Abstract
Objective
Catheterization to measure bladder sensitivity is aversive and hinders human participation in visceral sensory research. Therefore, we sought to characterize the reliability of sonographically-estimated female bladder sensory thresholds. To demonstrate this technique’s usefulness, we examined the effects of self-reported dysmenorrhea on bladder pain thresholds.
Methods
Bladder sensory threshold volumes were determined during provoked natural diuresis in 49 healthy women (mean age 24 ± 8) using three-dimensional ultrasound. Cystometric thresholds (Vfs – first sensation, Vfu – first urge, Vmt – maximum tolerance) were quantified and related to bladder urgency and pain. We estimated reliability (one-week retest and interrater). Self-reported menstrual pain was examined in relationship to bladder pain, urgency and volume thresholds.
Results
Average bladder sensory thresholds (mLs) were Vfs (160±100), Vfu (310±130), and Vmt (500±180). Interrater reliability ranged from 0.97–0.99. One-week retest reliability was Vmt = 0.76 (95% CI 0.64–0.88), Vfs = 0.62 (95% CI 0.44–0.80), and Vfu = 0.63, (95% CI 0.47–0.80). Bladder filling rate correlated with all thresholds (r = 0.53–0.64, p < 0.0001). Women with moderate to severe dysmenorrhea pain had increased bladder pain and urgency at Vfs and increased pain at Vfu (p’s < 0.05). In contrast, dysmenorrhea pain was unrelated to bladder capacity.
Discussion
Sonographic estimates of bladder sensory thresholds were reproducible and reliable. In these healthy volunteers, dysmenorrhea was associated with increased bladder pain and urgency during filling but unrelated to capacity. Plausibly, dysmenorrhea sufferers may exhibit enhanced visceral mechanosensitivity, increasing their risk to develop chronic bladder pain syndromes.
Keywords: cystometry, dysmenorrhea, bladder pain syndrome
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) and overactive bladder (OAB) affect approximately 15–20% of adult women in the U.S. 1, 2 Despite this high prevalence, our understanding of the underlying pathophysiology of these conditions is limited. Investigations surrounding the mechanisms responsible for increased bladder sensitivity would be useful for clarifying the primary causes of IC/BPS and OAB. However, it remains challenging to conduct large scale, longitudinal studies, particularly in patients with pelvic pain, using the conventional method of retrograde bladder cystometry 3 because catheterization is uncomfortable and can cause bladder infections. Furthermore, these evaluations may not accurately reflect bladder function in that they impose a retrograde source of stimulation onto the bladder’s afferent nerves and use higher filling rates than natural diuresis. Even worse, with IC/BPS patients who often have both enhanced sensory and pain thresholds in the urethra and bladder, there may be persistent discomfort or even pain flares induced during such diagnostic testing. Consequently, there is a need for a reliable, noninvasive and more physiologic method of quantifying visceral sensitivity in patients with abnormal bladder function.
Noninvasive bladder sensory testing would also be highly useful in screening asymptomatic, at-risk populations with latent bladder sensitivity. While the pathophysiology of IC/PBS remains enigmatic, several comorbid conditions are implicated as contributing factors. Most notably, 65% of women with chronic genitourinary pelvic pain also report moderate to severe menstrual pain. 4 Similarly endometriosis, closely associated with dysmenorrhea, increases the risk for IC/PBS by four fold. 5 Dysmenorrhea sufferers exhibit increased sensitivity to both somatic stimuli and to colorectal distension, suggesting that these findings might extend to other visceral pain conditions such as IC/PBS. 6, 7 In fact treating dysmenorrhea hormonally alleviates organ-specific pain in IBS and urinary calculosis. 8 Thus, to better understand the mechanism of heightened visceral sensitivity, studies are needed to determine if dysmenorrhea specifically produces alterations in bladder nociception, mechanoreception, or capacity.
The reliability and reproducibility of two- and three-dimensional ultrasound has been previously studied to some degree for measuring bladder volumes (predominantly to assess post-void residual), with three-dimensional techniques clearly more accurate. 9–11 We recognized this volume measurement technique could be modified into a noninvasive visceral pain test, with serial measurements of increasing bladder volume coupled with simultaneous acquisition of repeated report of relative bladder pressure and pain. Many prior mechanistic studies of bladder pain patients using cystometry have been fairly small, pointing out the need for broadly acceptable visceral pain measures.12, 13 In this project, we sought to estimate the validity and reliability of a more physiologic method for determining bladder sensory thresholds: three-dimensional transabdominal sonographic measurement of bladder volumes. The additional information regarding bladder nociception, mechanoreception, and capacity was subsequently used to examine the mechanism by which dysmenorrhea might increase bladder pain sensitivity.
Materials and Methods
Subjects
The primary aim of this study was to estimate physiological bladder sensory thresholds with three-dimensional transabdominal ultrasound and determine the validity and reliability of these measures. All study procedures were conducted between June and September 2009. Eligible women had to be 18 years of age or older and generally healthy. Exclusion criteria included pregnancy, an active bladder infection or suspected infection without a recent medical evaluation, Stage III or greater bladder prolapse (defined as the patient able to feel her vagina prolapsing outside of the vaginal opening in a resting state), and ongoing use of diuretic medications. Participants were recruited using Internet and paper-based community advertisement boards. Informed consent was obtained from all participants. The NorthShore University HealthSystem Institutional Review Board approved this study.
