Summary
Sleep duration in America has gradually declined over the last four decades and appears to have reached a plateau for the last six years, with recent studies reporting that the population's current average sleep duration is approximately 6 hours. In this paper, we examine epidemiologic and community-based data on sleep complaints reported by American adults, specifically addressing the role of race/ethnicity in the subjective report of sleep problems. Subjective and objective findings indicate that black1 Americans have higher rates of long (≥ 9 h) and short (≤5 h) sleep than their white counterparts, and this may mediate a higher risk of cardiovascular disease (CVD), obesity and diabetes among blacks. In addition, studies show mixed results on sleep complaints among blacks compared to those among other ethnicities. This paper explores factors that may contribute to racial/ethnic differences in sleep including intra-ethnic variation, cultural biases, genetics and psychosocial factors.
Keywords: sleep duration, sleep quality, sleep apnea, race/ethnicity, repressive coping, response bias, sleep complaints, education, gender, age, America
Decline in sleep duration in America
Epidemiologic and community-based studies have shown that nightly sleep duration in America declined from 1960 to 2000 and seems to have a reached a plateau at approximately 6–6.5 hours for the last six years (Table 1). In 1960, epidemiologic data from the American Cancer Society showed that the average sleep duration nationwide was 8.5 hours (1). The 1982 Cancer Prevention Study II found that among 1.1 million Americans, the modal sleep duration was 8 hours (2). Analysis of the 1982–1984 National Health and Examination Study (NHANES) I showed only 7.4% of participants reported sleeping ≤5 hours at baseline, whereas NHANES III (2005–2008) analysis found this baseline number was 33% (3;4). Community-based studies also show an increase in short sleepers (5;6). In a 2003–2004 follow-up wrist actigraphic study of Chicago residents (age 38–50) who participated in the 1986 Coronary Artery Risk Development in Young Adults (CARDIA) study, the mean nightly sleep duration was 6.1 hours (7). Similarly, a home-based actigraphic study showed the average adult (40–64 years old) slept 6.22 hours at night (8).
Table 1.
Summary of Epidemiological Studies in America that Examine Trends in Sleep over Time
| Source | Study Design | Sample | Results | Limitations |
|---|---|---|---|---|
| NSF, 200512 | Random interviews | Sleep in America poll | Americans sleeping ≤ 8 h in 2001, 38%; 2002, 30%; in 2005, 26% | Response bias of self-reports |
| Stamatakis et al, 20075 | Questionnaire data | Alameda County Health and Ways of Living Study (1965, 1974, 1983, 1994, 1999), n=6928 | Overall percentage of short sleepers increased over time for African Americans (26% in 1965 to 54% in 1999), Hispanics (12% in 1965 to 37% in 1999) and whites (15% in 1965 to 25% in 1999). | Response bias of self-reports |
| Knutson et al, 201017 | Meta-analysis of 8 national studies, 1975–2006 | American's Use of Time Series (1975,1985,199–99); Environmental Protection Agency Time Use Study (1992–94); American Time Use Survey (ATUS) (2003,2004,2005,2006) | Odds of short sleep higher for full-time workers, those with some college education, and African Americans. Short sleepers were 7.6% in 1975, 9.3% in 2006 | Time diaries, total daily sleep not limited to sleep at night |
| Luckhaupt, 201014 | Cross-sectional epidemiologic survey | 2004–07 National Health Interview Survey (NHIS);, employed adults; ages ≤18 y, n=66,099 | Proportion of 6 h sleepers was 30.7% in 2004 (95% CI: 1.03–1.13) and 28.4% in 2007; mean proportion of short sleepers 37.2% (95% Cl: 1.24–1.35) for blacks and 28.8% for whites | Sample limited to the employed; response bias of self-reports |
| Robinson et al, 201016 | Time diaries | 2003–2007 ATUS, ages 18–64 y | Sleep in America increased; in 1965, 56 hrs/week, in 2007, 59 hr/week | Weekly estimates, does not limit sleep to nightly sleep or does not account for making up for lost sleep during the weekend. |
Abbreviations: CI, confidence interval
Results from national polls converge with epidemiologic data, showing a similar trend. While this paper does not rely solely on these findings due to their inherent limitations such as deviation from scientific methodology and cohort inconsistencies (9), national polls provide a general impression about habitual sleep quantities in America since 1975. About 37 years ago, the national Gallup poll found that the modal sleep duration was ≥ 8 hours (10); by 2011, the National Sleep Foundation (NSF) Sleep in America poll showed that on weeknights, the average sleep duration was 6 hours and 55 minutes (11). With each successive NSF poll, a downward trend has been observed in habitual sleep duration. In 2001, the proportion of Americans sleeping 8 hours or longer on weeknights was 38%; in 2002, it was 30%, and in 2005, it was 26% (12).
The average sleep duration appears to have plateaued between 6 and 6.5 hours in recent years. Given the well-established association between low socioeconomic status and sleep problems (13), steep sleep decline in America could be a probable response to America's poor economic state and housing crisis, which began in 2007. However, the extant literature reveals nominal decreases in sleep duration with rates hovering around 6–6.5 hours.
Few studies have found an increase in sleep duration during the 2000s and over the last four decades (14;15;16); while one metaanalysis showed virtually no change in sleep duration over the last 31 years: a modest 1.7% increase in adult short sleepers (17). This discrepancy in results is in part due to cohort differences, as might be the case in employment status. Numerous studies have found that more unemployed persons report short sleep durations than those who are employed (13;18). This may account for the finding that among employed participants of the National Health Interview Surveys (NHIS), the proportion of 6-hour sleepers decreased from 30.7% to 28.4% from 2004 to 2007 (14). Knutson's metaanalysis, in contrast, showed an increase in the odds of short sleep for full-time workers only, not the unemployed, a difference possibly due to study design; studies based entirely on time diaries, such as the Robinson and Knutson studies, determined daily sleep as the total amount of sleep per day, perhaps accounting for naps taken throughout the day, which may compensate for sleep loss at night; whereas responses to surveys such as the Sleep in America poll or the NHIS specify nightly sleep duration.
