Abstract
Objectives
Infrapopliteal angioplasty (PTA) is routinely used to treat critical limb ischemia (CLI) despite limited data on long-term outcomes.
Methods
We reviewed all patients undergoing infrapopliteal PTA for CLI from 2004–2012 stratified by TASC class. Outcomes included restenosis, primary patency, reintervention (w/ PTA or bypass), amputation, procedural complications, wound healing, and survival.
Results
Infrapopliteal PTA (stenting 14%, multilevel intervention 50%) was performed in 459 limbs of 413 patients (59% male) with technical success of 93% and perioperative complications in 11%. TASC class was 16% A, 22% B, 27% C, and 34% D. Multilevel interventions were performed in 50% of limbs and were evenly distributed among all TASC classes. All technical failures were TASC D lesions. Mean follow-up was 15 months. 5-year survival was 49%. One- and 5-year primary patency was 57% & 38% and limb salvage was 84% & 81%. Restenosis was associated with TASC C (HR 2.2, 95% CI 1.2–3.9, P=.010) and TASC D (HR 2.4, 95% CI 1.3–4.4, P=.004) lesions. Amputation rates were higher in patients who were not candidates for bypass (HR 4.4, 95% CI 2.6–7.5, P<.001) and with TASC D lesions (HR 3.8, 95% CI 1.1–12.5, P=.03). Unsuitability for bypass was also predictive of repeat PTA (HR 1.8, 95% CI 1.0–3.4, P=.047). Postoperative clopidogrel use was associated with lower rates of any revascularization (HR .46, 95% CI .25–.83, P=.011).
Conclusions
Infrapopliteal PTA is effective primary therapy for TASC A, B, and C lesions. Surgical bypass should be offered to patients with TASC D disease who are suitable candidates. Multilevel intervention does not adversely affect outcome.
INTRODUCTION
Percutaneous transluminal angioplasty (PTA) is an established alternative to surgical bypass for the treatment of infrainguinal peripheral arterial disease in patients of limited life expectancy, poor surgical candidates, or those who lack adequate venous conduit1–3. Citing lower perioperative morbidity and mortality, high technical success, and equivalent rates of limb salvage, some groups now endorse PTA as a comparable, if not preferred first-line therapy for patients with critical limb ischemia (CLI)4–8. Guidelines for PTA of the infrapopliteal vessels are less well defined than those for the iliac or femoropopliteal segments and in fact, the original TransAtlantic Inter-Society Consensus (TASC) I classification of infrapopliteal lesions9 are notably absent from the TASC II guidelines, which offer no specific recommendations on the management of these lesions10.
While early success with infrapopliteal PTA has been reported8, 11, 12, little data are available on mid- and long-term outcomes. As evidence emerges to suggest that primary treatment with PTA may threaten the success of subsequent bypass13,14, 15 and may result in higher costs and more frequent reinterventions than bypass16, a more complete understanding of the outcomes following infrapopliteal PTA is needed. Our objective was therefore to describe our institution’s experience with and mid-term outcomes of the use of infrapopliteal PTA for limb salvage.
METHODS
Subjects & Settings
The Beth Israel Deaconess Medical Center institutional review board approved this study protocol. We performed a retrospective chart review using prospectively collected data in a computerized registry on all consecutive patients undergoing an attempt at infrapopliteal angioplasty for critical limb ischemia or bypass graft outflow vessel stenosis from February 2004 to February 2012. Patients were identified using CPT codes for infrapopliteal revascularization (angioplasty and atherectomy, ± stenting). In order to capture patients in whom an intervention was attempted but aborted due to failure to cross a lesion, we also searched for these codes, as well as those for diagnostic lower extremity angiogram, in combination with the CPT modifier 52. This work builds upon and includes 176 patients from our previous manuscript,11.
Indications for intervention included tissue loss, rest pain, or stenosis within the outflow vessel of a tibial bypass, as determined by low graft flow velocities on duplex ultrasound (DUS). For patients with more than one indication for intervention, indication was assigned according to the following hierarchy: tissue loss, acute limb ischemia (ALI), rest pain, and graft outflow stenosis. Cases were performed using 5F or 6F sheaths preferentially through retrograde contralateral and occasionally antegrade ipsilateral access. No brachial or other upper extremity access was used. Starting around September of 2007, ultrasound guidance has routinely been used by the vascular surgeons at our institution for gaining percutaneous access for catheter-based interventions. In this series, ultrasound-guidance was used on 225 patients (50%). Stents were placed only for flow-limiting dissections or residual stenosis >30%. The type of stent used varied with availability and surgeon preference. All patients were discharged on aspirin and clopidogrel unless they had a contraindication or if a bypass was planned.
Measurements
Determination of lesion severity was based on the TASC I classification for infrapopliteal lesions9 since TASC II did not include an anatomic classification of tibial disease10. If more than one vessel had a successful intervention, the limb was assigned the worst of the TASC classes for lesions in series and the lesser class for lesions in parallel. Patients were categorized as unsuitable for bypass at the time of angiography if they lacked or had a wound over a distal target, had no or inadequate conduit, were non-ambulatory, had a medical contraindication, or had severe dementia. Preoperative ambulatory status was determined by reviewing preoperative clinic and admission notes. Ambulatory status was categorized as independent ambulation, ambulation with assistance (e.g. cane or walker), wheelchair-bound, or bedridden.
Outcome Variables
Technical success was defined as a residual stenosis <30% as assessed on single-view completion angiography. Adjunct procedures included stent placement, mechanical atherecomy, thrombectomy, thrombolysis, and intra-arterial nitroglycerin infusion. Multilevel interventions involved concomitant treatment of proximal (e.g. iliac, femoral, popliteal) lesions. The TASC classes for these proximal lesions were not quantified. Standard follow-up visits for all patients were at 2 weeks, then every 3 months for 1 year, and every 6 months thereafter or more frequently as needed.
Patency and restenosis were determined using DUS, Doppler waveforms, segmental pressures, pulse volume recordings, and when indicated, angiography. On DUS, restenosis was defined as a peak systolic velocity ratio >3.0 or angiographic restenosis >70%. Freedom from secondary restenosis was based on the last available vascular laboratory study, using the same DUS threshold for angiography. Limb salvage was defined as freedom from major (below or above knee) amputation. Reintervention was defined as either repeat PTA on the same lesion previously intervened on or bypass graft to an infrapopliteal target encompassing the previously treated area. The decision for repeat PTA vs. bypass was at the discretion of the surgeon caring for the patient. Mortality data was determined using the Social Security Death Index. Discharge disposition was divided into 4 categories: home, home with services (e.g. visiting nurse, home physical therapy, or infusion services), extended care (e.g. skilled nursing, long-term acute care, or rehabilitation facility), or in-hospital death. Postoperative ambulatory status and wound healing were assessed based on findings documented by the surgeon in clinic at 6- and 12-month follow-up or by other providers included in the patient’s online medical record as close to these time points as possible. Wound healing was rated as complete, improved, stable, or worse as judged by the operating surgeon. Long-term living situation was categorized as dependent (i.e. nursing home, long-term rehab) or independent (living independently, with family members, or at an assisted living facility) and was also determined at 6- and 12-months from follow-up.
