Abstract
Introduction
Topiramate (TPM), an anti-epileptic drug with >4 million users, increases renal stones in adults. We screened outpatient TPM-treated children without history of stones to estimate the prevalence of renal stones and to characterize urine stone-risk profiles.
Methods
Children taking TPM ≥1 month underwent an interview, renal ultrasound, and spot urine testing in this prospective study. Normal spot urine values were defined as: calcium/creatinine ratio ≤0.20 mg/mg (>12 months) or ≤0.60 mg/mg (≤12 months), citrate/creatinine ratio >0.50 mg/mg, and pH ≤6.7.
Results
Of 41 patients with average age of 9.2 years (range 0.5–18.7), mean TPM dose of 8.0 mg/kg/day (range 1.4–23.6), and mean treatment duration of 27 months (range 1–112), two (4.9%) had renal stones. The majority of children taking TPM had lithogenic abnormalities on spot urine testing, including 21 (51%) with hypercalciuria, 38 (93%) with hypocitraturia, and 28 (68%) with pH≥6.7. Hypercalciuria and hypocitraturia were independent of TPM dose and duration; urine pH increased with dose. 24-hour urine parameters improved in 1 stone-former once TPM was weaned.
Conclusions
Asymptomatic stones were found in 2/41 (4.8%) children taking TPM. Risk factors for stones were present in the spot urine of most children, including hypocitraturia (93%) and hypercalciuria (51%), independent of TPM dose and duration. High urine pH, found in 68%, correlated with TPM dose. Pediatric specialists should be aware of increased risks for stones, hypercalciuria, hypocitraturia, and alkaline urine in children taking TPM.
Introduction
Topiramate (TPM) is a neuromodulatory agent increasingly used in children for a wide range of conditions including migraine prophylaxis, [1] various forms of pediatric epilepsy,[2–4] Tourette syndrome,[5] bipolar disorder,[6] and autistic disorder.[7] TPM has been shown to cause a metabolic acidosis,[8–12] presumably by inhibiting carbonic anhydrase activity in the kidney.[13] Metabolic acidosis related to TPM is associated with the formation of alkaline urine, hypocitraturia and hypercalciuria in adults.[14–18] TPM use is also associated with an increased risk of calcium stones in adults, demonstrating a stone prevalence of 20% among long-term users compared to an expected prevalence of 5–8% in the general population.[19–20] Limited reports of the prevalence of kidney stones in children taking TPM have shown variable results, ranging from 0–54%, with minimal information about urinary risk factors for stone formation.[1,3,4,20,21] We hypothesized that children on TPM would be at increased risk for stones and might exhibit similar stone-risk urine profiles to adults taking TPM. Thus, we screened a cohort of outpatient TPM-treated children without known history of stones to estimate the prevalence of asymptomatic renal stones by ultrasound scan and to characterize spot urine stone-risk profiles.
Methods
Patients
This study was reviewed and approved by the institutional review board of Children’s Medical Center at Dallas and UT Southwestern Medical Center with primary caregivers signing informed consent and children aged 10 and older giving assent. An electronic database of pediatric patients with active TPM prescriptions was obtained from the Children’s Medical Center (CMC) at Dallas Department of Neurology. The parents of patients were mailed invitations to participate in this cross-sectional study or they were invited by the patient’s neurologist during routine visits to the CMC outpatient Neurology clinic if they were actively receiving TPM (minimum one month). Interested parents received an interview by telephone or in clinic, which included questions regarding their child’s age, demographics, height, weight, total daily dosage and duration of TPM, urinary symptoms, and any history of stones. Exclusion criteria included subjects with a preexisting history of kidney stone disease, use of other carbonic anhydrase inhibitors, antacids and/or diuretics, recurrent urinary tract infections, chronic diarrhea, and/or ketogenic diet. We did not exclude neurologically-impaired and/or immobilized children since such these children make up a substantial portion of outpatients taking TPM and may be at particularly high risk for developing stones on TPM due to their underlying condition.
Imaging
All eligible subjects were invited to undergo renal-bladder ultrasound and spot urine testing via bag or clean catch collection. Renal-bladder ultrasounds were interpreted by both a pediatric radiologist and a pediatric urologist specifically evaluating for the presence of stones. Screening computed tomography (CT) and abdominal radiographs were not performed due to unnecessary radiation risk. A suspected stone (mobile echogenic focus with shadowing on ultrasound) was confirmed with abdominal radiograph.