Overall protocol
An example timeline is shown in Figure 1 and general description of the protocol sequence is described in the figure legend. Prior to the visit, participants were asked to hydrate with 12 ounces of water one hour before the visit and abstain from caffeine the day of testing. After reading and signing the consent form, participants completed a brief demographic and general health questionnaire. Menstrual cycle phase was estimated based on self-reported last menstrual period, as prior studies have shown a relationship between cycle phase and self-reported visceral sensitivity. 14 Next, they emptied their bladders and received a baseline ultrasound scan in supine position. Following this initial scan, participants were asked to drink 20 ounces of water within 5 minutes to further encourage diuresis. They began observation seated in a private room, and were offered Sudoku puzzles and popular magazines to peruse while waiting. They were instructed not to do other tasks, such as make phone calls. Participants received a list of verbal descriptors of the three levels of bladder urgency, first sensation (Vfs), first urge (Vfu), and maximum tolerance (Vmt), to permit consistent awareness and recognition of reaching each level of relative discomfort throughout the study session. Definitions were as follows: 1) First sensation – When riding in a car, the driver pulls over to a rest stop to urinate, you would go as well, 2.) first urge- When riding in a car, you would initiate the request to find a rest stop to urinate, 3.) maximum tolerance - When riding in a car, you would urinate on the side of the road in bumper to bumper traffic. These detailed descriptors were used on the recommendation of our urogynecology clinicians, as they employ in clinical practice (Dr. Peter Sand, personal communication, November 2011). These descriptor variables operationalize the International Continence Society’s published definitions. 15 At each of these three descriptor thresholds a volumetric scan was performed. A prior study estimated these thresholds in healthy women as Vfs = 180 mL, Vfu = 270 mL, and Vmt = 430 mL, and similar ranges are used widely in clinical practice. 16 When participants reached each descriptor, they rated their level of pain and urgency using a 10 cm visual analogue scale (VAS). The urgency scale was anchored at opposite ends with the descriptors “no urgency” and “worst urgency imaginable.” Similarly, the pain scale was anchored at opposite ends with the descriptors “no pain” and “worst pain imaginable.” Additionally, every 15 minutes from the time that participants finished drinking the priming dose of water, they were instructed to evaluate their current level of pain and urgency using the same VAS measures. If a participant did not reach maximum tolerance at 45 and 60 minutes, respectively, she was asked to drink an additional ten ounces of water (maximum of twenty additional ounces) to encourage diuresis. The study was capped at two hours. All bladder scans were performed in a consistent manner by the sonographer (DS). The participants repeated this procedure 7–28 days after the first visit in order to establish within-individual stability of the bladder sensory thresholds. Scheduling was based on availability and subjects could participate at any point during their menstrual cycle for each visit.
Figure 1. Example timeline of bladder testing protocol.
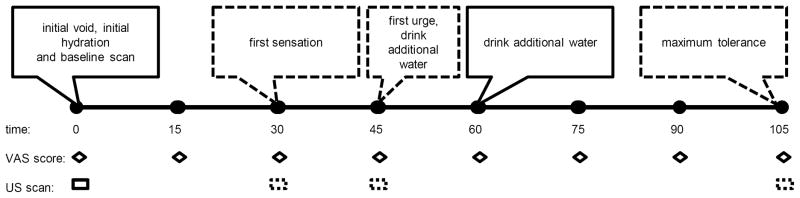
The events fixed in 15 minute intervals are shown in solid lines. Ultrasound scans dependent on subject descriptors of bladder capacity are shown in dashed lines. At the beginning of the study, all of the subjects voided, began hydration and underwent a baseline scan. Every 15 minutes subsequently, subjects indicated urgency and pain on a VAS scale. Once the subject had attained first sensation of bladder distension, an ultrasound scan was performed. Additional scans were performed as the subjects reached the prespecified, verbally anchored cystometric thresholds. If study was not completed by 45 and 60 minutes, the subject was instructed to drink an additional 10 oz of water at each point. The duration of the study was capped at 120 minutes.
Volume measurements
Bladder volume was measured using a Voluson 730 three-dimensional transabdominal 5.0 MHz ultrasound transducer (GE Healthcare, Wauwatosa, WI). The transducer was applied in a coronal axis against the abdomen just above the symphysis pubis. Measurement of bladder volumes was performed using the scanner’s onboard Virtual Organ Computer-aided Analysis (VOCAL™) software. First, the rendered image encompassing the bladder was sectioned into six planes separated by 30°. The perimeter of the bladder on each section was electronically outlined using a computer trackball, and the total three-dimensional bladder volume reconstructed automatically from the collective tracings. For any boundary of the bladder that fell outside the transducer’s capture window, we measured that boundary at the edge of the capture window. Techniques and criteria for defining the bladder boundary using the onboard software were determined and agreed upon through discussion between the PI and sonographer and practiced in pilot studies. Research assistants were then trained on these methods, and selected scans were reviewed throughout the study for quality checks. To test interrater reliability, sixteen sets of patient measurements were completed by both research assistants (AE, AM) blinded to each other’s measurements. Once interrater reliability had been determined, the measurements used for the final descriptive analysis were chosen from a single set of measurements done by either the first author or one research assistant (AE).
Statistical analysis
We performed a univariate analysis of data that included means, medians, and standard deviations. Normality of data was confirmed by the Shapiro-Wilks test and visual inspection. Bivariate comparisons for mean bladder volumes and potential predictors was performed for age, race, BMI, menstrual cycle phase, urine production rate, and the serially reported VAS scores for pain and sensory urgency using t-tests, ANOVA, and the Kruskal-Wallis test as appropriate. We used intraclass correlations (ICC’s) to assess the reliability of repeat bladder volume measurement between sessions and between examiners, and report 95% confidence intervals to estimate retest reliability (one month). Calculations were performed either using Microsoft Excel XP (Richmond, VA) or STATA version 11.0 (College Station, TX).
