Abstract
Benzoquinone (BQ) is an extremely potent electrophilic contact allergen that haptenates endogenous proteins through Michael addition (MA). It is also hypothesized that BQ may haptenate proteins via free radical formation. The objective of this study was to assess the inductive effects (activating and deactivating) of substituents on BQ reactivity and mechanistic pathway of covalent binding to a nucleophilic thiol. The BQ binding of Cys34 on human serum albumin was studied and for reactivity studies, nitrobenzenethiol (NBT) was used as a surrogate for protein binding of the BQ and benzoquinone derivatives (BQD). Stopped flow techniques were used to determine pseudo-first order rate constants (k) of methyl, t-butyl and chlorine substituted BQD reactions with NBT whereas electron pair resonance (EPR) studies were performed to investigate the presence a free radical mediated binding mechanism of BQD. Characterization of adducts was performed using mass spectrometry and nuclear magnetic resonance spectroscopy (NMR). The rate constant values demonstrated the chlorine substituted (activated) BQD to be more reactive toward NBT than the methyl and t-butyl substituted (deactivated) BQD, and this correlated with the respective EPR intensities. The EPR signal, however, was quenched in the presence of NBT suggesting MA as the dominant reaction pathway. MS and NMR results confirmed adduct formation to be a result of MA onto the BQ ring with vinylic substitution also occurring for chlorine substituted derivatives. The binding positions on BQ and NBT:BQ(D) stoichiometric ratios were affected by whether the inductive effects of the substituents on the ring were positive or negative. The reactivity of BQ and BQD is discussed in terms of the potential relationship to potential allergenic potency.
INTRODUCTION
Benzoquinone (BQ) belongs to a large class of quinonic compounds commonly used for dye production. Quinones have been used as analytical reagents,1 polymer modifiers,2 photoresistors,3 catalysts,4 herbicides,5 fungicides,5,6 and plant-growth regulators.7 BQ is also broadly used in tanning, dyes,1 cosmetics8 and non-prescription forms of skin lightening cosmetics.9–11 The widespread domestic and industrial use of BQ and its products results in a significant human population being exposed to quinones. Exposure to BQ leads to allergic contact dermatitis (ACD),12 which is the clinical manifestation of contact allergy that is estimated to affect a significant proportion (1–4%) of the general population.13
Chemical contact allergens must either directly or after metabolic or abiotic activation, bind covalently to skin protein to be recognized by the immune system and become allergenic. Early evidence demonstrating chemical reactivity as underpinning the sensitizing potency of a chemical was reported by Landsteiner and Jacobs in 1936.14 The rationalization of skin sensitizer properties in terms of electrophilicity or proelectrophilicity of the chemicals, enabling them to bind to proteins is supported by observations from Rosenkranz et al.15 where 30–40% out of 355 randomly chosen chemicals that are human sensitizers were electrophilic. While the majority of electrophilic chemicals are known to exist within the five common mechanistic applicability domains (i.e. Michael addition (MA), Schiff base formation (SBF), nucleophilic substitution (SN1/2), nucleophilic aryl substitution (SNAr) and acylation (AA)) other less apparent mechanistic domains may contribute to the reactivity of chemicals and their ultimate allergenicity. We have previously reported the haptenation of mercaptobenzothiazole to proteins via disulfide formation as an example of non-electrophile-nucleophile interaction.16 Binding of the skin sensitizer 7-oxodehydroabietic acid to proteins has been reported to be via a free radical mechanism 17 showing it as a viable protein modification pathway. Diverse BQ-protein haptenation pathways/mechanism may result in different adducts with varying immunogenicity. Understanding and characterization of the dominant reaction pathways will facilitate the filling of knowledge gaps in quantitative mechanistic models for identifying and potency ranking of skin sensitizers.
The need for reliable non-animal based methods for identification of chemical sensitizers and the evaluation of their sensitization capacity has led to the development of a number of in vitro and in chemico methods. New legislation (e.g., the REACH initiative in the European Union) has led to several initiatives to increase acceptance of reactivity and (quantitative) structure-activity relationships (QSARs) based methods to reduce reliance on animal testing. The present study utilizes NBT in an in chemico assay that was developed for screening thiol reactive haptens for their skin sensitizing potency.18 In this article, we investigate reactivity based QSAR analysis for BQ and BQD (Table 1) which have been hypothesized to react via MA. We also hypothesize that BQ and BQD may also react to NBT via an alternate mechanism involving BQ free radicals.19 Using BQ as the central molecule we present reactivity of BQ and BQD to NBT where we investigate the effect of activating and deactivating substituents on the reactivity and mechanistic pathways of BQ and BQD. BQ-protein haptenation is discussed in terms of the dominant reaction mechanism between Michael addition and a free radical mediated mechanism, and the influence of substituent effects on the binding.
Table 1.
Benzoquinone and Benzoquinone Derivatives that were reacted with NBT.

|
EXPERIMENTAL SECTION
Chemicals
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) and were used without further purification unless otherwise noted.
Thiol Binding Determination
The method reported by Schultz et. al. 20 was used with slight modification. Briefly, Ellman’s reagent (5,5′-dithiobis-(2-nitrobenzoic acid; DTNB) and cysteine (5 mM) stock solutions were prepared in a 50 mM ammonium carbonate buffer (NH4)2CO3; pH 7.4) buffer. Cysteine (Cys) standards from 50 to 250 μM were reacted with 1 mM DTNB at room temperature in a total volume of 2 mL. Absorbance readings taken at 412 nm (ε412 nm = 14,150 M−1cm−1) after 15 min were plotted against the final cysteine concentration to give the standard curve. This calibration curve was used to determine Cys and glutathione (GSH) binding to BQ following reaction of 0.125 mM Cys/GSH with 0.625 mM mM BQ for 2 h with subsequent addition of 0.625 mM DTNB for 15 min. The calibration curve was used to determine human serum albumin (HSA) reduced sulfhydryl concentration ([SH]/[HSA]) following reaction of 0.8 mg/mL (≈ 11.9 μM) HSA with 0.625 mM DTNB for 15 min with subsequent absorbance readings at 412 nm. BQ (0.625 mM) was incubated with 0.8 mg/mL (≈ 11.9 μM) HSA for 2 h with subsequent addition of 0.625 mM DTNB to measure the loss of Cys34 reactivity.
Preparation of Reduced Thiols
The reconversion of the Cys34 residue on HSA to the free sulfhydryl form was performed by incubating equal volumes of 4 mg/mL HSA with 4 mM dithiothreitol (DTT) in (NH4)2CO3 buffer at room temperature for 3 h, followed by dialysis (molecular mass cutoff of 3500 Da) in 2 L of 50 mM (NH4)2CO3 for 48 h with three buffer changes during that period to ensure complete removal of DTT. The post-dialysis HSA concentrations were determined using the Bradford assay21 with human serum albumin (HSA) as the standard protein. HSA thiol concentrations were determined using Ellman’s reagent as outlined above and the extent of thiol blocking with BQ was measured with Ellman’s reagent and compared with blocking performed with the thiol specific reagent, N-ethylmaleimide (NEM).
Extent of BQ Binding to Cys34 in HSA
A stock solution consisting of 4 mg/mL HSA was prepared in 50 mM (NH4)2CO3. Then, 0.8 mg/mL (≈ 11.9 μM) HSA was incubated with 250 μM BQ for 1 h on a shaker at 37 °C followed by dialysis for 48 h. Loss of DTNB reactivity (as described above) was used to determine loss of thiol reactivity as a result of BQ covalent binding. As controls, HSA thiol concentrations were determined for (i) fresh HSA in buffer that was not incubated or dialyzed, (ii) HSA (in the absence of BQ) that had been incubated under the same conditions (as the BQ-treated HSA) and dialyzed, and (iii) HSA which had been reduced by dithiothreitol (DTT) and dialyzed. A comparison of the kinetics of the reaction of DTNB with (i) reduced HSA, (ii) reduced and dialyzed HSA, and (iii) reduced HSA incubated with BQ with subsequent dialysis was performed. Human albumin has only one free thiol, Cys34. To ascertain the Cys34 as the BQ-targeted residue, the thiol specific blocking reagent N-ethylmaleimide (NEM) was reacted with reduced HSA (as a positive control) prior to reactions with DTNB. DTT-reduced HSA (0.8 mg/mL, 11.9 μM) was reacted with 250 μM NEM for 30 min in a pH 7.4 (NH4)2CO3 buffer at room temperature with subsequent dialysis. Aliquots were taken for [SH]/[HSA] determination with DTNB. HSA pretreated with BQ and incubated with DTNB was also subjected to the same analysis.
