Abstract
Context:
Anovulation is likely responsible for 20% of female infertility. Although clomiphene citrate remains the first-line therapy for ovulation induction in anovulatory patients who are not estrogen-deficient and to treat unexplained infertility, there remains a discrepancy between ovulation and conception rates with its use, attributed to its antiestrogenic effects on cervical mucus and the endometrium. Alternative agents, including aromatase inhibitors, have been used that have not been associated with these side effects.
Evidence Acquisition:
A literature search was conducted to specifically explore the use of aromatase inhibitors for ovulation induction and superovulation.
Evidence Synthesis:
Recent studies have found that aromatase inhibitors may be safe and useful agents for ovulation induction in patients with polycystic ovarian syndrome as well a treatment option for superovulation in patients with either unexplained infertility or endometriosis.
Conclusions:
Aromatase inhibitors may be an effective alternative treatment to clomiphene citrate for both ovulation induction and superovulation.
Anovulation is thought to be responsible for about 20% of female infertility. Clomiphene citrate (CC) is the first-line therapy for ovulation induction in patients with euestrogenic anovulation. Ovulation occurs in 60–85% of anovulatory patients treated with CC, resulting in a 10–20% pregnancy rate per cycle (1–6). There is a discrepancy between ovulation and conception rates with CC use, part of which can be attributed to its antiestrogenic effects on the cervical mucus and endometrium (2, 3, 7). Thus, alternative agents to induce ovulation have been used, including aromatase inhibitors (AIs).
AIs were originally developed for the treatment of advanced breast cancer in postmenopausal women. Because of their ability to suppress plasma estrogen concentrations, they have been increasingly used in the area of reproductive medicine; the use of AIs in gynecology, however, is “off label” (8).
This paper will review the scientific basis of using AIs in reproductive medicine and discuss the current literature on the use of AIs for ovulation induction and superovulation. The use of AIs for in vitro fertilization and fertility preservation is reviewed elsewhere (9, 10).
Pharmacology
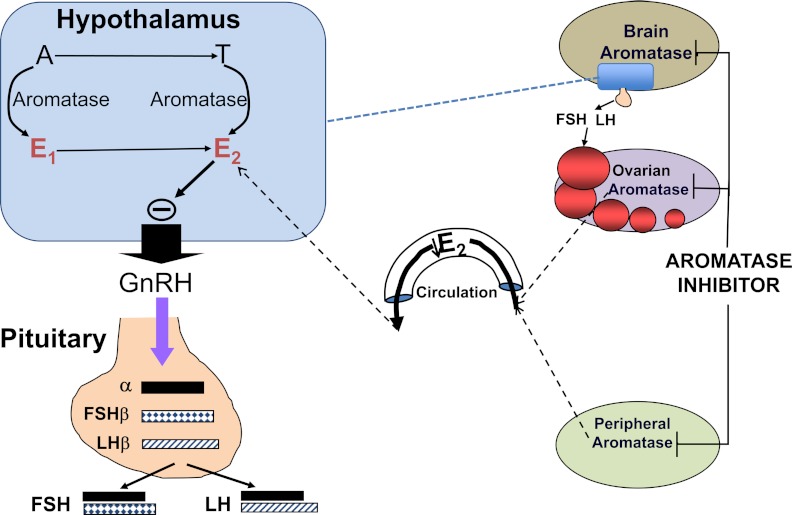
Aromatase is part of the cytochrome P450 enzyme family. It is expressed in human cells including the ovarian granulosa cell, the placental syncytiotrophoblast, the testicular Leydig cell, as well as in other extraglandular sites including adipose tissue, brain, and skin fibroblasts (11, 12). The highest levels of aromatase are found in the ovarian granulosa cells of premenopausal women, whereas adipose tissue is a major site of aromatase expression in postmenopausal women (13, 14).