Statistical analysis
All analyses were performed on a per-limb and intention-to-treat basis. Patient demographics and outcomes were reported as absolute numbers and percentages. Pearson χ2 and Fisher’s exact test were used for comparisons of categorical variables. Means of continuous variables were compared using student’s t-test assuming equal variances while medians of continuous variables were compared using the Wilcoxon rank sum test. Primary outcomes included survival, patency, and freedom from restenosis, secondary restenosis, repeat PTA, bypass, any revascularization, and limb salvage. Both bivariate and multivariable predictors of these outcomes were identified using the log-rank test and Cox proportional hazards regression modeling. For the purposes of building the multivariable models, age was divided into 4 subcategories: age < 60, age 60–69, age 70–79, and age ≥ 80. The designated referent groups for categorical variables were age <60 and TASC A lesions. Patients who underwent infrapopliteal angioplasty for ALI were excluded in the Kaplan-Meier analyses. All statistical analyses were performed using STATA 12.0 (StataCorp, College Station, TX).
RESULTS
Demographics
Infrapopliteal angioplasty was performed on 459 limbs in 413 patients. Average follow-up was 15 months (range 0–85 months). Patient demographics and comorbidities are shown in Table I. 59% of patients were male and the median age was 71 years (range, 31–96 years). There was a relatively even distribution of lesion severity across the four TASC groups, with TASC D lesions being the most prevalent. 149 (32%) of patients had undergone at least one prior procedure (PTA, bypass graft, or minor amputation) on the ipsilateral limb. Of the 38 who had undergone a previous PTA, only 2 were infrapopliteal.
TABLE I.
Patient demographics, comorbidities, TASC class, and other baseline variables.
| Variable | No. | % |
|---|---|---|
| Age (years), median [IQR] | 73 | [63, 81] |
| Age (years), mean [SD] | 71 | 12 |
| Male gender | 271 | 59 |
| Comorbidities | ||
| HTN | 386 | 84 |
| DM | 342 | 75 |
| HPL | 279 | 61 |
| CAD | 229 | 50 |
| Dialysis-dependent renal failure | 71 | 15 |
| Cr > 2.0 mg/dL | 102 | 22 |
| Prior MI | 84 | 18 |
| CHF | 120 | 26 |
| CVA | 74 | 16 |
| COPD | 37 | 8 |
| Current or prior smoker | 203 | 58 |
| TASC Class: | ||
| TASC A | 75 | 16 |
| TASC B | 101 | 22 |
| TASC C | 126 | 27 |
| TASC D | 157 | 34 |
| Prior procedure on ipsilateral limb | ||
| Prior PTA | 37 | 8 |
| Prior bypass graft | 102 | 22 |
| Prior minor amputation | 47 | 10 |
| Indication: | ||
| Tissue loss | 363 | 79 |
| Rest pain | 57 | 12 |
| Acute limb ischemia | 16 | 3 |
| Threatened graft | 28 | 6 |
| Not bypass candidate: | 111 | 24 |
| No target | 44 | 10 |
| No conduit | 24 | 5 |
| Non-ambulatory | 23 | 5 |
| Medical contraindication | 29 | 6 |
| Wound over potential target | 6 | 1 |
| Severe dementia | 1 | 0.2 |
Indications for Intervention
The majority (79%) of interventions were performed for tissue loss with fewer performed for rest pain (12%), ALI (3%), or to treat a stenosis in the native outflow vessel of a previous bypass graft performed for CLI (6%). One hundred eleven patients (24%) had at least one relative contraindication to bypass, most frequently due to a medical contraindication (n=29) such as malignancies, recent stroke or myocardial infarction, a severe cardiac history, or most frequently, multiple concurrent comorbidities (e.g. a patient with significant heart disease, congestive heart failure, and chronic obstructive pulmonary disease).
Procedural details
Procedural details are shown in Table II. Antegrade access was used in 8% of cases. Multilevel intervention (concomitant angioplasty ± stenting of the iliac, femoral, or popliteal vessels) was performed in 50% of all limbs and was more common in patients of advanced age (40% in patients age <60 years, 44% in patients age 61–70 years, 51% in patients age 71–80 years, and 60% in patients >80 years, P=.025) and women (61% vs. 42%, P<.001). Multilevel interventions were generally evenly distributed among the four TASC classes. Overall, 86 infrapopliteal stents were placed in 63 limbs (14%). Adjunct procedures were performed in 67 (15%) patients. Fourteen patients (3%) had intraprocedural tPA injection, 13 patients (3%) had catheters placed for overnight tPA thrombolysis. Mechanical thrombectomy was performed in 34 (7%) patients. Atherectomy was performed in 6 (1%) as an adjunct procedure. A concurrent ipsilateral minor amputation during the same admission was performed in 60 patients (13%).
TABLE II.
Procedural Details
| Variable | No. | % |
|---|---|---|
| Antegrade access | 37 | 8 |
| Number of infrapopliteal vessels treated | ||
| One | 311 | 68 |
| Two | 37 | 8 |
| Three | 1 | 0.2 |
| Only TPT* | 31 | 7 |
| One + TPT* | 71 | 15 |
| Two + TPT* | 7 | 2 |
| Three + TPT* | 1 | 0.2 |
| Multilevel intervention | 229 | 50 |
| w/ TASC A | 30 | 7 |
| w/ TASC B | 51 | 11 |
| w/ TASC C | 72 | 16 |
| w/ TASC D | 76 | 17 |
| Proximal vessel intervened on | ||
| Iliac | 7 | 2 |
| SFA | 169 | 37 |
| Popliteal | 167 | 36 |
| Vein graft | 46 | 10 |
| Stent | ||
| Infrapopliteal | 63 | 14 |
| Femoropophteal | 95 | 21 |
| Iliac | 6 | 1 |
| Graft | 6 | 1 |
| Adjunct procedure | ||
| Procedural tPA infection | 27 | 6 |
| Overnight tPA thrombolysis | 13 | 3 |
| Mechanical thrombectomy | 34 | 7 |
| Atherectomy | 6 | 1 |
| Ipsilateral minor amputation | 57 | 12 |
| Technical success | 427 | 93 |
| Procedural events | ||
| Dissection | 69 | 15 |
| Spasm | 29 | 6 |
| Procedural complications | ||
| AVF | 6 | 1 |
| Thromboembolism | 17 | 4 |
| Rupture | 1 | 0.2 |
TPT = tibioperoneal trunk
Technical success
Technical success was achieved in 427 of 459 (93%) limbs. All of the 32 technical failures involved TASC D lesions and 26 were due to inability to cross an occlusion. There was no correlation between technical failure and adjunct procedures, multilevel intervention, or stent placement. Technical failures were not more frequent in patients who were poor candidates for bypass.
Intraprocedural events & complications
Flow-limiting dissections occurred in 69 (15%) of vessels. These were all successfully treated with stent placement. Vessel spasm occurred in 29 (6%) of vessels. Resolution of vasospasm was achieved in all but one patient treated with nitroglycerin whose angiogram at the end of the case showed vessel patency but persistently sluggish flow. However, this patient experienced no adverse sequelae. Six arteriovenous fistulas were created and all were successfully treated with stenting. There were 17 cases (4%) of distal embolization. The majority of these were successfully managed with different combinations of mechanical or suction/aspiration thrombectomy, thrombolysis, and re-PTA. Two were beyond reach or were deemed minor enough to not significantly affect pedal perfusion. One minor vessel rupture occurred and was successfully treated with a bare-metal self-expanding stent.