Sleep duration: evidence supporting racial/ethnic differences
Concomitant with data showing sleep decline in America is a recent wave of findings showing that blacks have the highest prevalence of long (≥ 9 h) and short sleep (≤ 5 h) in America (5;19;20). In light of such findings, this paper focuses on comparisons of sleep between blacks and other ethnicities, primarily whites, as these two groups have been extensively studied. The 2010 Sleep in America poll showed that blacks reported the least amount of sleep on weekdays, averaging 38 minutes less sleep than whites (21). Community studies show the odds of short sleep in black Americans are higher than in their white counterparts. Previous data from a study using telephone interviews documents a greater prevalence of short sleep among blacks compared with whites living in Detroit, Michigan (18.7% vs. 7.4%) (22). Actigraphic data obtained from adults residing in San Diego showed that white individuals slept an average of 6.32 hours, whereas black individuals averaged 5.9 hours a night (23). A similar observation was made in a sample of Chicago residents, for whom actigraphic sleep duration among white women was 6.7 hours; white men, 6.1 hours; black women, 5.9 hours; and black men, 5.1 hours (7).
Factors contributing to sleep loss include cell phone usage before going to bed, ambient temperature, carbohydrate consumption, socioeconomic status, exercise, noise, light, use of certain drugs, and the phase of the circadian pacemaker (11;20;24–29). It is also well established that physical health, anxiety, and depression are all significant correlates of impaired sleep (30–32).
Early epidemiologic data show that a larger proportion of black adults in America compared with whites slept > 8 hrs (33;34). This is consistent with other data indicating blacks are more likely than individuals of other races to be long sleepers as well. Analysis of 2005 NHIS data indicated that independent of the influence of demographic and medical factors, blacks were more likely to report both sleeping ≤ 5 hrs and ≥ 9 hrs than white respondents, suggesting greater variation in their habitual sleep time (19). Such extreme sleep durations are within the sleep time intervals commonly associated with early mortality and ill health (30;31), with a greater association between long sleep and mortality in older adults (35;36).
Consequences of sleep problems: Evidence supporting racial/ethnic differences
Residual daytime sleepiness, a direct result of sleep loss, has become a serious problem in the United States, negatively affecting cognitive performance. Residual daytime sleepiness is linked to decreased psychomotor and cognitive speed, attention/concentration, memory, and ability to drive (18), and increased risk for mortality in adults >65 when combined with sleep disordered breathing (37). This decline in cognitive function occurs in a sleep dose-dependent manner (38;39). Researchers found speed decreased on a psychomotor vigilance task following one day of sleep restriction; and continued to decrease over a seven-day period for participants sleeping 3–5 hours per night, with those sleeping 3 hours exhibiting the steepest decline in speed. Speed increased after the first night of returning to normal sleep, with those 3-hour sleepers experiencing the highest rate of increase. By the third night of normal sleep, speed stabilized at levels lower than baseline for all groups, indicating that full recovery of cognitive function requires many days of healthy sleep (39). While Horne interprets this stabilization as evidence of individual adaptation within the range (6–9 hours) with the least deleterious health effects (40), we posit that it may be difficult to conceive that adaptation could be observed over such short periods of time. Unless, incontrovertible evidence is available, sleepiness resulting from curtailed sleep durations remains an important public health risk (41).
Sleep insufficiency is associated with chronic diseases, such as type 2 diabetes, cardiovascular disease (CVD), depression, and obesity; heart attack; stroke; and mortality (18;41–44). While there is a breadth of research studying sleep in patients with chronic psychiatric disorders such as depression and schizophrenia, this paper focuses, rather, on the relationship between sleep and components of the metabolic syndrome as the former has been reviewed elsewhere. Shedding light on the association between sleep loss and obesity, researchers have shown that a single night of sleep deprivation results in higher food intake in healthy men (45). A more recent study found that when compared with normal sleep, chronic short sleep restriction (4 hour/night for five nights) resulted in higher fat and energy intake but similar energy expenditures for normal-weight males and females (46). In addition, while there is no evidence that insufficient sleep directly causes type II diabetes, previous studies show that short sleep is associated with increased levels of vispatin and leptin, markers of inflammation, and insulin resistance (47). Glucose levels and insulin resistance have also been found to be increased in patients with obstructive sleep apnea, a condition characterized by closure of the upper airway during sleep resulting in sleep fragmentation (48;49). Similarly, a causal relationship between sleep and depression has not been proven, although studies show that insomnia can predict future depression (50–52).
Compared with studies on short sleep, fewer studies focus on the consequences of long sleep. One study showed that one week of 9 hours of sleep had no significant effect on cognitive function (39). Both long sleep and short sleep are associated with components of the metabolic syndrome (34;42;53). However, there is limited research to determine systematically relationships between long sleep and symptoms characteristic of these medical conditions. Most recently, a study found a positive association between hypercholesterolaemia and short sleep in females, while negative associations were noted for males (54).
It appears that on either side of the population distribution of sleep durations, extreme values are more prevalent among blacks. This thus suggests greater relative risk for health problems among blacks (19;55). Compared with whites, blacks are at greater risk for sleep-disordered breathing (12;20;56–58), type 2 diabetes, CVD, obesity and stroke (59).
Does sleep mediate racial/ethnic differences in disease risk?
Recent evidence begins to shed light on the role of sleep in several health disparities. A multivariate analysis of the NHIS (ages18–85 years old) showed that the adjusted risk of obesity associated with short sleep for blacks and whites were 1.78 (95% CI: 1.30–2.45) and 1.43 (95% CI: 1.24–1.66), respectively. Thus, an excess of 35% of blacks were shown to have a greater risk for obesity associated with short sleep, compared with their white counterparts (60). This finding is important in view of the bidirectional causal relationships of obesity to sleep apnea (61;62) and CVD (63;64).
Research shows that sleep duration modifies risk of symptoms of CVD and type 2 diabetes in black Americans. In an ancillary CARDIA study of 578 Chicago residents (ages 33–45), sleep duration significantly predicted increased odds of incident hypertension (OR 1.37, 95% CI: 1.05, 1.78) and adjusting for sleep duration significantly reduced the diastolic blood pressure change over 5 years in blacks and whites (6). Knutson's group also found that predictors of glycemic control were sleep duration, in non-diabetics, and sleep quality, among those with at least one diabetic complication (65). In addition to sleep duration, sleep quality is emerging as a key contributing factor to quality of life (66).
Do variable sleep architecture and/or sleep pathways mediate racial/ethnic differences in disease?