Extent of BQ Binding to primary amines in HSA
A stock solution consisting of 0.8 mg/ml HSA prepared in PBS (pH 7.4) was incubated with 250 μM BQ for 1 h at room temperature followed by dialysis for 48 h against PBS buffer. The trinitrobenzene sulfonic acid (TNBS) amine specific probe assay was performed on HSA and HSA-BQ conjugates. HSA (500, 400, 200, 100, 50 μg/mL) were prepared in PBS buffer (pH 7.4). TNBS (5% w/v) was diluted 1: 5.48 with 0.1 M borate buffer (pH 9.3). To 500 μL of HSA and HSA-BQ, 12.5 μL of TNBS was added, mixed well and left to react for 30 minutes and absorbance was measured at 420 nm.
Whole Human Fresh Blood Collection and Serum Separation
Whole blood was drawn into 10 mL BD Vacutainer Serum Separation Tubes containing gel and clot activator transport and inverted four times for the blood to mix with the tube additives. The blood was allowed to sit for 30 min at 37°C for clotting to occur. Serum was separated by centrifugation of the tubes at 1000 g for 10 min at 4°C. The serum was transferred into plastic tubes using glass pasteur pipettes and aliquoted before determination of protein concentration. The aliquoted samples were stored at −20°C. The Cys-DTNB calibration curve was used to determine serum total sulfhydryl concentration ([SHT]) following reaction of 0.1 ml of 0.1 mg/mL serum protein with 0.4 ml of 0.5 mM DTNB for 15 min with subsequent absorbance readings at 412 nm. The calibration curve was also used to determine loss of [SHT] and the ratio [SHr]/[SHT] (SHr = blocked thiol) when serum was incubated with BQ prior to DTNB reactivity. To measure binding of BQ and NEM to high molecular weight thiols dialysis of the serum samples was performed for 48 h against PBS to remove low molecular weight thiols prior to incubation with BQ/NEM and subsequent determination of thiol binding with DTNB. The ratio of blocked thiol post dialysis (SHd) to total thiol of the control post dialysis SHTd (= [SHd]/[SHTd]) was also determined.
BQ and BQD Binding Kinetic Studies
Reaction kinetics were measured on a Hitech Scientific (Bradford-on-Avon, UK) SF-61DX2 double-mixing stopped-flow spectrophotometer with an F/4 Czerny-Turner MG-60 monochromator and a spectra scan control unit. The signal from the spectrophotometer was amplified and digitized via an Omega Engineering DAS-50/1 16-bit A/D board interfaced to a computer for storage and data analysis. Reaction progress was followed by monitoring the loss of free thiol on NBT at 412 nm, where it has its highest molar absorptivity coefficient (ε) as previously described. 18
Test chemicals were dissolved in acetonitrile at concentrations ranging from 0.01 to 10 mM. These solutions (5 μL) were combined with 5 μL of 0.1 mM NBT in phosphate buffer (pH 7.4) in a sealed reaction cell with rapid mixing. Absorbance readings were collected after a dead time of 1 ms. Control experiments contained test chemical in acetonitrile/phosphate buffer to determine background absorbance. Five replicates were performed for each chemical at each concentration. The temperature was maintained at 25°C in the observation cell with a VWR International (Radnor, PA) circulating water bath.
Electron Paramagnetic Resonance (EPR) Spectroscopy
EPR spectroscopy was carried out using a Bruker (Fremont, CA) EMX spectrometer equipped with a high-sensitivity cavity and an Aqua-X sample holder. Spectra were obtained at room temperature. Typical EPR parameters were as follows: 100 G sweep width (for the spin trapping experiments with 5,5-dimethyl-1-pyrroline N-oxide, (DMPO)); 9.77 GHz microwave frequency; 32 mW power; 2×105 receiver gain; modulation frequency of 86 kHz; modulation amplitude of 2 G; with the conversion time 5 ms and time constant being 10 ms with 200 X-scans for each 512 point spectrum. The final concentrations of BQD derivatives were 50 μM and DMPO was 100 mM unless specifically mentioned. Buffer solutions were prepared by adjusting with 5 M sodium hydroxide solution or 1 M hydrochloric acid. The effect of NBT on the generation of free radicals was studied by incubating 0.1 mM NBT with 1 mM BQD + 100 mM DMPO. Spectral simulations of EPR spectra were performed using WinEPR program developed by Bruker and the coefficients of simulated spectra were > 0.96.
Nuclear Magnetic Resonance (NMR) and Mass Spectroscopy
Reagents were from Sigma Aldrich and used as supplied. The reaction mixture of six-fold molar excess of NBT samples were incubated in phosphate buffer pH 7.4 for 24 hours. The reaction mixture was purified by preparative TLC (10–20% EtOAc/80–90% n-hexane) for samples of compounds 3-7 (Scheme 1) to obtain the products in a pure state whilst compound 8 was separated using Teledyne Isco Combflash Rf 200 PSI. After completion, solvents were evaporated using a rotor evaporator, and the products were dissolved in appropriate solvent for spectroscopy analysis. Mass spectra of products from reaction mixtures were taken on a high-resolution (m/Δm = 30 000) Thermo Scientific LTQ-Orbitrap Discovery mass spectrometer (San Jose, CA) equipped with an electrospray ionization source. All mass spectrometer samples were dissolved in MeOH/H2O (1:1) mixture. The MS ESI source parameters were set as follows: spray voltage (kV); 2.5, spray current (μA); 1.96, sheath gas flow rate; 20, auxiliary gas flow rate; 0.01, capillary voltage (V); −16, capillary temperature (°C); 300 and tube lens (V); −115. Detection was carried out in the negative ionization mode (-ESI) for 2 minutes in mass range m/z of 100–1000. The detection parameters were set up as follows: Analyzer; FTMS, negative polarity; mass range; normal, resolution; 30 000, scan type; centroid.1H-NMR spectra were recorded with a Bruker AMX-400MHz spectrometer using CDCl3 and D2O as solvents. Chemical shifts were reported as values in ppm relative to CHCl3 (7.26) in CDCl3 and TMS which was used as an internal standard.22
Scheme 1.
Reaction mechanisms for electron withdrawing and electron donating substituted benzoquinones with NBT
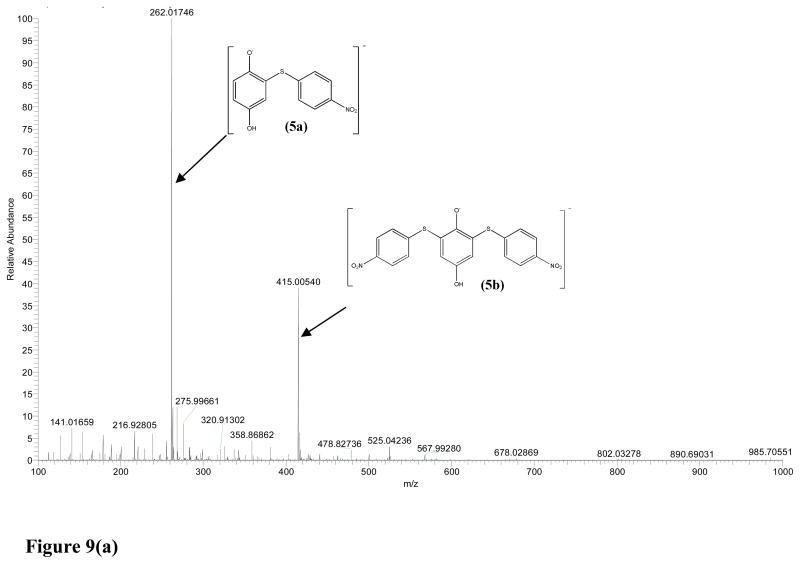
2-((4-nitrophenyl) thiol) benzene-1, 4-diol(2a and 5a), was a product of both BQ and CBQ reaction with NBT. 2a was recovered by solvent extraction using n-hexane which was evaporated using a rotor evaporator. 1H NMR (CDCl3, 400 MHz) δ 8.10 (d, J = 9.0 Hz, 2H), 7.14 (d, J = 9.0 Hz, 2H), 7.05 – 6.94 (m,3H). ESI-MS exact mass calculated for [M–H]− requires m/z 262.01795, found 262.01779 2a and 262.01746 for 5a and ESI-MS data was recorded using a scan range of m/z 100–600.