Postoperative complications
Perioperative and postoperative outcomes are shown in Table III. Postoperative complications developed in 52 patients (11%). These included 20 cases of access site arterial injuries that required blood transfusion, thrombin injection, or operative repair. There were 11 cases of acute kidney injury (defined as a creatinine increase of ≥20%), 4 myocardial infarctions, 4 congestive heart failure exacerbations, 5 cases of hemodynamically significant cardiac dysrhythmias, 5 pulmonary complications (respiratory failure or pneumonia), 5 cases of gastrointestinal bleeding or hematemesis, and 3 acute cerebrovascular accidents (2 ischemic, 1 hemorrhagic). Conditions that correlated with higher rates of postoperative complications on bivariate analysis were hyperlipidemia (15% vs. 6%, P=.001), preoperative statin use (14% vs. 8%, P=.037), preoperative warfarin use (23% vs. 8%, P<.001), and antegrade access (24% vs. 10%, P=.025). The mean INR of patients on preoperative warfarin was 1.7 while that of those not on preoperative warfarin was 1.2.
TABLE III.
Postoperative outcomes.
| Variable | No. | % |
|---|---|---|
| Mortality | ||
| In-hospital | 11 | 2 |
| 30-day | 26 | 6 |
| Postoperative complications | 52 | 11 |
| Access site arterial injury | 20 | 4 |
| Acute kidney injury | 11 | 2 |
| Acute myocardial infarction | 4 | 1 |
| Congestive heart failure | 4 | 1 |
| Dysrhythmia | 5 | 1 |
| Respiratory failure or pneumonia | 5 | 1 |
| GI bleed/hematemesis | 5 | 1 |
| Cerebrovascular accident | 3 | 1 |
| Length of stay (days), median [IQR] | 5 | [2, 10] |
| Discharge disposition: | ||
| Home | 110 | 24 |
| Home w/ services | 114 | 25 |
| Rehab/nursing facility | 142 | 31 |
| Unanticipated readmission for related problem | ||
| 30-day | 41 | 12 |
| 1-year | 140 | 41 |
Early mortality
In-hospital mortality occurred in 11 patients (2%). Five patients developed cardiac arrest, three patients had uncontrolled hemorrhage from a retroperitoneal or intra-abdominal hematoma and three patients were made comfort measures only (CMO): one patient declined above knee amputation after an unsuccessful attempt at thrombolysis for ALI, one patient developed contrast-induced nephropathy requiring dialysis, and one patient developed an intracranial hemorrhage and the family withdrew care.
Fifteen more patients died after discharge within 30 days of their index procedure resulting in a 30-day mortality rate of 6%. One patient was readmitted with massive gastrointestinal bleeding and was made CMO. This patient had been on Coumadin for atrial fibrillation and had a supratherapeutic INR. Another patient had dialysis-dependent renal failure, was found unresponsive on rounds, and diagnosed with encephalopathy. This patient was made CMO and discharged to hospice. The precise cause of death could not be identified in the remainder of the patients. However, we noted that of these 15 patients, 11 were age >80 years, a third had end-stage renal disease and were dialysis-dependent, a third had CHF, a third had experienced a postoperative complication, and half were not bypass candidates.
On bivariate analysis, thirty-day (including in-hospital) mortality was higher among patients who were older (age <60 years: 2%; age 61–70 years: 1%; age 71–80 years: 4%; age >80 years: 13%, P=.001), those who experienced postoperative complications (21% vs. 4%, P<.001), those not discharged on clopidogrel postoperatively (12% vs. 3%, P=.010), and those who were not bypass candidates (10% vs. 4%, P=.034), including the subgroup of those who had a medical contraindication to bypass (21% vs. 5%, P=.004).
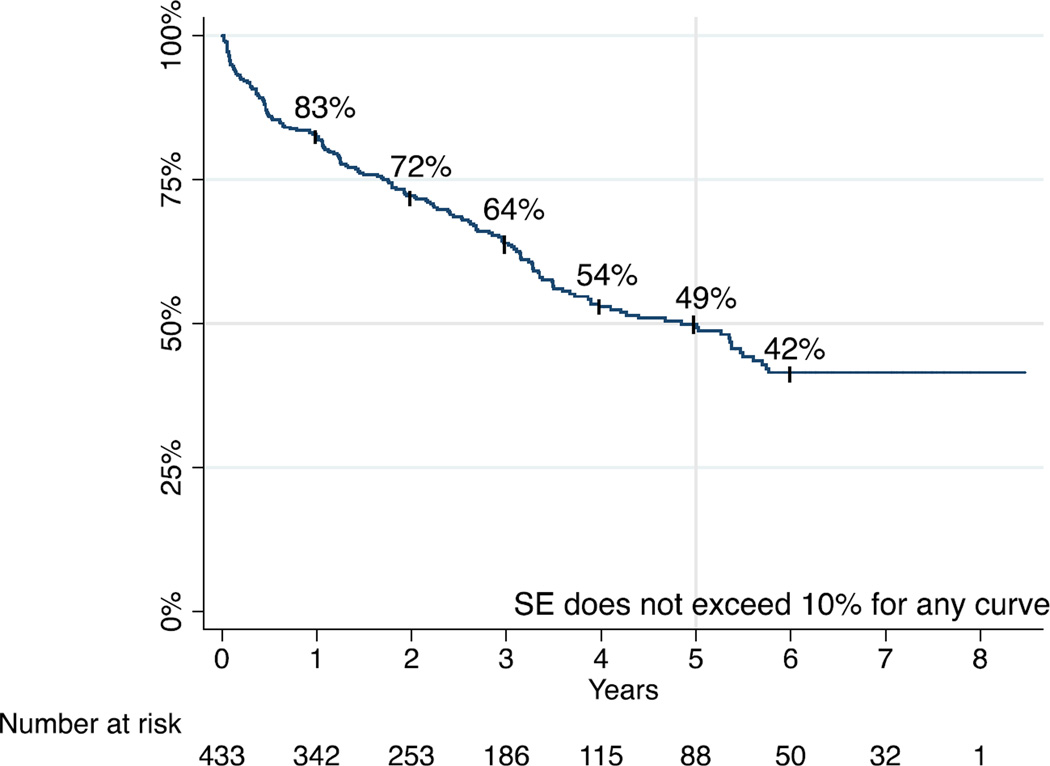
Late survival
Survival at 1, 3, and 5 years was 83%, 64%, and 49%, respectively (Figure 1). Median survival was 4.8 years. TASC class was not associated with long-term mortality rates. Multivariable predictors of mortality were age 71–80 years (HR 1.67, 95% CI 1.02–2.75, P=.042), age >80 years (HR 2.64, 95% CI 1.60–4.36, P<.001), serum creatinine >2.0mg/dl (1.71, 95% CI 1.03–2.85, P=.038), CHF (HR 1.45, 95% CI 1.02–2.07, P=.041), and not being a candidate for bypass (HR 1.78, 95% CI 1.24–2.54, P=.002).
FIGURE 1.