Because validation of the sonographic estimates could not be estimated from the initial dataset, it was characterized subsequently in a different, prospectively recruited set of participants. This group of women were involved in a separate, IRB-approved prospective study of bladder sensitivity and pelvic pain. This cohort contained healthy controls (n=8), subjects with chronic pelvic pain (n=3) and subjects with painful bladder syndrome (n=5). In this subset 63% were Caucasian while 32% were African American (1 subject did not provide race information), the average age was 31 years [standard deviation (SD) 8] and BMI was 28 (SD 8). They voided at perceived maximal capacity into a calibrated container immediately after undergoing sonographic estimation using the same technique. Weighed urine volume was then compared with the sonographic estimates.
To demonstrate the utility of this novel measure, we also assessed the relationship between self-reported menstrual pain and these bladder sensitivity measures in the original cohort. Subjects were asked to “indicate with one, single tick mark the amount of pain you have with your average period” on a 10 cm VAS scale with the anchors “No pain” and “Worst pain imaginable.” The specific effect of self-reported menstrual pain on bladder pain, urgency and capacity was evaluated at the previously described time points (first sensation, first urge, and maximum tolerance). One subject who was menopausal was excluded from this analysis. For each participant the average bladder pain, urgency and capacity was calculated at each threshold. To isolate the effects of above-average and moderate-severe menstrual pain, respectively, the subjects were divided into quartiles. ANOVA and Kruskal-Wallis tests as appropriate were performed on the composite data. Multiple comparisons tests were performed with the appropriate Holm-Sidak’s (parametric) or Dunn’s (non-parametric) method to identify whether an individual quartile was significantly affected by pain compared to the reference group of women experiencing below average menstrual pain (the 25–49% quartile).
Results
Sonographic bladder measurements are attractive to volunteers
A total of 49 participants were enrolled with an average age of 24 years (SD = 8). Two-thirds were Caucasian, and the vast majority had normal BMI (Table 1). The majority of the subjects (65%) were young (<21 yo) students from a nearby university. Subjects were asked regarding current medications and 39% subjects reported using oral contraceptives. Subjects also reported taking antidepressants (8%), anticonvulsants (6%), or antianxiety medications (6%). None of the subjects reported usage of pain medications or other medication suggestive of serious systemic disease other than insulin (n=1) or atorvastatin (n=1). The participants reported minimal bladder pain at baseline; using the first 15 minute assessment after water ingestion as a measure for initial bladder pain, only 3/49 women rated their pain VAS > 1 (maximum 4.4), and among the 10 women who were on their menses during the first visit, all women reported VAS less than 0.5/10. The menstrual phase at time of study varied with the luteal phase being most frequent (47%); 20% presented during their menses, and 25% in the follicular phase (8% could not be assigned a category). Only one participant was menopausal (2%). Age, race, BMI, and menstrual cycle phase had no effect on any of the mean bladder thresholds, self-rated pain, or self-rated urgency (p > 0.05 for all volumes). Forty-seven participants returned for follow-up visits within a month, with the majority occurring within two weeks (90%). One subject was unreachable, and another could not physically return within the month. While acceptability was not systemically surveyed, none of the subjects requested to stop participation in this study.
Table 1.
Participant demographics for sonographic cystometry validation
| Characteristic* | Subjects |
|---|---|
|
| |
| Age | 24.2 (8.2) |
| Race | |
| Caucasian | 32/49 (65.3%) |
| Asian | 13/49 (26.5%) |
| BMI kg/m2 (median, 25–75%) | 22.86 (20.1, 22.9) |
| Dysmenorrhea rating (0–10, VAS) | 2.76 (2.3) |
| Point in cycle visit 1, (n = 49) | |
| Unsure (hormonal suppression/menopausal) | 4/49 (8.2%) |
| Menses (day 0–5) | 10/49 (20.4%) |
| Follicular (day 6–14) | 12/49 (24.5%) |
| Luteal (day 15–35) | 23/49 (46.9%) |
| Point in cycle visit 2, (n = 47) | |
| Unsure (hormonal suppression/menopausal) | 6/47 (12.8%) |
| Menses (day 0–5) | 9/47 (19.1%) |
| Follicular (day 6–14) | 14/47 (29.8%) |
| Luteal (day 15–35) | 18/47 (38.3%) |
| Reached MT < 2 hours, Visit 1 | 42/49 (85.7%) |
| Reached MT < 2 hours, Visit 2 | 43/47 (91.5%) |
results display means (standard deviations) or count (percentages), BMI = body mass index, VAS = Visual Analog Scale, MT = maximum tolerance
Sonographic measurement is reliable and reproducible
The vast majority of participants reached maximum tolerance before the two hour mark (86% at first visit and 91% at second visit). Mean time (± SD) to first sensation, first urge and maximum tolerance was 35 ± 16, 59 ± 20, and 84 ± 24 minutes, respectively. Estimated average bladder filling rates (calculated from post-void to each sensory threshold) for the first visit increased over time (Vfs = 4.4 ± 2.5, Vfu = 5.5 ± 2.2, and Vmt = 6.0 ± 1.8 mL/min, respectively) and were very similar at the second visit. Mean bladder volumes (mL) from each subject’s first visit included: Vfs (160 ± 100), Vfu (310 ± 130), and Vmt (500 ± 180); results were very similar at the second visit. No significant post-void residual (PVR) was noted (mean PVR = 10 ± 10 mL, range 0–50 mL) based on the measurements obtained at the start of the study. The complete results are listed in Table 2. One-week retest reliability was ICC = 0.76 for Vmt (95% CI 0.64–0.88); values were lower for Vfs (0.62, 95% CI 0.44–0.80) and Vfu (0.63, 95% CI 0.47–0.80). Interrater reliability ranged from 0.99–1.0 for sixteen overlapping measurements between two research assistants (AE, AM). Age, race and BMI were not associated with sensory thresholds. Bladder filling rate was shown to positively correlate with all bladder sensory thresholds: Vfs r = 0.53, Vfu r = 0.59, Vmt r = 0.64 (p < 0.0001 for all).
