Abstract
Objectives
Endothelial dysfunction has been studied in animal models. However, direct evidence of endothelial function from human vessels is limited. Our objectives were to optimize methods in harvesting human arteries from amputation specimens, determine endothelial function, and measure responsiveness to the nitric oxide precursor, L-arginine (L-arg).
Methods
Fresh amputation specimens were transferred expeditiously from the operating room to the bench laboratory for dissection and arterial harvest in an IRB-approved protocol. Popliteal and tibial vessels were examined in pilot experiments leading to the use of the anterior tibial artery in consecutive experiments. Human lower extremity anterior tibial artery segments were harvested from amputation specimens (N=14). Specimens were rapidly collected and divided for endothelial dependent relaxation (EDR) studies in a tissue bath apparatus, immunohistochemistry, and intravascular ultrasound derived virtual histology (IVUS-VH). A total of 47 ring segments were studied. The data were compared with two-way ANOVA.
Results
Human lower extremity arteries exhibited low responsiveness to acetylcholine (Ach, EDR=24.9%, Ach 10−4). L-arg supplementation enhanced EDR by 38.5% (P<.0001). L-NAME (N-nitro-L-arginine methyl ester) abrogated EDR (P<.0001) in vessels exposed to L-arg. Arterial responsiveness was intact in all vessels (endothelial independent relaxation to sodium nitroprusside, 113.2 ± 28.1%). Histology and immunohistochemistry confirmed intact endothelium by morphometric analysis, CD31, eNOS, and arginase II staining. IVUS-VH indicated atheroma burden was 11.9 ± 4.7 mm3/cm, and plaque stratification indicating fibrous morphology predominant (59.9%; necrotic core, 16.9%; calcium, 11.2%). Variations in plaque morphology did not correlate with endothelial function nor responsiveness to L-arg.
Conclusions
Human lower extremity arteries demonstrate low baseline endothelial function in patients requiring amputation. Endothelial dysfunction is improved by L-arginine supplementation in an ex vivo model. These results support strategies to increase local levels of nitric oxide in human vessels.
INTRODUCTION
The seminal observation that endothelium is a key mediator of vascular vasomotor reactivity was made in 1980 by Furchgott and Zawadzki.1 The ability of the artery to relax was attributed to the elusive substance endothelium-derived relaxing factor (EDRF) which was identified as nitric oxide (NO) by Ignarro and Moncada.2,3 NO is thought to play a pivotal role in the regulation of endothelial function and to serve a cardioprotective role via its antithrombotic, anti-inflammatory, and antiatherosclerotic properties. Consistent with this construct, endothelial dysfunction is believed to represent the earliest abnormality in the development of atherosclerosis.4
Endothelial dysfunction studies in animal models have been attributed to decreased nitric oxide production by endothelial NO synthase (eNOS). Vasorelaxation can occur through two different mechanisms: endothelial-dependent vasorelaxation (EDR) and endothelial-independent vasorelaxation (EIR). Through interaction with endothelial cell surface receptors, mediators of endothelial dependent vasorelaxation (acetylcholine, bradykinin, etc.) cause NO release. NO then diffuses to the underlying smooth muscle cells and induces vasorelaxation. This process can be quantitatively assessed in the laboratory and the measurement of EDR of an artery reflects endothelial function. Isolated vessel ring studies remain a straightforward, simple method of assessing EDR secondary to NO.1 This method has been used reliably in previous animal models.5 Agonists for EDR (acetylcholine, bradykinin, calcium ionophore, etc.) or EIR (sodium nitroprusside, nitroglycerin, etc.) can be used to determine the level of endothelial and smooth muscle cell function. Furthermore, antagonists, such as L-NMMA (N-monomethyl L-arginine) and L-NAME (N-nitro-L-arginine methyl ester) can be used as artificial inhibitors of eNOS and allow specific controls for experimental findings and constructing of dose-response curves.
The direct assessment of endothelial function in patients affected by peripheral arterial disease (PAD) has been scant. Targeted therapies to alleviate endothelial dysfunction may significantly impact long-term clinical outcomes in patients with PAD. Thus, we proceeded to investigate the feasibility of measuring endothelial function in human lower extremity arteries harvested after amputation. Our objectives were to optimize methods in harvesting human arteries from amputation specimens, determine endothelial function using a tissue bath apparatus, and measure responsiveness to the nitric oxide precursor, L-arginine (L-arg). We also sought to determine the feasibility of using IVUS and immunohistochemistry staining in this ex-vivo model, and determine any correlation to EDR measurement. These direct measurements may lead to insights into biological therapies for PAD that target the endothelium.
MATERIALS AND METHODS
This protocol was approved by the Cleveland Clinic Institutional Review Board as a consent-exempt protocol with evaluation of discarded pathological tissue only. Patient-level information, clinical and demographic variables could not be obtained under these conditions.
Human lower extremity arterial segments were obtained from amputation specimens. Our pilot experiments of 7 limbs (3 popliteal, 4 tibial) led to the following methodological observations.
Initially, we attempted to harvest arteries from both above-knee and below-knee amputation specimens. Below-knee amputation specimens yielded very little usable arterial tissue for investigation. Thus, above-knee specimens were used uniformly leading to the data in this study.
