Risk factors in the relationship between gender and crack/cocaine. Exp Clin Psychopharmacol. 2007;15:165–75. doi: 10.1037/1064-1297.15.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saunders JB, Aasland OG, Babor TF, Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption- II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 74.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P, Version 2.0) New York, NY: New York State Psychiatric Institute; 1996. Unpublished measure. [Google Scholar]
- 75.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 76.Curran PJ, West SG, Finch JF. The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychol Methods. 1996;1:16–29. [Google Scholar]
- 77.Kline RB. Principles and practices of structural equation modeling. New York: Guilford; 1998. [Google Scholar]
- 78.Hamilton LC. Regression with graphics: A second course in applied statistics. Belmont, CA: Duxbury; 1992. [Google Scholar]
- 79.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publishing; 1991. [Google Scholar]
- 80.Maina G, Palmas A, Bovenzi M, Filon FL. Salivary cortisol and psychosocial hazards at work. Am J Ind Med. 2009;52(3):251–60. doi: 10.1002/ajim.20659. [DOI] [PubMed] [Google Scholar]
- 81.Chen EY, Brown MZ, Lo TT, Linehan MM. Sexually transmitted disease rates and high-risk sexual behaviors in borderline personality disorder versus borderline personality disorder with substance use disorder. J Nerv Ment Dis. 2007;195:125–9. doi: 10.1097/01.nmd.0000254745.35582.f6. [DOI] [PubMed] [Google Scholar]
- 82.Hull JW, Clarkin JF, Yeomans F. Borderline personality disorder and impulsive sexual behavior. Hosp Community Psychiatry. 1993;44:1000–2. doi: 10.1176/ps.44.10.1000. [DOI] [PubMed] [Google Scholar]
- 83.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus—pituitary—adrenal axis. Psychosom Med. 1999;61:154–62. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 84.Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in posttraumatic stress disorder: An animal model. Psychol Bull. 1992;112:218–38. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- 85.Hetzel-Riggin MD, Wilber EL. To dissociate or suppress? Predicting automatic vs. conscious cognitive avoidance. J Trauma Dissociation. 2005;11:444–57. doi: 10.1080/15299732.2010.495376. [DOI] [PubMed] [Google Scholar]
- 86.Simeon D, Guralnik O, Knutelska M, Hollander E, Schmeidler J. Hypothalamic-pituitary-adrenal axis dysregulation in depersonalization disorder. Neuropsychopharmacology. 2001;25:793–5. doi: 10.1016/S0893-133X(01)00288-3. [DOI] [PubMed] [Google Scholar]


