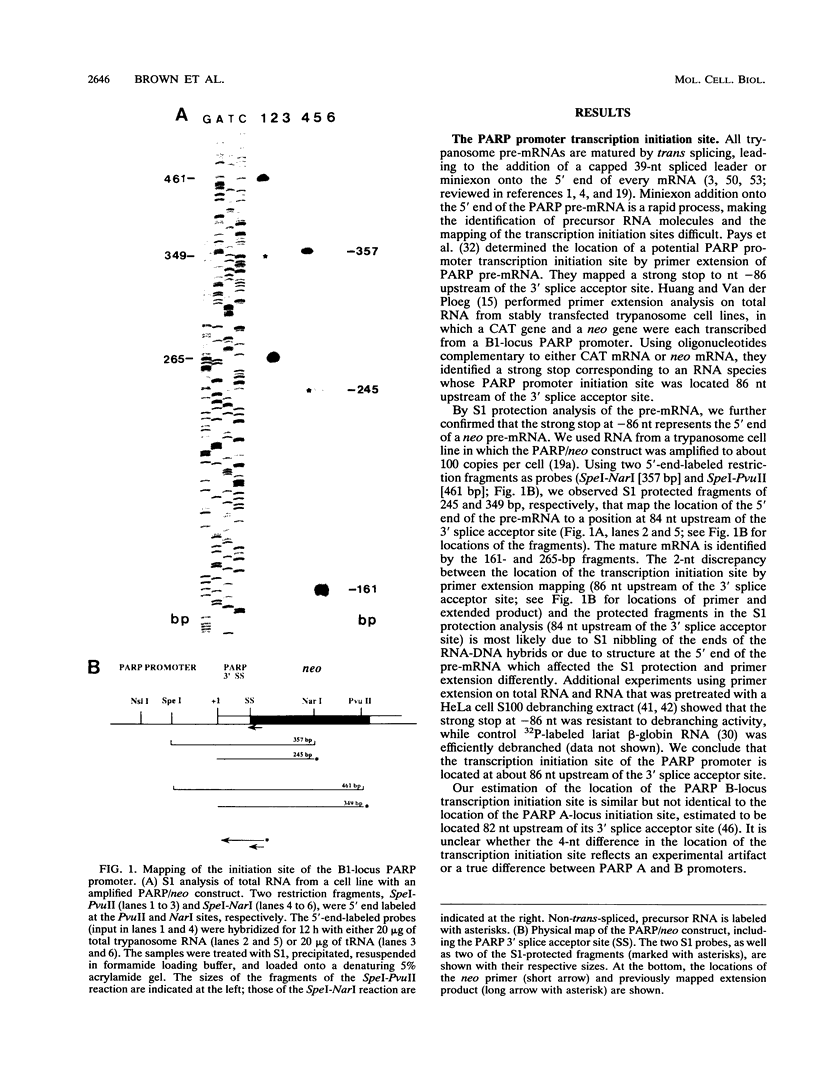
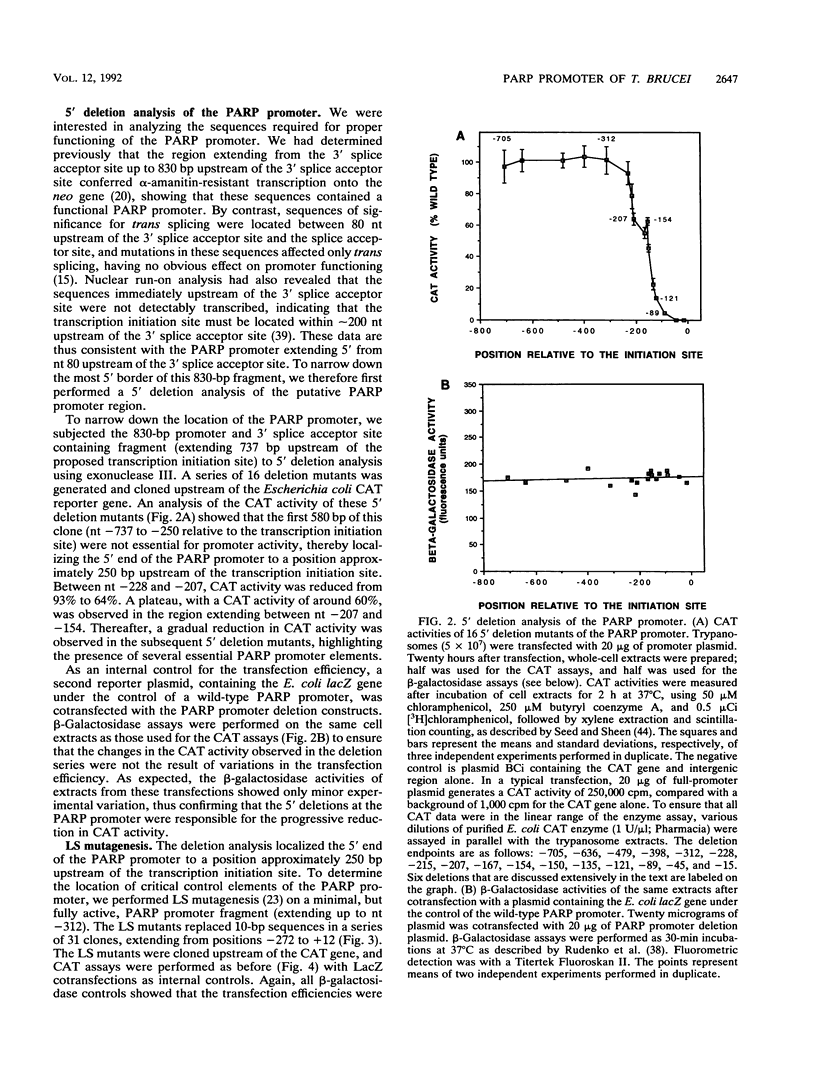
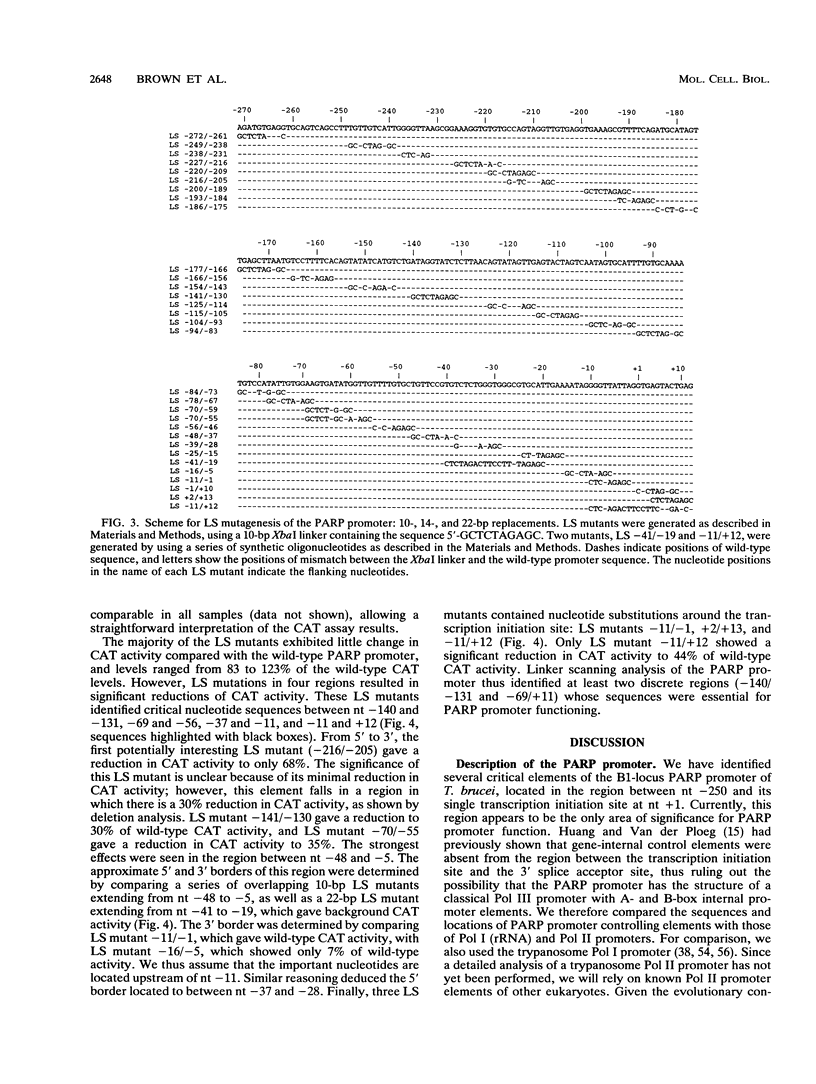
Abstract
All eukaryotic protein-coding genes are believed to be transcribed by RNA polymerase (Pol) II. An exception may exist in the protozoan parasite Trypanosoma brucei, in which the genes encoding the variant surface glycoprotein (VSG) and procyclic acidic repetitive protein (PARP) are transcribed by an RNA polymerase that is resistant to the Pol II inhibitor alpha-amanitin. The PARP and VSG genes were proposed to be transcribed by Pol I (C. Shea, M. G.-S. Lee, and L. H. T. Van der Ploeg, Cell 50:603-612, 1987; G. Rudenko, M. G.-S. Lee, and L. H. T. Van der Ploeg, Nucleic Acids Res. 20:303-306, 1992), a suggestion that has been substantiated by the finding that trypanosomes can transcribe protein-coding genes by Pol I (G. Rudenko, H.-M. Chung, V. P. Pham, and L. H. T. Van der Ploeg, EMBO J. 10:3387-3397, 1991). We analyzed the sequence elements of the PARP promoter by linker scanning mutagenesis and compared the PARP promoter with Pol I, Pol II, and Pol III promoters. The PARP promoter appeared to be of limited complexity and contained at least two critical regions. The first was located adjacent to the transcription initiation site (nucleotides [nt] -69 to +12) and contained three discrete domains in which linker scanning mutants affected the transcriptional efficiency: at nt -69 to -56, -37 to -11, and -11 to +12. The second region was located between nt -140 and -131, and a third region may be located between nt -228 and -205. The nucleotide sequences of these elements, and their relative positioning with respect to the transcription initiation site did not resemble those of either Pol II or Pol III promoter elements, but rather reflected the organization of Pol I promoters in (i) similarity in the positioning of essential domains in the PARP promoter and Pol I promoter, (ii) strong sequence homology between the PARP core promoter element (nt -37 to -11) and identically positioned nucleotide sequences in the trypanosome rRNA and VSG gene promoters, and (iii) moderate effects on promoter activity of mutations around the transcription initiation site.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N. Trans splicing of nuclear pre-mRNAs. Cell. 1990 Jun 29;61(7):1157–1160. doi: 10.1016/0092-8674(90)90674-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Boothroyd J. C., Cross G. A. Transcripts coding for variant surface glycoproteins of Trypanosoma brucei have a short, identical exon at their 5' end. Gene. 1982 Dec;20(2):281–289. doi: 10.1016/0378-1119(82)90046-4. [DOI] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Brun R., Schönenberger Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 1979 Sep;36(3):289–292. [PubMed] [Google Scholar]