Table 2.
Bladder volume, urgency scores, and pain scores reported at each bladder sensory threshold
| Post-void* | First Sensation (FS) | First Urge (FU) | Maximum Tolerance (MT)† | |
|---|---|---|---|---|
| Visit 1, n = 49 | ||||
|
| ||||
| Volumes mL x ± SD | 11 ± 12.53 | 157.33 ± 96.48 | 314.85 ± 130.16 | 501.49 ± 179.48 |
| VAS Urgency (median, 25–75%) | 2.00 (1.1, 2.6) | 5.02 (4.5, 6) | 8.57 (8.1, 9.4) | |
| VAS Pain (median, 25–75%) | 0.34 (0, 0.4) | 1.38 (0, 1.9) | 3.61 (0.4, 6.4) | |
|
| ||||
| Visit 2, n = 47 | ||||
|
| ||||
| Volumes mL x ± SD | 10.37 ± 15.03 | 163.74 ± 114.04 | 306.52 ± 137.21 | 479.89 ± 185.75 |
| VAS Urgency (median, 25–75%) | 2.03 (1.4, 2.4) | 4.67 (4.1, 5.6) | 8.39 (7.7, 9.5) | |
| VAS Pain (median, 25–75%) | 0.66 (0, 0.6) | 1.41 (0, 2.7) | 3.43 (0.3, 7.2) | |
Pain and urgency were not assessed at baseline;
maximum tolerance includes women who reached two hours without reaching maximum tolerance
Self-reported urgency and pain correlates with calculated bladder volume
Self-reported pain and urgency scores, using VAS, positively correlated with sensory thresholds (pain, r = 0.25, urgency = 0.65; both p < 0.0001). Predictably, pain and urgency scores steadily increased as the bladder filled (Table 2). Neither age nor menstrual phase predicted sensory thresholds. Bladder filling rate was positively associated with all sensory thresholds as seen in Figure 3 [using rate calculated from post-void to maximum tolerance, Vfs r = 0.47 (95% CI 0.21–0.67), Vfu r = 0.59 (95% CI 0.37 – 0.75), Vmt r = 0.66 (95% CI 0.46–0.79), all p-values < 0.0001]. This was similar regardless of which interval (up to first sensation or first urge) was used to calculate rate. We examined correlations between estimated bladder filling rate (based on maximal tolerance interval) and self-reported pain at all sensory thresholds and did not find any significant relationships (r = 0.03–0.10). Additionally, we performed a regression analysis between urgency VAS and pain VAS across all 15 minute intervals, to provide a method of sensory examination independent of threshold criterion. Self-report of urgency was correlated to pain report (r = 0.54, p < 0.001).
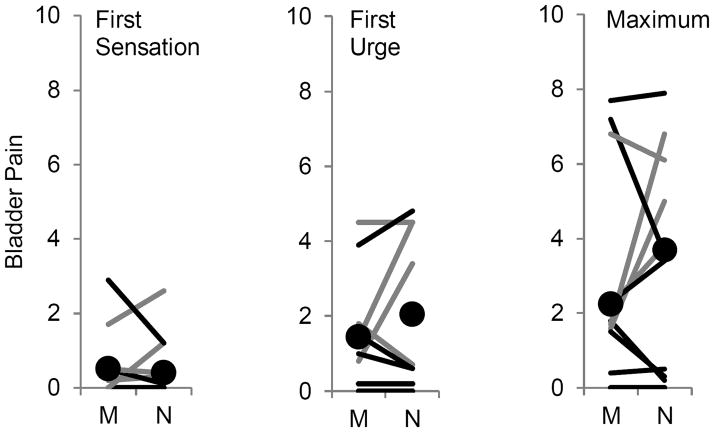
Figure 3.
Within-subjects examination of bladder pain during menses
Legend: Gray lines represent subjects with severe menstrual pain (upper quartile) and black lines represent the rest of subjects in which it was possible to obtain data measurements during menses (M) and at another portion of their menstrual cycle (N). Black circles indicate median results from subjects reporting pain during diuresis during first sensation, first urge and maximum tolerance.
Voided volumes correlate with calculated bladder volume
To validate the accuracy of these sonographically measured volumes, particularly because a subset of bladder cross-sections had to be estimated at the transducer window limit, we performed additional post-hoc analyses. As described above sixteen participants from an ongoing bladder pain evaluation study (healthy controls and women with IC/BPS or chronic pelvic pain) agreed to void into a urine collection canister upon reaching maximum bladder capacity. They all underwent sonographic estimate of bladder volume immediately prior to voiding as part of the overall study. One woman with >100 mL post void residual was excluded from analysis; the remaining 15 had minimal post void residuals (maximum 50 mL, mean PVR 10 ± 20 mL). Estimated maximal capacity was 410 ± 160 mL, while actual voided volume was 460 ± 180 mL, and voided volume ranged from 60–700 mL. Sonographically measured volumes underestimated actual voided volume by an average of 70 ± 30 mL. Underestimation was reduced at actual volumes below 400 mL (on average 50 mL at true volumes < 400 ml vs. 70 mL for true volumes > 400 mL). The estimated and actual volumes over this broad range were highly correlated (R2 = .94, p < 0.0001).