2-methyl-6-((4-nitrophenyl)thio)benzene-1,4-diol (3a) was a produced as yellow crystals from the reaction of MBQ with NBT. The reaction mixture was purified by preparative TLC with the eluent ethyl acetate/n-hexane (1.5:8.5 ratio). 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.32 (d, J = 8.9 Hz, 9H), 7.71 (d, J = 8.9 Hz, 11H), 6.56 (s, 1H), 5.90 (s, 2H), 2.12 (s, 7H). For MS analysis ESI-MS solvent was 50:50 water: methanol which resulted in the m/z 276.03259, against the exact mass m/z = 276.03360 calculated for [M–H]−.
2,5-bis((4-nitrophenyl)thio)benzene-1,4-diol (7a) Data: The reaction mixture was purified by preparative TLC, eluent ethyl acetate/n-hexane (1:9 ratio) and orange crystals were obtained. 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.13 (d, J = 9.1 Hz, 2H), 7.30 (s, 1H), 7.17 (d, J = 9.1 Hz, 2H).
2,5-dimethyl-3-((4-nitrophenyl)thio)benzene-1,4-diol (8) Data: Flash chromatography eluent, n-hexane =100% with reaction time of 1 min, then ethyl acetate/n-hexane = 1:1 for 12 min. Fine yellow crystals after drying the solvent on rotor vapor. 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.08 (d, J = 8.7 Hz, 2H), 7.09 (d, J = 8.7 Hz, 2H), 6.82 (s, 1H), 4.77 (s, 2H), 2.35 – 2.15 (m, 6H). 13C NMR (CDCl3, 100 MHz) δ (ppm) 125.76 (d, J = 7.3 Hz), 124.30, 121.14, 77.34, 16.39, 13.79.
RESULTS
HSA Thiol-BQ Reactions
Loss of thiol reactivity to DTNB was observed with both fresh HSA and DTT reduced HSA when they were incubated with BQ before reacting with DTNB. In order to confirm the thiol residue as the target for BQ, the thiol blocking reagent N-ethylmaleimide (NEM) was incubated with DTT-reduced HSA followed by dialysis before addition of DTNB. Figure 1 shows that BQ and NEM resulted in 72.3 ± 2.6% and 90.4 ± 2.3% loss, respectively, of HSA thiol-DTNB reactivity after 5 min at 21:1 molar ratios of BQ:HSA and NEM:HSA. A paired t-test of the thiol losses demonstrated a significant difference (α < 0.05) between BQ and NEM induced thiol loss. Dialysis of both the HSA-BQ and HSA-NEM adducts did not result in recovery of Cys34 reactivity confirming that both BQ and NEM bind irreversibly to Cys34 on HSA. There was, however, a slight dilution of the protein concentration after dialysis. Preincubation of HSA for 30 min with BQ or NEM with subsequent DTNB reactivity resulted in 85.4 ± 3.7% and 91.5 ± 4.2% thiol loss, respectively (Figure 1). The thiol loss between BQ and NEM were not statistically significant (α = 0.16) after 30 min.
Figure 1.
Measurement of BQ binding to HSA Cys34 by loss of DTNB reactivity. HSA was treated with DTT, dialyzed and reacted with BQ. NEM was used as a positive control. (a) 0.8 mg/mL HSA reacted with 1 mM DTNB, (b) 0.8 mg/mL HSA preincubated with 1 mM BQ reacted with 1 mM DTNB, (c) 0.8 mg/mL HSA preincubated with 1 mM NEM reacted with 1 mM DTNB, (d) 1 mM DTNB.
BQ reactivity to primary amines in HSA
TNBS reactivity of accessible primary amines on non-conjugated HSA was compared to HSA that had been conjugated with BQ. There was 18.4 ± 2.9% loss of TNBS amine reactivity when BQ was incubated with HSA in 50 mM (NH4)2CO3, pH 7.4 buffer. Interestingly, BQ amine binding was enhanced to 35.6 ± 6.6 % of TNBS reactive HSA amines when BQ conjugation was performed in PBS (pH 7.4). Dialysis of the HSA-BQ adduct did not result in recovery of primary amine reactivity confirming that BQ also binds irreversibly to primary amines on HSA.
Serum Thiol – BQ Reactivity
The serum total sulfhydryl concentration ([SHT]) following reaction with DTNB for 15 min was 221 μM. Dialysis of the serum samples removed low molecular weight thiols with resultant [SHTd] being 117 μM. For the undialysized serum BQ and NEM thiol loss ([SHr]/[SHT]) after 5 min was 74.4 ± 5.6 and 89.2 ± 3.7%, respectively, showing significant difference (α < 0.05) between BQ and NEM. Preincubation for 30 min resulted in 90.7 ± 6.7 and 93.5 ± 5.9% thiol loss, respectively, which was not statistically significant (α > 0.05). Dialysis of the serum samples incubated with BQ and NEM resulted in reduced DTNB reactivity for the control serum sample and all other samples even though the post-dialysis thiol loss [SHd] per total serum thiol of the control post dialysis [SHTd] did not change. The thiol loss was reduced to 67.9 ± 4.7 for BQ and 70.1 ± 3.9% for NEM 5 min preincubations. When the BQ and NEM preincubation period with the dialyzed serum samples was extended to 30 min the thiol losses were 76.9 ± 5.3% and 82.6 ± 7.1%, respectively.
NBT-BQ/BQD Reactions
The previously reported nitrobenzenethiol assay for screening thiol reactive haptens18 was used in this study to determine the reactivity of BQ(D) electrophilic species (E). The decrease in absorption of NBT at 412 nm (ε412nm = 11068 ± 126 M−1cm−1) was measured after rapid mixing with BQ(D). As an example, Figure 2 shows the consumption of NBT with MBQ. The NBT-E reactions were measured at different time intervals from 0.1 s to 10 min under pseudo-first order conditions ([NBT] ≪ [E]).
Figure 2.
Reaction of MBQ with NBT in 50% acetonitrile in a pH 7.4 phosphate buffer at 25 °C. Absorbance readings were performed at 412 nm. Using [NBT]0 = 0.1 mM. BQ concentrations were varied from (a) 0.0 mM, (b) 0.0125 mM, (c) 0.025 mM, (d) 0.05 mM, (e) 0.1 mM, (f) 0.2 mM, (g) 0.4 mM and (h) 0.8 mM.
| (1) |
The absorbance of the remaining free thiol at time t, was used to calculate [NBT]t using the molar absorptivity of NBT.
| (2) |
Figure 3 shows how the apparent rate constant (k = −ka[NBT]0) and order of reactions with respect to electrophile were determined from the slope of the at least five initial rates (Ri) against five concentration variations of E for a fixed [NBT]. Linear plots which were obtained with all NBT- E reactions (Figure 3, r2 = 0.997) confirmed that the reactions were pseudo first order with respect to BQ(D). The order of reactions of BQ(D) and the apparent rate constants (k) are listed in Table 2. Equation 3 for initial rates, derived from Equation 1, was integrated to give Equation 4 for the overall reaction where k′=ka[E]0;
Figure 3.
Initial rate vs [MBQ]. Rate constants are calculated from the slope of the [E] vs. initial rate for NBT depletion.
Table 2.