- Clayton C. E., Fueri J. P., Itzhaki J. E., Bellofatto V., Sherman D. R., Wisdom G. S., Vijayasarathy S., Mowatt M. R. Transcription of the procyclic acidic repetitive protein genes of Trypanosoma brucei. Mol Cell Biol. 1990 Jun;10(6):3036–3047. doi: 10.1128/mcb.10.6.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn P. L., Aman R. A., Boothroyd J. C. Inhibition of protein synthesis results in super-induction of procyclin (PARP) RNA levels. Mol Biochem Parasitol. 1991 Jan;44(1):133–139. doi: 10.1016/0166-6851(91)90229-y. [DOI] [PubMed] [Google Scholar]
- Evers R., Hammer A., Köck J., Jess W., Borst P., Mémet S., Cornelissen A. W. Trypanosoma brucei contains two RNA polymerase II largest subunit genes with an altered C-terminal domain. Cell. 1989 Feb 24;56(4):585–597. doi: 10.1016/0092-8674(89)90581-3. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Gottesdiener K., Chung H. M., Brown S. D., Lee M. G., Van der Ploeg L. H. Characterization of VSG gene expression site promoters and promoter-associated DNA rearrangement events. Mol Cell Biol. 1991 May;11(5):2467–2480. doi: 10.1128/mcb.11.5.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondal E. J., Evers R., Kosubek K., Cornelissen A. W. Characterization of the RNA polymerases of Trypanosoma brucei: trypanosomal mRNAs are composed of transcripts derived from both RNA polymerase II and III. EMBO J. 1989 Nov;8(11):3383–3389. doi: 10.1002/j.1460-2075.1989.tb08502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I., Roth E., Paule M. R. Ribosomal RNA transcription in vitro is species specific. Nature. 1982 Mar 11;296(5853):173–174. doi: 10.1038/296173a0. [DOI] [PubMed] [Google Scholar]
- Haltiner M. M., Smale S. T., Tjian R. Two distinct promoter elements in the human rRNA gene identified by linker scanning mutagenesis. Mol Cell Biol. 1986 Jan;6(1):227–235. doi: 10.1128/mcb.6.