Abstract
Objective
To evaluate and compare antioxidant activities of the aqueous extracts of unripe plantain (Musa paradisiaca), assess their inhibitory action on sodium nitroprusside induced lipid peroxidation in rat pancreas in vitro and to characterize the main phenolic constituents of the plantain products using gas chromatography analysis.
Methods
Aqueous extracts of plantain products (raw, elastic pastry, roasted and boiled) flour of 0.1 g/mL (each) were used to determine their total phenol, total flavonoid, 1,1 diphenyl-2 picrylhydrazyl (DPPH) and hydroxyl (OH) radical scavenging ability. The inhibitory effect of the extracts on sodium nitroprusside induced lipid peroxidation was also determined.
Results
The results revealed that all the aqueous extracts showed antioxidant activity. The boiled flour had highest DPPH and OH radical scavenging ability while raw flour had the highest Fe2+ chelating ability, sodium nitroprusside inhibitory effect and vitamin C content. The antioxidant results showed that elastic pastry had the highest total phenol and total flavonoid content. Characterization of the unripe plantain products for polyphenol contents using gas chromatography showed varied quantity of apigenin, myricetin, luteolin, capsaicin, isorhaemnetin, caffeic acid, kampferol, quercetin, p-hydroxybenzoic acid, shogaol, glycitein and gingerol per product on the spectra.
Conclusions
Considering the antioxidant activities and ability to inhibit lipid peroxidation of unripe plantain, this could justify their traditional use in the management/prevention of diseases related to stress.
Keywords: Polyphenols, Antioxidants, Musa paradisiacal, Sodium nitroprusside, Lipid peroxidation
1. Introduction
The oxidative properties of oxygen in diverse biological functions has been reported to play double edged properties of either a decrease of cell antioxidant capacity or increased amount of reactive oxygen species (ROS) which amount to oxidative stress in organisms[1]. Biomedical research shows increasingly that ROS contributes to both the initiation and the promotion of many major diseases. Free radicals and ROS also contributes to many less serious but still troubling symptoms of aging[1]. Free radical species are also very reactive and unstable thus becoming stables by stealing electrons from, proteins, lipids, carbohydrates, nucleic acids, or any nearby molecule thereby causing lipid peroxidation as well as a cascade of damage and disease[2]. In addition to ROS, reactive nitrogen species has also been implicated in surplus of publications to play a significant role in incidence of degenerative diseases[3]. Sodium nitroprusside is an antihypertensive drug, which acts by relaxing smooth vascular muscle; consequently it dilates peripheral arteries and veins[4]. However, its cytotoxicity has been reported to involve the release of cyanide and/or nitric oxide (NO) and that NO, is involved in the pathophysiology disorders such as seizure disorders, trauma, stroke etc. NO could act independently or in association with other ROS[4]. One of the major ways of fighting against degenerative diseases is to improve body antioxidant status. This could be achieved by higher consumption of vegetables and fruits. Earlier researches had proved that foods from plant origin usually contain natural antioxidants that can scavenge free radicals[5].
Natural antioxidants are powerful substances that rare capable of scavenging ROS or neutralize free radicals before they damage the body's cells. Phenolic compounds are made up of a group of secondary metabolites, which are synthesized by plants as a result of a plant's adaptation to biotic and abiotic stresses. In recent years, phenolic compounds have attracted the interest of researchers because of their antioxidant capacity; to protect the human body from free radicals, whose formation has been associated with the natural metabolism of aerobic cells. The antioxidant activity of phenolics is mainly due to their redox properties, which gives them the capacity to act as reducing agents, hydrogen donors, metal chelators, free radical scavenger and singlet oxygen quenchers[6]. The antiradical activity of flavonoids and phenols is majorly based on the structural relationship between the functional groups on their chemical structure[3]. Various methods have been developed for screening antioxidant activity of various classes of compounds. The reason for this is to search for novel natural antioxidants in plants and vegetables that may be of importance in pathologies involving reactive oxygen species, as well as preservation of food substances against oxidation[7].
Plantain (Musa paradisiaca) is a tropical fruit that constitute a staple food crop in Central and West Africa. Over 2.11 million metric tons of plantains are produced in Nigeria annually which contributes substantially to the nutrition of sub tropical local populations. Starch is the main component of plantain, as well as proteins, fat, ash, and dietary fiber. Plantains are also reported to be a great source of calcium, vitamins A, B1, B2, B3, B6, C and minerals such as potassium and phosphorus[8]. Different varieties of plantain are consumed by the households in Nigeria but the most preferred (plantain) varieties are the false horn type (locally known as ‘Agbagba’). When processed into flour, it is used traditionally for preparation of gruel which is made by mixing the flour with appropriate quantities of boiling water to form a thick paste (locally known as ‘amala’). Fresh plantain pulp (ripe or unripe) can also be roasted or boiled. These dishes were generally accompanied by stew or vegetables sauces with fish or meat, depending on the income of the household[9]. Plantain is employed in the folklore management of diseases such as ulcer, wound healing and many others due to its anti-ulcerogenic, antimicrobial, anti urolithiatic activities, analgesic properties[10]. Recently, the flavonoid leucocyanidin has been identified as the active ingredient in plantain for its anti-ulcerogenic properties[11]. This indicates that as at the time of this research there are limited publications on the phenolic profile of plantain or its products. This study therefore seeks to determine the antioxidant properties, inhibitory effect of unripe plantain products (raw, roasted, elastic pastry and boiled) on sodium nitroprusside induced lipid peroxidation as well as identify their polyphenolic constituents.
2. Materials and methods
2.1. Sample collection and preparation
“False Horn” matured green plantain (Musa paradisiaca) were bought at Oja-Oba in Akure, Ondo State. Authentications of the unripe plantains were carried out at the Department of Biology, Federal University of Technology, Akure, Nigeria.