Severity of dysmenorrhea increases bladder pain during bladder distension
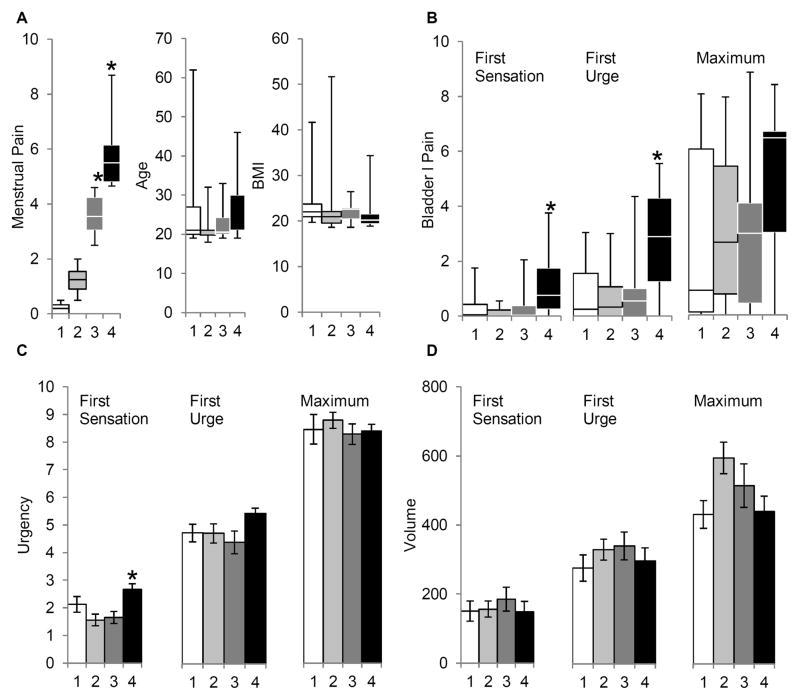
To evaluate the effect of menstrual pain on bladder pain sensitivity, the participants in the main study were divided into quartiles based on their self-reported level of menstrual pain. We verified the primary difference between these quartiles was the self-reported level of menstrual pain (Fig 2a, p < 0.001) and not age or BMI (p > 0.05). While most women in the bottom three quartiles of menstrual pain reported no bladder pain at first sensation, most subjects in the highest quartile reported significant bladder pain at first sensation (Fig 2b, p <0.05). Similarly, at first urge to void threshold, most women with moderate or more severe menstrual pain (pain above VAS 4.5 cm) were reporting a VAS bladder pain score of 3 cm or higher. In contrast the majority of subjects in the other three quartiles were reporting minimal bladder pain. ANOVA (p < 0.05) and post-hoc tests (p < 0.05) confirmed that women in the highest menstrual pain quartile reported elevated bladder pain at first urge. On the other hand, most women reported some degree of bladder pain at the maximum tolerance threshold. At this suprathreshold bladder distension level, no significant effect of menstrual pain on reported bladder pain was observed.
Figure 2. Dysmenorrhea is associated with increased bladder pain during natural diuresis.
Top: Box-plots (minimum, bottom quartile, median, top quartile, maximum) are shown for data that does not follow a normal distribution. A: Demographics of individual quartiles with differing levels of self-reported menstrual pain. B: Reports of bladder pain at first sensation, first urge and maximum tolerance in each individual quartile. Bottom: Bar graphs (average and standard error of the mean) are shown for C: urgency and D: sonographically measured volume at each bladder threshold. Menstrual pain was significant as a factor in bladder pain-first sensation & urge, and in bladder urgency-first sensation (p’s < 0.05). * indicates p <0.05 significant post-hoc difference compared to 2nd quartile.
To explain the mechanism underlying increased bladder pain in women with dysmenorrhea, the effects of menstrual pain on self-reported urgency and capacity were characterized. Notably, subjects in the most severe dysmenorrhea quartile had increased reported urgency (Figure 2c, p < 0.05), but no significant difference in bladder capacity at first sensation (Figure 2d). Urgency or capacity was unrelated to dysmenorrhea status at higher bladder sensory thresholds. In summary, these findings show moderate-to-severe menstrual pain in a healthy population is associated with a slight increase in reported pain and urgency at first sensation and pain at first urge without any measurable affect on bladder compliance.
To establish whether increased pain report during bladder filling in subjects with dysmenorrhea was influenced by being on their menses at the actual visit, the effects of menstrual cycle phase were examined. Twelve subjects were identified that participated during both menses and another portion of their menstrual cycle, permitting a within subjects analysis (Figure 3). Although the difference between self-reported bladder pain during menses and at other portions of the menstrual cycle are not statistically significant (p’s >0.4), subjects most often reported less pain during diuresis in their menstrual phase. To verify these are consistent with entire dataset, we performed multivariable regression analysis across self-reported pain intervals against the factors menstrual cycle phase, menstrual pain and urgency (r = 0.57, p <0.0001). Whereas menstrual cycle phase was not a contributing factor (β = −0.05 ± 0.9, p = 0.60), menstrual pain (β = 0.13 ± 0.03, p <0.0001) and urgency (β = 0.33 ± 0.02, p <0.0001) were significant factors. These findings refute the idea that increased pain during bladder filling occurs particularly during menstruation, and imply that severe dysmenorrhea is correlated with alterations in bladder pain sensitivity throughout the menstrual cycle.