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Van der Ploeg L. H. Requirement of a polypyrimidine tract for trans-splicing in trypanosomes: discriminating the PARP promoter from the immediately adjacent 3' splice acceptor site. EMBO J. 1991 Dec;10(12):3877–3885. doi: 10.1002/j.1460-2075.1991.tb04957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y., Safrany G., Hisatake K., Tanaka N., Maeda Y., Kato H., Kominami R., Muramatsu M. Structure of the core promoter of human and mouse ribosomal RNA gene. Asymmetry of species-specific transcription. J Mol Biol. 1991 Mar 5;218(1):55–67. doi: 10.1016/0022-2836(91)90873-5. [DOI] [PubMed] [Google Scholar]
- Jones M. H., Learned R. M., Tjian R. Analysis of clustered point mutations in the human ribosomal RNA gene promoter by transient expression in vivo. Proc Natl Acad Sci U S A. 1988 Feb;85(3):669–673. doi: 10.1073/pnas.85.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Nagamine M., Sasaki T., Takakusa N., Miwa T., Kominami R., Muramatsu M. Presence of a limited number of essential nucleotides in the promoter region of mouse ribosomal RNA gene. Nucleic Acids Res. 1985 May 24;13(10):3515–3532. doi: 10.1093/nar/13.10.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooter J. M., Borst P. Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res. 1984 Dec 21;12(24):9457–9472. doi: 10.1093/nar/12.24.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P. W. Trans splicing in trypanosomes--archaism or adaptation? Trends Genet. 1989 Jul;5(7):204–208. doi: 10.1016/0168-9525(89)90082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Van der Ploeg L. H. Homologous recombination and stable transfection in the parasitic protozoan Trypanosoma brucei. Science. 1990 Dec 14;250(4987):1583–1587. doi: 10.1126/science.2177225. [DOI] [PubMed] [Google Scholar]
- Margottin F., Dujardin G., Gérard M., Egly J. M., Huet J., Sentenac A. Participation of the TATA factor in transcription of the yeast U6 gene by RNA polymerase C. Science. 1991 Jan 25;251(4992):424–426. doi: 10.1126/science.1989075. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Tower J., Sollner-Webb B. A complex control region of the mouse rRNA gene directs accurate initiation by RNA polymerase I. Mol Cell Biol. 1985 Mar;5(3):554–562. doi: 10.1128/mcb.5.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig H., Schäffner A. R., Sieber H., Hartmann G. R. Primer-independent abortive initiation by wheat-germ RNA polymerase B (II). Eur J Biochem. 1985 Jun 3;149(2):337–343. doi: 10.1111/j.1432-1033.1985.tb08931.x. [DOI] [PubMed] [Google Scholar]