Rate Constants Derived from the Initial Rate Methods for NBT-BQ/BQD Reactions.
| Chemical | k(s−1) | r2 | Order |
|---|---|---|---|
| BQ (pH 7.4) | 6.96 × 10−1 | 0.996 | 1 |
| BQ (pH 5.5) | 1.622 | 0.989 | 1 |
|
| |||
| (EDG) pH 7.4 | |||
| MBQ | 7.75 × 10−3 | 0.994 | 1 |
| tBBQ | 1.91 × 10−3 | 0.991 | 1 |
| DMBQ | 7.22 × 10−5 | 0.994 | 1 |
|
| |||
| (EWG) pH 7.4 | |||
| CBQ | 1.023 | 0.996 | 1 |
| 2,6-DCBQ | 8.72 × 101 | 0.987 | 1 |
| 2,5-DCBQ | 1.97 ×102 | 0.989 | 1 |
|
| |||
| (EDG) pH 5.5 | |||
| MBQ | 1.31 × 10−1 | 0.997 | 1 |
| tBBQ | 3.32 × 10−2 | 0.999 | 1 |
| DMBQ | 2.30 × 10−3 | 0.995 | 1 |
| (3) |
| (4) |
The NBT depletion data for all initial concentrations of the BQD electrophiles (E) were fitted into the integrated rate law (Equation 4) and the k values given in Table 3 calculated for the given [E] variations for all BQD. NBT is amenable to oxidative reactions such as disulfide formation which can compete with NBT reactions with the BQD. The side reactions for NBT were minimized by varying initial [E]0 and plotting the slopes, (−ks[E]0), obtained from plots of data fitted into Equation 4 against [E]0. By assuming that the ki ≪ ks within the overall reaction time, where ki is the rate of loss of NBT due side reactions and ks is the rate of NBT reaction with E the Equation 5 is reduced to Equation 6;
Table 3.
Rate Constants and Half Lives for BQ and BQD on pH 7.4 and 5.5.
| Chemical | ka(s−1) | ks(s−1) | t½(s) | ki(s−1) |
|---|---|---|---|---|
| BQ (pH 7.4) | 1.66 × 103 | 1.50 × 103 | 4.44 × 10−4 | 1.06 × 10−2 |
| BQ (pH 5.5) | 2.61 × 104 | 2.26 × 104 | 3.07 × 10−5 | 6.94 × 10−1 |
|
| ||||
| (EDG) pH 7.4 | ||||
| MBQ | 3.87 × 102 | 3.23 × 102 | 2.14 × 10−3 | 5.74 × 10−3 |
| tBBQ | 2.39 × 101 | 2.31 × 101 | 3.00 × 10−2 | 8.06 × 10−4 |
| DMBQ | 7.47 × 10−1 | 7.27 × 10−1 | 9.54 × 10−1 | 2.93 × 10−4 |
|
| ||||
| (EWG) pH 7.4 | ||||
| CBQ | 2.18 × 105 | 2.62 × 105 | 2.64 × 10−6 | 3.78 ×10−1 |
| 2,6-DCBQ | 1.60 × 106 | 1.21 × 106 | 5.74 × 10−7 | 2.98 |
| 2,5-DCBQ | 2.92 × 106 | 2.83 × 106 | 2.45 × 10−7 | 2.99 × 10−1 |
|
| ||||
| (EDG) pH 5.5 | ||||
| MBQ | 1.51 × 103 | 1.60 × 103 | 4.34 × 10−4 | 1.71 × 10−3 |
| tBBQ | 3.60 × 102 | 3.37 × 102 | 2.06 × 10−3 | 3.33 × 10−2 |
| DMBQ | 1.05 × 101 | 1.25 × 101 | 5.56 × 10−2 | 1.48 × 10−3 |
| (5) |
| (6) |
Pseudo-first-order rate constants were calculated as the slopes of the resultant linear curves ([E] vs −ka[E]0), and intercept gave the pseudo-first-order rate constant ki for the side reaction. The rate constants ka, ks and ki, are listed in Table 3. The rate constant values for ka and ks were not significantly different for all BQD. There was an increase in the disulfide formation or loss of NBT for chlorine substituted BQ as indicated by higher ki values (ranging from 2.98 s−1 for CBQ to 0.387s−1 for 2,6-DCBQ ). The rate constants in Tables 2–3 demonstrate that reactivity of BQ decreased significantly when electron donating groups were added with the observed order (CH3)3C < CH3 < H. The reaction half-lives (t1/2) ranged from 0.4 ms (BQ) to 0.95 s (tBBQ). The reactivity of BQ was enhanced significantly when the substituents were electron withdrawing. The order of reactivity was BQ < CBQ < 2,6-DCBQ < 2,5-DCBQ as demonstrated by the k values in Table 2–3. In comparison, the rate constants for electron withdrawing substituents (EWG) substituents were much higher than those of electron donating substituents (EDG) at pH 7.4. For example the rate constant for CBQ was 132 fold higher than that for MBQ and that of 2,5- DCBQ was 2.5×106 higher than that of DMBQ. The kinetics at pH 5.5 for BQD with EWG were too fast to be measured on the stopped-flow spectrophotometer.
Benzoquinone Derivatives and Free Radical Formation
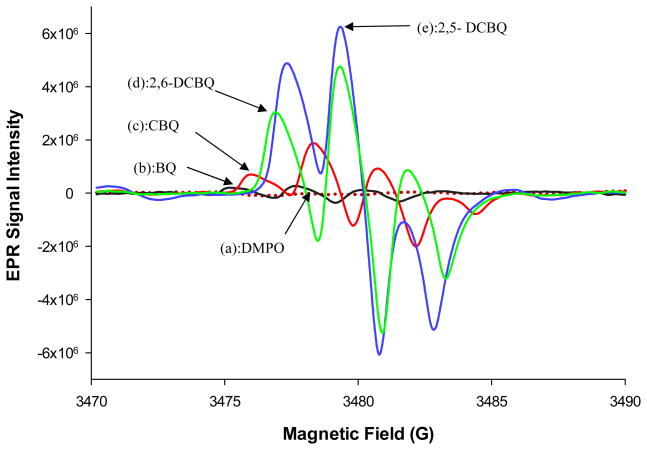
Figures 4(a–e) show the EPR signals that were observed with EWG substituted BQD. No EPR signals were observed with free DMPO and all EDG substituted BQD. The EPR signal intensity was in the order; 2,5-DCBQ > 2,6-DCBQ > CBQ > BQ. This trend in EPR intensity signal was the same trend which was observed with pseudo-first order rate constants. Reduction of the BQ resulted in a weak signal of approximate 1:2:1, triplet line spectrum (aH2 = aH3 = aH5 = aH6 = 2.4 G at pH 7.4). Under the same conditions (50 mM DMPO and pH 7.4) there was a 5 fold increase in SQ· yield from reduced CBQ compared to the reduced BQ radical. This demonstrated that the chlorine substituent has an effect on SQ radical yield. CBQ reduction gave a quartet of lines, with a peak height ratio of 1:3:3:1 and hyperfine splitting of aH3 = 2.2 G, aH5 = 2.4 G and aH6 = 2.3 G as shown in Figure 4. The addition of another chlorine atom (2,6-DCBQ) resulted in a 3 fold increase in signal intensity of the corresponding SQ radical compared to the EPR signal from CBQ. 2,6-DCBQ gave the expected triplet line spectrum (1:2:1), aH3 = aH5 = 2.5 G. There was an observed 1.3 fold increase in the signal intensity signifying an increase in stabilization effect when the second chlorine was put on the 5th position of the ring to form 2,5-DCBQ. A triplet line spectrum (1:2:1) was also observed with 2,5-DCBQ, with aH3 = aH6 = 2.2 G. There was an observed shift in peaks to higher magnetic field and g-value whenever a chlorine atom was added or changed positions on the BQ and the same trend of increase (depending on number and position of chlorine atom) was observed on EPR signal intensity.
Figure 4.
Effects of chlorine substitution on BQ on EPR signal intensity for (a) DMPO,(b) BQ (c) CBQ, (d) 2,6-DCBQ and (e) 2,5-DCBQ in 50 mM phosphate buffer, 100 mM DMPO and 50 μM of the corresponding BQD at 25 °C.