Discussion
Three-dimensional transabdominal ultrasound yields fairly accurate and reliable measure of bladder volumes at the thresholds of first sensation, first urge, and maximum tolerance. The mean volume measurements for each bladder sensory threshold resemble those determined by conventional cystometry, providing face validity for the technique. Compared to Wyndaele and De Wachter’s healthy volunteer study measured with cystometry, we found average thresholds to be slightly higher, and accounting for the sonographic underestimate of about 70 mL compared to actual volume we observed, noninvasive measurement of maximal bladder capacity likely is closer to 450–500 mL compared to the 429 mL they reported. 16 The one-week retest reliability results support temporal stability of bladder sensory thresholds and resemble the results reported by Broekhuis and colleagues using conventional cystometry. 17
Natural diuresis is more physiologically relevant for measuring pain thresholds than cystometry
We observed that the physiological bladder filling rate correlated with bladder sensory volumes. This supports a process of accommodation as noted by De Wachter et al, who looked at the effects of different cystometric filling rates on bladder afferent nerve firing in rats.18 Increased filling rate was associated with an increase in the activation pressure threshold for bladder afferents. Notably, this is the opposite of findings at supraphysiological filling rates used in retrograde cystometry. The underlying neural activation at bladder filling rates of 50–100 mL/min compared to 2–5 mL/min is likely fundamentally different, explaining this discrepancy. One interesting hypothesis worth pursuing from this observation is that individuals with chronicially lower urine production rates could be at higher risk for subsequent development of sensory urgency or bladder pain.
Increased nociceptive mechanosensation in dysmenorrhea could explain its role as a risk factor in IC/PBS
In our initial application of this methodology, we found that women with high comorbid menstrual pain exhibit visceral allodynia, as they report bladder pain at physiologically innocuous distension volumes. This finding is consistent with other studies showing viscero-visceral organ cross-sensitization and associations between dysmenorrhea and IC/PBS. 4, 8 What is interesting about this is none of these women reported clinical symptoms of bladder dysfunction. Conceivably women with dysmenorrhea who experience repeated bouts of cross-organ inflammation in the bladder adjacent to the uterus may eventually be at higher risk for IC/PBS, resembling findings by Winnard and colleagues in an animal uterine pain model. 19 This chronic exposure to uterine inflammation could produce bladder allodynia via the repeatedly demonstrated process in other pain models in which an adjacent tissue insult sensitizes mechanosensitive A fibers as well as increases convergence of A fibers onto C fibers.20–24 Specifically, the alterations in mechanosensitivity could be produced by an increase in TRPV expression in the inferior hypogastric nerve which projects to both the bladder and uterus. 25 TRPV channel expression is correlated with menstrual pain in humans 26, 27, and TRPV expression increases bladder afferent sensitivity in mice.28 However, it is quite likely that other ion channels such as TRPA are also contributing to increased visceral sensitivity simultaneously 29, but have not yet been fully characterized in bladder pain models.
Another possible explanation at the systems level is that alterations in descending inhibition are impaired in subjects with dysmenorrhea. Visceral distension is often sufficient to inhibit nociception in animals at other sites via the descending modulatory pathway. 30, 31 Indeed, rapid ingestion of large volumes of water in humans sufficient enough to produce stomach distension inhibits pain perception.32 Compared with that prior study, our water loading is substantially smaller and over a more extended period (1500 ml vs 600–1200 ml), but it is possible that bladder distension in healthy subjects is also capable of activating the descending modulatory pathway. In subjects with dysmenorrhea, accumulating evidence suggests that descending inhibition of nociception is impaired. 6, 33 Whereas women without dysmenorrhea significantly engage the periaqueductal gray during pain perception, a critical site in descending modulation, women with dysmenorrhea do not. 34 Although less likely, an alternative explanation would be that psychological factors such catastrophization could be escalating these women’s general perception of visceral pain. Indeed catastrophization, and factors associated with it such as anxiety and depression are associated with more severe menstrual pain. 35–37 From a prevention standpoint for chronic pelvic pain, this finding deserves further investigation.
Limitations and technical issues
It is possible that the specific wording descriptors regarding traffic scenarios contributed to the slightly higher thresholds obtained in this study and future studies should carefully consider the effects of urgency descriptors on bladder capacity measurements. This study also excluded women with vaginal prolapse and this group will need further evaluation in future studies, although for studying bladder pain there seems to be little overlap between these conditions. Likewise, this approach needs to be tested in men, where its noninvasive profile may prove an even more attractive alternative to catheterization of the longer male urethra. Other sources of possible error include distortion of the bladder image by the abdominal wall, which might obscure precise delineation of the vesico-uterine ultrasound interface. Our ongoing study is recruiting women with a broader range of BMIs which should address the issue of generalizability. For the measurements of maximum tolerance, estimates of the volume were necessitated by the boundary of the bladder extending past the scanned region. For studies of impaired bladder sensation this is less of an issue, as patients can be clearly confirmed as having a “normal” bladder capacity as long as it exceeds 400–600 mL. Our primary interest in developing this technique was to help profile IC/BPS patients by relative bladder sensitivity; at the low volumes of sensitivity typically seen in that population (<250 mL capacity), these measures perform well with only an average underestimate of 50 mL. In clinical settings where time constraints exist, clinicians might be able to also limit the evaluation to first urge (which was reached in 74 minutes by 75% of our participants), and have patients simply assess maximum capacity by voiding at home.