2.2. Chemicals and equipment
Folin-Ciocalteu's phenol reagent, gallic acid and anhydrous sodium carbonate used were products of Fluka (Buchs, Switzerland). Quercetin and DPPH (2,2-diphenyl-1picrylhydrazyl), ascorbic acid and starch were products of Merck (Darmstadt, Germany), Iron chloride, Iron (II) sulphate, H2O2 (Aldrich, USA) and trichloroacetic acid (Fisher products). All other chemicals used were purchased from Rovet Scientific Limited, Benin City, Edo State, Nigeria. The distilled water used was obtained from the Chemistry Department at Federal University of Technology, Akure. Optical absorbance was measured with a UV-Visible spectrophotometer (Model 6305; Jenway, Barloworld Scientific, Dunmow, United Kingdom).
2.3. Preparations of samples
The plantain fruits (33 kg) were divided into portions to be processed into 4 different products (raw, roasted, elastic pastry and boiled).
2.4. Raw flour
The first portion of the plantain fruits (14 kg) was peeled, sliced (10 mm) and sun-dried for about 3 weeks to a constant weight (10 kg) and ground into flour. The raw flour was passed through a local made hand sieve. The fine plantain flour got was kept in an air tight container at room temperature (25 °C) for future analysis.
2.5. Elastic pastry flour
The raw flour was divided into two (keeping a portion of the raw flour and kept as mentioned above) while the other portion of the raw flour was prepared to make the thick elastic paste locally known as ‘amala’. This was achieved by stirring the flour continuously in a pot of boiling water until it is well cooked to form a thick, smooth brown elastic paste. The elastic paste was later sun-dried for about 4 weeks to constant weight (2 kg), ground into flour and also kept in an air tight container for future analysis.
2.6. Boiled plantain flour
Fresh peeled plantain (8 kg) was boiled in 5 L of tap water for twenty minutes at a temperature of 100 °C. The water was drained off and the boiled plantains were sliced (10 mm), sun-dried for about 4 weeks to a constant weight, and ground into flour. The sample was kept in an air tight container for future analysis.
2.7. Roasted plantain flour
Fresh peeled plantain fruits (8 kg) were roasted using the Nigerian traditional method. This is done by putting the plantain on wire gauze over red hot charcoal. The roasting was carried out by frequently turning the plantain to maintain even browning. The roasted plantain is a traditional snack known as ‘booli’. The roasting was done for 10 min. The roasted plantains were also later sun-dried for about 3 weeks to a constant weight, and ground into flour. The sample was kept in an air tight container for future analysis.
2.8. Aqueous extract preparation
Ten grams of each milled sample (raw, elastic pastry, boiled and roasted plantain flour) was soaked in 100 mL distilled water for about 24 h. The mixture was filtered. In a situation where the filtrate appeared to be very cloudy, the filtrate was centrifuged to obtain a clear supernatant liquid, which was subsequently used for the various assays[12]. All antioxidant tests and analyses were performed in triplicate, and results were averaged.
2.9. Determination of total phenol content
The total phenol content was determined according to the method of Singleton et al[13]. Briefly, the aqueous extracts were oxidized with 2.5 mL 10% Folin-Ciocalteau's reagent (v/v) and neutralized by 2.0 mL of 7.5% sodium carbonate. The reaction mixture was incubated for 40 min at 45 °C and the absorbance was measured at 765 nm in the spectrophotometer (JENWAY 6305). The total phenol content was subsequently calculated as gallic acid equivalent.
2.10. Determination of total flavonoid content
The total flavonoid content of the unripe plantain extracts was determined using method of Meda et al[14]. The volume of 0.5 mL of sample/standard quercetin was mixed with 0.5 mL methanol, 50 µL of 10% AlCl3, 50 µL of 1 mol/L potassium acetate and 1.4 mL water. The reaction mixture was incubated at room temperature for 30 min. Thereafter, the absorbance of the reaction mixture was measured at 415 nm in the spectrophotometer (JENWAY 6305). Total flavonoid content was calculated using quercetin as a standard.
2.11. Determination of vitamin C content
Vitamin C content of the unripe plantain extracts was determined using the method of Benderitter et al[15]. A volume of 75 µL DNPH (2 g dinitrophenyl hydrazine, 230 mg thiourea and 270 mg CuSO4•5H2O in 100 mL of 5 mol/L H2SO4) was added to 500 µL reaction mixture (300 µL of the extracts with 100 µL 13.3% trichloroacetic acid and water). The reaction mixture was subsequently incubated for 3 h at 37 °C, then 0.5 mL of 65% H2SO4 (v/v) was added to the medium and the absorbance was measured at 520 nm using a spectrophotometer (JENWAY 6305). The vitamin C content of the extracts was subsequently calculated.
2.12. DPPH radical scavenging ability
The free radical-scavenging ability of the extracts against DPPH free radical was measured by measuring the decrease in absorbance of methanolic DPPH solution at 517 nm in the presence of each sample extract as described by Gyamfi et al[16]. Briefly, 1 mL of different concentrations (400, 300, 200 and 100 µL) of extracts were added to 1 mL of 0.4 mmol/L methanolic solution containing DPPH radicals. The mixture was left in the dark for 30 min and the absorbance was measured at 516 nm in the spectrophotometer using (JENWAY 6305). The DPPH free radical scavenging ability was subsequently calculated by comparing the results of the test with those of the control (not treated with the extract). The ability of the sample to scavenge was calculated relative to the control using the formula[17]:
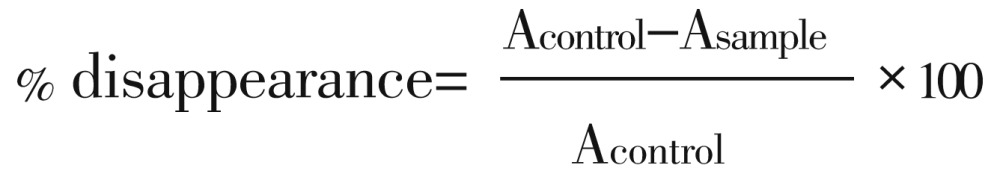 |
Where Acontrol represents absorbance of control, Asample represents absorbance of test sample and Acontrol represents the absorbance of control.