The Effect of pH on Radical formation
The determination of the effect of pH on radical formation was done using phosphate buffer from pH 5.5 to pH 8.6. Using 2,6-DCBQ as an example, Figure 5 shows that there was no EPR signal detected when pH was below 6.5. The increase in pH from 6.5 introduces more OH− ions into the system which act as a reducing agent to quinones giving semiquinone radicals.23 The peak signal intensity went up from pH 6.5 to 7.6 and then down after pH 7.6. Under the aerobic conditions of these experiments the oxygen acts as a fast and efficient radical quencher whilst forming a superoxide or peroxy radical (ROO·).23 The decrease in signal intensity peak after pH 7.4 is attributed to the peroxy radicals which are reported to form from dissolved oxygen24 The spectrum was composed of a quartet of lines, with a peak height ratio of 1:2:2:1, with parameters including hyperfine constants, aH = 14.8 G and was attributed to the DMPO-OH. These parameters are typical of a DMPO-OH adduct EPR signal generated by the reaction of OH with DMPO.25 The peaks at pHs > 7.6 increased in intensity then went down as the DMPO-OH adduct peaks appeared and increased in intensity. A proposed scheme leading to DMPO-OH formation is shown in Figure 6. The electron g-factors of BQD radicals changed with increasing number of chlorine atoms and position(s) of the chlorine substituents on the BQ ring (Table 4). Compared to BQ radical (g = 2.001382) the CBQ (g = 2.001534, Δg = 0.000152) and the 2,6-DCBQ (g = 2.001585, Δg = 0.000203) and changing chlorine position to 2,5-DCBQ (2.002077, Δg = 0.000695). This increase in g-value was harmonious with the increased spin orbit coupling that is expected with increasing number of chlorine atoms.
Figure 5.
The relationship between EPR signal intensity and rate constants due to pH variation for [2,6-DCBQ]0 = 50 μM in 50 mM of phosphate buffer and 100 mM DMPO at 25 °C. (a) EPR signal due to DMPO-SQ adduct formation (b) EPR signal due to DMPO-OH and trace (c) shows the effect of pH on the rate of reaction between 2,6-DCBQ and NBT. There is an inverse relationship between the rate constant and EPR DMPO-SQ signal.
Figure 6.
Schematic routes which lead to DMPO-SQ and DMPO-OH EPR active adduct formation.
Table 4.
Summary of peak shift and g-factor values.
| Chemical | Peak shift (mT) | g-factor |
|---|---|---|
| BQ | 0 (Standard) | 2.001382 |
| CBQ | 0.0782 | 2.001534 |
| 2,6-DCBQ | 0.176 | 2.001585 |
| 2,5-DCBQ | 0.215 | 2.002077 |
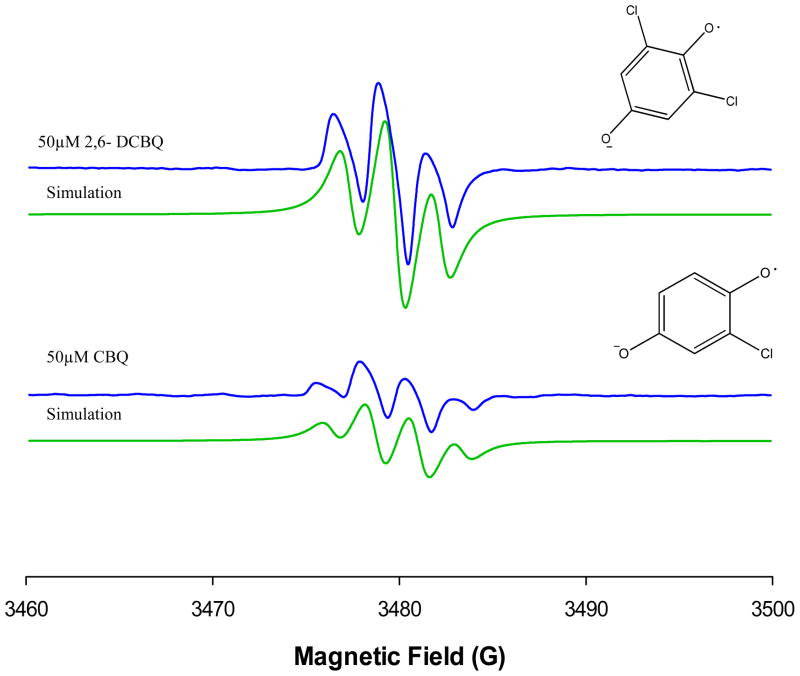
EPR Spectrum Simulations
The main objective with respect to spectrum simulations was to obtain a satisfactory simulation of the room-temperature EPR spectrum for BQ with EWG using CBQ and 2,6-DCBQ as examples (see Figure 7). The fitting of the data were simple and the best fit values were obtained with hyperfine splitting constant, aH3 = aH5 = 2.5 G for 2,6-DCBQ and aH3 = 2.2 G, aH5 = 2.4 G and aH6 = 2.3 G for CBQ. The adopted values for hyperfine splitting constant are most likely valid for the BQD radical formation at physiological pH.
Figure 7.
Computer simulations on the effects of chlorine substitution on BQ on EPR signal intensity data shown in Figure 5(c) CBQ and (d) 2,6-DCBQ. This model gave a reasonably good fit to the experimental data in both hyperfine splitting constants and the signal intensity.
NBT effect on SQ radical formation
Figure 8 shows that the addition of NBT to a DMPO and 2,6-DCBQ mixture decreased the amount of EPR signal formed even when the DMPO was in excess over both the NBT and the 2,6-DCBQ. Control experiments showed no reactivity between DMPO and NBT. Figure 8 shows that mixing NBT and 2,6-DCBQ in equimolar quantities resulted in about 50% loss of EPR signal. When NBT was 3-fold in excess over 2,6-DCBQ the EPR signal loss was > 90% in agreement with the NBT-2,6-DCBQ reactivity that was observed. The loss of EPR signal is due to NBT-BQ adduct formation (Scheme 2) overpowering the production of DMPO-SQ adduct formation. This shows that the rate of production of free radicals from 2,6-DCBQ is much slower than the rate of adduct formation between NBT and the 2,6-DCBQ. The NBT quenches the system of any available 2,6-DCBQ thus no radicals are produced. This suggests that the free radical mechanism is overshadowed by the MA and therefore has minimal contribution to the rate constants obtained for the rapid depletion of NBT.
Figure 8.
Plot of depletion of DMPO-SQ signal versus [NBT] at 25 °C. [DMPO]0 =100 mM, [2,6- DCBQ]0 = 0.05 mM, and varied [NBT]= (a) 0 mM, (b) 0.02 mM, (c) 0.04 mM, (d) 0.08 mM, (e) 0.16 mM, (f) 0.32 mM and 0.6 mM in 100 mM phosphate buffer of pH 7.4.
Scheme 2.