Variant anatomy, biomarkers, and genes in different ethnicities may indicate differential sleep pathways, which may influence disease. Studies of patients with depression have helped shed some light on this prospect, as there are data showing racial/ethnic differences in sleep regulation among patients with depression. Early studies showed that black patients with depression had more stage 2 sleep, longer rapid eye movement (REM) sleep latency, lower REM density, and less REM sleep than their white counterparts (67;68), pointing to differences in sleep architecture among blacks and whites. However, a meta-analysis of fourteen studies found psychosocial factors including mild depression may moderate ethnic differences in sleep continuity and duration between blacks and whites, not sleep architecture (20). While data is scarce and more research needs to be done to establish the role of sleep in regulating sleep architecture among depressed patients of different ethnicities, these observations imply that racial/ethnic factors, psychosocial, and/or genetic factors affecting health and disease states (69;70) may differentially influence sleep regulatory processes.
Differential anatomic risk factors and biomarkers among various ethnic groups have indicated possible racial differences in the genetic underpinnings of sleep (71–75). Research has shown that inflammatory markers are related to risk for type two diabetes, obesity and CVD. One study found that mid-life black women with high levels of inflammatory biomarkers also experienced short sleep at night, while another showed that sleep restriction in young adult black women resulted in increased levels of inflammatory hormone adiponectin. Together, these studies suggest that an inflammation pathway may mediate the link between reduced sleep duration and cardiometabolic diseases, and this pathway may function differently in blacks relative to whites (73;76).
Evidence of a genetic basis of anatomical differences comes from a sleep apnea study in which half of the participants were same-sex siblings. It showed that upper airway soft tissue structures have high levels of heritability as demonstrated by family aggregation (77). Further support of genetic differences as a mediating factor of racial/ethnic sleep disparities comes from research on the human leukocyte antigen DQB1*0602 allele, which is closely associated with narcolepsy, a neurologic disorder characterized by excessive daytime sleepiness. A recent study showed that DQB1*0602 may be a biomarker for predicting individual differences in normal and sleep loss conditions. When chronically deprived of sleep, healthy DQB1*0602-positive subjects showed more fragmented sleep, greater REM sleep latency reductions, smaller stage 2 reductions, and higher fatigue, compared to healthy DQB1*0602-negative subjects. However, a significant difference in DQB1*0602 allelic frequencies was not found in whites and blacks in their total sample of 129 participants (78); it is likely that epigenetic mechanisms involving environmental factors such as stress may influence the expression of these genes. Differential levels of these environmental factors among various ethnic groups may thereby mediate genetic regulation of sleep. More research is needed to determine if genetics plays an important role in racial/ethnic sleep disparities.
Sleep complaints: evidence supporting racial/ethnic differences
There are no nationwide epidemiological trend studies on sleep complaints commonly included in insomnia definitions: difficulty initiating sleep (DIS), difficulty maintaining sleep (DMS), and early morning awakening (EMA). Therefore, it is unclear whether sleep complaints reported by Americans have increased or decreased over the last four decades. It is likely that sleep complaints would increase, if in fact sleep durations have declined substantially.
Studies examining racial/ethnic differences in sleep complaints have yielded conflicting data, with some suggesting blacks report fewer sleep complaints than whites while others found the opposite is true. In the Duke Established Populations for Epidemiologic Studies of the Elderly, a longitudinal study of adults (ages ≥ 65 years) selected from urban and rural counties of North Carolina (79), 24% of the respondents complaining of wakeful sleep were black and 76% were white. Similarly, data from the Atherosclerosis Risk in Communities Study (ARIC), a prospective, population-based study of 13,563 participants (ages 47–69 years), showed that black participants reported fewer sleep complaints relative to whites (80).
Our research team has used community-based sleep data in Brooklyn, New York to examine racial/ethnic differences in sleep complaints (81–83) and to explore potential explanatory factors (84). Two waves of data were obtained to examine health characteristics and psychosocial factors among older adults in Brooklyn. In the first wave of data (n = 1118), men and women (mean age: 75 ± 6 years) with self-ascribed ethnicity as African Americans (21%), Caribbean Americans (39%), Eastern Europeans (15%), and European Americans (25%) participated. In the second wave of data (n = 1440), women (mean age: 60± 6 years) with self-ascribed ethnic identity as African Americans (22%), English-speaking Caribbeans (22%), Haitians (22%), Dominicans (12%), Eastern Europeans (11%), and European Americans (11%) participated. In both studies, participants were recruited using a stratified, cluster sampling technique. Details of the recruitment strategy and data collection procedures are published elsewhere (81;83).
Sleep complaints of older blacks in the Brooklyn data showed similarities to previous studies regarding DIS, DMS, and EMA: blacks tended to report fewer sleep complaints than their white counterparts. This was consistent with their report of greater satisfaction with sleep. (83) Similarly, a recent study analyzing the 2006 Behavioral Risk Factor Surveillance System (BRFSS) (n=159,856; mean age = 51.5 years) found African Americans had fewer sleep complaints than whites (13).
In contrast to these studies, a 2010 NSF poll (n=1,007, ages 25–60) showed that blacks were the least likely to report having a good night's sleep at least a few nights per week compared to other ethnic groups (Whites, 68%; Blacks, 66%; Asians 84%; Hispanics 76%) (21). A cross-sectional study of urban primary care patients (mean age 51.9 years old) also found that blacks had higher rates of sleep disturbance compared to whites (85). Similarly, a study by Patel and colleagues observed that African-Americans and Latinos were more likely to report poorer sleep compared to whites (26). Together, these findings show a discrepancy in self-reports of sleep complaints among blacks in America.
Sleep complaints: evidence supporting intra-ethnic differences
There are several studies showing intra-ethnic differences in sleep among Hispanics (86). Currently two large-scale NIH-sponsored studies are being done exploring sleep disorders, including sleep apnea, and sleep risk within Hispanic populations with different countries of origin. Few such intra-ethnic studies have been done among blacks in the United States.
Our study of older Brooklyn residents has allowed the recognition of intra-ethnic heterogeneity in sleep complaints (83). Analysis of the first wave of the Brooklyn sleep data indicated that the prevalence of sleep complaints differed significantly as a function of respondent's ethnic origin or place of birth. Examination of differences between white and black respondents showed that estimates of sleep complaints among African Americans and Caribbean Americans were 71% and 47%, respectively. Among European Americans and Eastern Europeans, rates were 70% and 77%, respectively (81). Hence, estimated rates of sleep complaints for African Americans were surprisingly similar to estimates provided by respondents of European descent.