Estradiol is the principal product of ovarian granulosa cells during the follicular phase of the menstrual cycle. The biologically active estradiol is produced from cholesterol through serial enzymatic actions in the ovarian theca and granulosa cells, cooperating in a paracrine manner (Figure 1) (11, 15). This process has 2 rate-limiting steps: entry of cholesterol into the mitochondria of theca cells, which is regulated by steroidogenic acute regulatory protein; and conversion of androstenedione to estrone by aromatase in granulosa cells (Figure 1). Because AIs cause a decrease in estrogen concentration, they have become useful for treating estrogen-dependent conditions including endometriosis (16).
Figure 1.
The inhibition of aromatization decreases the circulating estrogen produced from both ovarian follicles and from the peripheral conversion of androgens and also decreases the locally produced estrogen in the brain. When given early in the menstrual cycle, this would then release the hypothalamic-pituitary axis from estrogenic negative feedback resulting in an increase in gonadotropin secretion and ovarian follicular growth.
Table 1 shows the 3 generations of AIs. The first-generation inhibitor, aminoglutethimide, induces a medical adrenalectomy, which causes many side effects including lethargy, skin rashes, and nausea. The second-generation inhibitors, including fadrozole and formestane, are more selective and have fewer side effects. The route of administration for these early generation medications is im. The third generation of AIs, which include letrozole, anastrozole, and exemestane, are derivatives of triazole. These newer AIs are selective, reversible, and potent, making them better for use in clinical practice. At doses of 1–5 mg, letrozole and anastrozole have been found to inhibit estrogen levels by at least 97–99% (17–23). When given to premenopausal women, the decrease in estrogen levels causes an increase in secreted FSH from the pituitary gland. This increased FSH stimulates follicular development and is the basis for the use of AIs for ovulation induction (24). As shown in Figure 1, when given early in the menstrual cycle, the inhibition of aromatization would decrease the circulating estrogen produced from ovarian follicles and from the peripheral conversion of androgens and also decrease the locally produced estrogen in the brain. This would then release the hypothalamic-pituitary axis from estrogenic negative feedback, resulting in an increase in gonadotropin secretion and ovarian follicular growth (25).
Table 1.
Three Generations of AIs
| First Generation | Second Generation | Third Generation |
|---|---|---|
| Aminoglutethimide | Fadrozole | Letrozole |
| Formestane | Anastrozole | |
| Exemestane |
The half-life of letrozole is 45 hours. For ovulation induction, it is usually given for 5 days. The main side effects when used for ovulation induction or superovulation include mild headache and muscle or joint pains (26). Importantly, unlike CC, AIs have not been associated with poor cervical mucus or thinning of the endometrium (26).
Ovulation Induction in Women With Polycystic Ovarian Syndrome (PCOS)
The first papers on the use of the AI letrozole for ovulation induction were published over 10 years ago (27) (Table 2). Two early randomized trials in women with PCOS compared CC to letrozole (28, 29). The first showed comparable ovulation rates, live birth rates, and endometrial thickness, with a lower estradiol level in the letrozole group (28), whereas the second showed a significantly thicker endometrium, higher ovulation and pregnancy rate, with a lower number of mature follicles in the letrozole group (1). More recent studies in women with PCOS resistant to CC have shown that treatment with letrozole produced a significantly higher ovulation rate and comparable endometrial thickness when compared to both combined CC and metformin or placebo (30, 31).
Table 2.