- Mowatt M. R., Clayton C. E. Developmental regulation of a novel repetitive protein of Trypanosoma brucei. Mol Cell Biol. 1987 Aug;7(8):2838–2844. doi: 10.1128/mcb.7.8.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowatt M. R., Clayton C. E. Polymorphism in the procyclic acidic repetitive protein gene family of Trypanosoma brucei. Mol Cell Biol. 1988 Oct;8(10):4055–4062. doi: 10.1128/mcb.8.10.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musters W., Knol J., Maas P., Dekker A. F., van Heerikhuizen H., Planta R. J. Linker scanning of the yeast RNA polymerase I promoter. Nucleic Acids Res. 1989 Dec 11;17(23):9661–9678. doi: 10.1093/nar/17.23.9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape L. K., Windle J. J., Sollner-Webb B. Half helical turn spacing changes convert a frog into a mouse rDNA promoter: a distant upstream domain determines the helix face of the initiation site. Genes Dev. 1990 Jan;4(1):52–62. doi: 10.1101/gad.4.1.52. [DOI] [PubMed] [Google Scholar]
- Parent A., Zeitlin S., Efstratiadis A. Minimal exon sequence requirements for efficient in vitro splicing of mono-intronic nuclear pre-mRNA. J Biol Chem. 1987 Aug 15;262(23):11284–11291. [PubMed] [Google Scholar]
- Pays E., Coquelet H., Pays A., Tebabi P., Steinert M. Trypanosoma brucei: posttranscriptional control of the variable surface glycoprotein gene expression site. Mol Cell Biol. 1989 Sep;9(9):4018–4021. doi: 10.1128/mcb.9.9.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E., Coquelet H., Tebabi P., Pays A., Jefferies D., Steinert M., Koenig E., Williams R. O., Roditi I. Trypanosoma brucei: constitutive activity of the VSG and procyclin gene promoters. EMBO J. 1990 Oct;9(10):3145–3151. doi: 10.1002/j.1460-2075.1990.tb07512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder R. H. rRNA synthesis in the nucleolus. Trends Genet. 1990 Dec;6(12):390–395. doi: 10.1016/0168-9525(90)90298-k. [DOI] [PubMed] [Google Scholar]
- Richardson J. P., Beecroft R. P., Tolson D. L., Liu M. K., Pearson T. W. Procyclin: an unusual immunodominant glycoprotein surface antigen from the procyclic stage of African trypanosomes. Mol Biochem Parasitol. 1988 Dec;31(3):203–216. doi: 10.1016/0166-6851(88)90150-8. [DOI] [PubMed] [Google Scholar]
- Roditi I., Carrington M., Turner M. Expression of a polypeptide containing a dipeptide repeat is confined to the insect stage of Trypanosoma brucei. Nature. 1987 Jan 15;325(6101):272–274. doi: 10.1038/325272a0. [DOI] [PubMed] [Google Scholar]