Survival
Length of stay & discharge
Median length of stay (LOS) was 5 days (range 0–44 days, IQ range 2–10). On bivariate analysis, LOS was significantly longer in patients with dialysis-dependent renal failure (7 vs. 4 days; P=.006), serum Cr >2.0 (7 vs. 4 days; P=.001), and those taking warfarin preop (6 vs. 4 days, P=.028). LOS was also longer for patients who were not bypass candidates due to medical contraindication (8 vs. 5 days, P=.033) or due to wounds over potential targets (15 vs. 5 days, P=.013). Not surprisingly, LOS was also significantly higher in patients who experienced postoperative complications (10 vs. 4 days, P<.0001). In contrast, LOS was shorter for patients with rest pain (4 vs. 5 days, P=.024) and graft outflow stenosis (1 vs. 5 days; P<.001) than tissue loss. As a whole, discharge disposition was home for 27% of patients, home with services for 31%, and rehab or long-term care for 36%. Post-intervention, more patients were on anticoagulants and a statin than pre-intervention (Table IV).
TABLE IV.
Pre- and post-intervention medication utilization
| Medication | Pre-intervention (n=459) % |
Pre-intervention* (n=55) % |
Post-intervention (n=431) % |
Post-intervention* (n=55) % |
|---|---|---|---|---|
| Aspirin | 63 | 80 | 89 | 98 |
| Clopidogrel | 32 | 31 | 86 | 89 |
| Warfarin | 20 | 20 | 25 | 25 |
| Statin | 60 | 75 | 69 | 80 |
Only patients in the last 12 months of the study
Long-term qualitative outcomes
Unanticipated readmission related to the index operation occurred in 41 (12%) patients within 1 month. One hundred forty patients (41%) were readmitted for a related problem within one year. Among patients with available follow-up data, improvements in wound healing, ambulatory status, and independent living situation were noted at 6 months and 1 year post-intervention (Table V).
TABLE V.
Comparison of pre-intervention to post-intervention wound healing, ambulatory status and living situation
| Outcome | Pre-intervention % |
6-month Post-intervention % |
1-year Post-intervention % |
|---|---|---|---|
| Wound healing | (n=361) | (n=192) | |
| Complete | 15 | 63 | |
| Improved | 55 | 30 | |
| Stable | 27 | 8 | |
| Worse | 2 | 0.5 | |
| Ambulatory status | (n=310) | (n=354) | (n=183) |
| Ambulates independently | 51 | 56 | 63 |
| Ambulates with assistance | 39 | 32 | 30 |
| Wheelchair bound | 9 | 11 | 7 |
| Bedridden | 0.3 | 1 | 0 |
| Living situation | (n=331) | (n=381) | (n=177) |
| Independent | 72 | 63 | 80 |
| Dependent | 28 | 37 | 20 |
Patency
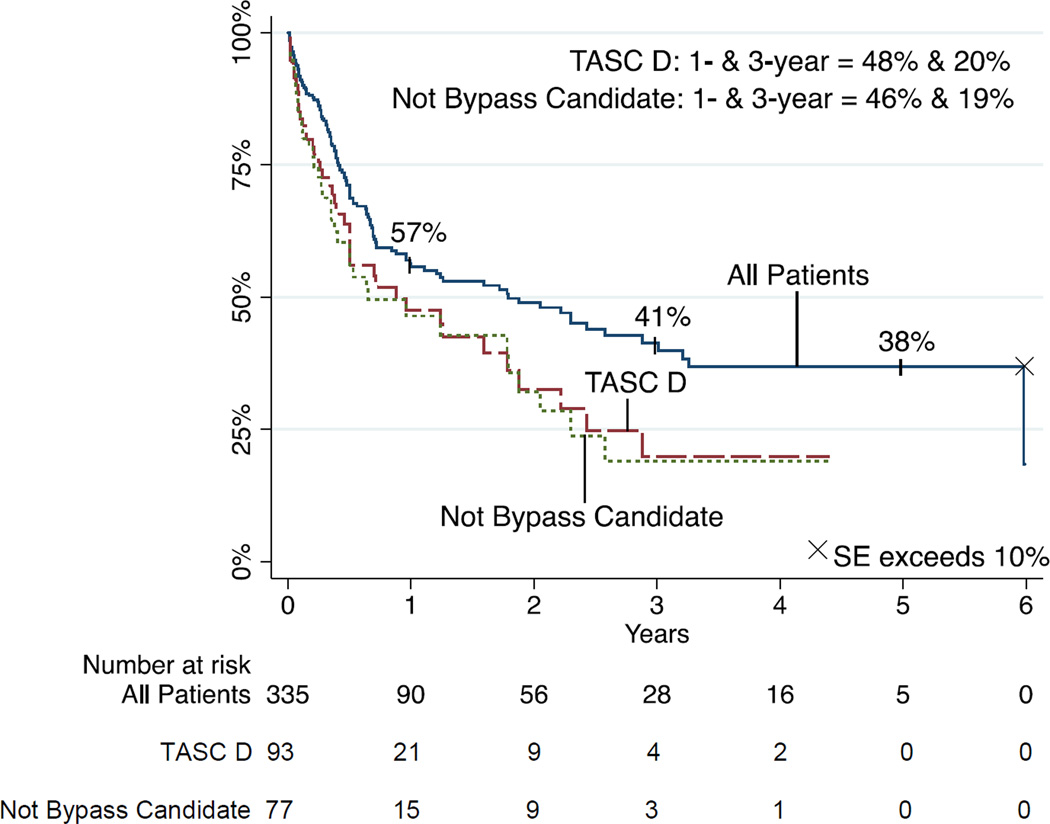
Primary patency was 57% and 38% at 1 and 5 years, respectively (Figure 2). TASC class was associated with patency (P=.002) with 1-year patency rates of 68% for TASC A, 62% for TASC B, 54% for TASC C, and 48% for TASC D lesions. Multivariable modeling indicated significant predictors of loss of patency to be TASC D lesions (HR 2.35, 95% CI 1.33–4.16, P=.003), and bypass non-candidacy (HR 2.01, 95% CI 1.33–3.033, P=.001).
FIGURE 2.
Primary patency
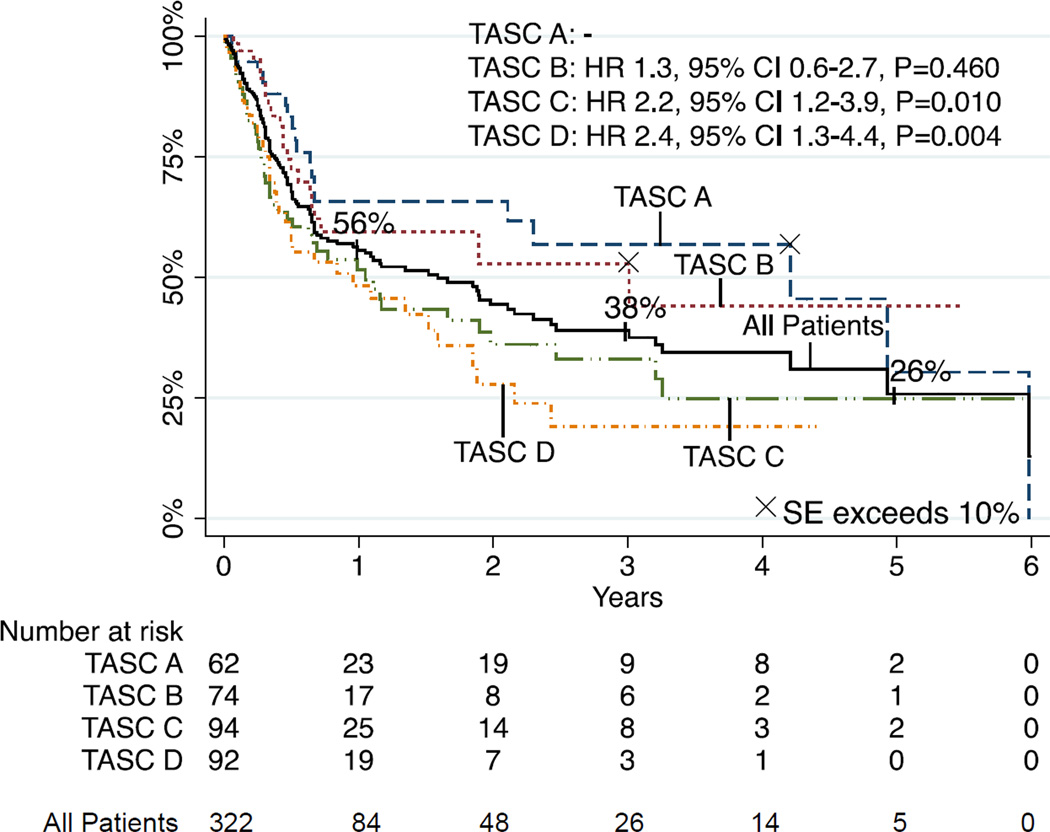
Freedom from Restenosis
Freedom from restenosis was 56% and 34% at 1 and 5 years, respectively (Figure 3). Multivariable predictors of restenosis were TASC C (HR 2.16, 95% CI 1.20–3.88, P=.010) and TASC D (2.42, 95% CI 1.32–4.43, P=.004) lesions.
