Abstract
The factors contributing to osteonecrosis of the jaw (ONJ) with anti-remodeling drug treatment are unclear. Both epidemiological and experimental studies have suggested the combination of bisphosphonates and dexamethasone results in ONJ more often than either agent alone. The goal of this study was to assess the combination of these two drugs in a large animal model previously shown to be susceptible to exposed bone in the oral cavity when treated with bisphosphonates. Skeletally mature beagle dogs were either untreated controls, or treated with zoledronic acid (ZOL), dexamethasone (DEX), or ZOL + DEX. Both zoledronic acid and dexamethasone were given at doses based on those used in humans. All animals underwent single molar extraction at both 7 and 8 months following the start of the study. Extraction sites were obtained at month 9 for assessment of osseous healing using micro-computed tomography and histology. No animals were observed to have exposed bone following dental extraction yet one animal treated with ZOL and one with ZOL+DEX had severely disrupted extraction sites as viewed by CT and histology. These two animals had intense periosteal reaction that was less obvious but still present on all ZOL-treated animals and absent from untreated animals. There was no significant difference in bone volume within the socket among groups at either 4 or 8 weeks post-healing yet the surface/volume ratio was significantly higher in animals treated with ZOL+DEX at 8 weeks compared to control animals. These findings suggest a more complex pathophysiology to ONJ than is implied by previous epidemiological studies as well as those in rodents and raise questions about the potential role of dexamethasone in its etiology.
Keywords: Bisphosphonate, osteonecrosis of the jaw, osseous healing, dental extraction
INTRODUCTION
For nearly a decade the condition known as osteonecrosis of the jaw (ONJ) has been as a rare but significant issue in the field of oral and maxillofacial surgery. First thought to be specific to bisphosphonates, it is now recognized to be generalizable to potent remodeling suppression drugs including denosumab [1]. The majority of ONJ cases have manifested in cancer patients [2–4] with a smaller number of cases reported in patients treated for other skeletal conditions such as osteoporosis [4, 5]. Despite a low incidence rate [4, 6], ONJ remains a significant concern due to its unknown pathophysiology and the millions of patients who are currently taking anti-remodeling agents.
Two risk factors common to epidemiological studies are dental extraction and dexamethasone treatment. Although ONJ has been documented to occur spontaneously, it is most commonly associated with trauma induced by a dental procedure. When the incidence of ONJ is separately assessed for various populations treated with BPs (osteoporosis, Paget’s disease, and cancer treatment patients), there is a consistent 10-fold increase in ONJ when dental extraction was concomitant with BP treatment. Corticosteroid treatment has also been repeatedly suggested as a potential contributor to ONJ.
The notion of dental extraction and dexamethasone as risk factors for ONJ has been supported by pre-clinical research. Using various animal models, dental extraction has been shown to increase the incidence of exposed bone [7]. Combining dental extraction and dexamethasone in these models exacerbates the number of animals that develop exposed bone. Since the first report of an ONJ animal model in 2008 which developed a model using both bisphosphonate and dexamethasone [8], the majority of studies have continued to use the combination of these drugs, along with dental extraction, to study the condition. One potential limitation of the studies to date is that they all utilize rodents, animals that lack significant intracortical remodeling under normal conditions [9]. To increase the translatability of work in rodents it is important to confirm the findings in a model that more closely mimics human bone physiology by undergoing intracortical remodeling.
The goal of this study was to document how the combination of bisphosphonates and dexamethasone affect the mandibular bone tissue following dental extraction in a large animal model previously shown to be susceptible to ONJ. Specifically, we tested the hypothesis that zoledronic acid and dexamethasone would significantly increase the number of animals that develop ONJ, and significantly compromise extraction site healing, compared to either treatment alone.
MATERIALS AND METHODS
Animals
Twenty-four skeletally mature female beagles (~ 1–2 years old) were purchased from Marshall Farms USA (North Rose, NY) and housed throughout the experiment in environmentally controlled rooms at Indiana University School of Medicine’s AAALAC accredited facility. All animal procedures were approved prior to the study by the IU School of Medicine Animal Care and Use Committee.
Experimental Design
Following two weeks of acclimatization, animals were assigned to untreated control (CON; n=6), zoledronic acid (ZOL; n=6), dexamethasone (DEX; n=6) or zoledronic acid plus dexamethasone (ZOL+DEX; n=6) treatment groups. ZOL was administered via intravenous infusion at a dose of 0.06 mg/kg, which corresponds to the 4 mg dose used in cancer patients, adjusted on a mg/kg basis [10], and work done previously in our lab [11]. ZOL was infused every 2 weeks, roughly twice as frequently as used clinically in order to maximize drug exposure during the experimental period. IV administration (40 mL total volume over 15 minutes) was done using an over-the-needle catheter in the leg vein (rotated for each infusion between the cephalic and saphenous). Dexamethasone treatment consisted of daily oral dosing (5 mg) for the first seven days of the 7th, 8th, and 9th months of the experiment. This dosing was based on a modified version of a low- dose protocol used clinically for multiple myeloma patients [12].
Eight months after the first ZOL dose, all animals underwent dental extraction of the 4th right premolar; one month later (start of the 9th month) all animals underwent extraction of the 4th left premolar. This design allows two different post-extraction times (four and eight weeks) to be studied in the same animals and matches those periods previously shown to detect treatment-related differences. Forth premolars were extracted as previously described [11]. Briefly, following pre-surgical antibiotics, animals were anesthetized, given an intra-oral regional block of the inferior alveolar nerve, and intubated with continuous isofluorane for the duration of the surgery. The epithelial attachment of the tooth was severed, the crown split down the middle and each of the two roots was removed individually by gripping forceps to the crown and then rocking back and forth in the buccal-lingual direction. In the case where the root was fractured during extraction the root was left intact and the socket was ignored for all analyses (Table 1). Following extraction the gingival tissue was closed using absorbable suture. Post-operative analgesia was given for 24 hours post-surgery.
Table 1.