A single above-knee amputation specimen may have a combination of patent tibial, popliteal, and femoral arteries for examination. Since we sought to keep the time elapsed from amputation to endothelial function measurement at a minimum, harvesting all of the patent arterial tree was too time-consuming. All studies were undertaken immediately upon vessel procurement minimizing any delay or endothelial deterioration.
We used a tissue bath chamber that was approximately 10 mL in size. The popliteal artery averaged 5–7 mm in diameter. Smaller diameter popliteal arteries were used but most were too large to mount in our system.
The anterior tibial artery was 2–3 mm in diameter and if patent became the preferential artery to study. Eight to 10 cm of this artery could be dissected quickly and patent segments were identified and mounted with minimal delay. Despite extensive plaque burden and calcification in most vessel segments, the small tungsten wires allowed for precise fitting into the lumen.
Human lower extremity artery segments (anterior tibial artery, 80%; popliteal artery, 20%) were harvested from patients undergoing lower extremity amputation (N=14). Specimens were rapidly collected and divided for EDR studies in a tissue chamber, immunohistochemistry, and intravascular ultrasound derived virtual histology (IVUS-VH). Adjacent vessel segments were used for EDR, IVUS, and IHC for ideal correlation. A total of 47 ring segments were studied. The data were compared with two-way ANOVA. Statistical significance was set at P<.05. Statistical analysis was performed using Prism software (GraphPad, San Diego, Calif). Results are expressed as Mean +/− SEM. No other laboratory or demographic data were obtained for our specimens. Our IRB approval for this pre-clinical study allowed us access to discarded tissue from human lower extremity amputations, but not for a full chart review. Accordingly, we were unable to account for clinical severity of disease, operative indications, or presence of infection.
Studies of Endothelial and Smooth Muscle Cell Function
The harvested arteries were sectioned into 4-mm segments and mounted on standard tungsten wire triangles (A-M Systems, Everett, WA), attached to isometric force displacement transducers (Radnoti 159920, Monrovia, Calif), and placed into tissue baths.6 The Radnoti apparatus could accommodate a maximum of 4 simultaneous studies, thus we took three or four segments from each vessel for a total of 47 rings for this experiment. The tissue baths were temperature controlled via a heated water jacket at 37° Celsius. Standard Krebs-Hensleit solution (NaCl 118mM, NaHCO3 25mM, glucose 5.6mM, KH2PO4 1.2mM, KCL 4.7mM, MgSO4 1.2mM, CaCl2 1.5mM) was used with a 95% oxygen, 5% carbon dioxide gas mixture bubbled into the baths.
Pre-load (2 grams) was applied to the arterial rings and the vessels were allowed to equilibrate for 1 hour using a standardized protocol adapted from previous animal studies (Figure 1).7–9 The arteries were then constricted using a high potassium solution (standard Kreb’s except KCl 122mM) to measure maximal contractile force for the artery (F-max). The baths were then emptied, rinsed three times with the Kreb’s solution, and again allowed to equilibrate. At this point, the arteries were constricted to 75% of F-max with 10−5 M norepinephrine (NE)(Sigma, St. Louis, MO). Acetylcholine (Ach)(Sigma) was then added in incremental log doses to achieve 10−7 M to 10−4 M bath concentrations for determination of EDR. The protocol was repeated with the addition of L-arginine (1mL of 1M solution, Sigma). EIR was measured in a similar fashion using sodium nitroprusside (NTP)(Sigma) in the 7 pilot vessels. Baths were rinsed and arterial segments brought to 75% F-max with NE between each reagent. Vessels were subjected to eNOS blockade by incubating the vessel segments (n=6) in N-monomethyl L-arginine (L- NAME, 1mL of 100 μM)(Sigma) for one hour during the equilibration period.
Figure 1.
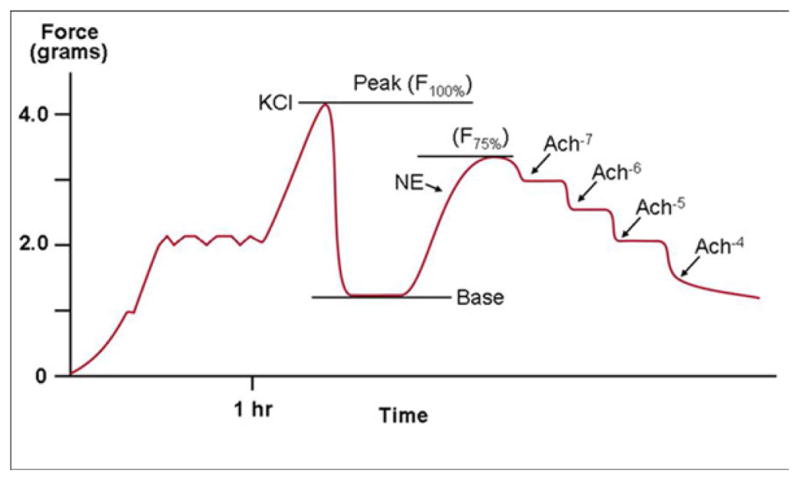
EDR curve is established using incremental doses of Acetylcholine.
Immunohistochemical Analysis
Intact endothelial presence and morphology were evaluated in a select number of specimens.