Urine
Urine was preserved with thymol and refrigerated, with testing performed within 48 hours. Urine pH was determined by pH electrode (Radiometer Analytical SAS, Lyon, France). Urine creatinine was analyzed by kinetic alkaline picrate method and citrate by citrate lyase procedure (Roche Diagnostics, Indianapolis, IN). Urinary calcium was analyzed by atomic absorption spectroscopy (Varian Inc, Palo Alto, CA). 24-hour urine testing was not performed because this was a pilot study designed to determine whether or not spot urine metabolic abnormalities were present in children taking TPM. In the absence of any known preliminary data, we could not justify invasive instrumentation with 24-hour indwelling catheter placement in the subset of TPM-prescribed children who are neurologically-impaired and incontinent. Normal spot and 24-hour urine values were defined as follows: calcium:creatinine (Ca/Cr) ratio ≤0.2 mg/mg for patients greater than12 months and <0.6 mg/mg for ages 6–12 months; citrate:creatinine (Cit/Cr) ratio as ≥0.5 mg/mg; and pH ≤6.7.[22–25] Patients with stones identified by renal ultrasound underwent abdominal radiograph, 24-hour urine testing, and stone treatment as deemed appropriate.
Statistical Analysis
The primary outcome variable was stone prevalence on ultrasound (i.e. the number of screened children with stones divided by the total number of screened children). The secondary measures were spot urine Ca/Cr ratio, Cit/Cr ratio, and urine pH. Spearman’s rank correlations were performed using GraphPad Prism (version 5.00 for Windows, GraphPad Software, San Diego California USA), with p<0.05 considered statistically significant.
Results
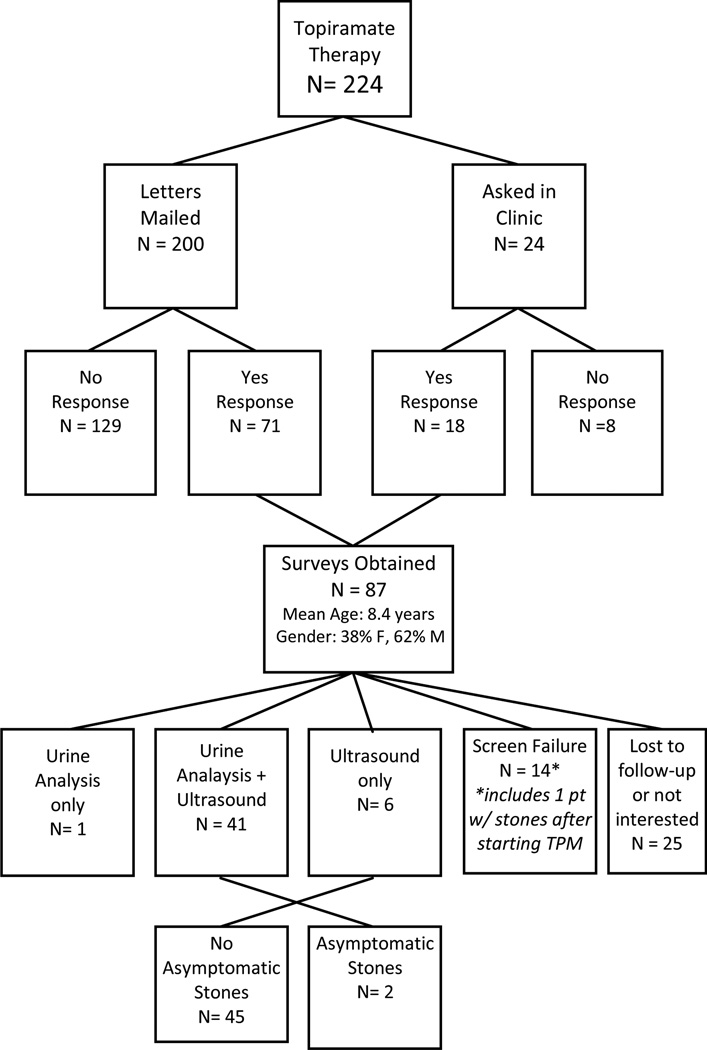
Telephone or in-person interviews were completed by 87 patients, and ultrasound and spot urine testing in 41 patients (Figure 1). All interviewed subjects had seizure disorder as the indication for TPM treatment. Demographics of interview-responders and the subset of ultrasound/spot urine-screened children are demonstrated in Table 1. The most common reasons interview-responders meeting inclusion criteria did not wish to participate in the screening study was parental desire for their children to forego any unnecessary testing followed by scheduling difficulties.
Figure 1.