Descriptive information from extraction sites
| Control | Dexamethasone | Zoledronate | Zoledronate + Dexamethasone |
|
|---|---|---|---|---|
| Root fractures | 2 of 24 | 0 of 24 | 4 of 24 | 4 of 24 |
| Periosteal reaction 4 wk | 0 of 6 | 0 of 6 | 3 of 6 | 5 of 6 |
| Periosteal reaction 8 wk | 0 of 6 | 0 of 6 | 4 of 6 | 3 of 6 |
All animals were administered calcein (5 mg/kg, intravenous) using a 2-12-2-5 schedule (labels were injected on two consecutive days, twelve days were allowed to pass, another two consecutive days of label were given, and then the animals were euthanized five days later) to allow dynamic histomorphometry analyses. At the start of the 10th month relative to the first ZOL dose, animals were euthanized by intravenous administration of sodium pentobarbital and the right and left mandibles, right 9th rib, and right tibia were dissected free and placed in 70% ethanol for analysis.
In vivo extraction site healing (oral lesion visualization)
All animals were assessed daily post-extraction for exposed bone in the oral cavity. Our criterion for classification of ONJ was if exposed bone existed at the extraction site within 4 weeks post-extraction. This time-period is two-times longer than the reported duration of normal epithelial closure in untreated beagles [13]. Our previous work has shown exposed bone and sequestration during this time frame in beagle dogs [11].
Micro-computed tomography of extraction site
The extraction sites of both the left and right hemimandibles were prepared and scanned as in previous studies [11]. Bone regions were isolated by making parallel buccal-lingual cuts with a diamond wire saw (Histosaw; Delaware Diamond Knives). Each entire extraction site was scanned using high-resolution micro-computed topography at a resolution of 8 microns (Skyscan 1172, Belgium) as previously described [11]. For each extraction site both sockets were assessed and the data averaged to get a single value for each site with the exception of cases where one root was fractured, for those animals only the socket with a successful complete extraction was analyzed. Volume and morphology of the bone within the socket was assessed in three dimensions by using a region of interest to isolate the socket from the surrounding bone followed by thresholding of this region to separate mineralized tissue from the marrow. Outcome parameters of the extraction socket bone consisted of bone volume/tissue volume (BV/TV, %) and bone surface/total volume (BS/TV, 1/mm).
Histological Processing
The extraction sites of both the left and right hemimandibles were processed for histological analyses following microCT analyses. A portion of the rib (located at the greatest curvature), and a portion of the tibia (~4 cm proximal to the distal end) were also prepared and analyzed to assess the treatment effects on non-oral high (rib) and low (tibia) remodeling sites.
All tissue segments were stained with basic fuchsin in order to assess bone matrix necrosis [14]. Using 1% basic fuchsin dissolved in increasing concentrations of ethanol (80%, 95%, and 100%), specimens were stained for 48 hours per step. Following basic fuchsin staining, bones were embedded in plastic and then sectioned (~100 µm thick) using a diamond wire saw (Histosaw; Delaware Diamond Knives). Two sections, separated by ~400 µm, were cut for each bone region; mandible sections were made as parallel buccal-lingual cuts near the center of one root socket.
Histological Assessment