We considered using direct catheterized volume measurement to validate the calculated volumes, but considerations for participant comfort led us to use voided estimates in normally voiding participants as an alternative preliminary validation. Our results show high correlation between the methods and acceptable clinical accuracy, particularly with the difference in volumes being < 50 mL when actual volume is below 400 mL. Retrograde cystometry remains a well-studied tool for voiding dysfunction, but in women with sensory disorders, particularly bladder pain, this technique should be attractive as a noninvasive tool, as the range of clinically relevant assessment should fall within this range. As we have demonstrated with our dysmenorrhea substudy, this methodology could be particularly useful to characterize preclinical visceral hyperalgesia that may evolve into a chronic pelvic pain state. Potential factors of interest testable with this methodology might include manipulating external distractions, diet, and emotional priming to see changes in visceral sensitivity. The high interrater reliability supports easy adoption by other visceral pain researchers of our cystometric measurement technique, and three-dimensional scanners are readily available in medical centers. Our naïve research assistants were able to complete these training sets by reviewing the stored digital images within a couple of hours, spread over a month, despite no prior familiarity with the ultrasound equipment. Using the ultrasound machine’s on-board VOCAL software, they were able to retrace the images in 2–3 minutes per set, using the archived images from previously evaluated participants. Although automated systems exist, they may misjudge female organs as part of the bladder and will ultimately require comparison with our manual approach to ensure optimal precision. Prior studies have shown that differences in volume of over 100 mL occur between true and estimated volume using the portable display-free methods. Moreover, they have been tested in a cohort of older male and female patients (mean age 70), where the confounding effect of a normal sized uterus is likely less apparent than the typical pelvic and IC/PBS sufferers who are of reproductive age. 9
In summary, these findings demonstrate the reliability and utility of this nociceptive diagnostic paradigm which has intriguing applications to the study of visceral pain and cross-organ sensitization. Women with moderate to severe dysmenorrhea report increased bladder urgency and noxious perception at low thresholds. Future work clarifying the physiological pathway responsible for visceral pain perception could utilize this model.
Acknowledgments
Sources of funding: The work was supported by NIH grant 5K23HD054645 to Dr. Tu. Dr. Hellman is supported by a Research Career Development Award from the NorthShore University HealthSystem Research Institute. Ms. Epstein’s time was supported by a Weinberg Undergraduate Summer Research Grant from Northwestern University. Ms. Melnyk’s effort was supported by the NorthShore University HealthSystem Research Institute Summer Fellows Program.
Footnotes
Disclosure: The authors report no conflict of interest.
Presented at the 59th American Congress of Obstetricians and Gynecologists Annual Clinical Meeting (San Francisco, CA 5/15/10), the 18th annual meeting of the International Pelvic Pain Society (Chicago, IL 10/22/10), and the 14th World Congress on Pain (International Association for the Study of Pain, Milan 08/30/12)
References
- 1.Clemens JQ, Meenan RT, O’Keeffe Rosetti MC, et al. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol. 2005;174:576–80. doi: 10.1097/01.ju.0000165170.43617.be. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–36. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 3.Kelly CE, Krane RJ. Current concepts and controversies in urodynamics. Curr Urol Rep. 2000;1:217–26. doi: 10.1007/s11934-000-0022-4. [DOI] [PubMed] [Google Scholar]
- 4.Zondervan KT, Yudkin PL, Vessey MP, et al. Chronic pelvic pain in the community--symptoms, investigations, and diagnoses. Am J Obstet Gynecol. 2001;184:1149–55. doi: 10.1067/mob.2001.112904. [DOI] [PubMed] [Google Scholar]
- 5.Warren JW, Howard FM, Cross RK, et al. Antecedent nonbladder syndromes in case-control study of interstitial cystitis/painful bladder syndrome. Urology. 2009;73:52–7. doi: 10.1016/j.urology.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj P, Bajaj P, Madsen H, et al. A comparison of modality-specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. Clin J Pain. 2002;18:180–90. doi: 10.1097/00002508-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Brinkert W, Dimcevski G, Arendt-Nielsen L, et al. Dysmenorrhoea is associated with hypersensitivity in the sigmoid colon and rectum. Pain. 2007 doi: 10.1016/j.pain.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Giamberardino MA, Costantini R, Affaitati G, et al. Viscero-visceral hyperalgesia: characterization in different clinical models. Pain. 2010;151:307–22. doi: 10.1016/j.pain.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Marks LS, Dorey FJ, Macairan ML, et al. Three-dimensional ultrasound device for rapid determination of bladder volume. Urology. 1997;50:341–8. doi: 10.1016/S0090-4295(97)00293-8. [DOI] [PubMed] [Google Scholar]
- 10.Farrell T, Leslie JR, Chien PF, et al. The reliability and validity of three dimensional ultrasound volumetric measurements using an in vitro balloon and in vivo uterine model. BJOG. 2001;108:573–82. doi: 10.1111/j.1471-0528.2001.00148.x. [DOI] [PubMed] [Google Scholar]