Arginase-1 and Arginase-2
Immunohistochemistry for Arginase-1 (1:200 dilution) and Arginase-2 (1:100 dilution) was performed using polyclonal rabbit anti-human antibodies. Tissue sections were subjected to heat-induced epitope retrieval pretreatment for 3 minutes at 95°C in 0.01M citrate buffer (pH 6.0), allowed to cool, immersed in 1% hydrogen peroxide/methanol before blocking with 5% skim milk, and incubated with primary antibody for one hour at room temperature. Slides were then developed for 5 minutes with 3-3′-diaminobenzidine (DAB) chromogen, counter-stained with hematoxylin, and coverslipped.
eNOS and CD31
Slides were treated with 5% normal goat serum in PBS for 30 min at room temperature. The slides were incubated for 30 min with a 1:100 dilution of primary anti-eNOS rabbit antibody. After three washes with PBS, the sections were incubated with biotinylated secondary antibody, and incubated with peroxidase substrate. Antibody-labeled specimens were rinsed with distilled water for 5 min, dehydrated, and mounted.
The endothelial marker CD31 was also visualized by immunoreactive (IR) staining. Sections were fixed in acetone for 10 minutes, and rinsed with PBS. The slides were incubated with 10% normal goat serum for 10 minutes in and then with primary mouse antihuman antibodies against CD31 for 60 minutes. The slides were then washed with PBS, and incubated with biotinylated secondary antibody along with peroxidase substrate. Antibody-labeled specimens were rinsed with distilled water for 5 min, dehydrated, and mounted.
Detection of Plaque Constituents by IVUS Derived Virtual Histology
The harvested arteries (n=9) were mounted in positions approximating their orientation in situ. The vessel was cannulated, secured with steel pins, and perfused with phosphate buffered saline solution at 100 mm Hg, This technique was used to simulate in-vivo conditions.10,11 An initial pullback was performed to qualitatively ascertain plaque composition and morphology. An IVUS catheter (Eagle Eye® Gold Catheter) was inserted into the vessel as far distally as possible and the catheter was pulled back (Trak Back® II) until a region of plaque was clearly visible on the IVUS monitor (s5™ Imaging System). At this location, a suture marker was attached to the vessel surface and clamped with a forceps visible in the IVUS image as a highly echoic streak. The IVUS screen was frozen and the image captured to videotape. This procedure was repeated as the catheter advanced toward the proximal end of the vessel for up to 10 markers. Due to the flexibility of this vessel testing apparatus, the geometrical, compositional, and mechanical data were obtained from the vessel. The luminal border was identified by visualization in the lab during post-imaging analysis. Once this border has been determined, several measurements were derived: 1) Lumen cross sectional area (CSA), the area bounded by the luminal border; 2) Calcific deposits, which appear as bright echoes that obstruct the penetrance of the ultrasound; 3) Fibrous plaques, which have an intermediate echogenicity; and 4) Necrotic core zones, which have reduced echogenicity.12 Areas comprising calcium, collagen fibers, and necrotic core were identified on the corresponding IVUS images via virtual histology (IVUS-VH). Virtual histology was performed using a modified coronary artery algorithm classified by color indicating calcium (white), necrosis (red), and fibrous (green) plaque components (Figure 2).