Intra-ethnic differences were also observed based on analysis of the second wave of data. This was a more comprehensive analysis, as it compared estimates of sleep complaints among women from six ethnic groups: African American, Dominican, Haitian, English-speaking Caribbean, European American, and Eastern European.(83) Again, the sleep complaints profile of African Americans was similar to that of European Americans for DIS (21% and 28%, respectively); DMS (63% and 59%, respectively); and EMA (45% and 48%, respectively). For Dominican women, estimates were remarkably similar to those of European and African American women. However, estimates for Haitian and English-speaking Caribbean women were substantially lower than all other groups (DIS=9% and 19%, respectively; DMS=32% and 26%, respectively; EMA=15% and 20%, respectively). Besides the obvious differences in cultural backgrounds, the only factors discriminating Dominican and African-American women from Haitian and English-speaking Caribbean women were greater risk profile (i.e., stress, smoking, and drinking) and more somatic complaints and respiratory diseases. (83) Discrepancies could not be explained by differences in socioeconomic status (24) or duration of residence in the United States (87).
Results of within-group analyses imply that reports of lower estimates of sleep complaints among blacks (79;80;83;88) may have been confounded by the presence of foreign-born blacks (i.e., Caribbean, African and others), and estimates of sleep complaints among African Americans derived from analysis of aggregate data might have, therefore, been underestimated. Plausibly, foreign-born blacks may be in fact experiencing less sleep problems than do American-born blacks. While we cannot discern the intra-ethnic composition of black strata that have been reported in previous studies, these data from intra-ethnic studies suggest that rigorous statistical analyses or interpretation of epidemiologic sleep data should consider the ethnic origin of study participants.
Psychosocial factors and sleep complaints
The role of repressive coping and response bias in underreporting of sleep complaints by black Americans
While there are no studies to date that examine the prevalence of underreporting of sleep complaints among black Americans over time, studies on perception of other health parameters suggest that blacks tended to report fewer self-perceived health problems than did whites. Of interest are epidemiologic and vital statistics data demonstrating that blacks have worse health outcomes than whites (59;89;90). The possibility of a bias in reporting among blacks may extend to subjective sleep data as well.
Response bias among older blacks may reflect positive reframing and repressive coping. Analysis of Brooklyn sleep data found that the relationship between race/ethnicity and sleep complaints is jointly dependent on the degree of repressive coping(84) . Studies comparing black caregivers with their white counterparts found that blacks used more positive reappraisal than did whites (91). Relative to whites (mean age=62.36), black caregivers (mean age=55.24) often appraised patient's problems as being less stressful and reported fewer depressed moods and higher self-efficacy in managing caregiving problems (92). Based on appraisal and coping research, several hypotheses have been advanced suggesting that older blacks might have developed effective strategies to deal with hardships due to poverty, racism, segregation, and other life stresses. These strategies over time would have fostered effective reframing of difficult life experiences that could not be easily changed (92).
Perhaps older blacks may modify their answers to sleep questionnaires in a manner they perceive is socially desirable, a possibility that we are currently investigating. Additionally, older blacks may cope with sleep problems within a positive self-regulatory framework, which allows them to deal more effectively with sleep-interfering psychological processes to stressful life events (93). While this positive reframing may be useful among blacks, among whites is not believed to be protective; rather, it may lead to increased psycho-physiological distress (93).
Whereas blacks reporting sleep complaints may benefit from this unique ability to cope with challenges posed by sleep disturbances, this may be maladaptive for those with sleep-disordered breathing or other medical conditions causing insomnia. The NSF 2010 Sleep In America Poll reported less blacks (76%) than whites (83%) believed that insufficient or poor sleep was linked to health problems. Furthermore, whites and Asians were slightly more likely than blacks and Hispanics to agree that shorter duration of sleep affects one's ability to care for family and relate well with family and friends (21). These findings indicate blacks may not regard inadequate sleep as a serious issue; hence, discrepancies between habitual sleep duration and perceived sleep need may not provoke any action toward increasing actual sleep duration.
The reluctance to address sleep problems might explain in part why sleep-disordered breathing is a public health problem in black communities (94;20). Data collected at a sleep clinic in Brooklyn suggests that only 38% of blacks are likely to adhere to recommendation for polysomnographic evaluations. This is alarming since 91% of black patients undergoing polysomnographic recordings received a diagnosis of sleep apnea (95).
Nonequivalent measures in surveys may lead to culturally-biased self-reports
Responding to questionnaires is not a simple task, requiring several cognitive decisions that can be influenced by the context within which responses are solicited (96). Perhaps cultural understandings of certain terms in the surveys may influence results (97). One study on responses to the Center for Epidemiological Studies Depression (CES-D) scale analyzed data from the New Haven Established Populations for Epidemiologic Studies of the Elderly (EPESE) and the five-state Hispanic-EPESE. Researchers found that among adults over 65, blacks were more likely than whites to endorse two interpersonal items (people are unfriendly and people do not like me) that researchers posited were a measure of perceived discrimination (98). These results demonstrate response bias among the elderly is linked to race/ethnicity. This is significant to sleep profiles in America because it implies that due to possible nonequivalent measures in established scales, self-reports on sleep may reflect cultural attributes rather than an accurate assessment of sleep. In an effort to eliminate response bias, surveys should reflect culturally appropriate measures that differ for various racial/ethnic groups (98).