Cohort information
Table 1.
Patient demographics, with data shown as mean (range, median) or number (%).
| All Surveyed (n=87) | Screened, Ultrasound & Urinalysis (n=41) |
|
|---|---|---|
| Age (years) | 8.4 (0.5–20.6, 6.8) | 9.2 (0.5–18.7,8.6) |
| Male | 53 (61%) | 20 (49%) |
| Weight (kg) | 35 (6–132, 26) | 38 (6–132, 29) |
| BMI (kg/m2) | 20 (7–42, 17) | 20 (7–42, 17) |
| TPM dose (mg/kg/day) | 7.8 (0.9–23.6, 6.3) | 8.0 (1.4–23.6, 6.3) |
| TPM duration (months) | 28 (1–157, 19) | 27 (1–112, 22) |
| Gastric Tube | 26 (30%) | 11 (27%) |
| Immobilization | 32 (37%) | 12 (29%) |
Renal ultrasound scans revealed asymptomatic renal stones in 2 (4.8%) children taking TPM, including a 7mm stone that was successfully treated with extra-corporeal shock wave lithotripsy in 1 patient and two 3mm stones in the other child, which were initially observed then later treated with ureteroscopy secondary to increasing stone size. The stones were radioopaque on abdominal radiographs, and stone analysis demonstrated calcium phosphate composition. TPM treatment duration was only 4 months in 1 patient, and 50 months in the other. Both stone-formers were ambulatory and healthy except for their seizure disorders.
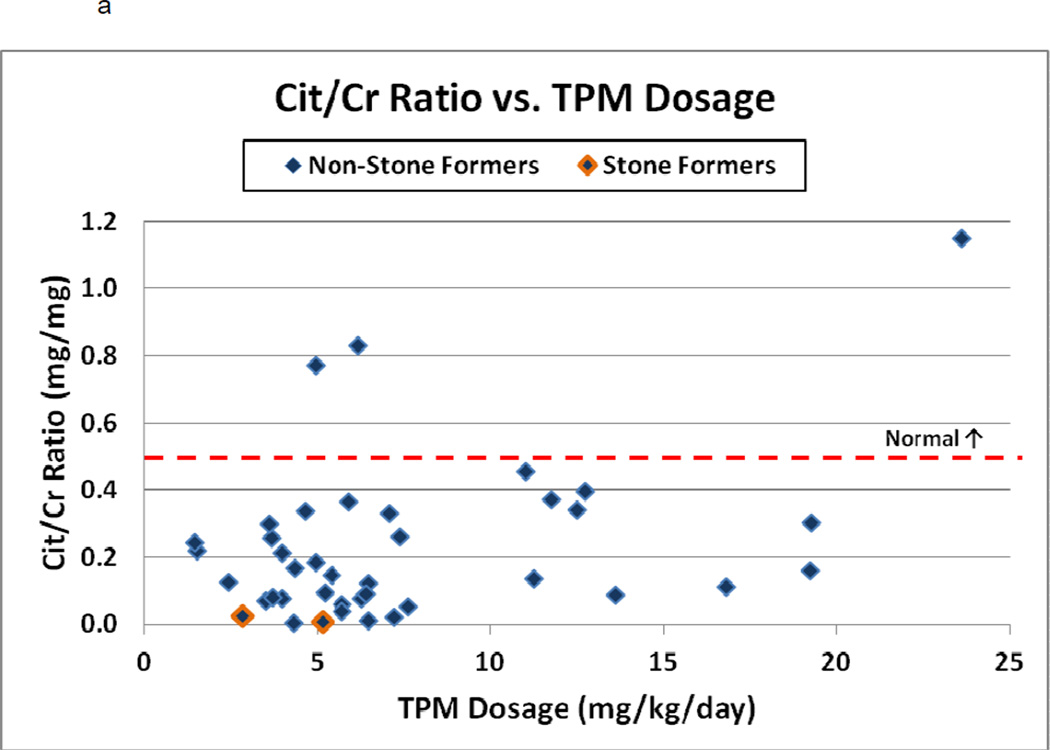
The most striking finding on spot urine testing was marked hypocitraturia (Figure 2a). The average urine Cit/Cr ratio was 0.22 mg/mg (range 0.01–1.15, median 0.18), with 93% demonstrating hypocitraturia (normal ratio >0.5 mg/mg). Both stone-formers were markedly hypocitraturic (0.02 and 0.06 mg/mg), a finding confirmed with 24-hour urine tests (0.02 and 0.04 mg/mg) and improved in 1 patient after shortly after stopping TPM (from 38 mgCit/24 hour to 250 mgCit/24 hours with a Cit/Cr ratio of 0.14 mg/mg). The second stone-forming patient was unable to be weaned off TPM due to worsening seizure activity. There were no significant correlations between the dose or duration of TPM and degree of hypocitraturia (r=0.26 and - 0.13, p=0.13 and 0.45 respectively).