- Roditi I., Schwarz H., Pearson T. W., Beecroft R. P., Liu M. K., Richardson J. P., Bühring H. J., Pleiss J., Bülow R., Williams R. O. Procyclin gene expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. J Cell Biol. 1989 Feb;108(2):737–746. doi: 10.1083/jcb.108.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G., Bishop D., Gottesdiener K., Van der Ploeg L. H. Alpha-amanitin resistant transcription of protein coding genes in insect and bloodstream form Trypanosoma brucei. EMBO J. 1989 Dec 20;8(13):4259–4263. doi: 10.1002/j.1460-2075.1989.tb08611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G., Chung H. M., Pham V. P., Van der Ploeg L. H. RNA polymerase I can mediate expression of CAT and neo protein-coding genes in Trypanosoma brucei. EMBO J. 1991 Nov;10(11):3387–3397. doi: 10.1002/j.1460-2075.1991.tb04903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G., Le Blancq S., Smith J., Lee M. G., Rattray A., Van der Ploeg L. H. Procyclic acidic repetitive protein (PARP) genes located in an unusually small alpha-amanitin-resistant transcription unit: PARP promoter activity assayed by transient DNA transfection of Trypanosoma brucei. Mol Cell Biol. 1990 Jul;10(7):3492–3504. doi: 10.1128/mcb.10.7.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G., Lee M. G., Van der Ploeg L. H. The PARP and VSG genes of Trypanosoma brucei do not resemble RNA polymerase II transcription units in sensitivity to Sarkosyl in nuclear run-on assays. Nucleic Acids Res. 1992 Jan 25;20(2):303–306. doi: 10.1093/nar/20.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin B., Green M. R. An RNA processing activity that debranches RNA lariats. Science. 1985 Jul 12;229(4709):135–140. doi: 10.1126/science.2990042. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Sentenac A. RNA polymerase B (II) and general transcription factors. Annu Rev Biochem. 1990;59:711–754. doi: 10.1146/annurev.bi.59.070190.003431. [DOI] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Shea C., Lee M. G., Van der Ploeg L. H. VSG gene 118 is transcribed from a cotransposed pol I-like promoter. Cell. 1987 Aug 14;50(4):603–612. doi: 10.1016/0092-8674(87)90033-x. [DOI] [PubMed] [Google Scholar]
- Sherman D. R., Janz L., Hug M., Clayton C. Anatomy of the parp gene promoter of Trypanosoma brucei. EMBO J. 1991 Nov;10(11):3379–3386. doi: 10.1002/j.1460-2075.1991.tb04902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Levin J. R., Ingles C. J., Agabian N. In trypanosomes the homolog of the largest subunit of RNA polymerase II is encoded by two genes and has a highly unusual C-terminal domain structure. Cell. 1989 Mar 10;56(5):815–827. doi: 10.1016/0092-8674(89)90686-7. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Mougey E. B. News from the nucleolus: rRNA gene expression. Trends Biochem Sci. 1991 Feb;16(2):58–62. doi: 10.1016/0968-0004(91)90025-q. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Tower J. Transcription of cloned eukaryotic ribosomal RNA genes. Annu Rev Biochem. 1986;55:801–830. doi: 10.1146/annurev.bi.55.070186.004101. [DOI] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. E., Steinberg T. H., Aronson D. B., Burgess R. R. Inhibition of in vivo and in vitro transcription by monoclonal antibodies prepared against wheat germ RNA polymerase II that react with the heptapeptide repeat of eukaryotic RNA polymerase II. J Biol Chem. 1989 Jul 5;264(19):11511–11520. [PubMed] [Google Scholar]
- Van der Ploeg L. H. Control of antigenic variation in African trypanosomes. New Biol. 1991 Apr;3(4):324–330. [PubMed] [Google Scholar]
- Van der Ploeg L. H., Liu A. Y., Michels P. A., De Lange T., Borst P., Majumder H. K., Weber H., Veeneman G. H., Van Boom J. RNA splicing is required to make the messenger RNA for a variant surface antigen in trypanosomes. Nucleic Acids Res. 1982 Jun 25;10(12):3591–3604. doi: 10.1093/nar/10.12.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. C., Rudenko G., Borst P. Three small RNAs within the 10 kb trypanosome rRNA transcription unit are analogous to domain VII of other eukaryotic 28S rRNAs. Nucleic Acids Res. 1986 Dec 9;14(23):9471–9489. doi: 10.1093/nar/14.23.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle J. J., Sollner-Webb B. Two distant and precisely positioned domains promote transcription of Xenopus laevis rRNA genes: analysis with linker-scanning mutants. Mol Cell Biol. 1986 Dec;6(12):4585–4593. doi: 10.1128/mcb.6.12.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomerdijk J. C., Kieft R., Shiels P. G., Borst P. Alpha-amanitin-resistant transcription units in trypanosomes: a comparison of promoter sequences for a VSG gene expression site and for the ribosomal RNA genes. Nucleic Acids Res. 1991 Oct 11;19(19):5153–5158. doi: 10.1093/nar/19.19.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomerdijk J. C., Ouellette M., ten Asbroek A. L., Kieft R., Bommer A. M., Clayton C. E., Borst P. The promoter for a variant surface glycoprotein gene expression site in Trypanosoma brucei. EMBO J. 1990 Sep;9(9):2791–2801. doi: 10.1002/j.1460-2075.1990.tb07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