Figure 2.
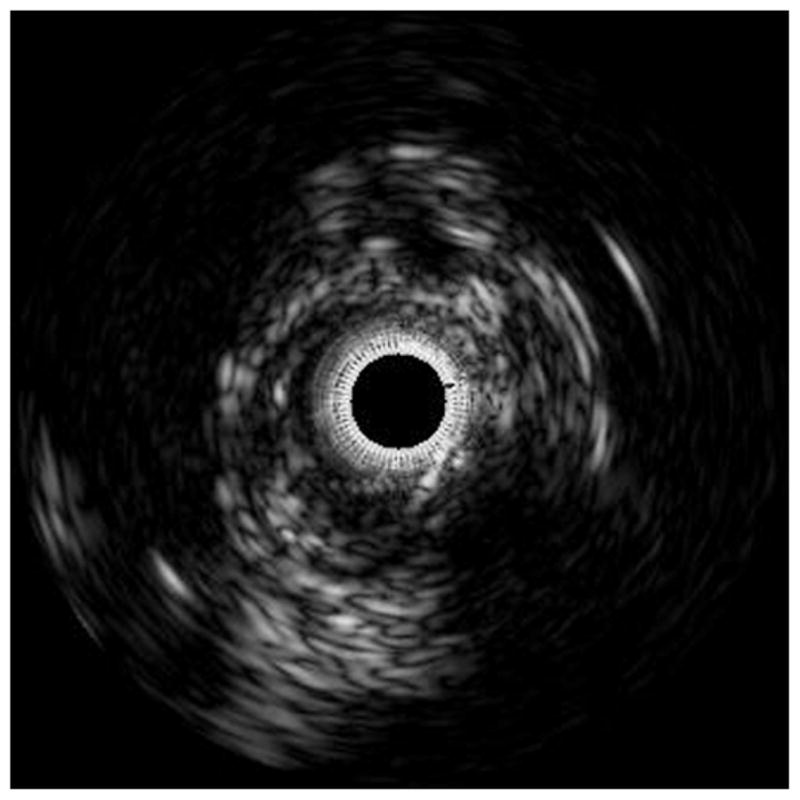
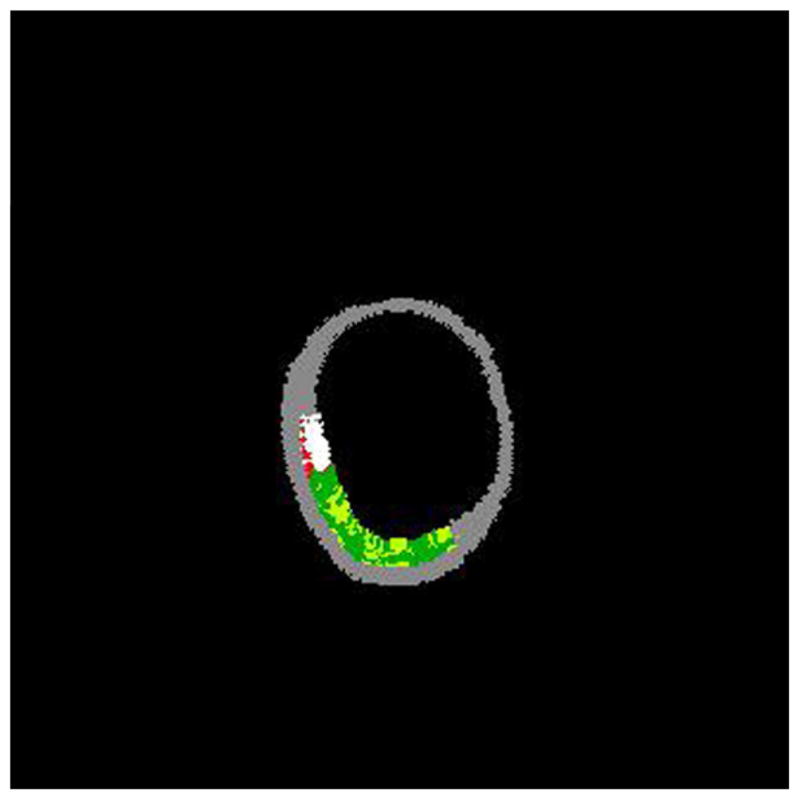
The accompanying figures demonstrate the ability to predict the composition of plaques using IVUS without any a priori information on the histology of this vessel. Figure 2a shows a gray-scale IVUS image from an anterior tibial artery. Figure 2b is the virtual histology using a modified coronary artery algorithm classified by color indicating calcium (white), necrosis (red), and fibrous (green) plaque morphology.
RESULTS
Endothelial function of diseased human arteries was assessed using a tissue chamber with endothelial-dependent and endothelial-independent agonists. EDR is expressed as a percentage secondary to acetylcholine-induced vasorelaxation. Human lower extremity arteries exhibited low EDR. At 10−7, EDR was 0.5% +/− 13.3, at 10−6,13.9% +/− 34.6, and at 10−5,17.9% +/− 34.8. Even at the highest molar dose of Ach 10−4, EDR was only 24.9% +/− 38.8. Pilot study popliteal segments (n=11 rings) were included in baseline EDR/EIR calculations and were not statistical outliers.
Endothelial dysfunction was ameliorated by L-Arginine supplementation in this ex-vivo model (n=18 rings). L-arg supplementation enhanced EDR by 38.5% (P<.0001, Figure 3). At 10−7, EDR was 26.6% +/− 29.2, at 10−6, 50.9% +/− 26, at 10−5, 60.3% +/− 27.6, and at 10−4, EDR was 63.4% +/− 27.1. The maximum relaxation more than doubled with L-arg supplementation. L-NAME abrogated EDR (P<.0001) in vessels exposed to L-arg. At 10−7, EDR was −4.3% +/− 10.6, at 10−6 −7.9 +/− 18.4, at 10−5 −11.4 +/− 24, and at 10−4 was −15.4 +/− 31.5. This indicated that the relaxation exhibited in the assay was secondary to the L-arg/eNOS mechanism with production of nitric oxide. Arterial responsiveness (EIR) was intact in all vessels tested (n=7) with relaxation to sodium nitroprusside at 113.2 ± 28.1% indicating intact smooth muscle response to a NO donor.
Figure 3.
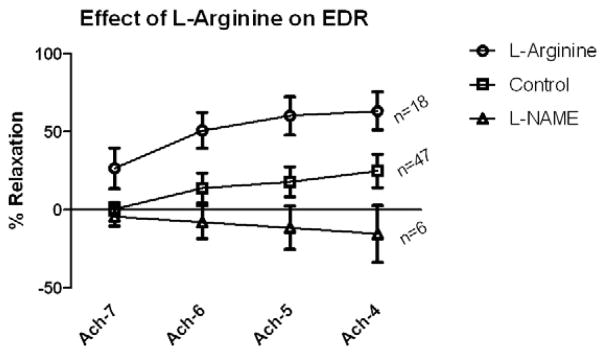
Effect of L-Arginine supplementation of human lower extremity arteries. EDR is augmented in the L-Arginine group (p<0.0001) by two-way analysis of variance. L-NAME blocked acetylcholine-induced EDR (p<0.0001). Values are shown +/−SEM. (*p<.05, **p<.01 with Bonferroni post-test comparisons for L-arg vs L-NAME groups)
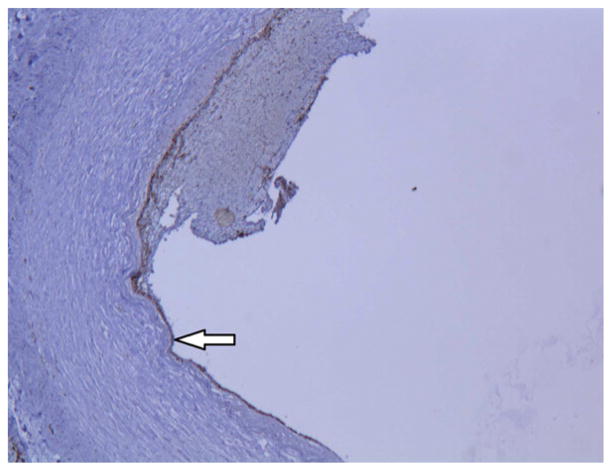
Histological analysis confirmed that diseased lower extremity arteries had intact endothelium in the segments adjacent to EDR evaluation. Immunohistology confirmed intact endothelium by morphometric analysis, CD31, eNOS, and arginase II staining (n=4 vessels) (Figure 4).