Summary of the Recent Studies Included in This Paper
| Study Type | Sample Size | Indication | Intervention | Outcome | |
|---|---|---|---|---|---|
| Badawy, 2008 (4) | Prospective, randomized | 220 | CC-resistant PCOS | Letrozole or anastrozole | No difference in pregnancy rate or miscarriage rate with either drug |
| Bayar, 2006 (28) | Prospective | 46 | Ovulatory infertility | CC vs letrozole | Comparable effectiveness |
| Atay, 2006 (29) | Prospective, randomized | 106 | PCO, ovulation induction | CC vs letrozole | Letrozole was associated with higher pregnancy rate |
| Kamath, 2010 (30) | Prospective | 36 | CC-resistant PCOS | Letrozole vs placebo | Letrozole treatment statistically increased ovulation rate |
| Abu Hashim, 2010 (31) | RCT | 250 | CC-resistant PCOS | Letrozole vs combined metformin-CC | Regimens equally effective for inducing ovulation and achieving pregnancy |
| Abu Hashim, 2010 (32) | RCT | 260 | CC-resistant PCOS | Letrozole vs LOD with 6-mo follow-up | Letrozole and LOD are equally effective for inducing ovulation and achieving pregnancy |
| Polyzos, 2008 (34) | Meta-analysis | 4 trials | PCOS | CC vs aromatase inhibitor | Significant advantage in pregnancy and delivery rates with AI compared with CC |
| Begum, 2009 (33) | Prospective, randomized | 64 | CC-resistant PCOS | Letrozole vs higher dose CC | Letrozole had better ovulation and pregnancy rate |
| Badawy, 2009 (••••) | Prospective, randomized | 280 | Unexplained infertility | Letrozole + FSH vs CC + FSH | No advantage of letrozole |
| Samani, 2009 (37) | Prospective | 64 | Unexplained infertility | CC→2 washout cycles→letrozole | Letrozole was associated with better follicular phase parameters |
| Fouda, 2011 (38) | RCT | 214 | Unexplained infertility | Extended letrozole vs CC | Extended letrozole found to have higher pregnancy rate per cycle and cumulative pregnancy rate |
| Abu Hashim, 2012 (39) | RCT | 136 | Endometriosis, no pregnancy 6–12 mo after laparoscopy | CC vs letrozole | No advantage of letrozole in overall pregnancy rate; total estradiol and number of follicles higher in CC group |
| Badawy, 2009 (35) | RCT | 796 infertile women and 200 spontaneous pregnant women | Safety | CC vs letrozole vs spontaneous pregnancy | Safety of drugs for both mother and fetuses was documented |
Letrozole has also recently been compared to laparoscopic ovarian diathermy (LOD) for ovulation induction in women with PCOS and CC resistance (32). In this study, 260 women were randomized to either letrozole daily for 5 days for up to 6 cycles (n = 128) or with 6 months of follow-up. Ovulation occurred in 335 of 512 cycles (65.4%) in the letrozole group and 364 of 525 cycles (69.3%) in the laparoscopy group without significant difference between the groups. Resumption of regular menstruation was similar in both treatment groups. A significant increase in midcycle endometrial thickness was observed in the letrozole group (8.8 ± 1.1 vs 7.9 ± 1.2 mm) (P < .05). Pregnancy rate was similar in both groups (15.6 vs 17.5%). There were no statistically significant differences with regard to miscarriage and live birth rates between the groups. No multiple pregnancy or ovarian hyperstimulation occurred in either group. This study concluded that letrozole and LOD are equally effective for inducing ovulation and achieving pregnancy in CC-resistant PCOS patients (32).
Another recent prospective, randomized, nonblinded trial compared the effectiveness of letrozole and CC in induction of ovulation in 64 anovulatory women with PCOS who failed to ovulate when taking 100 mg/d of CC in previous cycles (33). Patients were randomly divided into 2 groups, then treated with either 7.5 mg/d letrozole or 150 mg/d CC for 5 days starting from day 3 of the menstrual cycle. Twenty (62.5%) patients from the letrozole group and 12 (37.50%) patients from the CC group ovulated during the observation period, and this difference was statistically significant. This study found that there was a significantly lower estradiol level but greater endometrial thickness in the letrozole group on the day of human chorionic gonadotropin (hCG) administration. The pregnancy rate was also higher in the letrozole group (n = 13; 40.62%) in comparison with the CC group (n = 6; 18.75%), although this was not statistically significant. No multiple pregnancies occurred in either group (33).
Letrozole has also been compared to anastrozole for ovulation induction in CC-resistant women with PCOS (4). In this prospective randomized trial, a total of 220 infertile women (574 cycles) were randomized to treatment with 2.5 mg/d of letrozole (111 patients, 295 cycles) or 1 mg/d of anastrozole (109 patients, 279 cycles) for 5 days starting on menstrual cycle day 3. Both the total number of follicles and mature follicles were significantly higher in the anastrozole group. The endometrial thickness at the time of hCG administration was also significantly higher in the anastrozole group. Ovulation occurred in 183 of 295 cycles (62%) in the letrozole group and 177 of 279 cycles (63.4%) in the anastrozole group, whereas pregnancy occurred in 36 of 295 cycles (12.2%) in the letrozole group and 42 of 279 cycles (15.1%) in the anastrozole group, and these differences were not statistically significant. This study concluded that there were no significant differences in pregnancy or miscarriage rates between anastrozole and letrozole when used for ovulation induction in women with CC-resistant PCOS (4).