2.13. Fe2+ chelation assay
The Fe2+ chelating ability of the extracts was determined using a modified method of Minotti and Aust with a slight modification by Puntel et al[18],[19]. Freshly prepared 500 µmol/L FeSO4 (150 µL) was added to a reaction mixture containing 168 µL of 0.1 mol/L Tris-HCl (pH 7.4), 218 µL saline and the extracts (0-100 µL). The reaction mixture was incubated for 5 min, before the addition of 13 µL of 0.25% 1,10-phenanthroline (w/v). The absorbance was subsequently measured at 510 nm in a spectrophotometer. The Fe2+ chelating ability was subsequently calculated with respect to the control.
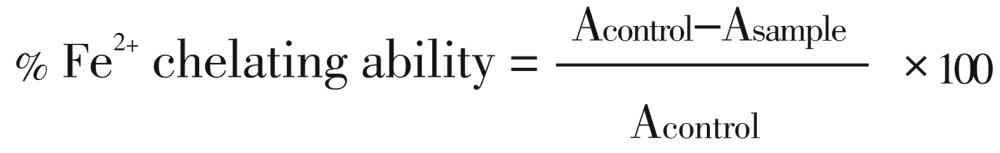 |
Where Acontrol represents absorbance of control, Asample represents absorbance of test sample and Acontrol represents the absorbance of control.
Iron II associates with ferrozine to form complexes. These can be prevented by chelating agents which works by decreasing the red color of the complexes. The measurement of the colour reduction determines the metal chelating activity and the estimation of the coexisting chelator activity[20].
2.14. OH radicals scavenging ability
The ability of plantain extracts to prevent Fe2+/H2O2−induced decomposition of deoxyribose was carried out using the method of Halliwell and Gutteridge[21]. Briefly, freshly prepared aqueous extract (0-100 mL) was added to a reaction mixture containing 120 mL 20 mmol/L deoxyribose, 400 mL 0.1 mol/L phosphate buffer, 40 mL 20 mol/L hydrogen peroxide and 40 mL 500 mmol/L FeSO4, and the volume was made to 800 mL with distilled water. The reaction mixture was incubated at 37 °C for 30 min, and the reaction was stopped by the addition of 0.5 mL of 2.8% trichloroacetic acid; this was followed by the addition of 0.4 mL of 0.6% thiobarbituric acid solution. The tubes were subsequently incubated in boiling water for 20 min. The absorbance was measured at 532 nm in spectrophotometer.
 |
Where Acontrol represents absorbance of control, Asample represents absorbance of test sample and Acontrol represents the absorbance of control.
2.15. Preparation of pancreas homogenates
The rats were decapitated under mild diethyl ether anesthesia, and the pancreas was rapidly dissected, placed on ice, and weighed. This tissue was subsequently homogenized in cold saline (1:10 w/v) with about 10 up-and-down strokes at approximately 1 200 r/min in a Teflon® (DuPont, Wilmington, DE) glass homogenizer. The homogenate was centrifuged for 10 min at 3 000 g to yield a pellet that was discarded, and the low-speed supernatant (S1) was collected and kept for lipid peroxidation assay[22].
2.16. Lipid peroxidation and thiobarbituric acid reactions
The lipid peroxidation assay was carried out using the modified method of Ohkawa et al[23]. S1 fraction (100 µL) was mixed with a reaction mixture containing 30 µL of 0.1 mol/L Tris-HCl buffer (pH 7.4), plantain extract (0-100 µL), and 30 µL of the pro-oxidant solution (7 µmol/L sodium nitroprusside). The volume was made up to 300 µL with water before incubation at 37 °C for 1 h. The colour reaction was developed by adding 300 µL 8.1% sodium dodecyl sulfate to the reaction mixture containing S1; this was subsequently followed by the addition of 600 µL of acetic acid/HCl (pH 3.4) and 600 µL 0.8% TBA. This mixture was incubated at 100 °C for 1 h. The thiobarbituric acid reactive species produced were measured at 532 nm.
2.17. Characterisation of phenolic constituent using gas chromatography (GC) analysis
The qualitative-quantitative analysis of the phenolic compounds of the samples was carried out using the method reported by Kelley et al[24]. The purified phenolic extracts (1 µL:10:1 splitless) was analyzed for composition by comparison with authentic standards (Aldrich, Milwaukee, WI) and with cochromatography with standards on a Hewlett-Packard 6890 gas chromatograph (Hewlett-Packard Corp., Palo Alto, CA) equipped with a derivatized, nonpacked injection liner, a Rtx-5MS (5% diphenyl-95% dimethyl polysiloxane) capillary column (30 m length, 0.25 mm column i.d., 0.25 µm film thickness), and detected with a flame ionization detector. The following conditions were employed for phenolics separation: injector temperature of 250 °C; temp. ramp, 80 °C for 2 min then ramped to 280 °C at 30 °C per minute and a detector temperature of 320 °C.
2.18. Data analysis
The triplicate readings of results were pooled and expressed as mean±SD. One way analysis of variance was used to analyze the results and Duncan test was used for the post hoc[25]. Findings were considered statistically significant when the P value was less than 0.05 (Microsoft Excel 2010; Redmond, WA, USA). EC50 (concentration of extract that will cause 50% concentration activity) was determined using linear regression analysis.