Figure 4.
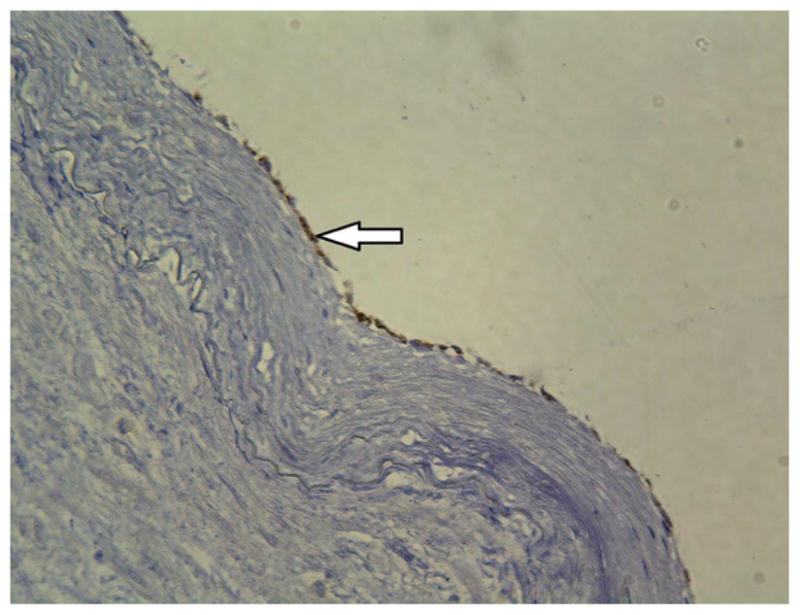
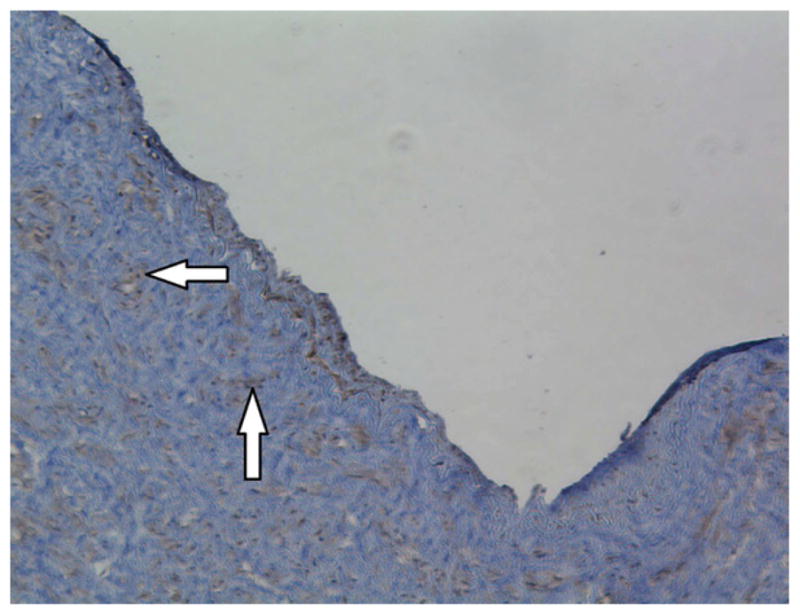
Immmunohistochemistry staining of CD31, eNOS, and arginase II demonstrates intact arterial endothelium. Images were taken at 10x magnification.