FIGURE 3.
Freedom from restenosis
Freedom from secondary restenosis
For patients who underwent revascularization, freedom from secondary restenosis was 55%, 49%, and 40% at 1, 2, and 3 years, respectively. Higher rates of secondary restenosis were associated with COPD (P=.018), history of prior BPG (P=.016), and graft outflow vessel stenosis (P=.009) as the initial indication for PTA. Lower rates of secondary restenosis were seen with tissue loss (P=.034) as the initial indication for PTA. There were no significant predictors of secondary restenosis on multivariable analysis.
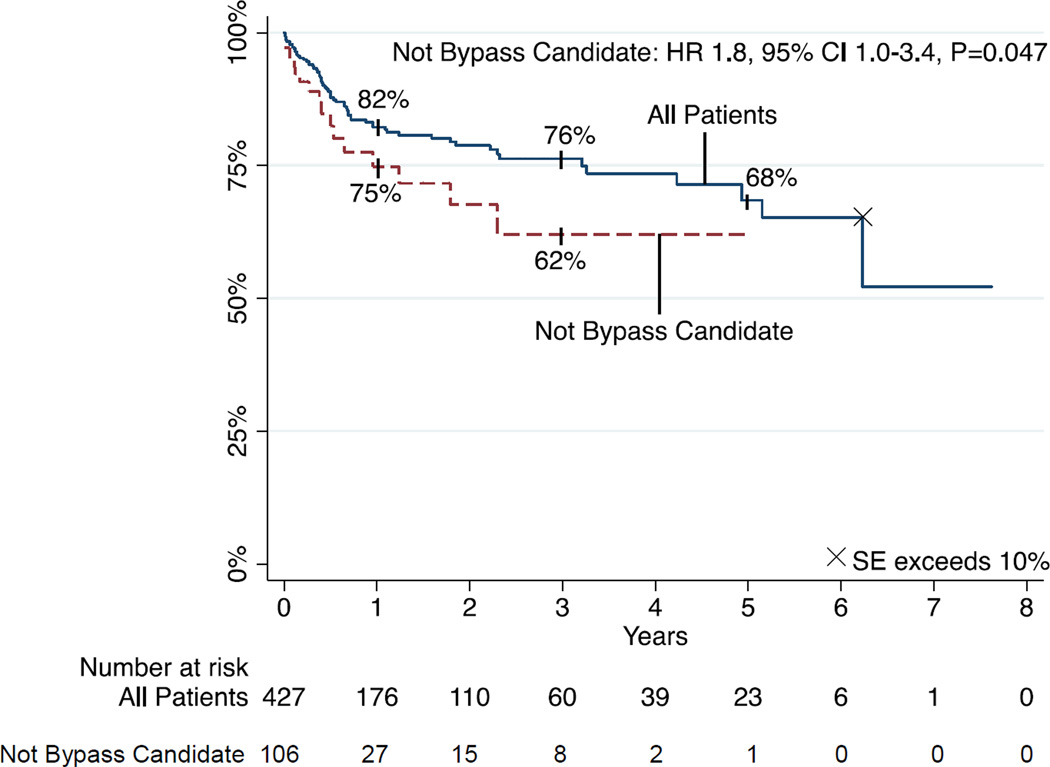
Freedom from repeat angioplasty
Freedom for repeat angioplasty (re-PTA) was 82% and 68% at 1 and 5 years, respectively (Figure 4). On multivariable analysis, bypass non-candidacy was a significant predictor of re-PTA (HR 1.84, 95% CI 1.01–3.35, P=.047). Of the 69 patients who underwent repeat PTA, 54 (78%) had one, 10 (14%) had two, and 5 (7%) had 3 or more repeat PTAs.
FIGURE 4.
Freedom from repeat angioplasty
Freedom from bypass
Freedom from subsequent bypass grafting (BPG) was 89 and 80% at 1 and 5 years, respectively (Figure 5). Multivariable predictors of freedom from bypass were bypass non-candidacy (HR .19, 95% CI .04–.80, P=.024) and technical success (HR .18, 95% CI .06–.52, P=.001).
FIGURE 5.
Freedom from bypass
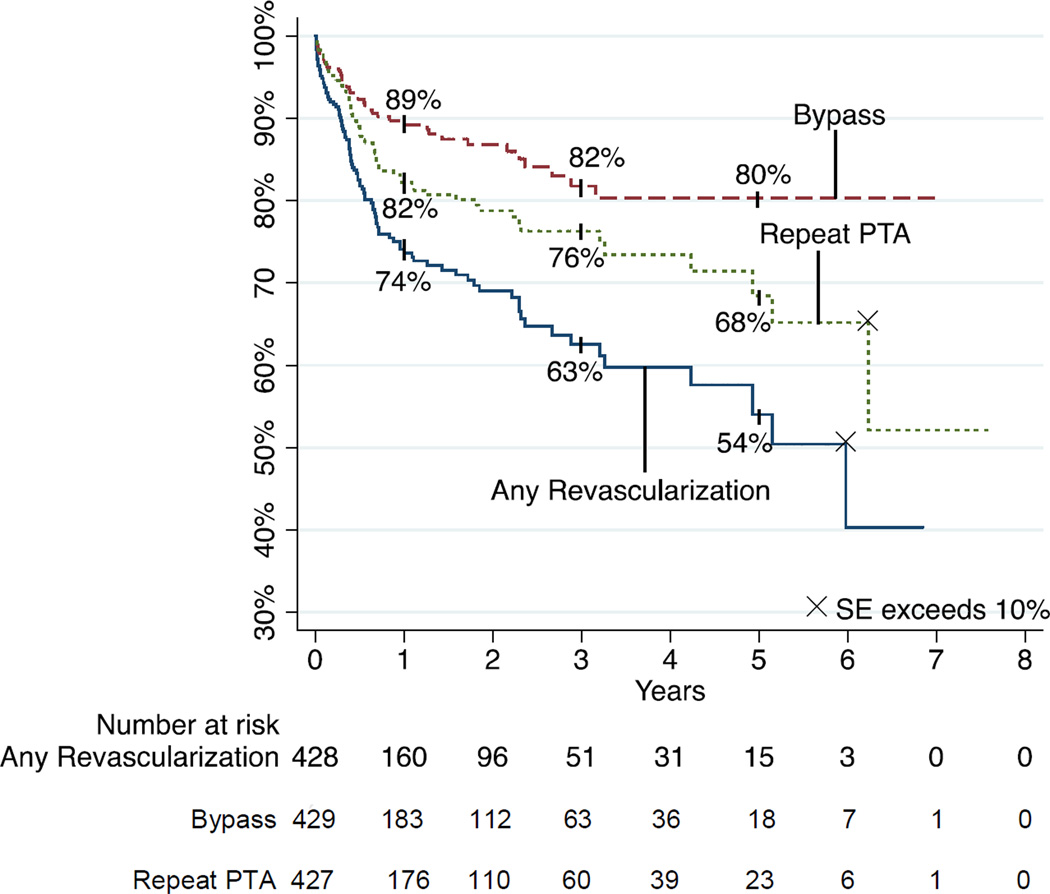
Freedom from any revascularization
(PTA and/or bypass). Freedom from any subsequent revascularization was 74% and 50% at 1 and 5 years, respectively (Figure 6). Freedom from revascularization was improved in patients discharged on clopidogrel (P<.001), patients in whom technical success was achieved (P=.003), and was worse in patients whose initial indication was bypass graft outflow vessel stenosis (P=.032). Multivariable modeling revealed that rates of revascularization were lower in patients discharged on clopidogrel (HR .52, 95% CI .30–.92, P=.025). Even when technical failures were excluded from the model, postoperative clopidogrel use was still predictive of higher freedom from revascularization (HR 0.46, 95% CI 0.25–0.83, p=0.011).