3. Results
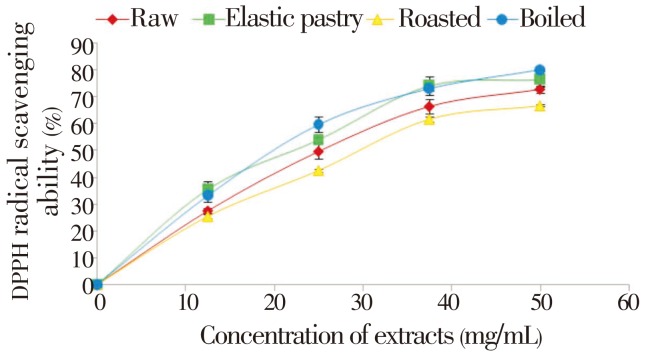
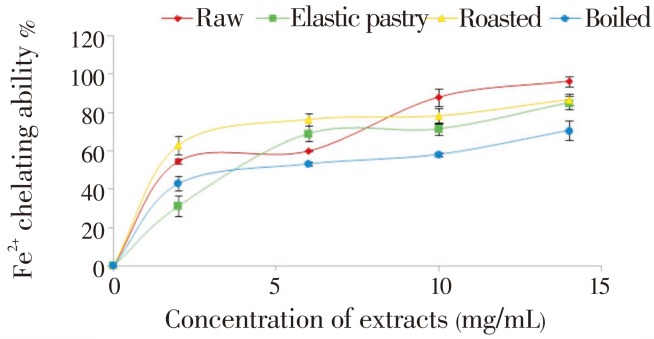
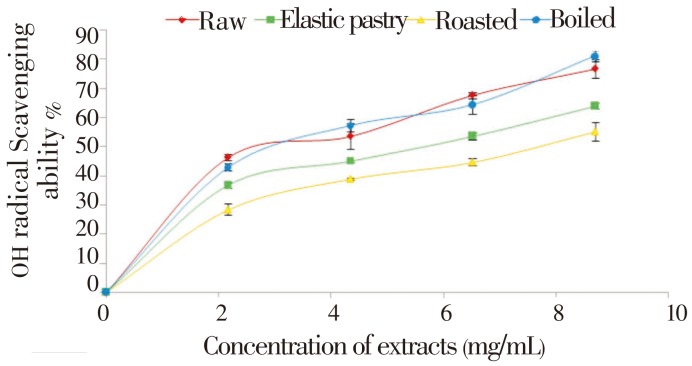
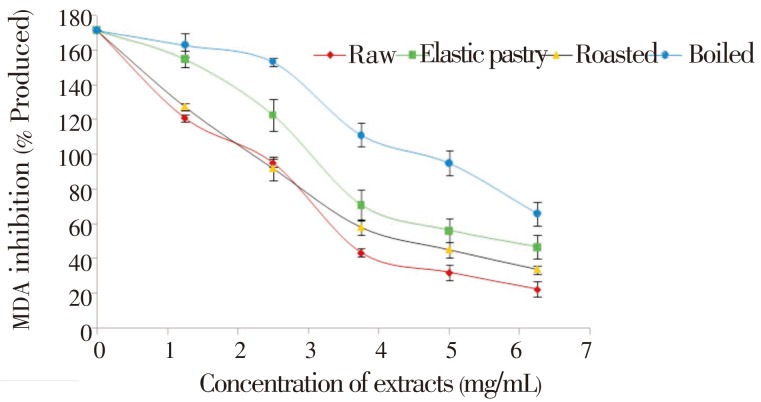
The DPPH free radical scavenging ability of the aqueous extracts of the plantain products as presented in Figure 1 revealed that all the aqueous extracts scavenged DPPH radicals in a dose-dependent manner in the range of 0-50 mg/mL. However, boiled extract has the highest DPPH scavenging ability. Considering the EC50 of the aqueous extract having the highest inhibition i.e. the lowest EC50 value (24.76) of boiled extract has the highest inhibitory activity. The unripe plantain products aqueous extracts were also assessed for their Fe2+ chelating ability. The result is presented in Figure 2 and their EC50 values (Table 1) revealed that all the aqueous extracts exhibited iron chelating ability in a dose dependent manner in the range of 0.2-14.0 mg/mL. However, raw extract had the highest Fe2+ chelating ability (P<0.05). Furthermore, Figure 3 depicts the OH radical scavenging ability. The extracts exhibited OH radical scavenging activity in a dose dependent manner in the range of 0-9 mg/mL. However, the highest activity was found in the boiled extract considering its EC50 (4.35) value. The trend of this result followed that of the DPPH radical scavenging ability. Contrary to the antioxidant activity results, the phenolic content (total phenol and total flavonoid) determination of the extracts revealed that elastic pastry had the highest total phenol (1.09±0.04 mg/g) and total flavonoid (0.73±0.01 mg/g) content. The vitamin C result showed that raw extract has the highest vitamin C content (37.1±0.27 mg/g) which is also in line with the Fe2+ chelating ability. The protective ability of the aqueous extracts of plantain products to act against sodium nitroprusside induced lipid peroxidation in cultured rats' pancreas is presented in Figure 4. There was significant (P<0.05) increase in the MDA (171.42) content on the addition of sodium nitroprusside during incubation while all the extracts exhibited inhibitory effect on sodium nitroprusside. However, the raw extract had significantly highest inhibitory effect in the lipid peroxidation in the isolated pancreas tissues (P<0.05). The trend of this result is in line with that of Fe2+ chelating ability and vitamin C content.
Figure 1. DPPH radical-scavenging ability of aqueous extracts of unripe plantain products.
Figure 2. Fe2+ chelating ability of aqueous extracts of unripe plantain products.
Table 1. Total phenolic contents, total flavonoid contents, vitamin C contents and EC50 of antioxidant activities of aqueous extracts of the unripe plantain products.
| Sample | Total phenol (mg/g) | Total flavonoid (mg/g) | Vitamin C (mg/100g) | EC50 of antioxidant activities (mg/mL) |
||
| DPPH radical scavenging ability | OH radical scavenging ability | Fe2+ chelating ability | ||||
| Raw | 0.94±0.02b | 0.71±0.02a | 37.1±0.27a | 33.58±4.53a | 5.14±0.30c | 5.68±0.80d |
| Elastic pastry | 1.09±0.04a | 0.73±0.01a | 24.7±0.05b | 27.44±0.65b | 6.26±0.33b | 7.64±0.83b |
| Roasted | 0.89±0.04c | 0.48±0.03c | 25.9±0.22b | 31.77±2.21a | 7.31±0.33a | 6.88±0.49c |
| Boiled | 0.93±0.02b | 0.61±0.01b | 30.3±0.03a | 24.76±3.26b | 4.35±0.69d | 9.37±1.22a |
Data represent mean±SD, n=3. Values with the same superscript letter along the same row are not significantly different (P>0.05).