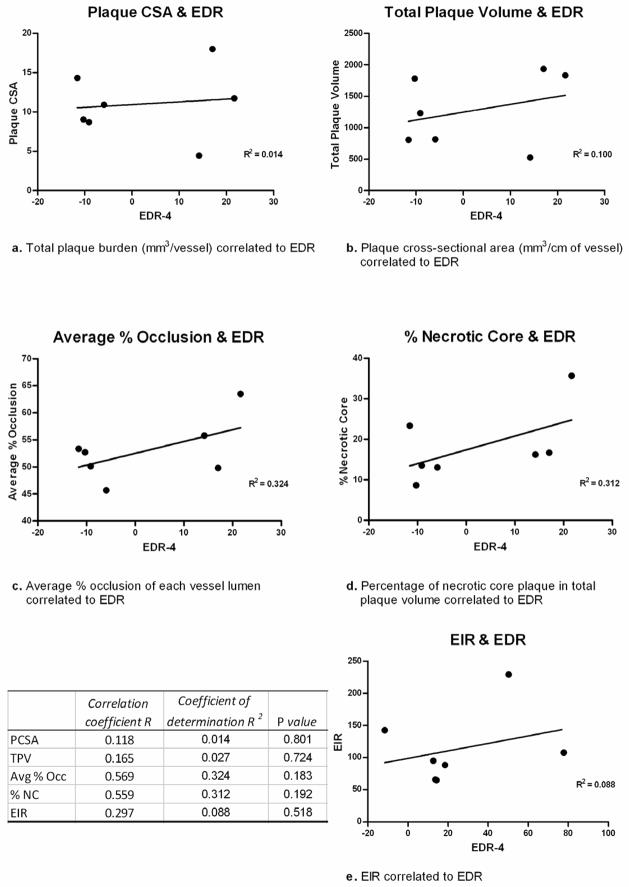
IVUS-VH was used to characterize plaque burden. A total of 9 vessels were subjected to IVUS-VH. Plaque cross sectional area (CSA) was 11.9 ± 4.7 mm3/cm of vessel. Multiplying the plaque CSA by the vessel length revealed an average total plaque volume of 1288.9 mm3/vessel. Plaque stratification indicating fibrous morphology as the predominant type (59.9%). Arteries also had necrotic core (16.9%) and calcium (11.2%) elements in the plaque. Average vessel CSA was 22.0 mm2, and average lumen CSA was 10.1 mm2, representing an average vessel lumen occlusion of 54.3%. EDR response of individual vessels did not correlate with plaque burden in our studies. Scatter plot analysis (Figure 5) showed poor correlation of EDR to total plaque volume, plaque CSA, average percentage occlusion, average percentage necrotic core, and EIR.
Figure 5.
Scatter plot analysis displays correlation of EDR to total plaque volume, plaque CSA, average percentage occlusion, average percentage necrotic core, and EIR. R, R2, and p values are shown for each plot.
DISCUSSION
This is the first study that we know of using harvested lower extremity arteries for assessing endothelial function. The results of our studies of endothelial function are both novel and compelling.
Previous studies have examined arteries in pre-clinical models or from explanted normal arteries unaffected by atherosclerosis. We examined EDR in diseased arteries harvested from amputated limbs presumably for severe atherosclerosis, gangrene or pedal sepsis. Endothelial-dependent relaxtion was improved with the addition of L-arginine. Furthermore, we were able to show that the addition of L-NAME, an inhibitor of eNOS, abrogated endothelial dependent relaxation and established a specific control for this experiment. The vessels retained the ability to vasodilate with the nitric oxide donor sodium nitroprusside, which indicated normal smooth muscle cell responsiveness to NO. The ex-vivo assessment of plaque burden and vessel characteristics by IVUS-VH were concordant with in-vivo imaging of human lower extremity arteies.13. We had hypothesized that increasing necrotic plaque would lead to decreased EDR which was not found in this limited sample. The ex-vivo model described offers a flexible assay to study pharmaceutical and biological agents that may improve endothelial function in diseased human vessels. This platform could be used for other novel agents prior to embarking on costly clinical trials.
In humans, measurement of endothelial function has been mostly done with quantification via angiography in the coronary circulation. Endothelial dysfunction is thought to be an early shift in balance in the vessel wall that makes it vulnerable to vasospasm, thrombosis, and lesion formation. Endothelial dysfunction also appears to be a characteristic feature of patients with coronary artery disease. Several groups have shown that epicardial coronary arteries in patients with coronary artery disease constrict both at sites of angiographically demonstrated obstructive atherosclerotic disease and at sites of plaque formation in response to acetylcholine.14–17 The same dose of acetylcholine causes vasodilation in coronary arteries of patients without demonstrable evidence of coronary artery disease. In contrast, the responses to nitroglycerin (a nitric oxide donor) are similar in individuals with and without coronary artery disease, indicating intact smooth muscle responsiveness to nitric oxide, at least in mildly atherosclerotic coronary arteries. Individuals with normal appearing coronary angiograms and risk factors (hypercholesterolemia, male sex, advancing age, smoking, diabetes mellitus, and family history of coronary artery disease) for atherosclerosis also manifest a vasoconstrictor response of the epicardial coronary arteries in response to acetylcholine.18,19 These observations suggest that endothelial dysfunction, mediated by diminished nitric oxide production by epicardial coronary arteries, precedes the development of angiographically demonstrable atherosclerotic disease.
Previous attempts at understanding endothelial function using noninvasive methods have relied on measures of brachial artery reactivity. This approach relies on the evaluation of flow-mediated vasodilation, which partly represents endothelium-dependent function. The brachial artery is occluded for five minutes using a blood pressure cuff inflated to suprasystolic levels, followed by deflation and measurement of brachial artery dilation by spectral Doppler ultrasound. Increases in arm blood flow are related to resistance vessel dilation (i.e. reactive hyperemia). Because the increase in flow and shear stress stimulates the release of NO, brachial artery diameter changes are believed to be secondary to NO release. This technique is attractive because it is non-invasive and allows repeated measurement. Despite its widespread use, there are both technical and interpretive limitations.20 Some of the changes in brachial artery diameter may be more related to the performance of reactive hyperemia and outflow vasodilation and resistance than brachial artery vasomotor function. Furthermore, this test assays an artery that rarely is affected by atherosclerosis and may not be a relevant indicator of lower extremity endothelial function.