Conclusion
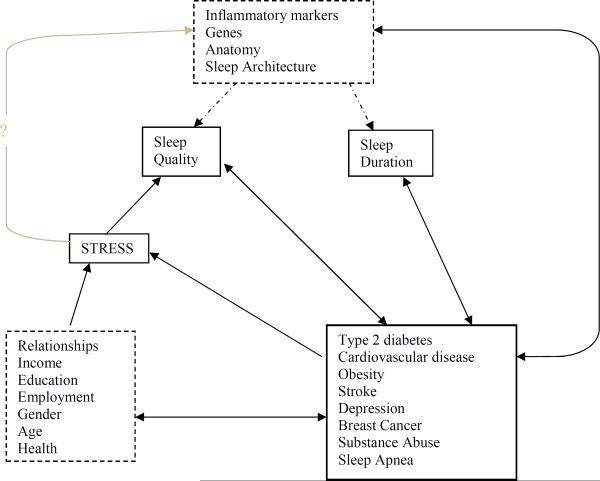
The preponderance of evidence suggests that sleep duration in America has been declining gradually, at least up to 2000's. Blacks seem to be characterized by a higher prevalence of long and short sleep compared with other ethnicities. Although further research is warranted, current studies show that poor sleep may mediate increased risk of type 2 diabetes, CVD, and obesity among blacks (fig. 1). Studies point to differences in anatomy, sleep architecture and genetics as a biological basis for racial/ethnic sleep disparities. In addition, psychosocial factors (e.g., stress over finances, health, relationships and employment), which are better documented, have a contributory role in understanding of sleep disparities in America. Figure 1 represents these concepts in a hypothetical model with dashed boxes and arrows symbolizing potential pathways. While race/ethnicity appears to be associated with disease risk linked to sleep, it is possible that genetics or socioeconomic status could be latent variables actually mediating the racial/ethnic differences seen in disease risk linked to sleep. Rather than performing analysis adjusting for individual manifest variables, as is commonly done, mediation analysis such as structural equation modeling, should be used to determine latent variables responsible for the racial/ethnic phenomenon observed in sleep-associated disease risk.
Fig. 1.
Hypothetical Model of Sleep Pathways to Racial/Ethnic Disparities in Disease. Few studies suggest inflammatory markers, genes, anatomy, and sleep architecture may vary between ethnic groups and mediate differential sleep pathways that have a bidirectional causal relationship to various diseases. Socioeconomic factors, gender, and age are variables that affect sleep quality and duration. Future research is needed to determine if these variables are associated with or are causes of physiologically or genetically mediated sleep pathways.
Intra-ethnic differences further influence sleep studies in America as country of origin seems to play a role in self-reporting of sleep. Future research is needed to ascertain means of adjusting study designs to ensure accurate self-reports of sleep and limit the possibility of response bias. Accurate assessment of sleep is important as it may be a relatively easy way for physicians to determine if patients are at a greater risk for diseases of the metabolic syndrome: CVD, obesity and diabetes.
Practice points
Assessment of sleep duration and quality should be routine at every regular physical exam especially for black American patients with metabolic syndrome.
Regulating the daily hours of sleep to 7–9 hours should be recommended for patients with metabolic syndrome.
Using culturally appropriate measures on questionnaires administered at the doctor's office during regular checkups may reduce response bias associated with self reports, particularly among black Americans, and may assess sleep in patients more accurately.
Culturally targeted education on the effect of sleep on health should be readily accessible in medical offices.
Research agenda.
Further research in this area should focus on:
-
1)
Using structural equation modeling to distinguish between manifest and latent variables that could be mediating racial/ethnic differences associated with disease risk linked to sleep. This will enable researchers to generate a more accurate model of associations between sleep, race/ethnicity, and disease.
-
2)
Using an intra-ethnic heterogeneous sample, determine whether estimates of sleep complaints observed among women are similar for men from the same ethnic groups.
-
3)
Intra-ethnic studies with black samples that include African-born immigrants would provide a more comprehensive sleep profile of blacks in the United States and indicate the extent to which genes, culture and environment may impact sleep.
-
4)
To determine the role of sleep in mediating obesity in different ethnicities, researchers should perform multivariate analysis of obese blacks and whites on a weight loss regiment of exercise, dietary changes, and sleep regulation (whereby participants sleep 7 hours a night). If adjusting for sleep does not significantly affect the efficacy of the weight loss regimen, one can infer sleep regulation may not be considered effective treatment of obesity.
-
5)
Clinical study can be done to determine if levels of inflammatory biomarkers are characteristically higher in black American patients with cardiometabolic disease than in other ethnicities with the same disease.
-
6)
Prospective or longitudinal randomized cohort study following American teenagers of different ethnicities into their young adult years should be done and objective measures of adiposity, nightly sleep duration (recorded via a time diary), physical activity and diet can be collected to determine if age and ethnicity affect short sleep associated obesity.
-
7)
A cross-sectional study enrolling men and women in urban American cities should be performed using multivariate logistic regression to test effects of age, gender, education, intra-ethnicity, income, sleep quality and sleep duration on the risk of metabolic syndrome.
Table 2.
Summary of Epidemiological Studies in America that Examine Sleep as a Mediating Factor for Ethnic Differences in Disease Risk
| Source | Study Design | Sample | Results |
|---|---|---|---|
| Brown, 200960 | Cross-sectional household interview survey | 2005 National Health Interview Survey (NHIS); n=29818, aged 18–85 y | Adjusted risk of obesity associated with short sleep higher for blacks than whites (1.78, 95% CI: 1.30–2.45; 1.43, 95% CI: 1.24–1.66) |
| Knutson et al, 20096 | Wrist actigraphy for 3 consecutive days from 2003–05 | Ancillary Coronary Artery Risk Development in Young Adults study of Chicago residents, n=578, aged 33–45 | Short sleep duration predicted incident hypertension (OR 1.37, 95% CI: 1/05, 1.78); sleep duration mediated difference between black and white Americans in diastolic blood pressure change over time (P=.02); short sleep and poor sleep maintenance predicted higher systolic and diastolic pressure and more adverse changes in systolic and diastolic blood pressure over 5 y (all P<.05) after adjusting for age, race, and sex and excluding those on anti-hypertensive medication |
| Knutson et al, 200665 | Cross-sectional study | Black American volunteers with type 2 diabetes interviewed at the University of Chicago Hospitals, n=161; HbAlc levels from medical charts to measure glycemic control; PSQI to measure sleep quality | In patients without diabetic complications, perceived sleep debt, not PSQI, was a significant predictor of HbAlc level (r=.51, P=.04); in patients with at least 1 complication, PSQI score, not sleep debt, was a significant predictor of HbAlc level (r=.043, P=.002) |
Abbreviations: CI, confidence interval
Acknowledgements
This research was supported by funding from the NIH (R01MD004113 and R01HL095799).We thank Dr. Carol Magai, the Director of the Intercultural Institute at Long Island University, for providing access to the Brooklyn sleep data reviewed in this paper.