Figure 3. OH radical scavenging ability of aqueous extracts of unripe plantain products.
Figure 4. Inhibition of sodium nitroprusside induced lipid peroxidation of pancreas by aqueous extracts of unripe plantain products.
The qualitative-quantitative analysis of the phenolic compounds of the samples using gas chromatography revealed main phenolic contents of varied quantity per product sample. The highest quantities of phenolics reported in Table 2 with revealed that the polyphenol-rich extract of raw flour contained the highest quantity of apigenin (4.29 mg/100 g), myricetin (4.74 mg/100 g), luteolin (6.63 mg/100 g), capsaicin (16.61 mg/100 g), and isorhaemnetin (6.55 mg/100 g). Elastic pastry flour contained the highest quantity of caffeic acid (1.48 mg/100 g). Roasted flour contained highest quantity of kampferol (4.86 mg/100g) and quercetin (4.88 mg/100 g). Boiled flour contained highest p-hydroxybenzoic acid (16.66 mg/100 g), shogaol (5.18 mg/100 g), glycitein (4.03 mg/100 g) and (14.38 mg/100g).
Table 2. Main constituents of phenolics present in unripe plantain products (mg/100 g).
| Phenolics identified | Raw | Elastic pastry | Roasted | Boiled |
| p-hydroxylbenzoic ccid | 1.43 | 11.36 | 1.37 | 16.66 |
| Shogaol | 8.39×10−3 | 4.58×10−3 | 4.81×10−3 | 5.18 |
| Glycitein | 1.02×10−3 | 3.50×10−3 | 3.60×10−3 | 4.03 |
| Gingerol | 2.00×10−2 | 1.20×10−2 | 1.00×10−2 | 14.38 |
| Caffeic acid | 1.82×10−1 | 1.48 | 1.52×10−3 | 1.61×10−2 |
| Apigenine | 4.29 | 1.62 | 2.89 | 1.84×10−3 |
| Kaempferol | 3.83 | 3.32 | 4.86 | 3.88×10−3 |
| Myricetin | 4.74 | 3.13 | 2.91 | 1.41×10−2 |
| Luteolin | 6.63 | 5.73×10−3 | 1.61 | 1.03×10−2 |
| Capsaicin | 16.61 | 1.46×10−2 | 12.13 | 1.71×10−2 |
| Quercetin | 7.82×10−3 | 6.89×10−3 | 4.88 | 8.07×10−3 |
| Isorhamnetin | 7.82×10−3 | 6.38×10−3 | 4.16 | 7.84×10−3 |
4. Discussion
Antioxidant activity in higher plants has often been associated with phenolic compounds[26]. In addition to their roles in plants, phenolic compounds in our diet may provide health benefits associated with reduced risk of degenerative diseases[6]. DPPH is a stable nitrogen-centered free radical. In DPPH assay the lower the EC50 the better it is able to scavenge the radicals, particularly peroxy radicals which are the propagators of the autoxidation of lipid molecules and there by break the free radical chain reaction[16]. Substances which are able to perform this reaction can be considered as antioxidants[26]. The result of DPPH agreed with earlier reports where plant phytochemicals scavenged DPPH[12],[16],[27]. Hence, the DPPH radical scavenging ability of the aqueous extract could be attributed to the hydrogen donating ability of the hydroxyl groups of the phenolics[28] hence, plantains could have hydrogen donating ability.
The mechanism of action of antioxidants chelate and deactivate transition metals thereby prevent such metals from participating in the initiation of lipid peroxidation and oxidative stress through metal catalysed reaction[12]. Overproduction of reactive oxygen species (ROS) can also have a direct attack on the polyunsatureated fatty acids of the cell membrane to induce lipid peroxidation[12]. The deleterious effect caused by iron is done by reacting with hydrogen peroxide to produce hydroxyl radical (OH.) through Fenton reaction. Superoxide can also react with Fe3+ to regenerate Fe2+ which can participate in the Fenton reaction[2]. Up till this present time, there are consistent suggestions that ROS induce lipid peroxidation in cell membranes and all effort is concentrated on terminating the deleterious effects of free radicals. The measurement of H2O2 radical scavenging activity is one of the designed methods of estimating the ability of anti-oxidants to reduce the level of pro-oxidants[29]. Hydrogen peroxide is not very reactive all by iself, but could be toxic to cells when there is an increase in its concentration in the cells[29]. In this study boiled flour has the highest OH. radical scavenging ability while the roasted flour has the least. Hence, there is a clear indication that all the aqueous extracts of plantain products were able to chelate and/or scavenge the Fe and OH. and this adds to the fact that the plantain products have Fe2+ chelating ability.
The introduction of a functional group containing two catenated oxygen atoms O-O in a free radical reaction lead to the generation of lipid peroxidation which have both direct and indirect effects in organisms. Its binding process has been connected with malonaldehyde formation which has been designated a biomarker of free radical-mediated damage and oxidative stress[3]. The release of cyanide and/or nitric oxide (NO) in sodium nitroprusside can cause cytotoxicity[29]. NO is a free radical with a short half-life (<30 s). Although, the independent action of NO may cause neuronal damage in cooperation with other reactive oxygen species such as superoxide radical to form peroxynitrite radical[29]. However, the result revealed that raw had higher MDA inhibitory activity. Hence, unripe plantain is suggested to partake in carrying out inhibitory activity by preventing cellular peroxidation of lipids.