Histological measurements were made using a semiautomatic analysis system (Bioquant OSTEO 7.20.10, Bioquant Image Analysis Co.) attached to a microscope (Nikon Optiphot 2 microscope, Nikon) with a fluorescent light source. For assessment of intra-cortical bone formation rate, one cross-section was assessed at each skeletal site in accordance with our previous work [11, 14]. For mandible sections, data were collected separately for alveolar bone regions (defined as bone above the most distally observed portion of the tooth root or extraction socket) and non-alveolar bone regions (below the tooth root). The cortical bone of the entire cross section of the rib and tibia was assessed. All measures and calculations are similar to those reported previously [11] and are in accordance with ASBMR recommended standards [15]. Bone matrix necrosis of the cortical bone was assessed by bright-field microscopy as previously described [14]. Regions of bone void of basic fuchsin stain >500 µm2 were considered necrotic.
Statistics
Statistical tests were performed using SAS software (SAS Institute, Inc.). Parameters were compared between groups using one-way ANOVA with pLSD post-hoc tests. Differences in extraction site properties were compared between time-points (4- and 8- weeks post-extraction) within each treatment group using paired t-tests. For all tests, p < 0.05 was considered to be statistically significant.
RESULTS
Absence of exposed bone and matrix necrosis
All extraction sites healed without noticeable visual incident and no exposed bone or sequestra were observed. There was also no evidence of matrix necrosis, as defined by histological analyses of basic fuchsin stained tissue, in any mandible sections.
MicroCT imaging reveals severe bone destruction in select zoledronate animals
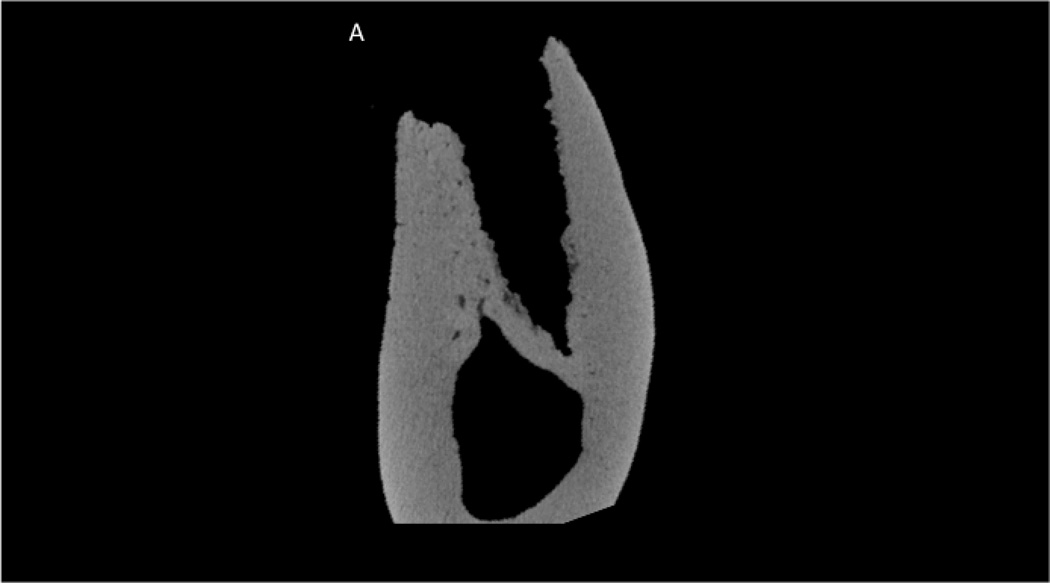
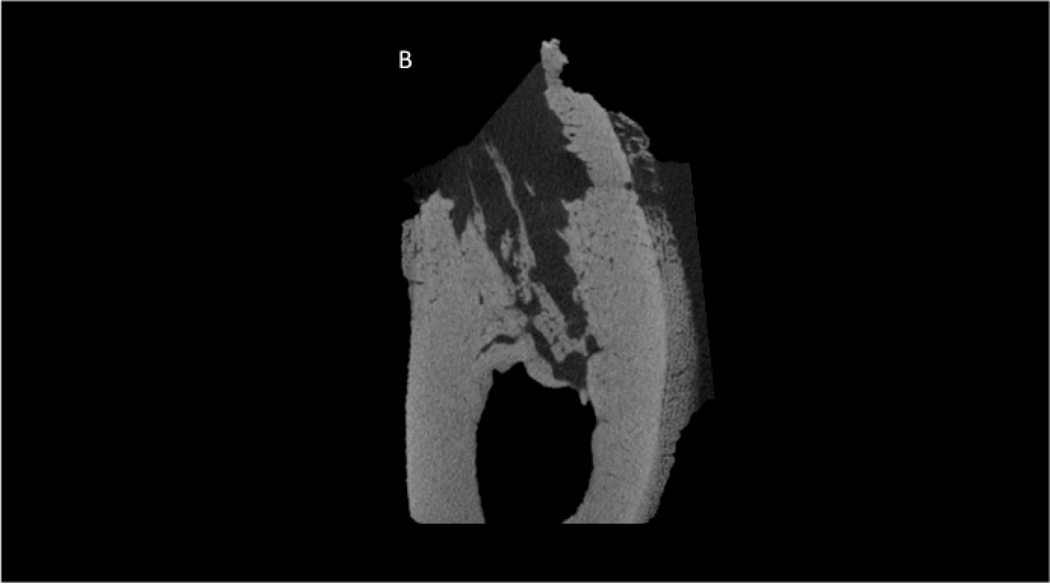
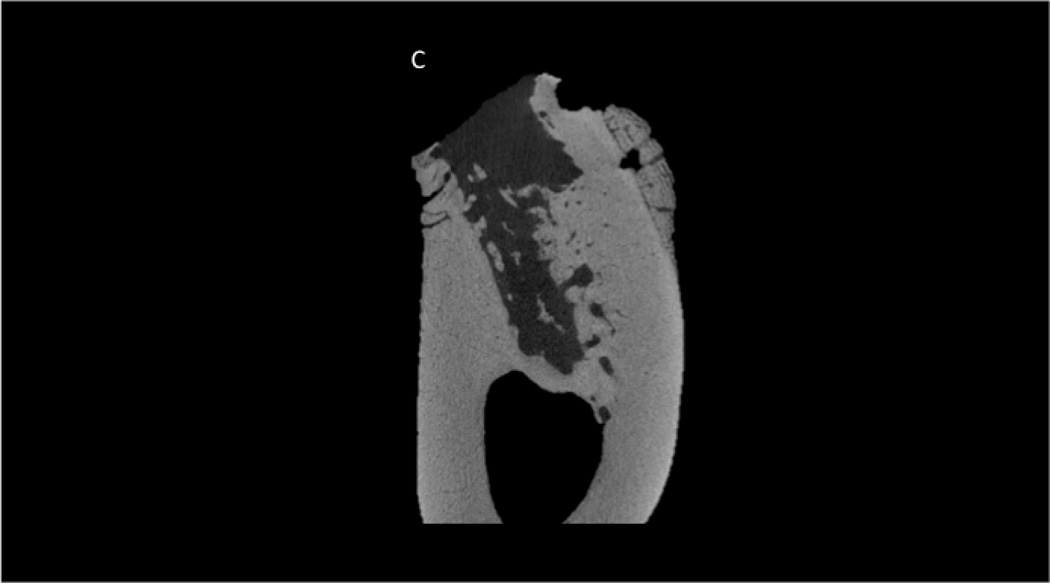
Although visual observation suggested normal healing in all animals, CT scanning ex vivo revealed two animals with severely compromised socket healing. One animal treated with ZOL and one treated with ZOL+DEX had extraction socket morphology that clearly differed from all other animals both within their own treatment groups, as well as the other two treatment groups (Figure 1). These sockets had minimal new tissue formation within the socket and clear destruction of the pre-existing alveolar bone tissue. There was also periosteal reaction (new bone formation) noted in both these animals. Histological sections confirmed CT morphologies in which both animals had destruction of alveolar bone and abundant periosteal reaction (Figure 2). The two animals with compromised healing were excluded from all subsequent analyses.
Figure 1.
Micro-computed tomography assessment of the extraction site in a zoledronate-treated animal (A) and each of the two zoledronate-treated animals that had abnormal healing (B and C). Images represent the mid-socket cross-section at 8 weeks post-extraction. Clear morphological differences can be observed between those that have abnormal healing compared to the zoledronate-treated animals including lack of alveolar socket woven bone, highly irregular/scalloped surfaces, and intense periosteal bone formation on the buccal surface.
Figure 2.
Histological appearance of non-healing socket in zoledronate-treated animal. Consistent with the CT images, the histological section revealed dramatic destruction of the alveolar bone (A) and dramatic periosteal bone formation (A,B,C). The periosteal bone was continuing to form even 8 weeks post-extraction as evident by the osteoblasts and osteoid (C).