Abbreviations
- DIS
difficulty initiating sleep
- DMS
difficulty maintaining sleep
- EMA
early morning awakening
- CVD
Cardiovascular disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Throughout the text, we use the term black in lieu of African American for there are instances where we refer to individuals with self-ascribed race/ethnicity as black, African American, African, or Caribbean American; the term white is used to denote individuals of European descent
Disclosure No potential conflicts of interest relevant to this article were reported.
Reference List
- (1).Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–16. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- (2).Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- (3).Bansil P, Kuklina EV, Merritt RK, Yoon PW. Associations between sleep disorders, sleep duration, quality of sleep, and hypertension: results from the National Health and Nutrition Examination Survey, 2005 to 2008. J Clin Hypertens (Greenwich) 2011;13:739–43. doi: 10.1111/j.1751-7176.2011.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Short Sleep Duration as a Risk Factor for Hypertension: Analyses of the First National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- *(5).Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: population. Ann Epidemiol. 2007;17:948–55. doi: 10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(6).Knutson KL, Van CE, Rathouz PJ, Yan LL, Hulley SB, Liu K, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–61. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(7).Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- *(8).Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber M, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: Effects of gender and ethnicity. Biol Psychiatry. 2000;47:921–7. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- (9).Webb WB. Opinion Polls and Science. Sleep. 2010;33:865–6. doi: 10.1093/sleep/33.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Gallup The Gallup Study of Sleeping Habits. The Gallup Organization. 1979:1–60. [Google Scholar]
- (11).National Sleep Foundation (NSF) Summary findings of 2011 Sleep in America Poll. 2011. [Google Scholar]
- *(12).National Sleep Foundation (NSF) Summary findings of 2005 Sleep in America Poll. 2005. [Google Scholar]
- (13).Grandner MA, Patel NP, Gehrman PR, Xie D, Sha D, Weaver T, et al. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med. 2010;11:470–8. doi: 10.1016/j.sleep.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Luckhaupt SE, Tak S, Calvert GM. The prevalence of short sleep duration by industry and occupation in the National Health Interview Survey. Sleep. 2010;33:149–59. doi: 10.1093/sleep/33.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Bin YS, Marshall MS, Glozier N. Secular Trends in adult sleep duration: a systematic review. Sleep Med Rev. doi: 10.1016/j.smrv.2011.07.003. in Press. [DOI] [PubMed] [Google Scholar]
- (16).Robinson JP, Michelson W. Sleep as a victim of the “time crunch” -A multinational analysis. electronic International Journal of Time Use Research. 2010;7:61–72. [Google Scholar]
- (17).Knutson KL, Van CE, Rathouz PJ, DeLeire T, Lauderdale DS. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep. 2010;33:37–45. doi: 10.1093/sleep/33.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Center for Disease Control and Prevention. Morbidity and Mortality Weekly Report. 2011;60:233–67. [Google Scholar]
- *(19).Nunes J, Jean-Louis G, Zizi F, Casimir GJ, von GH, Brown CD, et al. Sleep duration among black and white Americans: results of the National Health Interview Survey. J Natl Med Assoc. 2008;100:317–22. doi: 10.1016/s0027-9684(15)31244-x. [DOI] [PubMed] [Google Scholar]
- *(20).Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med. 2010;8:246–59. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- (21).National Sleep Foundation (NSF) Summary findings of 2010 Sleep in America Poll. 2010. [Google Scholar]
- (22).Singh M, Drake CL, Roehrs T, Hudgel DW, Roth T. The association between obesity and short sleep duration: a population-based study. J Clin Sleep Med. 2005;1:357–63. [PubMed] [Google Scholar]
- (23).Jean-Louis G, Mendlowicz MV, Gillin JC, Rapaport MH, Zizi F, Landolt HP, et al. Sleep Estimation from Wrist Activity in Patients With Major Depression. Physiol Behav. 2000;70:49–53. doi: 10.1016/s0031-9384(00)00228-6. [DOI] [PubMed] [Google Scholar]
- (24).Gellis LA, Lichstein KL, Scarinci IC, Durrence HH, Taylor DJ, Bush AJ, et al. Socioeconomic status and insomnia. J Abnorm Psychol. 2005;114:111–8. doi: 10.1037/0021-843X.114.1.111. [DOI] [PubMed] [Google Scholar]
- (25).Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Patel NP, Grandner MA, Xie D, Branas CC, Gooneratne N. “Sleep disparity”in the population: poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health. 2010;10:475. doi: 10.1186/1471-2458-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Goodin BR, McGuire L, Smith MT. Ethnicity moderates the influence of perceived social status on subjective sleep quality. Behav Sleep Med. 2010;8:194–206. doi: 10.1080/15402002.2010.509193. [DOI] [PubMed] [Google Scholar]
- (28).Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol. 2007;72:579–97. doi: 10.1101/sqb.2007.72.064. [DOI] [PubMed] [Google Scholar]
- (29).Libert JP, Bach V, Johnson LC, Ehrhart J, Wittersheim G, Keller D. Relative and combined effects of heat and noise exposure on sleep in humans. Sleep. 1991;14:24–31. doi: 10.1093/sleep/14.1.24. [DOI] [PubMed] [Google Scholar]
- (30).Kuppermann M, Lubeck DP, Mazonson PD, Patrick DL, Stewart AL, Buesching DP, et al. Sleep problems and their correlates in a working population. J Gen Intern Med. 1995;10:25–32. doi: 10.1007/BF02599573. [DOI] [PubMed] [Google Scholar]
- (31).Bliwise DL, King AC, Harris RB. Habitual sleep durations and health in a 50–65 year old population. J Clin Epidemiol. 1994;47:35–41. doi: 10.1016/0895-4356(94)90031-0. [DOI] [PubMed] [Google Scholar]
- (32).Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–8. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- (33).Schoenborn CA. Health habits of U.S. adults, 1985: the “Alameda 7”revisited. Public Health Rep. 1986;101:571–80. [PMC free article] [PubMed] [Google Scholar]