The proposed MA reaction between MBQ and p-nitrobenzenethiol
Characterization of BQD-NBT Adducts
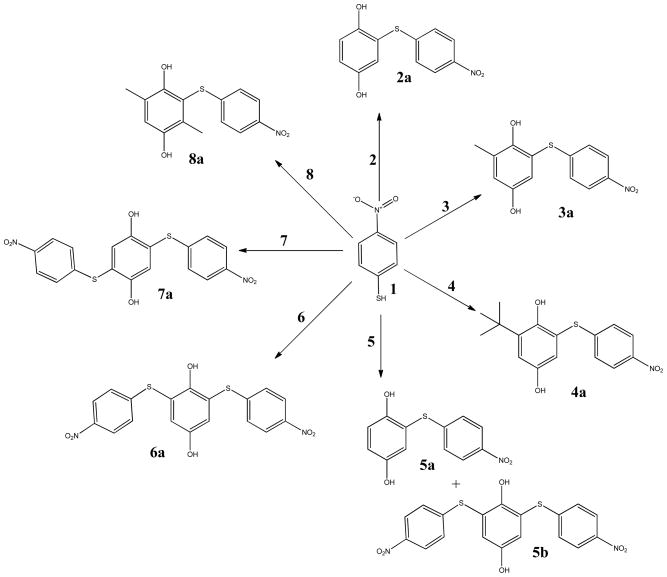
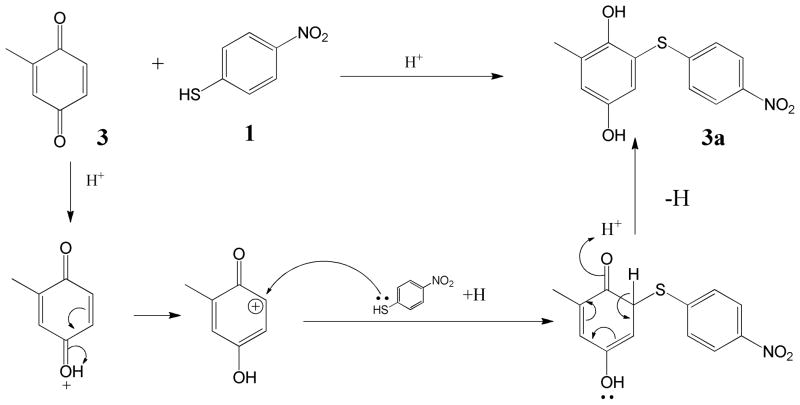
Based on NMR and MS characterization of the NBT-BQ(D) reaction products NBT was shown to react with BQ 2 to give a 1:1 ratio product 2-((4-nitrophenyl) thiol) benzene-1, 4-diol 2a in phosphate buffer at pH 5.5 after 1 h. The electron-donating group substituted benzoquinones reacted through MA (Scheme 2) giving a 1:1 ratio with NBT; MBQ 3 gave 2-methyl-6-((4-nitrophenyl)thio)benzene-1,4-diol 3a, tBBQ 4 gave 2-(tert-butyl)-6-((4-nitrophenyl)thio)benzene-1,4-diol 4a and DMBQ 8 gave 2,5-dimethyl-3-((4-nitrophenyl)thio)benzene-1,4-diol 8a. The reaction between CBQ 5 and NBT was involved in 2 reaction mechanisms in the following order; MA reaction followed by vinylic substitution (Scheme 3). Reaction between NBT and CBQ gave 2 products as confirmed by ESI-MS (Figure 9(a)); a monosubstituted benzoquinone, 2-((4-nitrophenyl)thio)benzene-1,4-diol 5a, and disubstituted benzoquinone; 2,6-bis((4-nitrophenyl)thio)benzene-1,4-diol 5b. The reaction between the di-substituted chloro-benzoquinones 2,5-DCBQ and 2,6-DCBQ gave corresponding di-substituted benzoquinone with chlorines being substituted by NBT in a 1:2 ratio (BQD:NBT). Reaction between 2,5-DCBQ 6 and NBT gave 2,5-bis((4-nitrophenyl)thio)benzene-1,4-diol 6a whilst 2,6-DCBQ 7 gave a corresponding di-substituted benzoquinone,2, 5-bis((4-nitrophenyl)thio)benzene-1,4-diol 7a.
Scheme 3.
The proposed reaction between CBQ and p-nitrobenzenethiol
Figure 9.
Negative ESI-MS spectra of a the p-nitrobenzenethiol adduct(s) formation with (a) CBQ giving a mono-NBT-hydroxyl BQ adduct (m/z 415.00540) and a di-NBT-hydroxyl BQ adduct (m/z 262.01746) and spectrum (b) MBQ giving a mono-NBT-hydroxyl MBQ adduct (m/z 276.03259).
The BQ(D)-NBT reaction is acid catalyzed as shown by the faster reaction rate at lower pH. Acid facilitated the creation of a carbocation center on which the nucleophile attacks (Schemes 3 and 4). The reactions between electron donating substituted benzoquinone and NBT were direct 1:1 ratio MA reactions (Schemes 3). NBT went onto position 6 for mono-substituted BQ and position 3 for di-substituted BQ (Scheme 1), and this was confirmed by ESI-MS (Figure 9(b)). For electron withdrawing substituted benzoquinones, the carbocation center created on the carbon on which chlorine atom was attached and this was the target for the –SH group on NBT followed by the substitution of the chlorine atom. In contrast to the methyl and tert-butyl substituted BQ the mechanism for the chlorine substituted BQs the reaction mechanism was direct nucleophilic vinylic substitution (SNV). As a result of the electron withdrawing substituents CBQ gave 2-((4-nitrophenyl)thio)benzene-1,4-diol 5a as a product and 2,5-DCBQ gave 2, 5-bis((4-nitrophenyl)thio)benzene-1,4-diol 7a.
DISCUSSION
Murine local lymph node (LLNA) studies rank BQ as an extreme dermal sensitizer with an EC3 (threshold) value of 0.013%.26 BQ covalent binding to proteins resulting in immunogenic complexes has been attributed to BQ’s reactivity as a Michael acceptor. New evidence27,28 on both the quinone’s unique electrophilic chemistry and high reactivity, however, provides alternative hypothesis on the possible contributory role of other reaction pathways to BQ’s overall reactivity towards nucleophiles such as cysteinyl amino acids on proteins.29 The impact of substituents on BQ’s reactivity and ultimate in vivo allergenicity has not been previously assessed.
Binding experiments with HSA demonstrated that Cys34 (the only reduced thiol in HSA) on the protein is the preferred target for BQ binding and the bond formed is an irreversible covalent bond. BQ was shown to have high affinity for thiols when serum was used as the source for thiols. Comparison of BQ and NEM blocked thiols pre- and post-dialysis showed that the ratios [SHr]/[SHT] and [SHd]/[SHTd] were equal demonstrating the binding of BQ to both high and low molecular weight thiols such as cysteine and glutathione. The binding of BQ to cysteine and glutathione may result in reduced BQ available for protein binding, but its extreme potency26 in the LLNA assay shows that the concentrations absorbed through the skin during the in vivo assay are high enough to overcome any clearance which may result from low molecular weight thiol binding. The observed 85.5 % loss of reactivity to thiols compared to 18 % loss of reactivity to primary amines under identical conjugation conditions, demonstrates that BQ is much more reactive to thiols than amines, in agreement with previously reported observations.30
The thiol nucleophile, NBT, was used to measure reactivity of six BQD. NBT has previously been utilized as a model nucleophile where relative reactivity of electrophilic allergens to NBT was highly correlated to LLNA EC3 values.18 All the BQD tested rapidly reacted with NBT. There was, however, a 90 fold decrease in reactivity of the BQ ring when MBQ was reacted with NBT. The methyl group donates electrons into the BQ π system leading to an increase in electron density on the π system with consequent reduction of the partial positive charges on the electrophilic carbons on BQ. The π electrons also repelled the electrons on the –SH group on NBT thus slowing down the reaction. The positive inductive effect became more apparent when the methyl group was replaced with the tert-butyl group. The reaction dropped by approximately 4 fold from MBQ to tBBQ. This was due to more electron donating ability of tert-butyl compared to methyl group. The steric hindrance offered by tert-butyl, to the incoming NBT, compared to the methyl group could also have contributed the decreased reactivity of tBBQ. For NBT-DMBQ reactions, the further 26-fold loss of reactivity relative to tBBQ can be attributed to the increased positive inductive effect of two methyl groups in close proximity to the reacting β carbocations. The repulsive force of π electron cloud around the electrophilic carbon was increased by having two methyl groups on the ring.
With BQD with electron withdrawing substituents the pseudo-first order rate constants for NBT reactivity at pH 7.4 increased in the following order BQ < CBQ < 2,6-DCBQ < 2,5-DCBQ (Table 4). The effect of the increased negative inductive effect on the rate constants is apparent from CBQ to the dichloro-substituted BQD. When 2,6-DCBQ was reacted with NBT there was an 85-fold increase in NBT reactivity compared to CBQ. There was an observed increase in rate constants by 2 fold between 2,6-DCBQ and 2,5-DCBQ with the latter being faster. The difference in reactivity between the dichloro substituted derivatives may be attributed to the fact the 2,5-DCBQ has its chlorine atoms in para positions to each other which makes the carbon atoms on which the chlorine atoms are attached more partially positive due to inductive withdrawal of electrons, unlike the 2,6-DCBQ which has chlorine atoms on meta positions to each other and do not influence each other by inductive electron withdrawal. This makes 2,5-DCBQ more reactive than 2,6-DCBQ. This strongly agrees with the findings of Enoch et al. 31 in which a direct relationship between electrophilic effects of a chemical and rate reaction by Michael addition reaction were observed and used in an electrophilicity index for prediction of skin sensitizaztion potential. For both 2,6-DCBQ and 2,5-DCBQ, the –SH on NBT adds on to position on which the chlorine atom is attached by MA and then nucleophilic elimination of the chlorine atoms (reaction of 1st mole on Scheme 3).