FIGURE 6.
Freedom from subsequent revascularization by reintervention type
Limb salvage
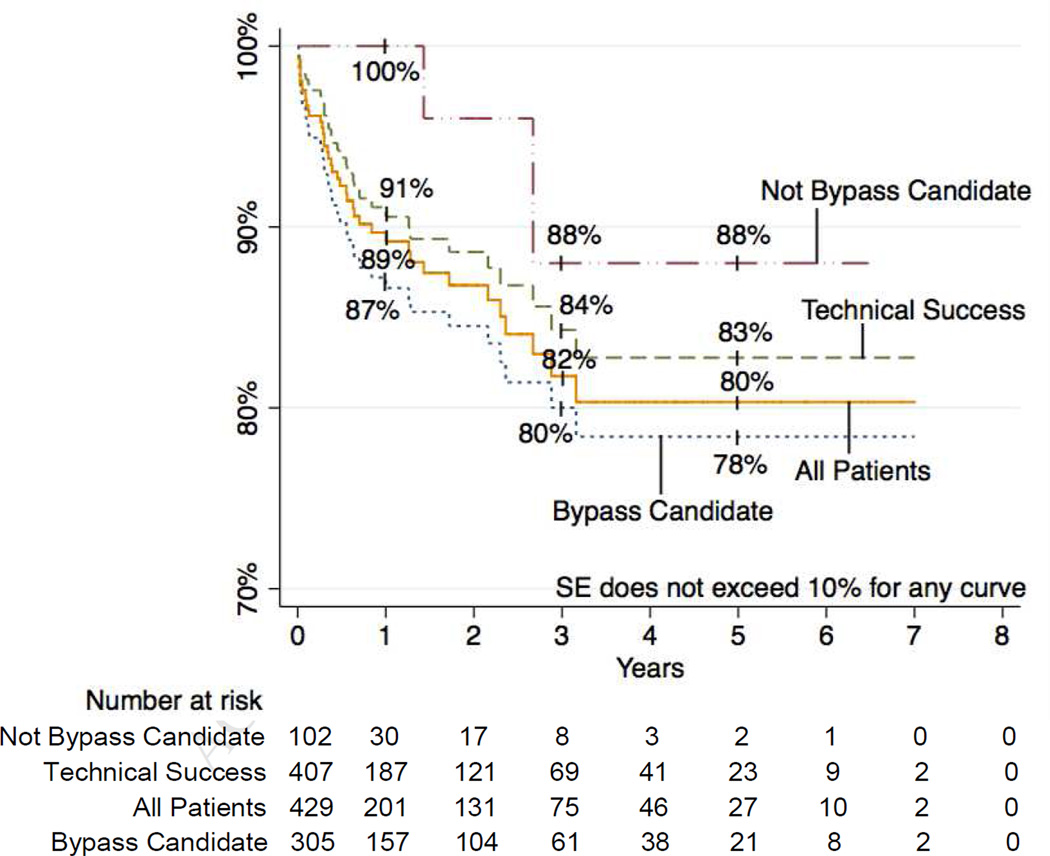
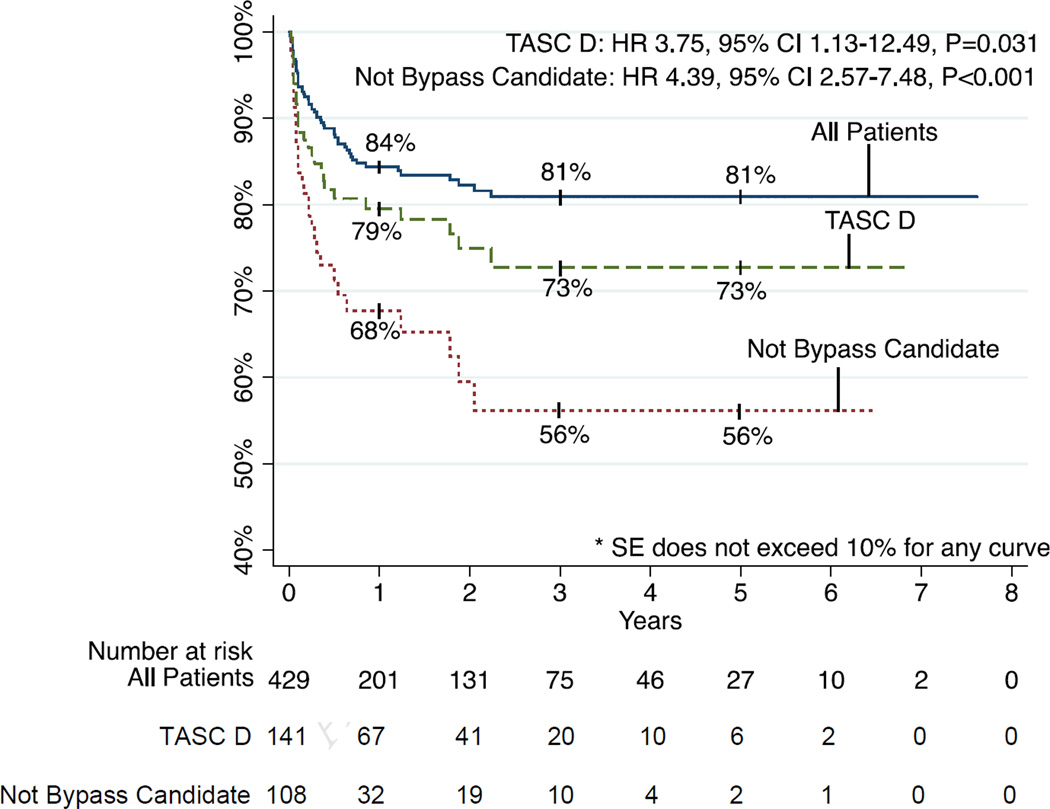
Limb salvage was achieved in 84% and 81% at 1 and 5 years and was sustained at 81% to 7 years (Figure 7). Thirty-day major amputation rate was 4%. Freedom from amputation was associated with TASC class (P=.006) with 1-year limb salvage rates of 95% for TASC A, 87% for TASC B, 81% for TASC C, and 80% for TASC D. Limb salvage was invariably worse for patients who were not bypass candidates (P<001). Specifically, lower rates of limb salvage were seen in patients with a wound over a distal bypass target (P<.001), those who were non-ambulatory (P=.005), those without a distal target (P<.001) those for whom an adequate venous conduit was not available (P=.005). Of note, multilevel intervention was not associated with significant differences in amputation rates compared to interventions on the infrapopliteal vessels only (12% vs. 19% at 1 year, 17% vs. 21% at 5 years, P=0.197). Amputation rates were also higher in patients on dialysis (P<.001) and those whose baseline serum Cr was > 2.0mg/dl (P=.002). Multivariable predictors of amputation were TASC D lesions (HR 3.75, 95% CI 1.13–12.49, P=.031) and not being a bypass candidate (HR 4.39, 95% CI 2.57–7.48, P<.001). Of the 62 patients who underwent subsequent major amputation, 6 patients had lost a distal target in the interval between their last PTA and amputation. However, it was not clear in these cases whether this was due to the intervention vs. subsequent progression of disease. Of the remaining patients, 36 were not candidates for bypass, 9 underwent a bypass that ultimately failed, 3 had a bypass outflow vessel stenosis treated with PTA but the bypass eventually failed anyway, 6 later presented with wet gangrene or had tissue destruction too extensive for salvage, one patient underwent BKA at an outside hospital, and one patient became non-ambulatory due to progressive dementia and contracture of a prior BKA on the contralateral side.