- 11.Byun SS, Kim HH, Lee E, et al. Accuracy of bladder volume determinations by ultrasonography: are they accurate over entire bladder volume range? Urology. 2003;62:656–60. doi: 10.1016/s0090-4295(03)00559-4. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald MP, Koch D, Senka J. Visceral and cutaneous sensory testing in patients with painful bladder syndrome. Neurourol Urodyn. 2005;24:627–32. doi: 10.1002/nau.20178. [DOI] [PubMed] [Google Scholar]
- 13.Ness TJ, Richter HE, Varner RE, et al. A psychophysical study of discomfort produced by repeated filling of the urinary bladder. Pain. 1998;76:61–9. doi: 10.1016/s0304-3959(98)00023-2. [DOI] [PubMed] [Google Scholar]
- 14.Heitkemper MM, Cain KC, Jarrett ME, et al. Symptoms across the menstrual cycle in women with irritable bowel syndrome. Am J Gastroenterol. 2003;98:420–30. doi: 10.1111/j.1572-0241.2003.07233.x. [DOI] [PubMed] [Google Scholar]
- 15.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–26. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 16.Wyndaele JJ, De Wachter S. Cystometrical sensory data from a normal population: comparison of two groups of young healthy volunteers examined with 5 years interval. Eur Urol. 2002;42:34–8. doi: 10.1016/s0302-2838(02)00221-x. [DOI] [PubMed] [Google Scholar]
- 17.Broekhuis SR, Kluivers KB, Hendriks JC, et al. Reproducibility of same session repeated cystometry and pressure-flow studies in women with symptoms of urinary incontinence. Neurourol Urodyn. 2010;29:428–31. doi: 10.1002/nau.20783. [DOI] [PubMed] [Google Scholar]
- 18.De Wachter S, De Laet K, Wyndaele JJ. Does the cystometric filling rate affect the afferent bladder response pattern? A study on single fibre pelvic nerve afferents in the rat urinary bladder. Neurourol Urodyn. 2006;25:162–7. doi: 10.1002/nau.20157. [DOI] [PubMed] [Google Scholar]
- 19.Winnard KP, Dmitrieva N, Berkley KJ. Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1592–601. doi: 10.1152/ajpregu.00455.2006. [DOI] [PubMed] [Google Scholar]
- 20.Woolf CJ, Shortland P, Sivilotti LG. Sensitization of high mechanothreshold superficial dorsal horn and flexor motor neurones following chemosensitive primary afferent activation. Pain. 1994;58:141–55. doi: 10.1016/0304-3959(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 21.Woolf CJ, King AE. Dynamic alterations in the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat spinal cord. J Neurosci. 1990;10:2717–26. doi: 10.1523/JNEUROSCI.10-08-02717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–8. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 23.Koerber HR, Mirnics K, Kavookjian AM, et al. Ultrastructural analysis of ectopic synaptic boutons arising from peripherally regenerated primary afferent fibers. J Neurophysiol. 1999;81:1636–44. doi: 10.1152/jn.1999.81.4.1636. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99:244–53. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauroy B, Demondion X, Bizet B, et al. The female inferior hypogastric (= pelvic) plexus: anatomical and radiological description of the plexus and its afferences--applications to pelvic surgery. Surg Radiol Anat. 2007;29:55–66. doi: 10.1007/s00276-006-0171-3. [DOI] [PubMed] [Google Scholar]
- 26.Rocha MG, e Silva JC, Ribeiro da Silva A, et al. TRPV1 expression on peritoneal endometriosis foci is associated with chronic pelvic pain. Reprod Sci. 2011;18:511–5. doi: 10.1177/1933719110391279. [DOI] [PubMed] [Google Scholar]
- 27.Nie J, Liu X, Guo SW. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am J Obstet Gynecol. 2010;202:346, e1–8. doi: 10.1016/j.ajog.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 28.Daly D, Rong W, Chess-Williams R, et al. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2007;583:663–74. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackshaw LA, Brierley SM, Hughes PA. TRP channels: new targets for visceral pain. Gut. 2010;59:126–35. doi: 10.1136/gut.2009.179523. [DOI] [PubMed] [Google Scholar]
- 30.Brink TS, Hellman KM, Lambert AM, et al. Raphe magnus neurons help protect reactions to visceral pain from interruption by cutaneous pain. J Neurophysiol. 2006;96:3423–32. doi: 10.1152/jn.00793.2006. [DOI] [PubMed] [Google Scholar]
- 31.Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. II. Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexes. J Neurophysiol. 1991;66:29–39. doi: 10.1152/jn.1991.66.1.29. [DOI] [PubMed] [Google Scholar]
- 32.Sedan O, Sprecher E, Yarnitsky D. Vagal stomach afferents inhibit somatic pain perception. Pain. 2005;113:354–9. doi: 10.1016/j.pain.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Lundeberg T, Bondesson L, Lundstrom V. Relief of primary dysmenorrhea by transcutaneous electrical nerve stimulation. Acta Obstet Gynecol Scand. 1985;64:491–7. doi: 10.3109/00016348509156727. [DOI] [PubMed] [Google Scholar]
- 34.Vincent K, Warnaby C, Stagg CJ, et al. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain. 2011;152:1966–75. doi: 10.1016/j.pain.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 35.Walsh TM, LeBlanc L, McGrath PJ. Menstrual pain intensity, coping, and disability: the role of pain catastrophizing. Pain Med. 2003;4:352–61. doi: 10.1111/j.1526-4637.2003.03039.x. [DOI] [PubMed] [Google Scholar]
- 36.Latthe P, Mignini L, Gray R, et al. Factors predisposing women to chronic pelvic pain: systematic review. BMJ. 2006;332:749–55. doi: 10.1136/bmj.38748.697465.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silberg JL, Martin NG, Heath AC. Genetic and environmental factors in primary dysmenorrhea and its relationship to anxiety, depression, and neuroticism. Behav Genet. 1987;17:363–83. doi: 10.1007/BF01068137. [DOI] [PubMed] [Google Scholar]