- (34).Qureshi AI, Giles WH, Croft JB, Bliwise DL. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10-year follow-up from NHANES I. Neurology. 1997;48:904–11. doi: 10.1212/wnl.48.4.904. [DOI] [PubMed] [Google Scholar]
- (35).Kripke DF, Langer RD, Elliott JA, Klauber MR, Rex KM. Mortality related to actigraphic long and short sleep. Sleep Med. 2011;12:28–33. doi: 10.1016/j.sleep.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Youngstedt SD, Jean-Louis G. Long sleep a greater mortality risk than short sleep in older adults. J Am Geriatr Soc. 2011;59:957–8. doi: 10.1111/j.1532-5415.2011.03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Gooneratne NS, Richards KC, Joffe M, Lam RW, Pack F, Staley B, et al. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep. 2011;34:435–42. doi: 10.1093/sleep/34.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Mollicone DJ, Van Dongen HP, Rogers NL, Banks S, Dinges DF. Time of day effects on neurobehavioral performance during chronic sleep restriction. Aviat Space Environ Med. 2010;81:735–44. doi: 10.3357/asem.2756.2010. [DOI] [PubMed] [Google Scholar]
- (39).Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J. Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- (40).Horne J. The end of sleep: `Sleep debt' versus biological adaptation of human sleep to waking needs. Biol. Psychol. 2011;87:1–14. doi: 10.1016/j.biopsycho.2010.10.004. [DOI] [PubMed] [Google Scholar]
- (41).Institute of Medicine . Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. The National Academies Press; Washington, DC: 2006. [PubMed] [Google Scholar]
- * (42).Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–36. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- (43).Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath. 2010;14:63–70. doi: 10.1007/s11325-009-0281-3. [DOI] [PubMed] [Google Scholar]
- (44).Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab. 2010;24:687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- (46).St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, RoyChoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Hayes AL, Xu F, Babineau D, Patel SR. Sleep duration and circulating adipokine levels. Sleep. 2011;34:147–52. doi: 10.1093/sleep/34.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Zizi F, Jean-Louis G, Brown CD, Ogedegbe G, Boutin-Foster C, McFarlane SI. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep. 2010;10:43–7. doi: 10.1007/s11892-009-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Pandey A, Demede M, Zizi F, Al Haija'a OA, Nwamaghinna F, Jean-Louis G, et al. Sleep apnea and diabetes: insights into the emerging epidemic. Curr Diab Rep. 2011;11:35–40. doi: 10.1007/s11892-010-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Mezick EJ, Matthews KA, Hall M, Strollo PJ, Jr., Buysse DJ, Kamarck TW, et al. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med. 2008;70:410–6. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Stevens RG. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol. 2009;38:963–70. doi: 10.1093/ije/dyp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Leproult R, Van CE. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA. 2011;305:2173–4. doi: 10.1001/jama.2011.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Lopez-Acevedo MN, Torres-Palacios A, Elena Ocasio-Tascon M, Campos-Santiago Z, Rodriguez-Cintron W. Overlap syndrome: an indication for sleep studies? : A pilot study. Sleep Breath. 2009;13:409–13. doi: 10.1007/s11325-009-0263-5. [DOI] [PubMed] [Google Scholar]
- (54).Sabanayagam C, Shankar A. Sleep duration and hypercholesterolaemia: Results from the National Health Interview Survey 2008. Sleep Medicine. 2012;13:145–150. doi: 10.1016/j.sleep.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Zizi F, Pandey A, Murrray-Bachmann R, Vincent M, McFarlane S, Ogedegbe G, Jean-Louis G. Race/Ethnicity, Sleep Duration, and Diabetes Mellitus: Analysis of the National Health Interview Survey. The Am J of Med. 2012;125:162–7. doi: 10.1016/j.amjmed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152:1946–9. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- (57).Gottlieb DJ, Whitney CW, Bonekat WH, Iber C, James GD, Lebowitz M, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–7. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- (58).Demede M, Pandey A, Zizi F, Bachmann R, Donat M, McFarlane SI, et al. Resistant hypertension and obstructive sleep apnea in the primary-care setting. Int J Hypertens. 2011;2011:340929. doi: 10.4061/2011/340929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57(Suppl 1):108–45. doi: 10.1177/1077558700057001S06. [DOI] [PubMed] [Google Scholar]
- (60).Brown CD, Jean-Louis G, Zizi F, von Gizycki H, Ogedegbe G, McFarlane SI. Short Sleep Duration and the Risk of Obesity among Black and White Americans. Sleep. 2009;32:153. [Google Scholar]
- (61).Yu JC, Berger P., III Sleep apnea and obesity. S D Med. 2011 Spec No:28–34. [PubMed] [Google Scholar]
- (62).Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17:533–40. [PubMed] [Google Scholar]
- (63).Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- (64).Jean-Louis G, Zizi F, Clark LT, Brown CD, McFarlane SI. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med. 2008;4:261–72. [PMC free article] [PubMed] [Google Scholar]
- (65).Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of Sleep Duration and Quality in the Risk and Severity of Type 2 Diabetes Mellitus. Arch Intern Med. 2006;166:1768–74. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- (66).Jean-Louis G, Kripke DF, Ancoli-Israel S. Sleep and Quality of Well-Being. Sleep. 2000;23:1115–21. [PubMed] [Google Scholar]
- (67).Poland RE, Rao U, Lutchmansingh P, McCracken JT, Lesser IM, Edwards C, et al. REM sleep in depression is influenced by ethnicity. Psychiatry Res. 1999;88:95–105. doi: 10.1016/s0165-1781(99)00080-3. [DOI] [PubMed] [Google Scholar]
- (68).Giles DE, Perlis ML, Reynolds CF, 3rd, Kupfer DJ. EEG sleep in African-American patients with major depression: a historical case control study. Depress Anxiety. 1998;8:58–64. [PubMed] [Google Scholar]
- (69).Kleinman A. Culture and patient care: psychiatry among the Chines. Drug Therapy. 1981;11:134–40. [Google Scholar]