The structure for 2,5-DCBQ is such that it will undergo MA and then nucleophilic substitution onto position 2 and 5 which have the higher electrophilicity. 2,6-DCBQ where positions 2 and 6 are not influenced by the negative inductive effect of the chlorine atoms possess a lesser partial positive charge on these positions This makes 2,5-DCBQ more reactive than 2,6-DCBQ. Increased rate constants increased several-fold for MBQ, DMBQ and tBBQ when NBT reactivity was measured at pH 5.5 suggesting that the MA reactions are acid catalyzed, Figure 5c shows an inverse relationship between rate constant and pH. The acid catalysis is more pronounced for CBQ, 2,5-DCBQ and 2,6-DCBQ reactions performed at pH 5.5 which could not be measured using the stopped flow technique. The fact that rate constants for MBQ, DMBQ and tBBQ had increased several-fold under pH 5.5 but were still lower than those of CBQ, 2,5-DCBQ and 2,6-DCBQ at pH 7.4 demonstrates the agreement on the effect of electron donating versus electron withdrawing substituents on BQ reactivity under pH’s 7.4 and 5.5.
BQ has been hypothesized to haptenate proteins via MA. The production of semiquinone (SQ) radicals as a mechanism of BQ(D) protein haptenation was investigated and EPR signal intensity was of the order; 2,5-DCBQ > 2,6-DCBQ > CBQ > BQ. No EPR signals were observed with methyl- and tert-butyl-substituted BQD. BQ generated radicals are known to form through the one electron reduction of BQ, followed by hemolytic cleavage of the O-O bonds on the carbonyl functional group. The resulting SQ radical may potentially bind directly to a protein.32 Thus the presence of the SQ radical indicated the potential of free radical mechanisms as another mechanism of BQ(D) covalent modification of proteins. Increased production of free radicals from chloro-substituted BQD (Figure 4) is a result of the high negative inductive effect arising from the highly electronegative chlorines. This activates the BQ in contrast to the positive inductive effect of the electron donating groups. The increase in g-values from the mono-chloro-substituted to the dichloro-substituted BQD is harmonious with the increased spin orbit coupling that will be expected with increasing number of chlorine atoms. Halogenation of BQ has been known to increase the persistence of SQ radicals.33 The EPR results show that the yield of the SQ radicals resulting from chlorine substituted BQD depend on pH and on the position and number of chlorines on the SQ ring.
The trend in EPR intensity signal agreed with the observed order of reactivity indicating that inductive effects have a dominant influence on the chemistry of the BQD compared with other factors such as steric hindrance. The effect of pH on radical formation (Figure 5) highlights the sensitivity of the SQ radicals to either acidic or basic conditions. The increase in pH from 6.5 to 7.6 might have introduced more OH− ions into the system which act as a reducing agent to quinones giving semiquinone radicals.23 Beyond pH 7.6 the OH− effect might have been overcome by the presence of produced oxygen which has been reported to act as a fast and efficient SQ radical quencher, forming a superoxide radical or peroxy radical (ROO·)23 in the process (Figure 6). Peroxy radicals have been shown to produce dissolved oxygen in alkaline conditions thus availing more oxygen which quenches the SQ radical. The observed decrease in SQ radical peaks intensity at pH > 7.6 (Figure 4) as the peaks of DMPO-OH adduct simultaneously started appearing can explain the minimum or no role of OH− and oxygen quenching in the temporary increase in SQ radical intensity at pHs 7.6 and below followed by oxygen quenching at pHs beyond 7.6.
The contribution of a free radical pathway to protein haptenation may not be significant because of lack of production of SQ radicals at acidic pH, in contrast with the increased NBT reactivity which was observed at pH 5.5. Skin pH is around 5.5 and lack of formation of radicals shown herein means that BQ and BQD binding to proteins will occur via MA with additional vinylic substitution where the substituents such as chlorine are good leaving groups. The dominance MA versus free radical formation was also demonstrated with the inclusion of NBT to (DMPO + BQ) mixture (pH 7.4) which resulted in quenching of the EPR signal even when the DMPO ≫ NBT and the BQD. The loss of EPR signal was due to NBT-BQ adduct formation (Scheme 2) which formed at rate that was faster than the production of DMPO-SQ adduct formation. It may have been possible that NBT quenching of the EPR signal was due to the SQ radical forming quickly and then reacting with NBT to form an EPR silent adduct. This argument was, however, dismissed when MS and NMR characterization of adducts formed in the reaction mixture showed no presence of NBT-SQ adducts. NBT mops the system of any available BQD thus no radicals are produced. This aspect proves that the free radical mechanism plays a minimal role, at best, in BQD binding. The dominance of MA as a pathway is further supported by quantitative mechanistic read-across based estimation of BQ EC3 values which utilized known potency data for sensitizers in the MA domain. A theoretical EC3 value of 0.013% was obtained in agreement with actual LLNA EC3 value of 0.01%.26
The inclusion of substituents on BQ has been shown to have a profound effect on the overall chemistry of BQ. This is manifested in an increased or decreased reactivity of BQD toward NBT and the emergence of other mechanistic pathways such as free radical mechanism and vinylic substitution (Scheme 3). NMR results also demonstrate the variations in the stoichiometry of the NBT-BQD reactions as well as the shifting of positions for nucleophilic addition. From the hapten hypothesis14 perspective the effect of vinylic substitution (Scheme 3) for the chloro-substituted BQD would not lead to any immunogenic adducts that are different from those formed with BQ as this step is subsequent to MA which results in adduct formation. The substituted chlorine leaves as a salt which would not be expected to modify any proteins.
CONCLUSION
It can be concluded that MA is the predominant mechanism for BQ and methyl-BQD haptenation of proteins and that MA followed by vinylic substitution is dominant for chlorine-BQD. Based on the findings in these studies SQ radical induced binding of proteins may play a minimal to no role in the development of BQD sensitization. Subsequent studies such as the LLNA will be able to confirm the effect of substituents on the allergenic potency of BQD.
Acknowledgments
Funding
This work was supported by an Interagency Agreement with the NIEHS (IAG#Y1-ES-0001-12) and Grant Number CHE 1056311 from the National Science Foundation.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
ABBREVIATIONS
- ACD
allergic contact dermatitis
- AA
acylation
- BQ
p- benzoquinone
- BQD
benzoquinone derivatives
- CBQ
chlorobenzoquinone
- Cys
cysteine
- 2, 5-DCBQ
2,5-dichlorobenzoquinone, 2,6-DCBQ, 2,6-dichlorobenzoquinone
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- DTNB
5,5′-dithiobis-(2-nitrobenzoic acid)
- DTT
dithiothreitol
- GSH
glutathione
- HSA
human serum albumin
- 2-MBQ
2 methyl- benzoquinone
- MA
Michael addition
- NEM
N-ethylmaleimide
- NBT
nitrobenzenethiol
- SNAr
nucleophilic aryl substitution
- SN1/2
nucleophilic substitution
- TNBS
trinitrobenzene sulfonic acid
- QSARs
quantitative structure-activity relationships
- 2-tBBQ
2-tertbutyl benzoquinone
- SQ
semiquinone
- SBF
Schiff base formation
- SS
skin sensitization
- SH
sulfhydryl
References
- 1.Al-Tamrah SA. Colorimeteric Determination of p-Benzoquinone, Hydroquinone and Paracetamol. Arab Gulf J Scient Res, Math Phys Sci. 1988;A6(3):363–375. [Google Scholar]