Zoledronate alters distribution of bone within healing socket and this is further altered by addition of dexamethasone
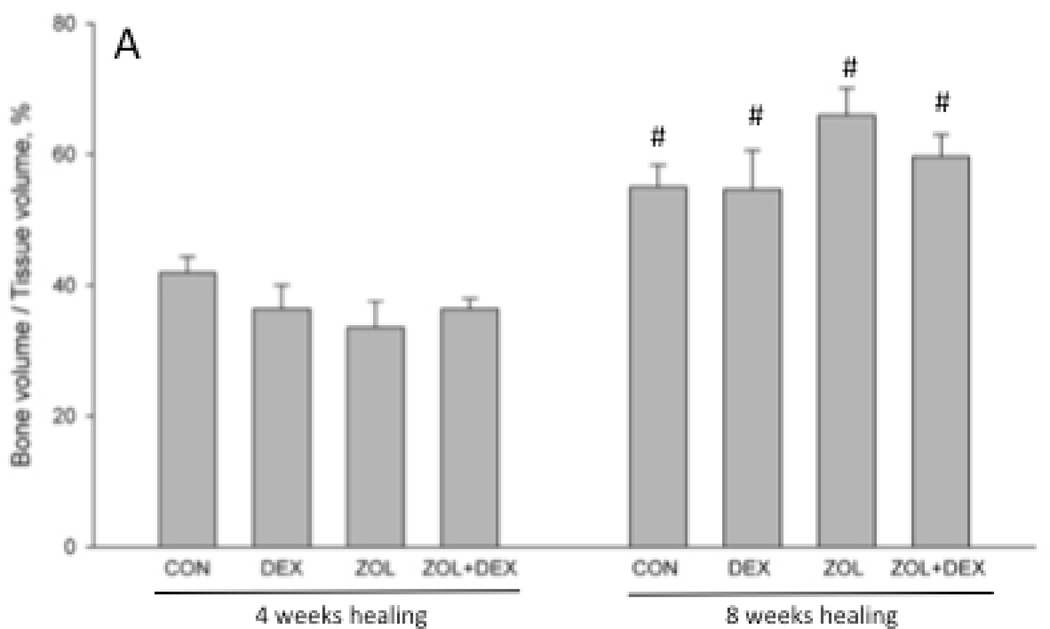
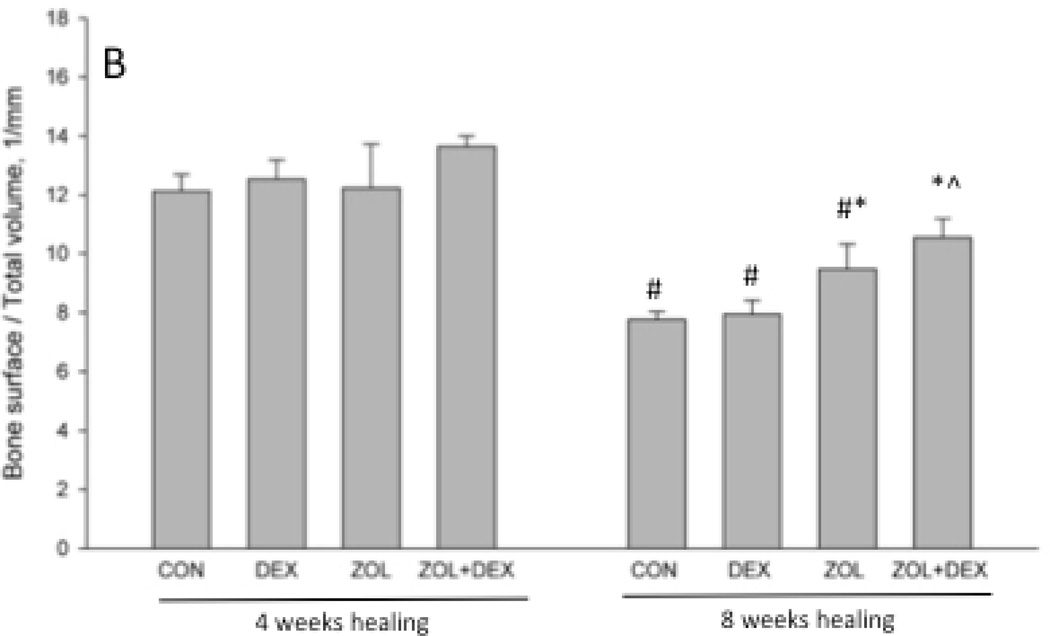
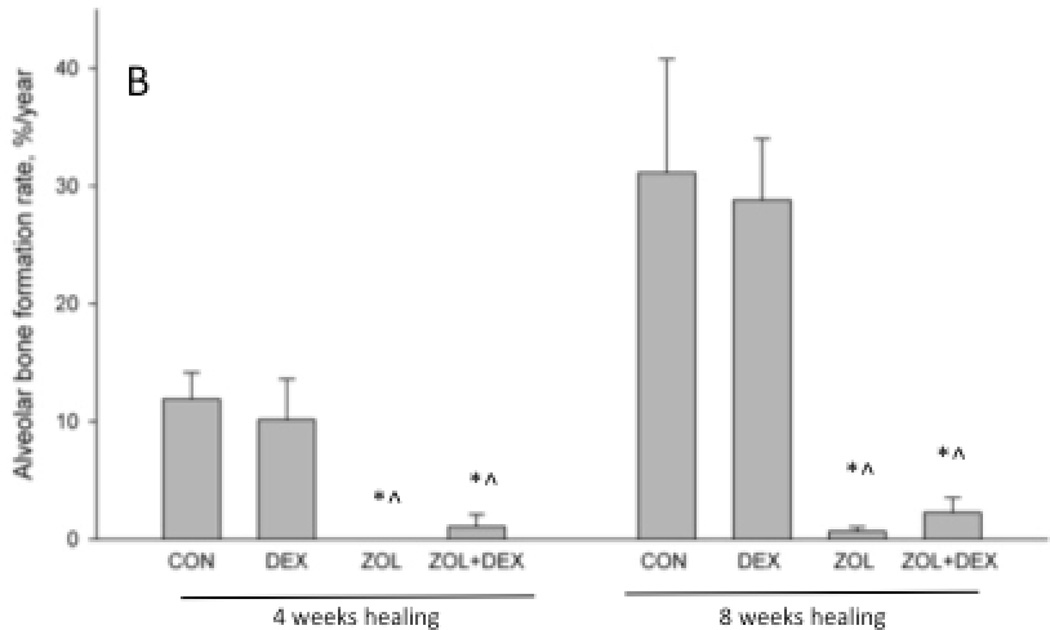
There was no significant difference in extraction socket bone volume (BV/TV) or morphology (BS/TV) among groups after 4 weeks of healing (Figure 3). By week 8 post-extraction, there was significantly higher bone volume compared to 4 weeks in all groups and this was not affected by treatment. At the eight-week time point ZOL and ZOL+DEX animals had significantly higher BS/TV compared to CON animals (+8 and +20%, respectively). BS/TV declined significantly between 4 and 8 weeks for all groups except ZOL+DEX. Qualitative assessment of CT images revealed modest periosteal reaction on the majority of ZOL and ZOL+DEX animals while such assessment was notably absent from CON and DEX only groups (Table 1).