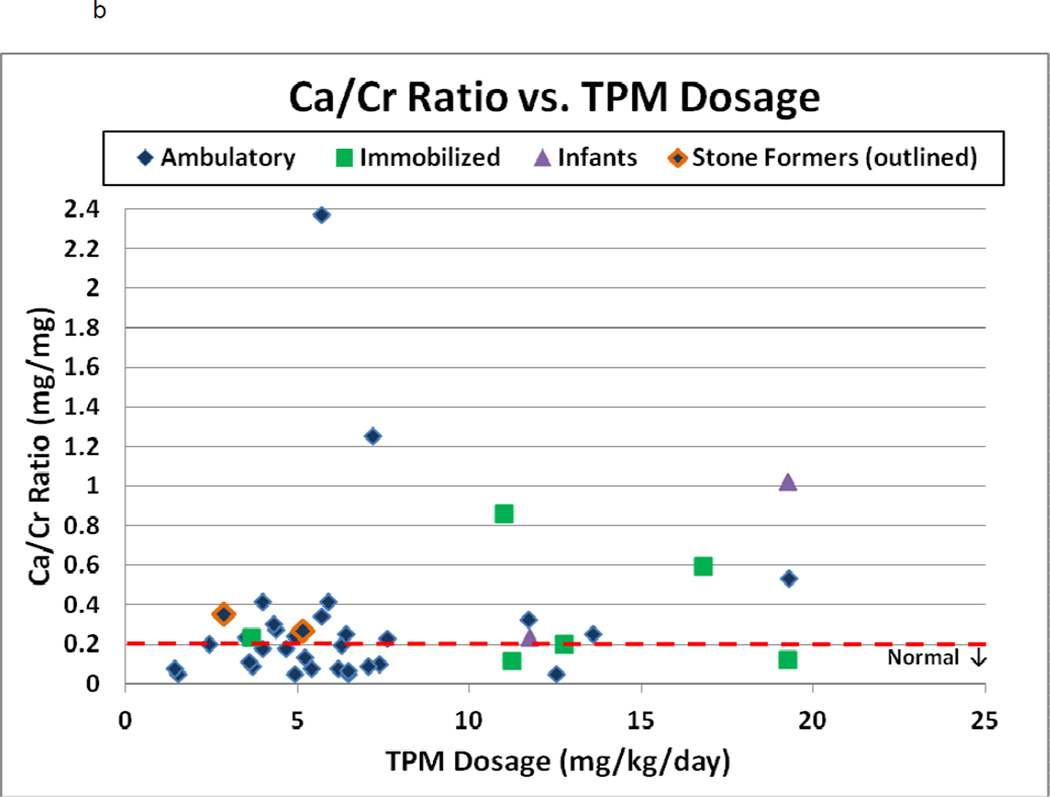
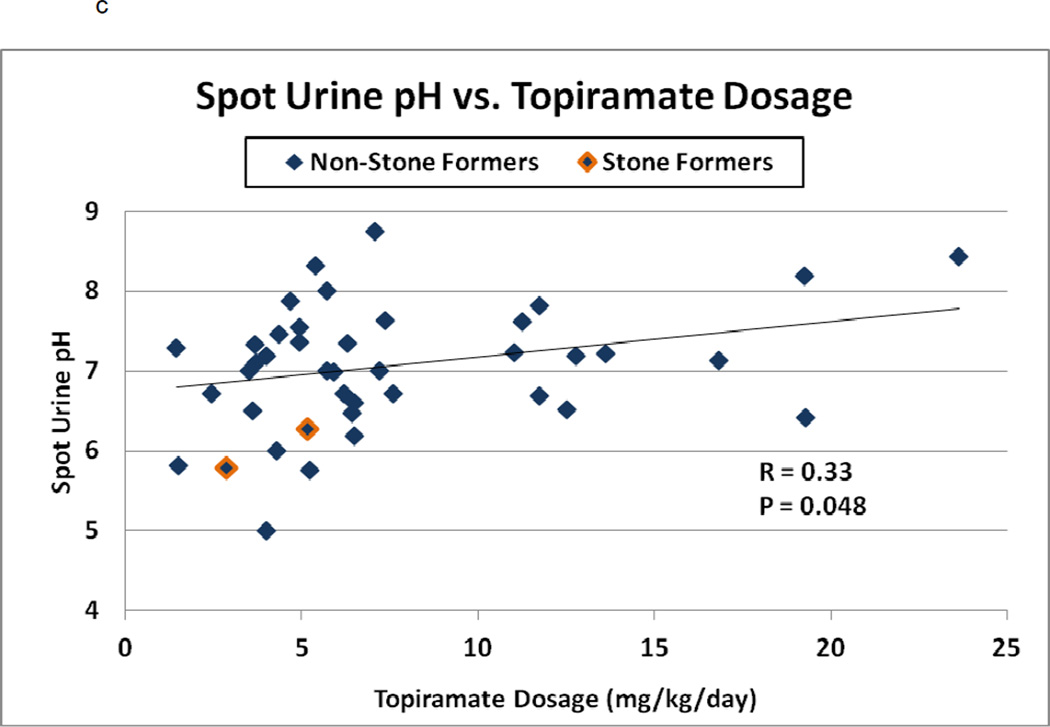
Figure 2.