FIGURE 7.
Limb salvage
DISCUSSION
Although initial technical success (93% in our 8-year experience) is achieved in the vast majority of patients in whom infrapopliteal PTA is undertaken, our study emphasizes the importance of appropriate patient selection in maintaining long-term success. Despite a high 5-year restenosis rate of 74%, we demonstrated that acceptable limb salvage rates could be achieved, with 81% of all patients free of amputation at 5-year follow-up. However, frequent reinterventions were performed, with nearly a third of patients undergoing repeat angioplasty and one fifth of patients undergoing bypass within 5 years. We found that TASC class was strongly predictive of primary patency, restenosis, and limb salvage. Specifically, TASC D lesions more than halved the odds of maintaining primary patency, doubled the odds of restenosis, and more than tripled the odds of major amputation.
Another significant and recurrent predictor of outcome was suitability for bypass. Patients who were poor candidates for surgical bypass had higher rates of repeat angioplasty, subsequent bypass, amputation, and mortality. As bypass non-candidacy was often a proxy for more advanced disease, the finding that more than a quarter of our patients were poor surgical candidates emphasizes the fact that CLI remains a challenging condition to treat given the burden of systemic atherosclerotic disease and multiple comorbidities inherent in this patient population. For individuals who are poor candidates for bypass, endovascular therapy offers a favorable alternative to amputation. However, their outcomes are nonetheless uniformly worse.
The results of this study reinforce our previously published short-term outcomes11, in which we similarly found the major determinants of our primary outcomes to be TASC D lesions and bypass candidacy. Comparing our primary outcomes then and now, we observed that we have made improvements in every category except limb salvage, which has remained relatively stable. Of note, the policy at our institution has always been to be aggressive with bypass when PTA has failed. In addition to advances in technology and cumulative surgeon experience, we surmise that our improvements in outcomes may be due to broader inclusion criteria when choosing patients for this procedure. Whereas we previously tended to reserve tibial PTA for patients with no other options, in more recent years, we undertake tibial PTA in patients with good bypass options.
As percutaneous transluminal angioplasty (PTA) is increasingly used as an alternative primary therapy for critical limb ischemia4–7, a growing body of literature has accumulated comparing PTA to bypass. To date, there has only been one large randomized control trial comparing PTA to bypass for CLI3, which suggested that bypass should be offered first over balloon angioplasty in patients with available venous conduit who are expected to live longer than 2 years. However, this study encompassed all infrainguinal disease and was not specific to infrapopliteal disease.
Our institution previously published its 10-year experience with more than 1,000 cases of dorsalis pedis bypass17. Over a mean follow-up of 24 months, 5-year primary patency, limb salvage, and survival were 57%, 78%, and 49% respectively. Postoperative complications occurred in 12% and 30-day mortality was observed in 0.9%. Though the comparison to our series of infrapopliteal PTA is not ideal, rates of postoperative complications, 5-year survival and limb salvage were similar between PTA and bypass. Thirty-day mortality was considerably higher in our PTA group. This is likely due in part to selection bias; however, when we considered only patients who were surgical candidates, our 30-day mortality was still comparatively high at four percent. This highlights the severity of the general medical condition of these patients selected for PTA but also challenges the notion that endovascular therapy is always associated with lower perioperative morbidity and mortality. In our study, age was a significant predictor of 30-day mortality with a rate as high as 13% in patients age >80 years. The mean age of the bypass patients was 4 years younger than that of our infrapopliteal PTA cohort.
The results of our study are consistent with those published by other groups. In a retrospective comparison of 48 patients undergoing PTA vs. 50 patients undergoing bypass for infrapopliteal lesions, Casella et al.18 similarly found that TASC D lesions were associated with inferior secondary patency and limb salvage. Conrad et al.8 found that one consistent predictor of worse outcomes in their series of 155 infrapopliteal PTA was chronic renal failure. Just as bypass non-candidacy was a surrogate of systemic morbidity in our series, chronic renal failure was a harbinger of worse outcomes in their study. In our series, dialysis-dependent renal failure and/or a baseline serum creatinine > 2.0 were also associated with higher rates of amputation and lower likelihood of receiving bypass.
Romiti et al. performed a meta-analysis of 30 articles on the topic of infrapopliteal angioplasty published between 1990 and 200619. To provide a comparison to bypass, they used a separate meta-analysis of popliteal-to-distal vein bypass20. Across the 30 studies, they calculated overall 1-year primary patency rates of 58.1% for PTA vs. 81.5% for bypass, secondary patency rates of 83.3% for PTA vs. 85.9% for bypass, limb salvage rates of 86.0% for PTA vs. 88.5% for bypass, and survival rates of 87.0% for PTA (no survival rate estimate was available for bypass). The authors concluded that although PTA was limited by lower durability, PTA nonetheless achieved limb salvage at comparable rates. Exact rates of subsequent bypass were not reported in this meta-analysis and therefore no comment can be made on how best to identify patients who will ultimately undergo bypass and/or those who will progress to amputation. Given our results, it is likely that the comparison of the two meta-analyses suffers from selection bias as well.
The only population-based study of infrapopliteal PTA that we are aware of was recently published by Vogel et al.21 using Medicare data. They reported comparable rates of an in-hospital mortality (2.8%) and 30-day mortality (6.7%). As with our study, they similarly found advancing age and renal failure to be the strongest predictors of mortality but reported significantly higher rates of 30-day re-hospitalization (30%), 30-day BKA rate (12%), and postoperative complications (14%). It is possible their numbers may have been exaggerated since administrative data cannot account for laterality and comorbidities may sometimes be confused with complications.