Figure 3.
Micro-computed tomography assessment of the extraction site osseous healing. Three-dimensional analysis of the entire socket region support document that after 4 weeks of healing there was no difference in bone volume/tissue volume (BV/TV, %) or bone surface/total volume among the groups. After 8 weeks of healing, BV/TV was significantly higher in all treatment groups compared to the 4 week time-point. Bone surface / total volume (BS/TV) was significantly lower at 8 weeks compared to 4 weeks in CON, DEX, and ZOL groups but not ZOL+DEX. At the 8-week time point BS/TV was significantly higher in ZOL and ZOL+DEX compared to CON. p < 0.05 versus 4 week time-point within group (#), versus CON-treatment within time point (*), or versus DEX- treatment within time point (^).
Zoledronate suppression of intracortical remodeling is not altered with the addition of dexamethasone
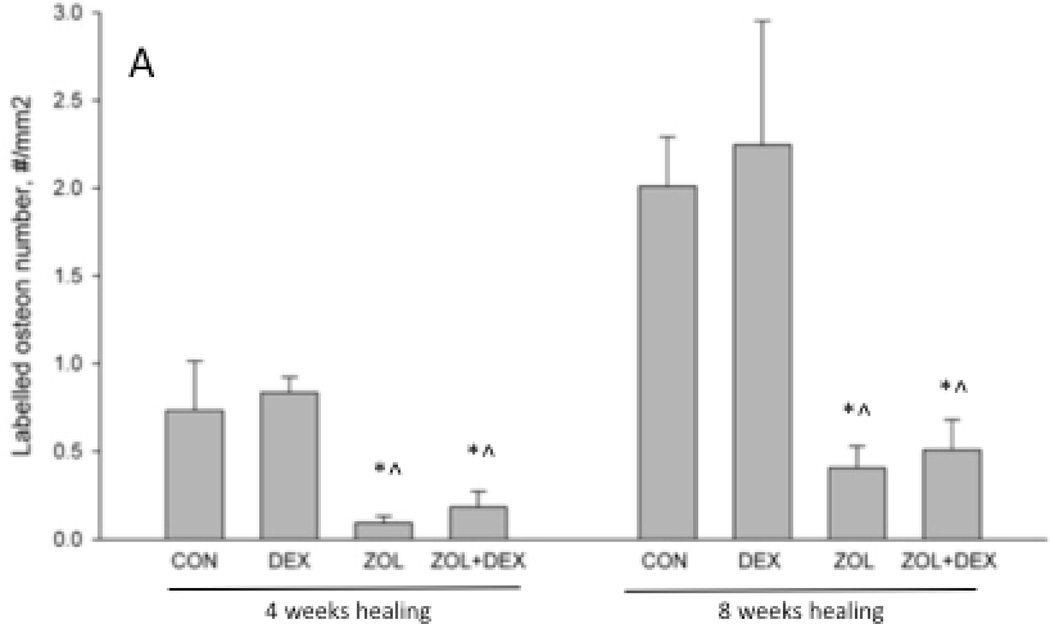
At 4-weeks post-extraction, alveolar bone intracortical remodeling was significantly lower in ZOL and ZOL+DEX groups compared to CON (Figure 4A and B). There was no effect of DEX compared to CON or of ZOL+DEX compared to ZOL. Similar patterns among the groups were observed at 8-weeks post extraction. Animals treated with ZOL, either with or without DEX, had significantly lower labeled osteon number and bone formation rate compared to CON and DEX.
Figure 4.
Histological assessment of intra-cortical bone formation rate in the alveolar region of the mandible. Labeled osteon number (A) and bone formation rate (B) were assessed in the alveolar bone adjacent to the extraction site by measuring fluorochrome labels injected at the end of the experiment. Both parameters were significantly lower at both 4 and 8 weeks post-healing in ZOL and ZOL+DEX groups compared to both CON and DEX groups. p < 0.05 versus CON-treatment within time point (*) or versus DEX- treatment within time point (^).
At the rib, a site with high bone remodeling rates comparable to alveolar bone, DEX produced a significantly lower number of labeled osteons relative to CON, although bone formation rate was not significantly different between groups (Table 2). Labeled osteon number was significantly lower in ZOL and ZOL+DEX compared to both CON and DEX. At the tibia, a site with low turnover, DEX, ZOL, and ZOL+DEX produced equivalent reductions in labeled osteon number compared to CON (Table 2).
Table 2.
Dynamic histomorphometry data of intra-cortical rib and tibial bone
| CON | DEX | ZOL | ZOL+DEX | |
|---|---|---|---|---|
| Rib | ||||
| Labeled osteon number, #/mm2 | 2.88 ± 0.51 | 1.26 ± 0.41 | 0.07 ± 0.02 | 0.05 ± 0.02 |
| Bone formation rate, %/year | 23.7 ± 4.6 | 14.6 ± 3.6 | NA | NA |
| Tibia | ||||
| Labeled osteon number, #/mm2 | 0.40 ± 0.10 | 0.07 ± 0.02 | 0.05 ± 0.01 | 0.03 ± 0.01 |
| Bone formation rate, %/year | 3.7 ± 1.3 | 0.3 ± 0.2 | 0.2 ± 0.1 | NA |
DISCUSSION
Bisphosphonates, dexamethasone, and dental extraction have each been identified as risk factors for ONJ in retrospective clinical studies and the combination of these three factors has produced a phenotype similar to ONJ in several rodent experiments. The results of the current study show that prolonged high dose zoledronic acid and short- term dexamethasone are not sufficient to produce exposed bone in the oral cavity even following dental extraction in a beagle dog.
We, and others, have previously documented that zoledronic acid produced rapid and profound reductions in bone remodeling of the dog mandible [11, 16, 17]. Furthermore, it has been shown that dental extraction produces regions of non-viable bone matrix, exposed bone, and sequestra formation in dogs treated for just one month at the same doses of zoledronate used in the current study [11]. It was therefore unexpected that in the current study, when zoledronate was given for 7–8 months prior to dental extraction, no animals deveioped exposed bone. Our findings question the concept that duration of anti-remodeling treatment is linked to the risk of developing exposed bone following dental extraction. Although epidemiological studies suggest treatment duration is a risk factor for ONJ, there are a number of reports of cases in patients treated for just a weeks/months who develop exposed bone [6, 18].