a–c: Spot urine Citrate/Creatinine ratio, Calcium/Creatinine ratio, and urine pH versus TPM dosage (mg/kg/day) among 41 children. In 2a–c, stone-formers are outlined and normal values are indicated by the dashed line. In 2b, patients are separated into ambulatory (diamond), ambulatory with stones (outlined diamond), immobilized patients (square) and infants (triangle). In 2c, Spearman’s correlation coefficient and p-value are indicated
Spot urine biochemistries also revealed hypercalciuria in 21 patients (51%, Figure 2b), including one stone-former with confirmation on 24-hour test (24-hour Ca/Cr ratio of 0.23 mg/mg on TPM, and 0.11 mg/mg after stopping TPM), and 2 infants aged 6–12 months with ratios greater than 0.6 mg/mg. The average urine Ca/Cr ratio was 0.22 mg/mg (range 0.05–2.37, median 0.20). Hypercalciuria was independent of both dose and duration of TPM use (r=0.26 and 0.06, p=0.13 and 0.74, respectively). It was seen in ambulatory as well as immobilized patients.
The average urinary pH among our pediatric patients was 7.02 (range 5.0–8.7, median 7.18; Figure 2c). Spot urine pH increased with TPM dose (Spearman’s r=0.33, p=0.048), but not duration (r=−0.18, p=0.28). The 24-hour urine pH decreased after TPM discontinuation in one stone-former (pH 6.75 on TPM and 5.62 off TPM).
Urinary symptom survey performed in 87 patients taking TPM revealed presence of gross hematuria in 5 (5.7%), dysuria in 5 (5.7%), and abdominal pain in 16 (18.4%) of these children after they started TPM.
Discussion
Although the prevalence of stones among North American children is poorly defined, estimates have placed it around 0.01–0.02%.[26] In this pilot cross-sectional study, we found a markedly higher prevalence of 4.8% among a subset of 41 children taking TPM who were screened for stones with ultrasound. Hypocitraturia, hypercalciuria, and alkaline urine were three prevalent risk factors for stone disease among these children.
Limited reports of stone prevalence in children taking TPM have shown ranges from 0–54%, varying based upon patient age, treatment dose, duration, and co-morbid conditions.[1,3,4,20] The 4.8% prevalence found in our population is greater than that of the general pediatric population.[26] Our results are similar to the 5.2% incidence of kidney stones found by ultrasound during a retrospective observational study in which 5 of 96 children with initially negative ultrasonography developed stones after TPM therapy for at least one year.[21] However, our result differs from a previous report of 54% prevalence among institutionalized, non-ambulatory children on TPM treatment.[22] The discrepant results may be due to the inclusion of children with indwelling catheters and persistent UTI, which increases risk for kidney and bladder stones. We excluded children with recurrent UTIs and included ambulatory children as well as neurologically-impaired children well enough to be treated as outpatients. No child in our study had an indwelling catheter or was on intermittent catheterization.