Vitamin C contributes majorly to the antioxidant activities of plant food. Ascorbic acid is a good reducing agent and exhibits its antioxidant activities by electron donation. This is done by oxidizing tocopheroxyl free radical into dehydroascorbate. Vitamin C (non-enzymatic) antioxidant reaction scavenges the free radical pathway by destroying both the initiation and propagation reactions that promotes lipid peroxidation. Eventually, this mechanism has been tagged a harmless one[2]. However, the result of the vitamin C for the plantain products is higher when compared with some natural fresh fruit juices[30]. The basis for the low concentration of vitamin C content could be attributed to the difference in processing techniques that each of the unripe plantain products was subjected to[30]. Hence, heat treatment would have destroyed more of the heat instable vitamin C in elastic pastry, boiled and roasted plantain pulp.
Phenolics are one of the largest and the most widely studied groups of phytochemicals. They are widely reported to possess remarkable antioxidant and medicinal properties. Flavonoids are very large in number. Most of the antioxidant and medicinal properties credited to phenolics have been attributed to the function of flavonoids. In addition, each group of flavonoid has the capacity to exert antioxidant properties[31]. The Total phenolic content measures the total amount of phenolics, which include flavonoids. Total flavonoid content is designed to quantify the amount of flavonoids. The total phenol and total flavonoid content of extracts have been often reported as promising medicinal and nutritional ingredients[32]. The total phenol content of the plantain products is higher than some commonly consumed tropical plants[33]. Furthermore, the trend of the total phenol content and total flavonoid content results are in contrast to the earlier report which revealed that there is a direct relationship between the total phenol content and the antioxidant activity in some plant foods[33]. The basis for the lack of agreement between phenolic content and antioxidant activities under study cannot be categorically stated, however it is worth noting. The major ingredients of nutraceuticals have been identified as flavonoids. The area of this field begins to attract the interest of researchers since 1990 and has been expanded based on the observation that many natural products have beneficial effects in scavenging reactive oxygen species which are detrimental to human health. As a result, various methods of characterizations have been improved[34].
Moreover, flavonoids have been reported to exert photoprotective effect on UVB-induced skin damage that results from increased expression level of procollagen type I and decreased expression level of matrix metallopro-proteinase-I[35]. Flavonoids such as quercetin, kaempferol, morin, myricetin and rutin act by exerting antioxidant effects such as anti-inflammatory, antiallergic, antiviral and anticancer activity. They have also been reported to protect against liver diseases, cataracts, and cardiovascular diseases. Quercetin for example have been revealed to also protect against liver reperfusion ischemic tissue damage[31] as well as a good inhibitor of α-amylase activity[36]. The chemical structures such as the unsaturated C ring, 3-OH, 4-CO, the linkage of the B ring at the 3 position and the hydroxyl substitution on the B ring of flavonoids have also been reported to be responsible for α-amylase and α-glucosidase activity[37]. Up till recently, very little informations have been reported about the phenolic content of unripe plantain. But this study has further characterized the quatitative amount of flavonoids in unripe plantain products. As observed, the presence of polyphenol constituents could have contributed immensely to the antioxidant properties of these products. Reports have shown that flavonoids could exert a better protection by preventing the progressive impairment of pancreatic beta-cell function due to oxidative stress and may thus reduce the occurrence of type 2 diabetes. This could be achieved by lowering the level of lipid, glycosylated hemoglobin and postprandial blood glucose as well as increasing the insulin sensitivity when compared with the effect of some commercial drugs like arcabose and viglibose[37]. P- hydroxybenzoic acid has been reported to exert antifungal, antimutagenic, antisickling, and antimicrobial activities[38]. This eventually supports the highest DPPH radical scavenging ability exhibited by the boiled flour which also has the highest quantity of characterized p-hydroxybenzoic acid content. The result of raw flour which exhibited the highest Fe2+ chelating ability agreed with the earlier report where phytochemicals of the functional groups such as OH and C=O present in apigenin and naringenin may be responsible for the Fe2+ chelating ability of the phenolic-rich extract[31]. In addition, glycosides of apigenin and luteolin have also been revealed to participate in the inhibition of LDL-oxidation[39]. This study revealed that raw flour contained highest quantity of apigenin, luteolin, myricetin and capsaicin and isorhaemnetin. Gingerols and their related dehydration products, shogaols have been identified in ginger to carry out some activities such as anti-thrombotic, anti-pyretic, anti-inflammatory, hypolipidemic, hypocholesterolemic and anagesic properties[40]. Our research on the characterisation of these plantain products revealed that the quantity of gingerol, shogaol and glycitein were higher in the boiled flour. Caffeic acid, along with p-coumaric, and ferulic acids, had been reported to exert the total radical-trapping antioxidative potential. They are shown to partake in hydrogen and electron transfer reactions[41]. However considerable quantity of caffeic acid has been identified in the unripe plantain products under study. It is important to note that all the identified polyphenols are distributed in all the plantain products but in varied quantities and which might have one way or the other influenced their antioxidant activities in various assays.
The results of the present work highlighted the polyphenol contents and antioxidant activities present in plantain products (raw, roasted, elastic pastry and boiled). The results also indicated that plantain products are protective against reactive oxygen species, but there is variability in the level of antioxidant capacity among the products. On the basis of the studied data on antioxidant activities and the polyphenol contents identified, it could be suggested that the consumption of unripe plantain products could render a multifaceted action that will terminate the instant generation of free radicals thereby setting the human system free from accumulation of radicals and also the awareness about the good state of health to be experienced in association with the consumption of unripe plantain should be made readily available to people.
Acknowledgments
The authors graciously acknowledge the financial support given by the Education Trust Fund, Nigeria (Grant number: VCPU/URGC/46).