Although no animals presented with exposed bone post-extraction, one animal treated with zoledronate and one treated with zoledronate plus dexamethasone had significantly compromised bone healing based on CT assessments at 8 weeks post-extraction. These two animals had alveolar sockets that were notably absent of bone compared to other animals in their treatment groups (Figure 1). They also had striking loss of alveolar bone, with clear resorption pits, as well as profound amounts of periosteal woven bone on the buccal surface.
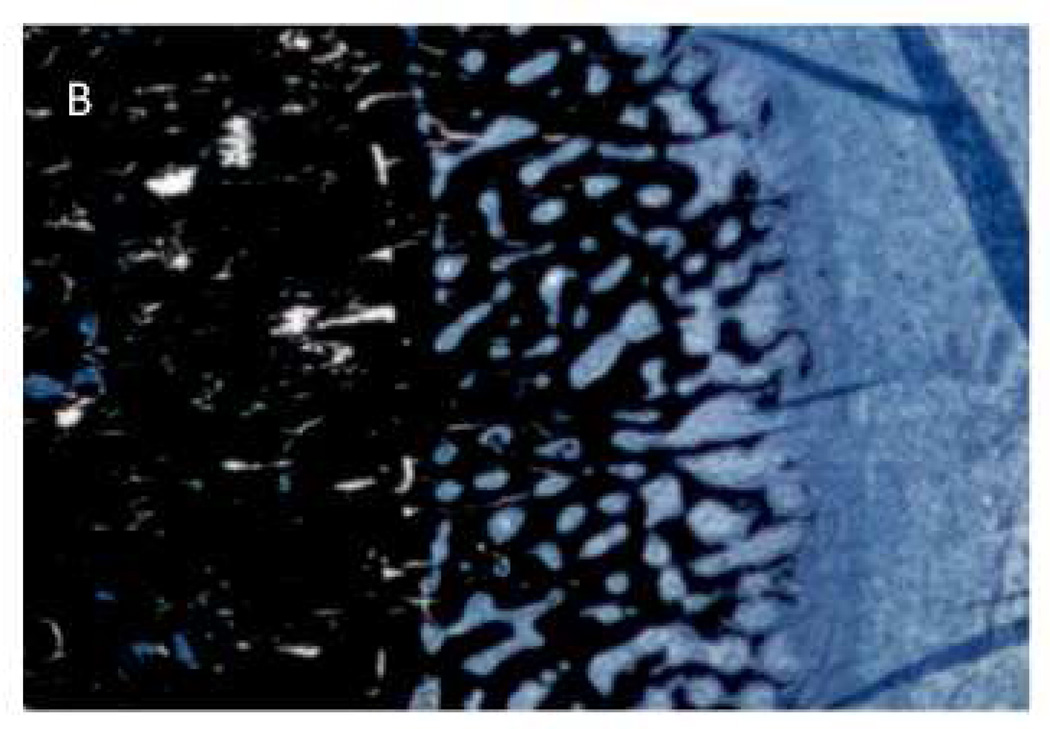
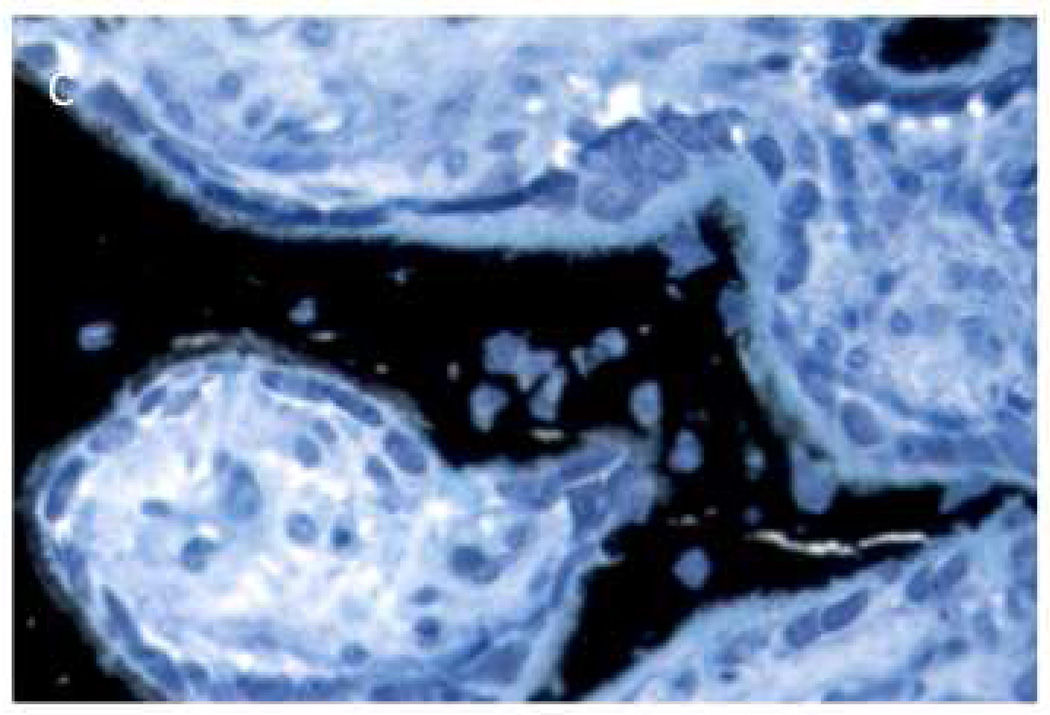
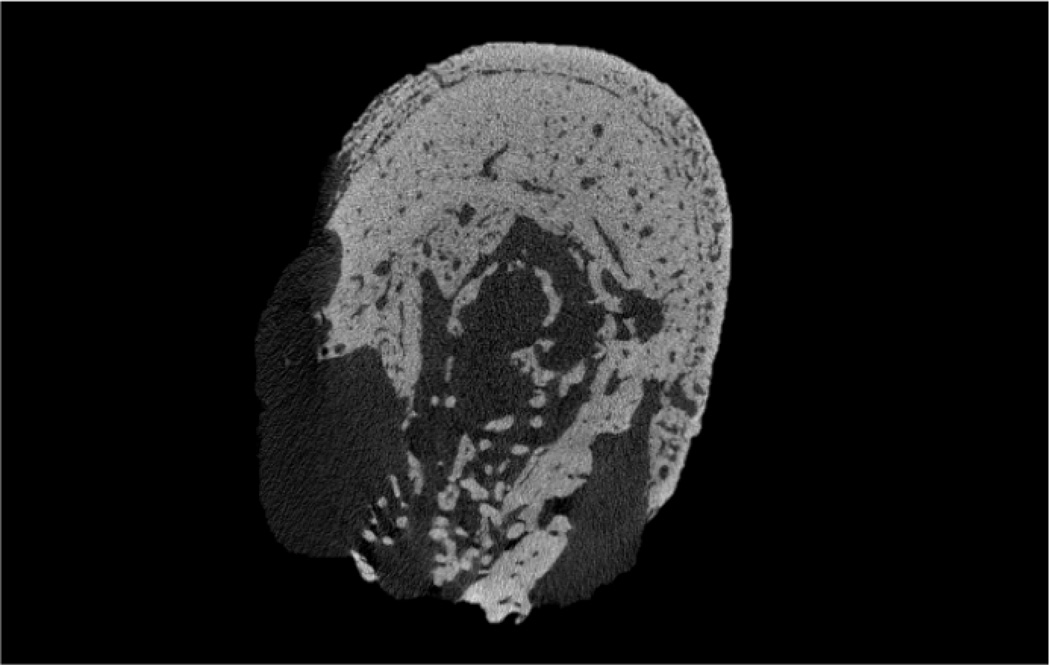
The observation of periosteal reaction is of interest given the recent report documenting periosteal reaction in a rat model of periodontal-induced ONJ as well as in patients with ONJ [24]. These studies showed 6 of 19 animals treated with zoledronate and induced to have periodontal disease had evidence of periosteal reaction. Although the two animals with abnormal osseous healing in the current work had profound periosteal reaction, re-examination of all animals in our study qualitatively revealed the presence of smaller amounts of periosteal reaction in a majority of animals treated with zoledronate. There was no sign of any periosteal reaction in non-zoledronate treated animals. Furthermore, retrospective examination of images from ONJ patient sequestra that our lab has scanned with high-resolution CT revealed a number of samples that contain tissue morphologically consistent with woven bone on a periosteal surface (Figure 5). The mechanism and implications of these findings are unclear. It has been suggested that extensive periosteal reactionary formation, such as can occur with osteitis, is mechanically mediated. In the two extreme cases where dramatic bone resorption was observed in the current study, the mechanical forces on the area were likely altered and the periosteal bone could have been an attempt to compensate. In the other zoledronate treated animals, the mechanical environment is likely was also altered because of the lack of cortical consolidation following dental extraction. Irrespective of the underlying reason, the emerging identification of periosteal reaction associated with ONJ in both animal models and humans suggests it may be an in-vivo biomarker for identification of abnormally healing sites that have potential for becoming ONJ. More work to understand the relationship, if any, between periosteal reaction and ONJ seems warranted.
Figure 5.
MicroCT images from human ONJ specimens that suggest a similar type of periosteal reaction as noted in the present study. Specimens were scanned and analyzed for a separate study [18].