The results of our present study are unique since we evaluated the spot urinary metabolic stone-risk profiles as a surrogate for kidney stone formation, which to our knowledge has not been evaluated in children taking TPM. Hypercalciuria is the most common metabolic cause of stones in children.[23,27–29] Using the commonly accepted normal calcium/creatinine ratio in children, defined as less than 0.6 mg/mg for age 6–12 months and 0.2 mg/mg for children over the age of 12 months, 51% of our TPM-treated population were hypercalciuric.[22]
Hypercalciuria has not only been associated with urolithiasis in children, but can also be found among children with dysuria, hematuria, and flank/abdominal pain without stones.[29] Survey results demonstrated that 16 (18.4%) and 5 (5.7%) of 87 children had abdominal pain and dysuria, respectively, after starting TPM. Further study among our TPM-treated patients will need to confirm spot urines with 24-hour urinalyses, address whether symptoms such as dysuria and abdominal pain are attributable to hypercalciuria, and evaluate if dietary and/or medical therapy for hypercalciuria is effective in treating the hypercalciuria. While immobilization likely contributes to hypercalciuria, both immobilized and ambulatory children had hypercalciuria on spot testing, and the majority of hypercalciuric children were ambulatory (Figure 2b). Longitudinal study before and after starting TPM may determine if hypercalciuria is induced or enhanced by TPM-administration in children, especially in immobilized children who may have an underlying predisposition to stone-disease and/or hypercalciuria even in the absence of TPM. Hypercalciuria on 24-hour urine testing resolved after stopping TPM in one ambulatory stone-forming child (24-hour Ca/Cr ratio of 0.23 mg/mg on TPM, and 0.11 mg/mg after stopping TPM).
Hypocitraturia is another important risk factor for developing kidney stones in children [23,27–29], with normal pediatric citrate/creatinine ratio defined as greater than 0.5 mg/mg.[23–25] Similar to adults taking TPM, we found hypocitraturia in nearly all of the children taking TPM [14–18], occurring even at low doses and with short duration of use. Hypocitraturia among TPM-users is thought to be due to metabolic acidosis, and consequently enhanced renal proximal tubular reabsorption of citrate, which can increase the risk of calcium oxalate and calcium phosphate stones.[15,31] The hypocitraturia markedly improved in 1 stone-former after stopping TPM (from 38 mgCit/24 hour to 250 mgCit/24 hours with a Cit/Cr ratio improving from 0.04 to 0.14 mg/mg). The other stone-former was unable to be weaned from TPM, had persistently low Cit/Cr ratios on spot and 24-hour urine testing (all <0.04), and had increasing size of her stones; thus, she was treated with potassium citrate supplementation. Unfortunately her urine pH increased to 8.5 on several spot checks after starting the potassium citrate and she has since begun hydrochlorothiazide with no new stones formed to date.
Highly alkaline urine was also found to be a urine stone risk factor in the adult TPM studies.[15–17] The average urinary pH among our pediatric TPM-treated patients was elevated at 7.02 (Figure 2c), and spot urine pH increased with TPM dose. Twenty-eight (68%) patients had a spot urinary pH exceeding 6.7. In this environment, the urine becomes supersaturated with respect to monohydrogen phosphate (pka 6.7) which ultimately increases the propensity for calcium phosphate crystallization and consequent calcium phosphate stone formation.[15.31] Stone analysis in the stone-former who underwent ureteroscopy demonstrated 90% calcium phosphate and 10% calcium oxalate monohydrate.
Limitations of our study include the small sample size, lack of longitudinal data in the non-stone-formers, and spot instead of 24-hour urine samples in the non-stone-formers. While our cross-sectional study design could not definitively prove that TPM use was the cause of stone formation or metabolic urine abnormalities, a longitudinal study in 7 adults showed hypocitraturia and alkaline urine were induced within 3 months after starting TPM.[17] The discontinuation of TPM improved hypocitraturia, resolved hypercalciuria, and lowered pH on 24-hour urine testing in one of our patients with kidney stones, and our stone prevalence of 4.8% was similar to the 5.2% incidence in Saudi Arabian children who developed stones after starting TPM.[21] Another limitation is that radiation risks in this population prompted the use of ultrasound instead of CT scans, and the utilization of ultrasound likely decreases sensitivity in detection of kidney stones. Despite these limitations, it is clear from this study that children taking TPM share not only the increased prevalence of stones seen in adult TPM-users, but also some of the same stone-risk urinary profiles, including hypocitraturia, hypercalciuria, and alkaline urine, suggesting a common mechanistic link. With our limited data, we also demonstrate that TPM dose and duration do not correlate with degree of hypercalciuria or hypocitraturia, thus all TPM-treated children should be considered at risk for stone disease. Future study may help identify children on TPM most at risk for stone formation who might benefit from screening, as well as ways to reduce this risk.