A recent meta-analysis of 4 trials showed a significant advantage for both pregnancy and delivery rates when AIs were compared to CC in women with PCOS (34). The odds ratio for pregnancies per patient was 2.0 (95% confidence interval, 1.1–3.8) (34). Further randomized trials comparing letrozole and CC as first-line treatment for ovulation induction in women with PCOS are needed.
AI and Unexplained Infertility
Several studies have compared CC to AIs for superovulation in unexplained infertility (8, 35–38) (Table 2). In one trial by Badawy et al (36), 412 women with unexplained infertility were randomized to treatment with 100 mg/d of CC (207 patients, 404 cycles) or 5 mg/d of letrozole (205 patients, 400 cycles) for 5 days starting on menstrual cycle day 3. The total number of follicles during stimulation and the serum estradiol and progesterone concentrations were statistically significantly higher in the CC group. There was no statistically significant difference in endometrial thickness at the time of hCG administration. Pregnancy occurred in 73 of 205 patients (400 cycles) in the letrozole group (35.6 and 18.2%, respectively) and 78 of 207 patients (404 cycles) (37.6 and 19.3%, respectively) in the CC group. These differences were not statistically significant. This study found no superiority between letrozole and CC for inducing ovulation in women with unexplained infertility before intrauterine insemination (IUI) (5).
Badawy et al conducted another prospective, randomized controlled trial (RCT) comprised of 796 infertile women completing 1100 cycles (35). Women were allocated to treatment with 100 mg/d of CC (420 patients, 634 cycles), or 5 mg/d of letrozole (269 patients, 323 cycles), or 1 mg/d of anastrozole (107 patients, 143 cycles) for 5 days starting on menstrual cycle day 3. Pregnancy occurred in 167 of 1398 cycles (11.9%) in total without significant differences between groups. This study concluded that AIs and CC resulted in favorable pregnancy outcomes and average miscarriage rates (8).
Another recent study by Samani et al (37) evaluated and compared follicular phase parameters during ovarian stimulation using CC or letrozole in couples with unexplained infertility who failed to achieve pregnancy with CC. A total of 64 unexplained infertile women who failed to get pregnant after CC-treated cycles were studied for 1 CC-treated cycle (100 mg/d), 2 washout cycles, and then 1 letrozole-treated cycle (5 mg/d). Letrozole-treated cycles were found to have a significantly greater number of follicles > 14 mm, a larger dominant follicle, thicker endometrium, and higher LH levels. The mean level of estradiol was significantly lower during the letrozole-treated cycle. This study concluded that treatment with letrozole resulted in overall better follicular phase parameters and endometrial development in women with unexplained infertility who failed to achieve pregnancy with CC (37).
Fouda and Sayed (38) published a recent RCT comparing the efficacy of extended letrozole regimen with CC in 214 women with unexplained infertility undergoing superovulation and IUI. Women were randomized to either letrozole 2.5 mg/d from cycle days 1 to 9 (211 cycles) or CC 100 mg/d from cycle days 3 to 7 (210 cycles). Both groups were comparable with regard to the number of mature follicles and the day of hCG administration. Serum estradiol was significantly greater in the CC group, and the endometrial thickness was significantly greater in the extended letrozole group. Both the pregnancy rate per cycle (18.96 vs 11.43%) and the cumulative pregnancy rate were significantly greater in the extended letrozole group (37.73 vs 22.86%). This study concluded that the extended letrozole regimen had a superior efficacy compared to CC in patients with unexplained infertility undergoing superovulation and IUI (38).