Our results regarding extraction site healing and alveolar remodeling activity are consistent with our previous studies and indicate that neither duration of zoledronate treatment (3 versus 9 months), nor adding dexamethasone to zoledronic acid dramatically alters these processes. Dexamethasone, zoledronate, or the combination did not affect the initial filling of the bone socket with bone, as evident by the similar bone volumes among groups at both 4 and 8 weeks. This is consistent with the idea that bisphosphonates to not alter osteoblast activity in vivo, yet contradicts the idea that dexamethasone induces osteoblast apoptosis, although the later findings are mostly based on high-dose chronic dosing. The effects of low-dose dexamethasone on osteoblasts have not been explored in detail. The combination of zoledronate and dexamethasone did compromise the remodeling of socket healing, as shown by the lack of reduction in bone surface/total volume, a measure of how the bone is distributed in the socket. The lack of significant decrease from 4 to 8 weeks suggest the bone that fills the socket is not being resorbed and replaced by cortical bone, as occurs in untreated animals [11]. Such findings are expected given the prominent inhibitory effect of zoledronate on resorption. The reason by zoledronate alone did not produce this effect is unclear, as it has been shown to previously [11].
The lack of exposed bone in the current work is in stark contrast to a number of studies in which the combination of zoledronate and dexamethasone produce exposed bone in rodents. The reason for this discrepancy is unclear, although several differences in models exist including drug dosing (rodent studies tend to use higher doses), post-extraction protocols (sites are sutured in dogs, but tend not to be in rodents), and dexamethasone protocol (the current study used low-dose intermittent treatment consistent with that used for multiple myeloma patients). Irrespective of the reason for differences among model systems there are several potentially important implications. Although there is an association between dexamethasone and ONJ in human studies the results from rodent studies should not be considered definitive evidence of a direct role of dexamethasone in the etiology of ONJ. Secondly, these conflicting results across models demonstrate the importance of pursing several model systems to study the pathophysiology of ONJ.
In conclusion, we document that prolonged treatment with high-dose zoledronate and dexamethasone does not produce exposed bone following dental extraction in a beagle dog model. These findings point to a more complex ONJ pathophysiology than is implied by previous studies in rodents and raise questions about the role of dexamethasone in its etiology. Furthermore our experiment shows a small cohort of zoledronate-treated animals without exposed bone yet with dramatic alterations in post- extraction healing which is accompanied by extensive periosteal reaction.
ACKNOWLEDGEMENTS
We would like to thank the following individuals who contributed to this work: Drew Brown and Tianyi Luo for assistance with histological analyses, Mark Koivuniemi and Lynette Jackson for CT scanning/analysis, Keith Condon for histological preparation, and Carrie Pell and her staff for assistance with animal care. This work was supported by grants from the NIH (R21-DE019686 and S10-RR023710). ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Ruggiero has served as a paid consultant for Amgen; no other authors have any conflicts to report.