Conclusions
There was a 4.8% prevalence of asymptomatic stones among 41 children taking TPM. Hypocitraturia and hypercalciuria were present on spot testing in 93% and 51%, respectively, independent of dose and duration. High urine pH, another risk factor for stones, correlated with increasing dose of TPM and was present in 28 (68%) children. Pediatric specialists should be aware of increased risks for stones, hypercalciuria, hypocitraturia, and alkaline urine in children taking TPM.
Acknowledgements
We would like to thank Medrith Greene for her help in patient recruitment and data collection, Beverly Huet for her help with statistical analysis, Tisha Franklin for her help with manuscript preparation, and the laboratory expertise of the Center for Mineral Metabolism & Clinical Research. This project was supported by Grant Number KL2 RR024983.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Funding: None
References
- 1.Lewis D, Winner P, Saper J, Ness S, Polverejan E, Wang S, et al. Randomized, doubleblind, placebo-controlled study to evaluate the efficacy and safety of topiramate for migraine prevention in pediatric subjects 12 to 17 years of age. Pediatrics. 2009;123:924–934. doi: 10.1542/peds.2008-0642. [DOI] [PubMed] [Google Scholar]
- 2.Girgis IG, Nandy P, Nye JS, Ford L, Mohanty S, Wang S, et al. Pharmacokinetic-pharmacodynamic assessment of topiramate dosing regimens for children with epilepsy 2 to <10 years of age. Epilepsia. 2010;51:1954–1962. doi: 10.1111/j.1528-1167.2010.02598.x. [DOI] [PubMed] [Google Scholar]
- 3.Coppola G, Caliendo G, Veggiotti P, Romeo A, Tortorella G, DeMarco P, et al. Topiramate as add-on drug in children, adolescents and young adults with Lennox-Gastaut syndrome: an Italian multicentric study. Epilepsy Res. 2002;51:147–153. doi: 10.1016/s0920-1211(02)00103-1. [DOI] [PubMed] [Google Scholar]
- 4.Coppola G, Capovilla G, Montagnini A, Romeo A, Spano M, Tortorella G, et al. Topiramate as add-on drug in severe myoclonic epilepsy in infancy: an Italian multicenter open trial. Epilepsy Res. 2002;49:45–48. doi: 10.1016/s0920-1211(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 5.Kuo SH, Jimenez-Shahed J. Topiramate in treatment of tourette syndrome. Clin Neuropharmacol. 2010;33:32–34. doi: 10.1097/WNF.0b013e3181c295c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wozniak J, Mick E, Waxmonsky J, Kotarski M, Hantsoo L, Biederman J. Comparison of open-label, 8-week trials of olanzapine monotherapy and topiramate augmentation of olanzapine for the treatment of pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2009;19:539–545. doi: 10.1089/cap.2009.0042. [DOI] [PubMed] [Google Scholar]
- 7.Rezaei V, Mohammadi MR, Ghanizadeh A, Sahraian A, Tabrizi M, Rezazadeh SA, et al. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1269–1272. doi: 10.1016/j.pnpbp.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Ko CH, Kong CK. Topiramate-induced metabolic acidosis: report of two cases. Dev Med Child Neurol. 2001;43:701–704. doi: 10.1017/s0012162201001268. [DOI] [PubMed] [Google Scholar]
- 9.Takeoka M, Holmes GL, Thiele E, Bourgeois BF, Helmers SL, Duffy FH, et al. Topiramate and metabolic acidosis in pediatric epilepsy. Epilepsia. 2001;42:387–392. doi: 10.1046/j.1528-1157.2001.04500.x. [DOI] [PubMed] [Google Scholar]
- 10.Philippi H, Boor R, Reitter B. Topiramate and metabolic acidosis in infants and toddlers. Epilepsia. 2002;43:744–747. doi: 10.1046/j.1528-1157.2002.37201.x. [DOI] [PubMed] [Google Scholar]
- 11.Belotti EA, Taddeo I, Ragazzi M, Pifferini R, Simonetti GD, Bianchetti MG, et al. Chronic impact of topiramate on acid-base balance and potassium in childhood. Eur J Paediatr Neurol. 2010;14:445–448. doi: 10.1016/j.ejpn.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Groeper K, McCann ME. Topiramate and metabolic acidosis: a case series and review of the literature. Paediatr Anaesth. 2005;15:167–170. doi: 10.1111/j.1460-9592.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 13.Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia. 2000;41(Suppl 1):S35–S39. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- 14.Vega D, Maalouf NM, Sakhaee K. Increased propensity for calcium phosphate kidney stones with topiramate use. Expert Opin Drug Saf. 2007;6:547–557. doi: 10.1517/14740338.6.5.547. [DOI] [PubMed] [Google Scholar]