Taken together, these recent studies suggest that overall pregnancy rates are similar for women with unexplained infertility being treated with either CC or AIs. There may be a benefit to the use of an extended letrozole regimen. Further randomized trials are needed to assess the efficacy of AIs for superovulation for the treatment of unexplained infertility.
AI and Endometriosis
Many studies have found that the combination of AI with conventional therapy can be used to alleviate endometriosis-related pain. However, few studies have been done using AI to treat endometriosis-related infertility (Table 2). One recent study by Abu Hashim et al (39) evaluated pregnancy rates with IUI combined with either letrozole or CC for superovulation in women who had recently undergone surgical treatment for minimal to mild endometriosis. A total of 136 women with primary infertility because of minimal to mild endometriosis who did not achieve pregnancy 6 to 12 months after laparoscopic treatment were randomized to 5 mg/d letrozole (69 women, 220 cycles) or 100 mg/d CC (67 women, 213 cycles) for 5 days, combined with IUI for up to 4 cycles. The clinical pregnancy rate per cycle and the cumulative pregnancy rate after 4 cycles were comparable in both groups. Two twin pregnancies occurred in the CC/IUI group. Miscarriage and live birth rates were also comparable between both groups. The total number of follicles and serum estradiol on the day of hCG administration were significantly increased in the CC group. This study concluded that superovulation with letrozole was equally as effective as CC for women with minimal to mild endometriosis who did not achieve pregnancy 6 to 12 months after laparoscopic treatment. Interestingly, pregnancy rates were similar between the groups, although follicular development was greater in the CC group (39). Molecular studies have found that eutopic endometrium of women with endometriosis as well as ectopic endometriotic lesions express aromatase (15). Perhaps this inhibition of aromatase expression improved pregnancy potential in patients with endometriosis, explaining why there were equal pregnancy rates in both groups, despite a decrease in follicular development.
Safety
Safety is a major concern in the use of AIs for ovulation induction or superovulation. There was a study presented and published only in abstract form that suggested an increase in congenital cardiac and bone malformations in pregnancies achieved using letrozole (40). This report was never fully published in a peer-reviewed journal, likely because of issues relating to the control group. Specifically, the control group used was not comparable to the treated group in terms of the patients' age and background risk of congenital malformations. Other studies failed to find an increased teratogenicity in pregnancies conceived using AIs (Table 2). A large multicenter retrospective study by Tulandi et al (41) found a significantly higher rate of congenital cardiac anomalies in the CC group compared to the AI-treated group. A recent randomized trial by Badawy et al (35) to assess the outcome of pregnancies after treatment with AIs also failed to detect a difference in malformation rate after treatment with either letrozole or anastrozole when compared with CC. More studies are needed to assess the safety of using AIs for either ovulation induction or superovulation.
Early studies suggested a lower multiple pregnancy rate, with more cycles resulting in monofollicular growth in women treated with letrozole 2.5 or 5 mg/d compared to CC (42, 43). However, a more recent randomized trial has questioned this finding, showing a comparable twin pregnancy rate between CC and letrozole (8.3 vs 9.1%) in women with unexplained infertility (35). In addition, a case report has been published describing the first reported case of a triplet pregnancy after using letrozole for ovulation induction in a woman with PCOS and primary infertility (44). Taken together, these studies suggest that the use of an AI does not eliminate the risk of either twins or higher order multiples, although it is associated with a higher rate of monofollicular growth.