Comments
Background
It is rare in Africa, Nigeria in particular, for any household not to consume any of the four forms (Amala, Booli, Boiled or Raw) of M. paradisiaca under study, either as breakfast, lunch or dinner. It is therefore necessary to study its constituents and various potential benefits to human health. Interestingly this study showed they contained vitamin C, flavonoids and polyphenols that possesses Fe-chelating, and scavenging/protective activities against DPPH and OH. radicals. This will go a long way to nutritionally reduce the various risks associated with degenerative diseases.
Research frontiers
This study was able to evaluate and compare the aqueous extracts of various forms of M. paradisiaca. The antioxidant and protective potentials as well as characterization of the phenolic components were extensively analyzed and discussed. By this study, we will now know that the unripe plantain he consumes every now and then, in whatever form, not only contain flavonoids and phenols as generally known, but can say categorically in detail, that they also contain various concentrations of luteolin, myricetin, kaempferol, glycitein, p-hydroxylbenzoic acid.
Related reports
The result of the DPPH assay is in agreement with works done by Gyamfi et al., 1999 and Oboh & Shodehinde, 2009. Fe chelating assay was supported by Moreira et al., 2010, Tapas et al., 2008, Sandhar et al., 2011, Min-ji et al., 2012 and Oboh et al., 2007. Studies carried out on vitamin C, a good reducing agent, were supported by Mahdavi et al., 2010. Phenolic and flavonoid data trend generated contradicts Iyawe and Azih, 2011 work, which revealed that there is direct relationship between the total phenol content and the antioxidant activity in some plant food. This was however supported by earlier work done by Akinmoladun et al., 2010 and Magalhaes et al., 2008.
Innovations and breakthroughs
Most studies on phytochemical screening in Nigeria usually simply identify qualitatively the presence of phenols, without going into details quantitatively, as to which of the phenols are actually present and in what quantity. This study has succeeded in identifying 12 different phenols in the four forms of M. paradisiaca and quantifies them.
Applications
Many people in Africa (healthy and sick) eat all the four forms of unripe plantain studied here at one time or the other with little or no knowledge of its compositions. The results generated were able to enlighten us with the fact that 12 different phenols can be identified, which confers on the plantain, Fe-chelating and scavenging properties, this explain why they are locally prescribed in Nigeria to patients with diabetes, carbohydrate disorders and other degenerative diseases.
Peer review
Two major findings were established by this study. The Polyphenol contents of the aqueous extracts of four forms M. paradisiaca were characterized, its antioxidant and protective potentials against ROS and DPPH were determined. The result suggests and encourages the consumption of unripe plantain to eradicate all kinds of radical accumulation and reduce the risk of degenerative diseases.
Footnotes
Foundation Project: Supported by the Education Trust Fund, Nigeria. Grant No. VCPU/URGC/46.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Mahantesh SP, Gangawane AK, Patil CS. Free radicals, antioxidants, diseases and phytomedicines in human health: Future perspects. World Res J Med Aromatic Plants. 2012;1(1):6–10. [Google Scholar]
- 2.Moreira da Silva F, Marques A, Chaveiro A. Reactive oxygen species: a double-edged sword in reproduction. The Open Vet Sci J. 2010;4:127–133. [Google Scholar]
- 3.Dauqan EMA, Abdullah A, Sani HA. Natural antioxidants: Lipid profile, lipid peroxidation, antioxidant enzymes of different vegetable oils. Adv J Food Sci Technol. 2011;3(4):308–316. [Google Scholar]
- 4.Oboh G, Rocha JBT. Antioxidant and neuroprotective properties of sour tea (Hibiscus sabdariffa, calyx) and green tea (Camellia sinensis) on some pro-oxidant-induced lipid peroxidation in brain in vitro. Food Biophys. 2008;3(4):382–389. [Google Scholar]
- 5.Gan RY, Kuang L, Xu XR, Zhang Y, Xia EQ, Song FL, et al. et al. Screening of natural antioxidants from traditional Chinese medicinal plants associated with treatment of rheumatic disease. Molecues. 2010;15:5988–5997. doi: 10.3390/molecules15095988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahdavi R, Nikniaz Z, Rafraf M, Jouyban A. Determination and comparison of total polyphenol and vitamin C contents of natural fresh and commercial fruit juices. Pak J Nutr. 2010;9(10):968–972. [Google Scholar]
- 7.Sarvajeet SG, Narendra T. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Bioch. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Osundahunsi OT. Scanning electron microscope study and pasting properties of ripe and unripe plantain. J Food Agric Environ. 2009;7(3&4):182–186. [Google Scholar]
- 9.Honfo FG, Tenkouano A, Coulibaly O. Banana and plantain-based foods consumption by children and mothers in Cameroon and Southern Nigeria: A comparative study. Afr J Food Sci. 2011;5:287–291. [Google Scholar]
- 10.Kumar RV, Venkatrajireddy G, Bikshapathi T, Reddy MK. Antioxidant-the maximum expressed activity among 63 medicinal plants. J Phyto Pharmacol. 2012;1(5):1–13. [Google Scholar]
- 11.Iweala EAA, Obichi IC, Omotosho OE. Biochemical and Histological responses of hepatoxic rat fed Musa paradisiaca L. supplemented diet. Int J Pharmacol. 2011;7(4):471–477. [Google Scholar]
- 12.Oboh G, Puntel RL, Rocha JBT. Hot pepper (Capsicum annuum, Tepin and Capsicum chinese, Habanero) prevents Fe2+-induced lipid peroxidation in brain-in vitro. Food Chem. 2007;102:178–185. [Google Scholar]