- (70).Flack JM, Amaro H, Jenkins W, Kunitz S, Levy J, Mixon M, et al. Epidemiology of minority health. Health Psychol. 1995;14:592–600. doi: 10.1037//0278-6133.14.7.592. [DOI] [PubMed] [Google Scholar]
- (71).Matthews KA, Zheng H, Kravitz HM, Sowers M, Bromberger JT, Buysse DJ, et al. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women?: Study of Women's Health across the Nation sleep study. Sleep. 2010;33:1649–55. doi: 10.1093/sleep/33.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Villaneuva AT, Buchanan PR, Yee BJ, Grunstein RR. Ethnicity and obstructive sleep apnoea. Sleep Med Rev. 2005;9:419–36. doi: 10.1016/j.smrv.2005.04.005. [DOI] [PubMed] [Google Scholar]
- (73).Buxbaum SG, Elston RC, Tishler PV, Redline S. Genetics of the apnea hypopnea index in Caucasians and African Americans: I. Segregation analysis. Genet Epidemiol. 2002;22:243–53. doi: 10.1002/gepi.0170. [DOI] [PubMed] [Google Scholar]
- (74).Colilla S, Rotimi C, Cooper R, Goldberg J, Cox N. Genetic inheritance of body mass index in African-American and African families. Genet Epidemiol. 2000;18:360–76. doi: 10.1002/(SICI)1098-2272(200004)18:4<360::AID-GEPI8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- (75).Palmer LJ, Buxbaum SG, Larkin EK, Patel SR, Elston RC, Tishler PV, et al. Whole genome scan for obstructive sleep apnea and obesity in African-American families. Am J Respir Crit Care Med. 2004;169:1314–21. doi: 10.1164/rccm.200304-493OC. [DOI] [PubMed] [Google Scholar]
- (76).Simpson NS, Banks S, Arroyo S, Dinges DF. Effects of sleep restriction on adiponectin levels in healthy men and women. Physiol Behav. 2010;101:693–8. doi: 10.1016/j.physbeh.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Schwab RJ, Pasirstein M, Kaplan L, Pierson R, Mackley A, Hachadoorian R, et al. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med. 2006;173:453–63. doi: 10.1164/rccm.200412-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Goel N, Banks S, Mignot E, Dinges DF. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology. 2010;75:1509–19. doi: 10.1212/WNL.0b013e3181f9615d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Blazer DG, Hays JC, Foley DJ. Sleep complaints in older adults: a racial comparison. J Gerontol A Biol Sci Med Sci. 1995;50:M280–M284. doi: 10.1093/gerona/50a.5.m280. [DOI] [PubMed] [Google Scholar]
- (80).Phillips B, Mannino D. Correlates of sleep complaints in adults: the ARIC study. J Clin Sleep Med. 2005;1:277–83. [PubMed] [Google Scholar]
- * (81).Jean-Louis G, Magai C, Cohen CI, Zizi F, von Gizycki H, DiPalma J, et al. Ethnic differences in reported sleep problems in older adults. Sleep. 2001;24:926–33. doi: 10.1093/sleep/24.8.926. [DOI] [PubMed] [Google Scholar]
- (82).Jean-Louis G, Magai C, Consedine N, Zizi F, Casimir GJ, Solomon W, et al. Cancer worry and insomnia complaints among American women. Behav Sleep Med. 2009;7:63–72. doi: 10.1080/15402000902762303. [DOI] [PubMed] [Google Scholar]
- * (83).Jean-Louis G, Magai C, Casimir GJ, Zizi F, Moise F, McKenzie D, et al. Insomnia symptoms in a multiethnic sample of American women. J Womens Health (Larchmt) 2008;17:15–25. doi: 10.1089/jwh.2006.0310. [DOI] [PubMed] [Google Scholar]
- (84).Jean-Louis G, Magai C, Consedine NS, Pierre-Louis J, Zizi F, Casimir GJ, et al. Insomnia symptoms and repressive coping in a sample of older Black and White women. BMC Womens Health. 2007;7:1. doi: 10.1186/1472-6874-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Pigeon WR, Heffner K, Duberstein P, Fiscella K, Moynihan J, Chapman BP. Elevated sleep disturbance among blacks in an urban family medicine practice. J Am Board Fam Med. 2011;24:161–8. doi: 10.3122/jabfm.2011.02.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Knutson KL. Association between sleep duration and body size differs among three Hispanic groups. Am J Hum Biol. 2011;23:138–41. doi: 10.1002/ajhb.21108. [DOI] [PubMed] [Google Scholar]
- (87).Frieden T. The Health of Immigrants in New York City: A Report from the New York City Department of Health and Mental Hygiene. 2006. [Google Scholar]
- (88).Jean-Louis G, Magai C, Cohen CI, Zizi F, von Gizycki H, DiPalma J, et al. Ethnic differences in reported sleep problems in older adults. Sleep. 2001;24:926–33. doi: 10.1093/sleep/24.8.926. [DOI] [PubMed] [Google Scholar]
- (89).Hendley Y, Zhao L, Coverson DL, Din-Dzietham R, Morris A, Quyyumi AA, et al. Exploring Differences in Weight Perception among Blacks and Whites. J Womens Health (Larchmt) 2011;20:1805–11. doi: 10.1089/jwh.2010.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Buster KJ, You Z, Fouad M, Elmets C. Skin cancer risk perceptions: A comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2011;66:771–9. doi: 10.1016/j.jaad.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Knight BG, McCallum TJ. Heart rate reactivity and depression in African-American and white dementia caregivers: reporting bias or positve coping? Aging and Mental Health. 1998;2:212–21. [Google Scholar]
- (92).Haley WE, Roth DL, Coleton MI, Ford GR, West CA, Collins RP, et al. Appraisal, coping, and social support as mediators of well-being in black and white family caregivers of patients with Alzheimer's disease. J Consult Clin Psychol. 1996;64:121–9. doi: 10.1037//0022-006x.64.1.121. [DOI] [PubMed] [Google Scholar]
- (93).Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- (94).Redline S, Tishler P, Hans M, Tosteson T, Strohl K, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- (95).Jean-Louis G, von GH, Zizi F, Dharawat A, Lazar JM, Brown CD. Evaluation of sleep apnea in a sample of black patients. J Clin Sleep Med. 2008;4:421–5. [PMC free article] [PubMed] [Google Scholar]
- (96).Shulruf B, Hattie J, Dixon R. Factors affecting responses to Likert type questionnaires: introduction of the ImpExp, a new comprehensive model. Soc Psychol Educ. 2008;111:59–78. [Google Scholar]
- (97).Breslau J, Javaras KN, Blacker D, Murphy JM, Normand SL. Differential item functioning between ethnic groups in the epidemiological assessment of depression. J Nerv Ment Dis. 2008;196:297–306. doi: 10.1097/NMD.0b013e31816a490e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Kim G, Chiriboga DA, Jang Y. Cultural equivalence in depressive symptoms in older white, black, and Mexican-American adults. J Am Geriatr Soc. 2009;57:790–6. doi: 10.1111/j.1532-5415.2009.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]