- 2.Cenas NK, Pocius AK, Kulys JJ. Bioelectrocatalytic Conversion of Substances on Polymer-Modified Electrodes. Bioelectrochem and Bioenerg. 1984;12(5–6):583–591. [Google Scholar]
- 3.Samsonova LV, Nikiforov GA. Direct and Sensitized Photolysis of 1,4-Benzoquinone Diazides in Organic-Solvents. Bull of the Acad of Scie of the Ussr Div of Chem Scie. 1984;33(5):943–947. [Google Scholar]
- 4.Kim SB, Cai C, Faust MD, Trenkle WC, Sweigart DA. A Water-Stable Organometallic Rhodium Quinone Catalyst and Its Recyclability. Organometallics. 2009;28(8):2625–2628. [Google Scholar]
- 5.Zweig G, Hitt JE, Cho DH. Mode of Action of Dipyridyls and Certain Quinone Herbicides. J Agri and Food Chem. 1969;17(2):176. [Google Scholar]
- 6.Meazza G, Dayan FE, Wedge DE. Activity of quinones on Colletotrichum species. Journal of Agricultural and Food Chemistry. 2003;51(13):3824–3828. doi: 10.1021/jf0343229. [DOI] [PubMed] [Google Scholar]
- 7.Wolf SJ, Timko MP. Invitro Root Culture - A Novel-Approach to Study the Obligate Parasite Striga-Asiatica (L) Kuntze. Plant Sci. 1991;73(2):233–242. [Google Scholar]
- 8.Hansson C, Ahlfors S, Bergendorff O. Concomitant contact dermatitis due to textile dyes and to colour film developers can be explained by the formation of the same hapten. Contact Derm. 1997;37(1):27–31. doi: 10.1111/j.1600-0536.1997.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 9.Wester RC, Melendres J, Hui XY, Cox R, Serranzana S, Zhai HB, Quan DY, Maibach HI. Human in vivo and in vitro hydroquinone topical bioavailability, metabolism, and disposition. J Toxicol and Environ Health-Part A. 1998;54(4):301–317. doi: 10.1080/009841098158863. [DOI] [PubMed] [Google Scholar]
- 10.Uddin S, Rauf A, Kazi TG, Afridi HI, Lutfullah G. Highly sensitive spectrometric method for determination of hydroquinone in skin lightening creams: application in cosmetics. Int J Cosm Scie. 2011;33(2):132–137. doi: 10.1111/j.1468-2494.2010.00599.x. [DOI] [PubMed] [Google Scholar]
- 11.DeCaprio AP. The toxicology of hydroquinone - Relevance to occupational and environmental exposure. Crit Rev Toxicol. 1999;29(3):283–330. doi: 10.1080/10408449991349221. [DOI] [PubMed] [Google Scholar]
- 12.Roberts DW, Aptula AO. Does the extreme skin sensitization potency of benzoquinone result from special chemistry? Contact Derm. 2009;61(6):332–336. doi: 10.1111/j.1600-0536.2009.01646.x. [DOI] [PubMed] [Google Scholar]
- 13.Johansson SGH, Emilsson K, Grotli M, Borje A. Structural Influence on Radical Formation and Sensitizing Capacity of Alkylic Limonene Hydroperoxide Analogues in Allergic Contact Dermatitis. Chem Res Toxicol. 2010;23(3):677–688. doi: 10.1021/tx900433n. [DOI] [PubMed] [Google Scholar]
- 14.Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds.. II. J Exp Med. 1936;64(4):625–639. doi: 10.1084/jem.64.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenkranz HS, Klopman G, Zhang YP, Graham C, Karol MH. Relationship between allergic contact dermatitis and electrophilicity. Environ Health Perspect. 1999;107(2):129–132. doi: 10.1289/ehp.99107129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chipinda I, Hettick JM, Simoyi RH, Siegel PD. Oxidation of 2-mercaptobenzothiazole in latex gloves and its possible haptenation pathway. Chem Res Toxicol. 2007;20(8):1084–1092. doi: 10.1021/tx700139g. [DOI] [PubMed] [Google Scholar]
- 17.Smith PA, Bowerbank CR, Savage PB, Drown DB, Lee ML, Alexander W, Jederberg WW, Still K. Conjugation of 7-oxodehydroabietic acid to lysine, a haptenation mechanism for an oxidized resin acid with dermal sensitizing properties. Appl Occup Environ Hyg. 1999;14(3):171–176. doi: 10.1080/104732299303133. [DOI] [PubMed] [Google Scholar]
- 18.Chipinda I, Ajibola RO, Morakinyo MK, Ruwona TB, Simoyi RH, Siegel PD. Rapid and Simple Kinetics Screening Assay for Electrophilic Dermal Sensitizers Using Nitrobenzenethiol. Chem Res Toxicol. 2010;23(5):918–925. doi: 10.1021/tx100003w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuzumi S, Nakanishi I, Maruta J, Yorisue T, Suenobu T, Itoh S, Arakawa R, Kadish KM. Formation of radical anions in the reaction of p-benzoquinone and C-60 with alkoxide ions. J Amer Chem Soc. 1998;120(27):6673–6680. [Google Scholar]
- 20.Schultz TW, Yarbrough JW, Johnson EL. Structure-activity relationships for reactivity of carbonyl-containing compounds with glutathione. SAR QSAR Environ Res. 2005;16(4):313–322. doi: 10.1080/10659360500204152. [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb HE, Kotlyar V, Nudelman A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J Org Chem. 1997;62(21):7512–7515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 23.Davies KJA. The evolution of Free Radical Biology & Medicine Still radical after a quarter of a century. Free Rad Biol and Med. 2010;49(12):1825–1833. doi: 10.1016/j.freeradbiomed.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Valgimigli L, Amorati R, Fumo MG, DiLabio GA, Pedulli GF, Ingold KU, Pratt DA. The unusual reaction of semiquinone radicals with molecular oxygen. J Org Chem. 2008;73(5):1830–1841. doi: 10.1021/jo7024543. [DOI] [PubMed] [Google Scholar]
- 25.Shinde SS, Hay MP, Patterson AV, Denny WA, Anderson RF. Spin Trapping of Radicals Other Than the (center dot)OH Radical upon Reduction of the Anticancer Agent Tirapazamine by Cytochrome P(450) Reductase. J Amer Chem Soc. 2009;131(40):14220. doi: 10.1021/ja906860a. [DOI] [PubMed] [Google Scholar]
- 26.Roberts DW, Patlewicz G, Kern PS, Gerberick F, Kimber I, Dearman RJ, Ryan CA, Basketter DA, Aptula AO. Mechanistic applicability domain classification of a local lymph node assay dataset for skin sensitization. Chem Res Toxicol. 2007;20(7):1019–1030. doi: 10.1021/tx700024w. [DOI] [PubMed] [Google Scholar]
- 27.Yu P, Strug I, Cafarella TR, Seaton BA, Krantz A. Site-specific crosslinking of annexin proteins by 1,4-benzoquinone: a novel crosslinker for the formation of protein dimers and diverse protein conjugates. Org Biomol Chem. 2012;10(23):4500–4504. doi: 10.1039/c2ob25460c. [DOI] [PubMed] [Google Scholar]
- 28.Chan K, Jensen N, O’Brien PJ. Structure-activity relationships for thiol reactivity and rat or human hepatocyte toxicity induced by substituted p-benzoquinone compounds. J Appl Toxicol. 2008;28(5):608–620. doi: 10.1002/jat.1312. [DOI] [PubMed] [Google Scholar]
- 29.Smith PA, Bowerbank CR, Savage PB, Drown DB, Lee ML, Alexander W, Jederberg WW, Still K. Conjugation of 7-oxodehydroabietic acid to lysine, a haptenation mechanism for an oxidized resin acid with dermal sensitizing properties. Appl Occ and Envir Hyg. 1999;3:171–176. doi: 10.1080/104732299303133. [DOI] [PubMed] [Google Scholar]
- 30.Gerberick GF, Vassallo JD, Foertsch LM, Price BB, Chaney JG, Lepoittevin JP. Quantification of chemical peptide reactivity for screening contact allergens: A classification tree model approach. Toxicol Sci. 2007;97(2):417–427. doi: 10.1093/toxsci/kfm064. [DOI] [PubMed] [Google Scholar]
- 31.Enoch SJ, Cronin MTD, Schultz TW, Madden JC. Quantitative and mechanistic read across for predicting the skin sensitization potential of alkenes acting via Michael addition. Chem Res Toxicol. 2008;21(2):513–520. doi: 10.1021/tx700322g. [DOI] [PubMed] [Google Scholar]
- 32.Karlberg AT, Bergstrom MA, Borje A, Luthman K, Nilsson JLG. Allergic contact dermatitis-formation, structural requirements, and reactivity of skin sensitizers. Chem Res Toxicol. 2008;21(1):53–69. doi: 10.1021/tx7002239. [DOI] [PubMed] [Google Scholar]
- 33.Song Y, Buettner GR, Parkin S, Wagner BA, Robertson LW, Lehmler HJ. Chlorination Increases the Persistence of Semiquinone Free Radicals Derived from Polychlorinated Biphenyl Hydroquinones and Quinones. J Org Chem. 2008;73(21):8296–8304. doi: 10.1021/jo801397g. [DOI] [PMC free article] [PubMed] [Google Scholar]