- 13.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Cioalteau reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 14.Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Faso honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- 15.Benderitter M, Maupoil V, Vergely C, Dalloz F, Briot F, Rochette L. Studies by electron paramagnetic resonance of the importance of iron in hydroxyl scavenging properties of ascorbic acid in plasma: Effects of iron chelators. Fundam Clin Pharmacol. 1998;12:510–516. doi: 10.1111/j.1472-8206.1998.tb00979.x. [DOI] [PubMed] [Google Scholar]
- 16.Gyamfi MA, Yonamine M, Aniya Y. Free radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally induced liver injuries. Gen Pharmacol. 1999;32:661–667. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- 17.Krings U, Berger RG. Antioxidant activity of some roasted foods. Food Chem. 2001;72:223–229. [Google Scholar]
- 18.Minotti G, Aust SD. An investigation into the mechanism of citrate-Fe2+ dependent lipid peroxidation. Free Radic Biol Med. 1987;3:379–387. doi: 10.1016/0891-5849(87)90016-5. [DOI] [PubMed] [Google Scholar]
- 19.Puntel RL, Nogueira CW, Rocha JBT. Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res. 2005;30:225–235. doi: 10.1007/s11064-004-2445-7. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi F, Ariga T, Yoshimira Y, Nakazawa H. Antioxidant and antilycation of carcinol from Garcinia indica fruit rind. J Agricult Food Chem. 2000;48:180–185. doi: 10.1021/jf990845y. [DOI] [PubMed] [Google Scholar]
- 21.Halliwell B, Gutteridge JMC. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: The role of superoxide and hydroxyl radicals. FEBS Lett. 1981;128:347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- 22.Belle NA, Dalmolin GD, Fonini G, Rubim MA, Rocha JB. Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res. 2004;1008:245–251. doi: 10.1016/j.brainres.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Kelley WI, Coffey DL, Mueller TC. Liquid chromatographic determination of phenolic acids in soil. J Ass Anal Commun Int. 1994;77:805–809. [Google Scholar]
- 25.Zar JH. Biostatistical analysis. USA: Prentice-Hall Incorporation; 1984. p. 620. [Google Scholar]
- 26.Adefegha SA, Oboh G. In vitro inhibition activity of polyphenol-rich extracts from Syzygium aromaticum (L.) Merr. & Perry (Clove) buds against carbohydrate hydrolyzing enzymes linked to type 2 diabetes and Fe2+-induced lipid peroxidation in rat pancreas. Asian Pac J Trop Biomed. 2012;2(10):774–781. doi: 10.1016/S2221-1691(12)60228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oboh G, Shodehinde SA. Distribution of nutrients, polyphenols and antioxidant activities in the pilei and stipes of some commonly consumed edible mushrooms in Nigeria. Bull Chem Soc Ethiop. 2009;23(3):391–398. [Google Scholar]
- 28.Eleazu CO, Amajor JU, Ikpeama AI, Awa E. Studies on the nutritional composition, antioxidant activities, functional properties and microbial loads of the flours of 10 elite cassava varieties. Asian J Clin Nutr. 2011;3(1):33–39. [Google Scholar]
- 29.Oboh G, Rocha JBT. Hot pepper (Capsicum spp.) protects brain from sodium nitroprusside- and quinolinic acid-induced oxidative stress in vitro. J Med Food. 2008;11(2):349–355. doi: 10.1089/jmf.2007.341. [DOI] [PubMed] [Google Scholar]
- 30.Mahdavi R, Nikniaz Z, Rafraf M, Jouyban A. Determination and comparison of total polyphenol and vitamin C contents of natural fresh and commercial fruit juices. Pak J Nutr. 2010;9(10):968–972. [Google Scholar]
- 31.Tapas AR, Sakarkar DM, Kakde RB. Flavonoids as nutraceuticals: A review. Trop J Pharm Res. 2008;7(3):1089–1099. [Google Scholar]
- 32.Akinmoladun AC, Obuotor EM, Farombi EO. Evaluation of antioxidant and free radical scavenging capacities of some Nigerian indigenous medicinal plants. J Med Food. 2010;13:444–451. doi: 10.1089/jmf.2008.0292. [DOI] [PubMed] [Google Scholar]
- 33.Iyawe HOT, Azih MC. Total phenolic contents and lipid peroxidation potentials of some tropical antimalarial plants. Eur J Med Plants. 2011;1(2):33–39. [Google Scholar]
- 34.Magalhães LM, Segundo MA, Reis S, Lima JLFC. Methodological aspects about in vitro evaluation of antioxidant properties. Anal Chim Acta. 2008;613:1–19. doi: 10.1016/j.aca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 35.Park HM, Moon E, Kim AJ, Lee S, Lee JB, Park YK, et al. et al. Extract of punica granatum inhibits skin photoaging induced by UVB irradiation. Int J Dermatol. 2010;49:276–282. doi: 10.1111/j.1365-4632.2009.04269.x. [DOI] [PubMed] [Google Scholar]
- 36.Li YQ, Gao F, Gao F, Shan F, Bian JS, Zhao CG. Study on the Interaction between 3 flavonoid compounds and r-amylase by fluorescence spectroscopy and enzymatic kinetics. J Food Sci. 2009;74(3):C199–C203. doi: 10.1111/j.1750-3841.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 37.Li YQ, Zhou FC, Gao F, Bian JS, Shan F. Comparative evaluation of quercetin and rutin as inhibitors of α-glucosidase. J Agric Food Chem. 2009;57:11463–11468. doi: 10.1021/jf903083h. [DOI] [PubMed] [Google Scholar]
- 38.Shahriar K, Robin JM. Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: occurrence and recent bioactivity studies. Molecues. 2010;15:7985–8005. doi: 10.3390/molecules15117985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandhar HK, Kumar B, Prasher S, Tiwari P, Salhan M, Sharma P. A review of phytochemistry and pharmacology of flavonoids. Int Pharm Sci. 2011;1(1):25. [Google Scholar]
- 40.Min-Ji B, Seon O, Mira J, Woo-Sik J. 6-Shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules. 2012;17:8037–8055. doi: 10.3390/molecules17078037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorinstein S, Lojek A, Ciz Milan, Pawelzik E, Delgado-Licon E, Medina OJ, et al. et al. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. Int J Food Sci Technol. 2008;43:629–637. [Google Scholar]