Summary
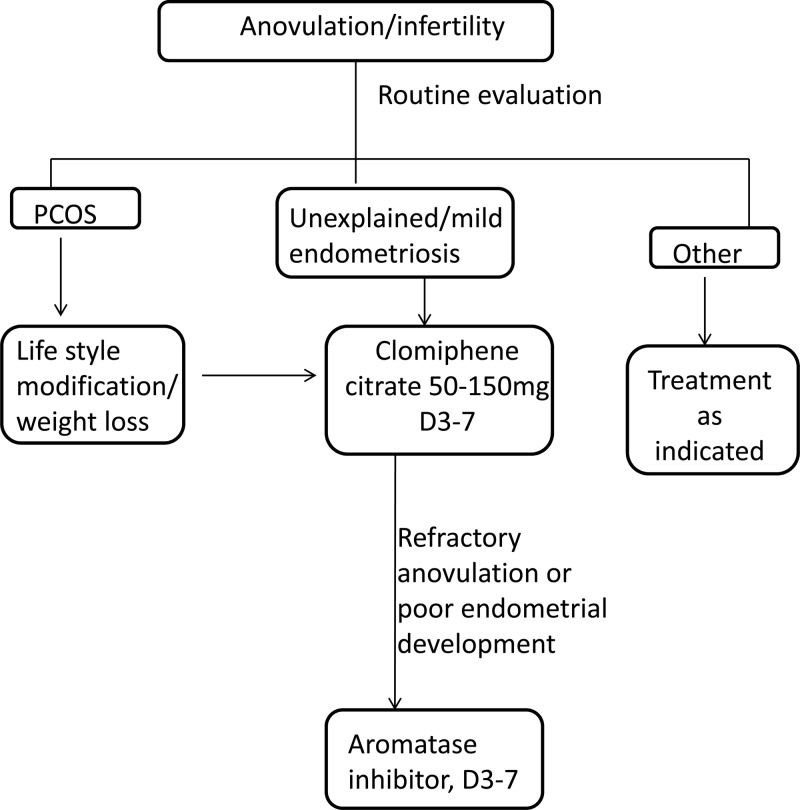
This review has focused on the use of AIs for ovulation induction and superovulation (Table 2). A treatment algorithm incorporating the use of AIs is shown in Figure 2. Recent studies have found that AIs may be useful agents for ovulation induction in women with PCOS as well a treatment option for superovulation in women with either unexplained infertility or endometriosis. Further studies to determine optimal dosage, duration of use, and safety data are needed. In summary, AIs may be an effective alternative treatment to CC for both ovulation induction and superovulation.
Figure 2.
Treatment algorithm for management of PCOS or unexplained infertility incorporating the use of AIs.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant K12HD050121, American Society for Reproductive Medicine career development award (to M.E.P.), and NIH Grant R37HD038691 (to S.E.B.).
Disclosure Summary: The authors report no conflicts of interest.
Footnotes
- AI
- aromatase inhibitor
- CC
- clomiphene citrate
- hCG
- human chorionic gonadotropin
- IUI
- intrauterine insemination
- LOD
- laparoscopic ovarian diathermy
- PCOS
- polycystic ovarian syndrome
- RCT
- randomized controlled trial.
References
- 1. Guzick D. Polycystic ovary syndrome: symptomatology, pathophysiology, and epidemiology. Am J Obstet Gynecol. 1998;179:S89–S93 [DOI] [PubMed] [Google Scholar]
- 2. Franks S, Adams J, Mason H, Polson D. Ovulatory disorders in women with polycystic ovary syndrome. Clin Obstet Gynaecol. 1985;12:605–632 [PubMed] [Google Scholar]
- 3. Kistner RW. Induction of ovulation with clomiphene citrate (clomid). Obstet Gynecol Surv. 1965;20:873–900 [DOI] [PubMed] [Google Scholar]
- 4. Badawy A, Mosbah A, Shady M. Anastrozole or letrozole for ovulation induction in clomiphene-resistant women with polycystic ovarian syndrome: a prospective randomized trial. Fertil Steril. 2008;89:1209–1212 [DOI] [PubMed] [Google Scholar]
- 5. Badawy A, State O, Abdelgawad S. N-Acetyl cysteine and clomiphene citrate for induction of ovulation in polycystic ovary syndrome: a cross-over trial. Acta Obstet Gynecol Scand. 2007;86:218–222 [DOI] [PubMed] [Google Scholar]
- 6. Badawy A, Baker El Nashar A, El Totongy M. Clomiphene citrate plus N-acetyl cysteine versus clomiphene citrate for augmenting ovulation in the management of unexplained infertility: a randomized double-blind controlled trial. Fertil Steril. 2006;86:647–650 [DOI] [PubMed] [Google Scholar]
- 7. Mitwally MF, Casper RF. Potential of aromatase inhibitors for ovulation and superovulation induction in infertile women. Drugs. 2006;66:2149–2160 [DOI] [PubMed] [Google Scholar]
- 8. Lee VC, Ledger W. Aromatase inhibitors for ovulation induction and ovarian stimulation. Clin Endocrinol (Oxf). 2011;74:537–546 [DOI] [PubMed] [Google Scholar]
- 9. Reddy J, Oktay K. Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertil Steril. 2012;98:1363–1369 [DOI] [PubMed] [Google Scholar]
- 10. Garcia-Velasco JA. The use of aromatase inhibitors in in vitro fertilization. Fertil Steril. 2012;98:1356–1358 [DOI] [PubMed] [Google Scholar]
- 11. Attar E, Bulun SE. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update. 2006;12:49–56 [DOI] [PubMed] [Google Scholar]