- 15.Welch BJ, Graybeal D, Moe OW, Maalouf NM, Sakhaee K. Biochemical and stone-risk profiles with topiramate treatment. Am J Kidney Dis. 2006;48:555–563. doi: 10.1053/j.ajkd.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Warner BW, LaGrange CA, Tucker T, Bensalem-Owen M, Pais VM Jr. Induction of progressive profound hypocitraturia with increasing doses of topiramate. Urology. 2008;72:29–32. doi: 10.1016/j.urology.2008.01.042. discussion 32-23. [DOI] [PubMed] [Google Scholar]
- 17.Lamb EJ, Stevens PE, Nashef L. Topiramate increases biochemical risk of nephrolithiasis. Ann Clin Biochem. 2004;41:166–169. doi: 10.1258/000456304322880104. [DOI] [PubMed] [Google Scholar]
- 18.Kaplon DM, Penniston KL, Nakada SY. Patients with and without prior urolithiasis have hypocitraturia and incident kidney stones while on topiramate. Urology. 2011;77:295–298. doi: 10.1016/j.urology.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 19.Maalouf NM, Langston JP, Van Ness PC, Moe OW, Sakhaee K. Nephrolithiasis in topiramate users. Urol Res. 2011;39:303–307. doi: 10.1007/s00240-010-0347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 21.Mahmoud AA, Rizk T, El-Bakri NK, Riaz M, Dannawi S, Al Tannir M. Incidence of kidney stones with topiramate treatment in pediatric patients. Epilepsia. 2011;52:1890–1893. doi: 10.1111/j.1528-1167.2011.03245.x. [DOI] [PubMed] [Google Scholar]
- 22.Goyal M, Grossberg RI, O'Riordan MA, Davis ID. Urolithiasis with topiramate in nonambulatory children and young adults. Pediatr Neurol. 2009;40:289–294. doi: 10.1016/j.pediatrneurol.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Gillespie RS, Stapleton FB. Nephrolithiasis in children. Pediatr Rev. 2004;25:131–139. doi: 10.1542/pir.25-4-131. [DOI] [PubMed] [Google Scholar]
- 24.Ertan P, Tekin G, Oger N, Alkan S, Horasan GD. Metabolic and demographic characteristics of children with urolithiasis in Western Turkey. Urol Res. 2011;39:105–110. doi: 10.1007/s00240-010-0306-1. [DOI] [PubMed] [Google Scholar]
- 25.Akcay T, Konukoglu D, Celik C. Hypocitraturia in patients with urolithiasis. Arch Dis Child. 1996;74:350–351. doi: 10.1136/adc.74.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belman AB, Wehle MJ, Segura JW. Clinical Pediatric Urology, Ch 38. 4th ed. Boca Raton, FL: Taylor and Francis; 2002. Pediatric Urolithiasis; pp. 1223–1246. [Google Scholar]
- 27.Alpay H, Ozen A, Gokce I, Biyikli N. Clinical and metabolic features of urolithiasis and microlithiasis in children. Pediatr Nephrol. 2009;24:2203–2209. doi: 10.1007/s00467-009-1231-9. [DOI] [PubMed] [Google Scholar]
- 28.Spivacow FR, Negri AL, del Valle EE, Calviño I, Fradinger E, Zanchetta JR. Metabolic risk factors in children with kidney stone disease. Pediatr Nephrol. 2008;23:1129–1133. doi: 10.1007/s00467-008-0769-2. [DOI] [PubMed] [Google Scholar]
- 29.Battino BS, De FW, Coe F, Tackett L, Erhard M, Wacksman J, et al. Metabolic evaluation of children with urolithiasis: are adult references for supersaturation appropriate? J Urol. 2002;168:2568–2571. doi: 10.1016/S0022-5347(05)64217-6. [DOI] [PubMed] [Google Scholar]
- 30.Parekh DJ, Pope JI, Adams MC, Brock JW., 3rd The role of hypercalciuria in a subgroup of dysfunctional voiding syndromes of childhood. J Urol. 2000;164:1008–1010. doi: 10.1097/00005392-200009020-00022. [DOI] [PubMed] [Google Scholar]
- 31.Pak CY, Adams-Huet B. Elucidation of factors determining formation of calcium phosphate stones. J Urol. 2004;172:2267–2270. doi: 10.1097/01.ju.0000140959.32579.44. [DOI] [PubMed] [Google Scholar]