REFERENCES
- 1.Aghaloo TL, Felsenfeld AL, Tetradis S. Osteonecrosis of the Jaw in a Patient on Denosumab. J Oral Maxillofac Surg. 2010 doi: 10.1016/j.joms.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo SB, Hellstein JW, Kalmar JR. Systematic review: Bisphosphonates and osteonecrosis of the jaws. Annals of Internal Medicine. 2006;144:753. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 3.Lacy MQ, Dispenzieri A, Gertz MA, Greipp PR, Gollbach KL, Hayman SR, Kumar S, Lust JA, Rajkumar SV, Russell SJ, Witzig TE, Zeldenrust SR, Dingli D, Bergsagel PL, Fonseca R, Reeder CB, Stewart AK, Roy V, Dalton RJ, Carr AB, Kademani D, Keller EE, Viozzi CF, Kyle RA. Mayo clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81:1047. doi: 10.4065/81.8.1047. [DOI] [PubMed] [Google Scholar]
- 4.Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in australia. J Oral Maxillofac Surg. 2007;65:415. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 5.Shane E, Goldring S, Christakos S, Drezner M, Eisman J, Silverman S, Pendrys D. Osteonecrosis of the Jaw: More Research Needed. Journal of Bone and Mineral Research. 2006;21:1503. doi: 10.1359/jbmr.060712. [DOI] [PubMed] [Google Scholar]
- 6.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu MM, Nooka A, Sayegh G, Guarneri V, Desrouleaux K, Cui J, Adamus A, Gagel RF, Hortobagyi GN. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. Journal of Bone and Mineral Research. 2008;23:826. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen MR, Ruggiero SL. Osteonecrosis of the Jaw: Recent Clinical and Preclinical Advances. BoneKEY. 2011:1. [Google Scholar]
- 8.Sonis ST, Watkins BA, Lyng GD, Lerman MA, Anderson KC. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncology. 2009;45:164. doi: 10.1016/j.oraloncology.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Kubek D, Burr DB, Allen MR. Ovariectomy Stimulates and Bisphosphonates Inhibit Intracortical Remodeling in the Mouse Mandible. Orthodontics and Craniofacial Research. 2010;13:214. doi: 10.1111/j.1601-6343.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemons MJ, Dranitsaris G, Ooi WS, Yogendran G, Sukovic T, Wong BYL, Verma S, Pritchard KI, Trudeau M, Cole DEC. Phase II Trial Evaluating the Palliative Benefit of Second-Line Zoledronic Acid in Breast Cancer Patients With Either a Skeletal-Related Event or Progressive Bone Metastases Despite First-Line Bisphosphonate Therapy. J Clin Oncol. 2006;24:4895. doi: 10.1200/JCO.2006.05.9212. [DOI] [PubMed] [Google Scholar]
- 11.Allen MR, Chu TG, Kubek D, Burr DB. Compromised Osseous Healing of Dental Extraction Sites in Zoledronic Acid-Treated Dogs. Osteoporos Int. 2011;22:693. doi: 10.1007/s00198-010-1268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV, Chanan-Khan AA, Lonial S, Yu Z, Patin J, Olesnyckyj M, Zeldis JB, Knight RD. Lenalidomide plus Dexamethasone for Relapsed Multiple Myeloma in North America. New England Journal of Medicine. 2007;357:2133. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 13.Cardaropoli G, Araujo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol. 2003;30:809. doi: 10.1034/j.1600-051x.2003.00366.x. [DOI] [PubMed] [Google Scholar]
- 14.Allen MR, Burr DB. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. Journal of Oral and Maxillofacial Surgery. 2008;66:987. doi: 10.1016/j.joms.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry - Standaridization of nomenclature, symbols, and units. Journal of Bone and Mineral Research. 1987;2:595. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 16.Huja SS, Mason A, Fenell CE, Mo X, Hueni S, D'Atri AM, Fernandez SA. Effects of Short-Term Zoledronic Acid Treatment on Bone Remodeling and Healing at Surgical Sites in the Maxilla and Mandible of Aged Dogs. J Oral Maxillofac Surg. 2010;29:29. doi: 10.1016/j.joms.2010.05.062. [DOI] [PubMed] [Google Scholar]
- 17.Allen MR, Kubek DJ, Burr DB. Cancer treatment dosing regimens of zoledronic acid result in near-complete suppression of mandible intracortical bone remodeling in beagle dogs. Journal of Bone and Mineral Research. 2010;25:98. doi: 10.1359/jbmr.090713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen MR, Pandya B, Ruggiero SL. Lack of Correlation Between Duration of Osteonecrosis of the Jaw and Sequestra Tissue Morphology: What It Tells Us About the Condition and What It Means for Future Studies. Journal of Oral and Maxillofacial Surgery. 2010;68:2730. doi: 10.1016/j.joms.2010.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. New England Journal of Medicine. 2009;360:53. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vigorita VJV, Silver JS, Eisemon EOE. Osteoclast abnormalities in fractured bone during bisphosphonate treatment for osteoporosis: a case report. Skeletal Radiology. 2012 doi: 10.1007/s00256-012-1407-4. [DOI] [PubMed] [Google Scholar]
- 21.Hayman A, Jones S, Boyde A, Foster D, Colledge W, Carlton M, Evans M, Cox T. Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disrupted endochondral ossification and mild osteopetrosis. Development. 1996;122:3151. doi: 10.1242/dev.122.10.3151. [DOI] [PubMed] [Google Scholar]
- 22.Hollberg K, Hultenby K, Hayman A, Cox T, Andersson G. Osteoclasts from mice deficient in tartrate-resistant acid phosphatase have altered ruffled borders and disturbed intracellular vesicular transport. Experimental Cell Reesarch. 2002;279:227. doi: 10.1006/excr.2002.5612. [DOI] [PubMed] [Google Scholar]
- 23.Pap T, Claus A, Ohtsu S, Hummel KM, Schwartz P, Drynda S, Pap G, Machner A, Stein B, George M, Gay RE, Neumann W, Gay S, Aicher WK. Osteoclast-independent bone resorption by fibroblast-like cells. Arthritis research & therapy. 2003;5:R163. doi: 10.1186/ar752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, Bezouglaia O, Dry SM, Tetradis S. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. Journal of Bone and Mineral Research. 2011;26:1871. doi: 10.1002/jbmr.379. [DOI] [PMC free article] [PubMed] [Google Scholar]