- 12. Simpson ER, Mahendroo MS, Nichols JE, Bulun SE. Aromatase gene expression in adipose tissue: relationship to breast cancer. Int J Fertil Menopausal Stud. 1994;39(suppl 2):75–83 [PubMed] [Google Scholar]
- 13. Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973;36:207–214 [DOI] [PubMed] [Google Scholar]
- 14. Bulun SE, Mahendroo MS, Simpson ER. Aromatase gene expression in adipose tissue: relationship to breast cancer. J Steroid Biochem Mol Biol. 1994;49:319–326 [DOI] [PubMed] [Google Scholar]
- 15. Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279 [DOI] [PubMed] [Google Scholar]
- 16. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis. Fertil Steril. 2012;98:1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polyzos NP, Fatemi HM, Zavos A, et al. Aromatase inhibitors in post-menopausal endometriosis. Reprod Biol Endocrinol. 2011;9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bajetta E, Zilembo N, Bichisao E, et al. Tumor response and estrogen suppression in breast cancer patients treated with aromatase inhibitors. Ann Oncol. 2000;11:1017–1022 [DOI] [PubMed] [Google Scholar]
- 19. Geisler J, King N, Anker G, et al. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res. 1998;4:2089–2093 [PubMed] [Google Scholar]
- 20. Geisler J, King N, Dowsett M, et al. Influence of anastrozole (Arimidex), a selective, non-steroidal aromatase inhibitor, on in vivo aromatisation and plasma oestrogen levels in postmenopausal women with breast cancer. Br J Cancer. 1996;74:1286–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plourde PV, Dyroff M, Dowsett M, Demers L, Yates R, Webster A. ARIMIDEX: a new oral, once-a-day aromatase inhibitor. J Steroid Biochem Mol Biol. 1995;53:175–179 [DOI] [PubMed] [Google Scholar]
- 22. Santen RJ, Manni A, Harvey H, Redmond C. Endocrine treatment of breast cancer in women. Endocr Rev. 1990;11:221–265 [DOI] [PubMed] [Google Scholar]
- 23. Dowsett M, Jones A, Johnston SR, Jacobs S, Trunet P, Smith IE. In vivo measurement of aromatase inhibition by letrozole (CGS 20267) in postmenopausal patients with breast cancer. Clin Cancer Res. 1995;1:1511–1515 [PubMed] [Google Scholar]
- 24. Ferrero S, Venturini PL, Ragni N, Camerini G, Remorgida V. Pharmacological treatment of endometriosis: experience with aromatase inhibitors. Drugs. 2009;69:943–952 [DOI] [PubMed] [Google Scholar]
- 25. Casper RF, Mitwally MF. Use of the aromatase inhibitor letrozole for ovulation induction in women with polycystic ovarian syndrome. Clin Obstet Gynecol. 2011;54:685–695 [DOI] [PubMed] [Google Scholar]
- 26. Casper RF, Mitwally MF. A historical perspective of aromatase inhibitors for ovulation induction. Fertil Steril. 2012;98:1352–1355 [DOI] [PubMed] [Google Scholar]
- 27. Mitwally MF, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305–309 [DOI] [PubMed] [Google Scholar]
- 28. Bayar U, Tanriverdi HA, Barut A, Ayoglu F, Ozcan O, Kaya E. Letrozole vs. clomiphene citrate in patients with ovulatory infertility. Fertil Steril. 2006;85:1045–1048 [DOI] [PubMed] [Google Scholar]
- 29. Atay V, Cam C, Muhcu M, Cam M, Karateke A. Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. J Int Med Res. 2006;34:73–76 [DOI] [PubMed] [Google Scholar]