Our current study has several limitations. As a retrospective study, the potential for selection and information bias exists. Since our data represent the experience of one group of surgeons at a single institution, our results are subject to the influence of specific referral patterns, surgeon experience, and patient selection preferences. Additionally, we did not perform a comparative cost analysis between endovascular and open therapies, which is of particular relevance given our finding that half our patients underwent reintervention by 5 years. Lastly, our method of identifying patients for study inclusion may have missed some attempts at crossing lesions and therefore the technical success may be somewhat overestimated.
CONCLUSION
Infrapopliteal angioplasty can achieve limb salvage and survival rates at 5 years comparable to those of surgical bypass and thus can be considered a reasonable first-line therapy in the treatment of TASC A, B, and perhaps C lesions even in a patient with adequate conduit available. TASC D lesions should preferably be treated with bypass in patients who are suitable candidates for surgery, with suitable distal targets, and adequate venous conduit. Patients who undergo infrapopliteal PTA because they are not bypass candidates do not achieve outcomes comparable to bypass and should be counseled about their higher risk for repeat PTA and/or amputation. Comparisons of PTA with bypass should account for this potential difference in patient populations.
Acknowledgments
This work was supported by the NIH T32 Harvard-Longwood
Research Training in Vascular Surgery grant HL007734.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures: R.C. Lo, none; R.P. Bensley, none; J Darling, none; S Dahlberg, none; A Hamdan, Endologix Consultant; M Wyers, Endologix Consultant, Boston Scientific Consultant; M.L. Schermerhorn, Endologix Consultant, Medtronic Consultant.
Presented at the 2012 Vascular Annual Meeting of the Society for Vascular 2 Surgery on 6/7/12.
REFERENCES
- 1.Beard JD. Which is the best revascularization for critical limb ischemia: Endovascular or open surgery? J Vasc Surg. 2008;48(6 Suppl):11S–16S. doi: 10.1016/j.jvs.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 2.Blevins WA, Jr, Schneider PA. Endovascular management of critical limb ischemia. Eur J Vasc Endovasc Surg. 2010;39(6):756–761. doi: 10.1016/j.ejvs.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery- first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess. 2010;14(14):1–210. doi: 10.3310/hta14140. iii-iv. [DOI] [PubMed] [Google Scholar]
- 4.Soderstrom MI, Arvela EM, Korhonen M, Halmesmaki KH, Alback AN, Biancari F, et al. Infrapopliteal percutaneous transluminal angioplasty versus bypass surgery as first-line strategies in critical leg ischemia: a propensity score analysis. Ann Surg. 2010;252(5):765–773. doi: 10.1097/SLA.0b013e3181fc3c73. [DOI] [PubMed] [Google Scholar]
- 5.Kudo T, Chandra FA, Ahn SS. The effectiveness of percutaneous transluminal angioplasty for the treatment of critical limb ischemia: a 10-year experience. J Vasc Surg. 2005;41(3):423–435. doi: 10.1016/j.jvs.2004.11.041. discussion 435. [DOI] [PubMed] [Google Scholar]
- 6.Haider SN, Kavanagh EG, Forlee M, Colgan MP, Madhavan P, Moore DJ, et al. Two-year outcome with preferential use of infrainguinal angioplasty for critical ischemia. J Vasc Surg. 2006;43(3):504–512. doi: 10.1016/j.jvs.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Dorros G, Jaff MR, Dorros AM, Mathiak LM, He T. Tibioperoneal (outflow lesion) angioplasty can be used as primary treatment in 235 patients with critical limb ischemia: five-year follow-up. Circulation. 2001;104(17):2057–2062. doi: 10.1161/hc4201.097943. [DOI] [PubMed] [Google Scholar]
- 8.Conrad MF, Kang J, Cambria RP, Brewster DC, Watkins MT, Kwolek CJ, et al. Infrapopliteal balloon angioplasty for the treatment of chronic occlusive disease. J Vasc Surg. 2009;50(4):799–805. doi: 10.1016/j.jvs.2009.05.026. e794. [DOI] [PubMed] [Google Scholar]
- 9.Dormandy JARB Rutherford. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31(1 Pt 2):S1–S296. [PubMed] [Google Scholar]
- 10.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter- Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Giles KA, Pomposelli FB, Spence TL, Hamdan AD, Blattman SB, Panossian H, et al. Infrapopliteal angioplasty for critical limb ischemia: relation of TransAtlantic InterSociety Consensus class to outcome in 176 limbs. J Vasc Surg. 2008;48(1):128–136. doi: 10.1016/j.jvs.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Bosiers M, Hart JP, Deloose K, Verbist J, Peeters P. Endovascular therapy as the primary approach for limb salvage in patients with critical limb ischemia: experience with 443 infrapopliteal procedures. Vascular. 2006;14(2):63–69. doi: 10.2310/6670.2006.00014. [DOI] [PubMed] [Google Scholar]
- 13.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Analysis of amputation free and overall survival by treatment received. J Vasc Surg. 2010;51(5 Suppl):18S–31S. doi: 10.1016/j.jvs.2010.01.074. [DOI] [PubMed] [Google Scholar]
- 14.Nolan BW, De Martino RR, Stone DH, Schanzer A, Goodney PP, Walsh DW, et al. Prior failed ipsilateral percutaneous endovascular intervention in patients with critical limb ischemia predicts poor outcome after lower extremity bypass. J Vasc Surg. 2011;54(3):730–735. doi: 10.1016/j.jvs.2011.03.236. discussion 735-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spinelli F, Stilo F, Benedetto F, De Caridi G, La Spada M. Early and one-year results of infrainguinal bypass after failure of endovascular therapy. Int Angiol. 2011;30(2):156–163. [PubMed] [Google Scholar]
- 16.Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg. 2011;54(4):1021–1031 e1021. doi: 10.1016/j.jvs.2011.03.281. [DOI] [PubMed] [Google Scholar]
- 17.Pomposelli FB, Kansal N, Hamdan AD, Belfield A, Sheahan M, Campbell DR, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37(2):307–315. doi: 10.1067/mva.2003.125. [DOI] [PubMed] [Google Scholar]
- 18.Casella IB, Brochado-Neto FC, Sandri Gde A, Kalaf MJ, Godoy MR, Costa VS, et al. Outcome analysis of infrapopliteal percutaneous transluminal angioplasty and bypass graft surgery with nonreversed saphenous vein for individuals with critical limb ischemia. Vasc Endovascular Surg. 2010;44(8):625–632. doi: 10.1177/1538574410373663. [DOI] [PubMed] [Google Scholar]
- 19.Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira N, De Luccia CA. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47(5):975–981. doi: 10.1016/j.jvs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Albers M, Romiti M, Brochado-Neto FC, De Luccia N, Pereira CA. Meta-analysis of popliteal-to-distal vein bypass grafts for critical ischemia. J Vasc Surg. 2006;43(3):498–503. doi: 10.1016/j.jvs.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Vogel TR, Dombrovskiy VY, Carson JL, Graham AM. In-hospital and 30-day outcomes after tibioperoneal interventions in the US Medicare population with critical limb ischemia. J Vasc Surg. 2011;54(1):109–115. doi: 10.1016/j.jvs.2010.12.055. [DOI] [PubMed] [Google Scholar]