Our results showed low EDR in response to acetylcholine compared to similar studies in animal models and human studies. An analysis of data collected by Padilla21 compared in vitro brachial and femoral artery endothelium-dependent and –independent relaxation between a group of pigs that exercise-trained for 16–20 wk and a group that remained sedentary. No differences in vasomotor function were found between the 2 groups, and their artery segments showed an EDR response of approximately 80–90% to equivalent doses of bradykinin. Woodman22 used vascular ring studies to test their hypothesis that exercise training preserves EDR in brachial arteries from hyperlipidemic pigs. Both normal-fat and high-fat diet pigs were subjected to either exercise or sedentary training schedules. Ach-induced relaxation was greatest in the normal-fat exercise group at 83.5%, with poor relaxation of 50.5% seen in the high-fat sedentary group. Similarly, Hutchison23 tested aorta ring segments from rabbits exposed to both high cholesterol diets and second hand smoke. The control group exhibited maximum EDR of approximately 90% in response to Ach. Hypercholesterolemia impaired maximal acetylcholine-induced relaxation. Second hand smoke further impaired maximal acetylcholine-induced relaxation to approximately 40%. Notably, chronic dietary L-Arginine improved endothelium-dependent relaxation and reduced the SHS-associated impairment of relaxation, reduced the effect of SHS to accelerate intimal lesion formation, and tended to reduce atherogenesis overall.
Patients scheduled for elective bypass surgery were randomized by Hambrecht24 into either a training or an inactive control group. Both in-vivo and ex-vivo assessment of endothelial function was performed in the Left internal mammary artery (LIMA). After 4 weeks of exercise training, the mean vasodilatory response to the maximum concentration of acetylcholine was significantly increased by 116%, whereas no significant change was detectable in patients of the control group. Ex-vivo ring studies showed showed EDR was preserved at >50% relaxation in human LIMA with similar concentrations of Ach in organ tissue chambers. Similar results were reported again in 2006.25 In patients with CAD, EDR of mammary arteries was significantly impaired in those positive for the T-786C or the G894T eNOS polymorphism. The maximal vasodilatory response to acetylcholine was 47% in patients with the T-786C polymorphism and 60% in patients positive for the G894T polymorphism. LIMA rings from wild-type variants responded to Ach with a 90% ring relaxation. The EPAS Trial26 studied the protective effects of angiotensin II type 1 receptor blockade and statin use on human coronary LIMA in patients undergoing CABG procedures. Maximal dilation in response to Ach 10−6 M was 51% for the control group, 69% for the statin-only group, 79% for the AT1 blocker group, and 86% for the dual-therapy group. Taken together, these studies have demonstrated retained endothelial function in both normal and disease states, and highlight the potential for treatment of endothelial dysfunction through various pharmacological modalities.
This work has several limitations. Given the constraints of the IRB approved protocol, no patient-level data could be obtained that may affect endothelial function. Thus, relevant variables of severity of ischemia, preoperative gangrene and patient clinical and demographic information were unable to be collected and correlated to the arterial results. The specimens obtained were limited to electively planned lower amputation specimens from a single institution and the results are based on a small number of harvested arteries. The time required between amputation, vessel harvest and tissue chamber analysis was kept at a minimum but could have led to vessel wall deterioration. Of note, “normal” arteries for comparison would be ideal but challenging to obtain. Amputations performed on trauma or cancer victims could provide additional data in “normal” arteries from younger, healthier patients. In general, there are very few amputations for oncologic or trauma indications at our institution. Comparing leg arteries to normal arteries trimmed from kidney or liver transplants was considered, but this idea was discarded because of the very different structure of the vessels in different arterial beds. In addition, any leg specimens with oncological pathology were not released in a timely fashion to allow endothelial function measurement. Correlating patient-level clinical factors with limb-based endothelial function and other parameters is the subject of future studies. Factors other than L-arginine availability may be playing a role in endothelial dysfunction and the ex vivo results presented herein may not completely reflect in vivo physiology.
Further ex vivo and in vivo studies are being planned to determine the effects of L-arginine supplementation and arginase blockade on lower extremity endothelial function. The effects of plaque burden, thrombus, and arginase II levels will be elucidated with additional arterial samples. The role of nitric oxide modulators in PAD as clinical adjuncts remains largely unknown. The ex-vivo model described offers a flexible assay to study pharmaceutical and biological agents that may improve endothelial function in diseased human vessels. We hope this platform will serve as a springboard to human clinical studies for patients with both acute and chronic limb ischemia.
CONCLUSIONS
The methods used in our study demonstrate an ex vivo model for IVUS-VH to establish plaque burden and composition, and to test EDR in an organ chamber apparatus of human arteries harvested from legs after amputation. Human lower extremity arteries demonstrate low baseline endothelial function in patients requiring amputation. Endothelial dysfunction is improved by L-arginine supplementation in an ex vivo model. These results support clinical strategies to increase local nitric oxide levels in atherosclerotic human vessels.
Acknowledgments
We thank the Lerner Research Institute Imaging Core (J. Drazba) for help with automated immunohistochemistry.