- 30. Kamath MS, Aleyamma TK, Chandy A, George K. Aromatase inhibitors in women with clomiphene citrate resistance: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2010;94:2857–2859 [DOI] [PubMed] [Google Scholar]
- 31. Abu Hashim H, Shokeir T, Badawy A. Letrozole versus combined metformin and clomiphene citrate for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: a randomized controlled trial. Fertil Steril. 2010;94:1405–1409 [DOI] [PubMed] [Google Scholar]
- 32. Abu Hashim H, Mashaly AM, Badawy A. Letrozole versus laparoscopic ovarian diathermy for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: a randomized controlled trial. Arch Gynecol Obstet. 2010;282:567–571 [DOI] [PubMed] [Google Scholar]
- 33. Begum MR, Ferdous J, Begum A, Quadir E. Comparison of efficacy of aromatase inhibitor and clomiphene citrate in induction of ovulation in polycystic ovarian syndrome. Fertil Steril. 2009;92:853–857 [DOI] [PubMed] [Google Scholar]
- 34. Polyzos NP, Tsappi M, Mauri D, Atay V, Cortinovis I, Casazza G. Aromatase inhibitors for infertility in polycystic ovary syndrome. The beginning or the end of a new era? Fertil Steril. 2008;89:278–280 [DOI] [PubMed] [Google Scholar]
- 35. Badawy A, Shokeir T, Allam AF, Abdelhady H. Pregnancy outcome after ovulation induction with aromatase inhibitors or clomiphene citrate in unexplained infertility. Acta Obstet Gynecol Scand. 2009;88:187–191 [DOI] [PubMed] [Google Scholar]
- 36. Badawy A, Elnashar A, Totongy M. Clomiphene citrate or aromatase inhibitors for superovulation in women with unexplained infertility undergoing intrauterine insemination: a prospective randomized trial. Fertil Steril. 2009;92:1355–1359 [DOI] [PubMed] [Google Scholar]
- 37. Samani FG, Farzadi L, Nezami N, Tarzamni MK, Soleimani F. Endometrial and follicular development following letrozole intervention in unexplained infertile patients failed to get pregnant with clomiphene citrate. Arch Gynecol Obstet. 2009;280:201–205 [DOI] [PubMed] [Google Scholar]
- 38. Fouda UM, Sayed AM. Extended letrozole regimen versus clomiphene citrate for superovulation in patients with unexplained infertility undergoing intrauterine insemination: a randomized controlled trial. Reprod Biol Endocrinol. 2011;9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abu Hashim H, El Rakhawy M, Abd Elaal I. Randomized comparison of superovulation with letrozole vs. clomiphene citrate in an IUI program for women with recently surgically treated minimal to mild endometriosis. Acta Obstet Gynecol Scand. 2012;91:338–345 [DOI] [PubMed] [Google Scholar]
- 40. Biljan M, Hemming R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole with gonadotropins. Fertil Steril. 2005;84(suppl 1):S95 [Google Scholar]
- 41. Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. 2006;85:1761–1765 [DOI] [PubMed] [Google Scholar]
- 42. Casper RF, Mitwally MF. Review: aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab. 2006;91:760–771 [DOI] [PubMed] [Google Scholar]
- 43. Mitwally MF, Biljan MM, Casper RF. Pregnancy outcome after the use of an aromatase inhibitor for ovarian stimulation. Am J Obstet Gynecol. 2005;192:381–386 [DOI] [PubMed] [Google Scholar]
- 44. Dicken CL, Nakhuda GS, Guarnaccia MM, Sauer MV, Lobo RA. Triplet pregnancy after ovulation induction with an aromatase inhibitor. Fertil Steril. 2008;90:1199.e9–e11 [DOI] [PubMed] [Google Scholar]