Footnotes
Presented in part at the Research Initiatives Conference/Arteriosclerosis, Thrombosis, and Vascular Biology (ATVB) Scientific Sessions, April 2011. Funded via the National Institutes of Health (K23 HL080247, V.S.K.) and the American Vascular Association (V.S.K.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 5.Reil TD, Moore WS, Kashyap VS, Nene SS, Gelabert HA, Quinones-Baldrich WJ. The effects of thrombus, thrombectomy and thrombolysis on endothelial function. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2000 Feb;19(2):162–168. doi: 10.1053/ejvs.1999.0977. [DOI] [PubMed] [Google Scholar]
- 6.Cohen RA, Shepherd JT, Vanhoutte PM. Inhibitory role of the endothelium in the response of isolated coronary arteries to platelets. Science. 1983 Jul 15;221(4607):273–274. doi: 10.1126/science.6574604. [DOI] [PubMed] [Google Scholar]
- 7.Kashyap VS, Reil TD, Moore WS, et al. Acute arterial thrombosis causes endothelial dysfunction: a new paradigm for thrombolytic therapy. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2001 Aug;34(2):323–329. doi: 10.1067/mva.2001.115004. [DOI] [PubMed] [Google Scholar]
- 8.Demougeot C, Prigent-Tessier A, Marie C, Berthelot A. Arginase inhibition reduces endothelial dysfunction and blood pressure rising in spontaneously hypertensive rats. Journal of hypertension. 2005 May;23(5):971–978. doi: 10.1097/01.hjh.0000166837.78559.93. [DOI] [PubMed] [Google Scholar]
- 9.Lewis C, Zhu W, Pavkov ML, Kinney CM, Dicorleto PE, Kashyap VS. Arginase blockade lessens endothelial dysfunction after thrombosis. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2008 Aug;48(2):441–446. doi: 10.1016/j.jvs.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002 Oct 22;106(17):2200–2206. doi: 10.1161/01.cir.0000035654.18341.5e. [DOI] [PubMed] [Google Scholar]
- 11.Bishop PD, Feiten LE, Ouriel K, et al. Arterial calcification increases in distal arteries in patients with peripheral arterial disease. Annals of vascular surgery. 2008 Nov;22(6):799–805. doi: 10.1016/j.avsg.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. Journal of the American College of Cardiology. 2001 Apr;37(5):1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 13.Arthurs ZM, Bishop PD, Feiten LE, Eagleton MJ, Clair DG, Kashyap VS. Evaluation of peripheral atherosclerosis: a comparative analysis of angiography and intravascular ultrasound imaging. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2010 Apr;51(4):933–938. doi: 10.1016/j.jvs.2009.11.034. discussion 939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egashira K, Inou T, Hirooka Y, et al. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. The Journal of clinical investigation. 1993 Jan;91(1):29–37. doi: 10.1172/JCI116183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodgson JM, Marshall JJ. Direct vasoconstriction and endothelium-dependent vasodilation. Mechanisms of acetylcholine effects on coronary flow and arterial diameter in patients with nonstenotic coronary arteries. Circulation. 1989 May;79(5):1043–1051. doi: 10.1161/01.cir.79.5.1043. [DOI] [PubMed] [Google Scholar]
- 16.Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. The New England journal of medicine. 1986 Oct 23;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 17.Quyyumi AA, Dakak N, Andrews NP, et al. Nitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. The Journal of clinical investigation. 1995 Apr;95(4):1747–1755. doi: 10.1172/JCI117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munro JM, Cotran RS. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Laboratory investigation; a journal of technical methods and pathology. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- 19.Quyyumi AA, Dakak N, Mulcahy D, et al. Nitric oxide activity in the atherosclerotic human coronary circulation. Journal of the American College of Cardiology. 1997 Feb;29(2):308–317. doi: 10.1016/s0735-1097(96)00472-x. [DOI] [PubMed] [Google Scholar]
- 20.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology. 2002 Jan 16;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 21.Padilla J, Newcomer SC, Simmons GH, Kreutzer KV, Laughlin MH. Long-term exercise training does not alter brachial and femoral artery vasomotor function and endothelial phenotype in healthy pigs. American journal of physiology Heart and circulatory physiology. 2010 Aug;299(2):H379–385. doi: 10.1152/ajpheart.00294.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodman CR, Turk JR, Williams DP, Laughlin MH. Exercise training preserves endothelium-dependent relaxation in brachial arteries from hyperlipidemic pigs. Journal of applied physiology. 2003 May;94(5):2017–2026. doi: 10.1152/japplphysiol.01025.2002. [DOI] [PubMed] [Google Scholar]
- 23.Hutchison SJ, Sudhir K, Sievers RE, et al. Effects of L-arginine on atherogenesis and endothelial dysfunction due to secondhand smoke. Hypertension. 1999 Jul;34(1):44–50. doi: 10.1161/01.hyp.34.1.44. [DOI] [PubMed] [Google Scholar]
- 24.Hambrecht R, Adams V, Erbs S, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003 Jul 1;107(25):3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 25.Erbs S, Mobius-Winkler S, Linke A, et al. Both T-786C and G894T polymorphism of endothelial nitric oxide synthase affect in-vitro endothelium-dependent relaxation of internal mammary artery rings from patients with coronary artery disease. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2006 Oct;13(5):826–831. doi: 10.1097/01.hjr.0000230100.70900.37. [DOI] [PubMed] [Google Scholar]
- 26.Morawietz H, Erbs S, Holtz J, et al. Endothelial Protection, AT1 blockade and Cholesterol-Dependent Oxidative Stress: the EPAS trial. Circulation. 2006 Jul 4;114(1 Suppl):I296–301. doi: 10.1161/CIRCULATIONAHA.105.001313. [DOI] [PubMed] [Google Scholar]